Changes in the Endophytic Bacterial Community of Brassica rapa after Application of Systemic Insecticides
Abstract
:1. Introduction
2. Results
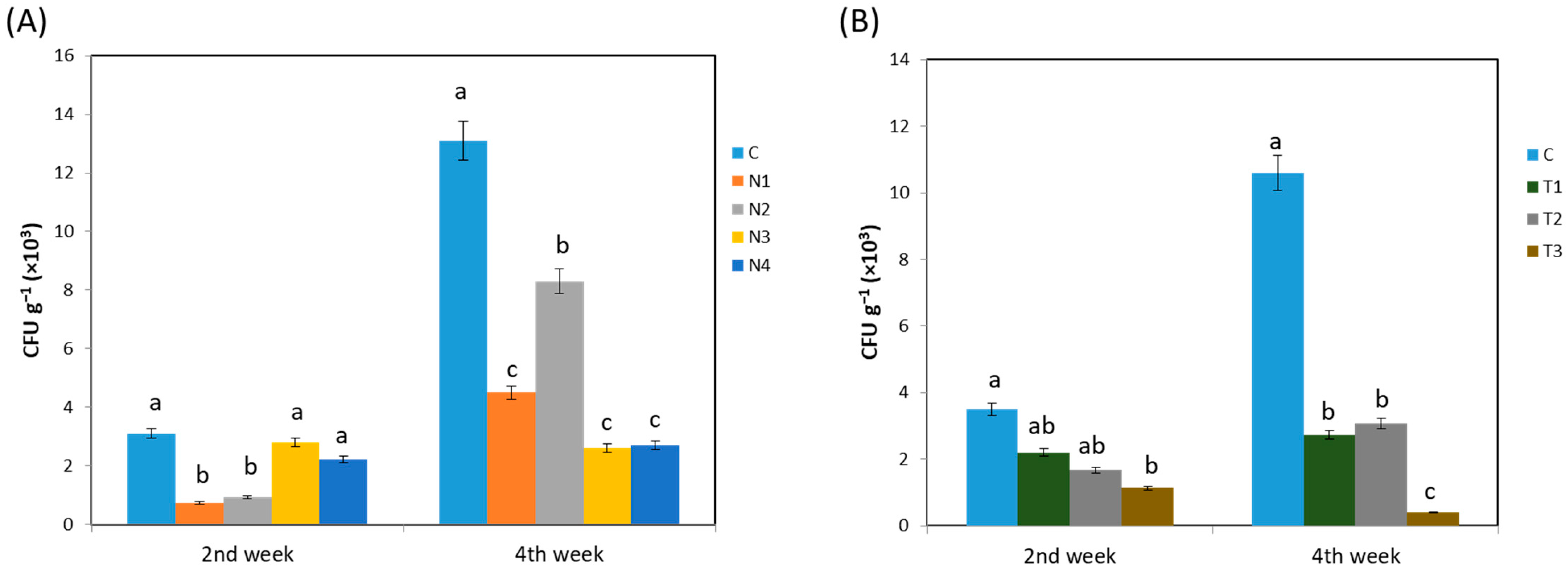
2.1. Changes in Bacterial Population and Diversity Caused by Insecticide Application
2.2. Impacts of Insecticides on Endophytic Bacterial Abundance and Richness
2.3. Taxonomic Changes after Insecticide Application
2.4. Multivariate Analysis to Assess the Impact of Insecticides on Endophytic Bacterial Community
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Plant Sample Preparation for Endophytic Bacteria
4.3. Isolation of Culturable Endophytic Bacteria
4.4. DNA Extraction and PCR Amplification
4.5. PCR-Restriction Fragment Length Polymorphism (RFLP) Determination
4.6. The 16S rRNA Gene Sequencing of Culturable Bacteria
4.7. Identification of Non-Culturable Endophytic Bacteria
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef]
- Wiewióra, B.; Zurek, G.; Zurek, M. Endophyte-mediated disease resistance in wild populations of perennial Ryegrass (Lolium perenne). Fungal Ecol. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Sessa, L.; Abreo, E.; Lupo, S. Diversity of fungal latent pathogens and true endophytes associated with fruit trees in Uruguay. J. Phytopathol. 2018, 166, 633–647. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- FAO (Food and Agricultural Organization of United Nations). Climate Change Fans Spread of Pests and Threatens Plants and Crops, New FAO Study. Available online: https://www.fao.org/news/story/en/item/1402920/icode/ (accessed on 28 September 2023).
- Pietrzak, D.; Kania, J.; Kmiecik, E.; Malina, G.; Wątor, K. Fate of selected neonicotinoid insecticides in soil–water systems: Current state of the art and knowledge gaps. Chemosphere 2020, 255, 126981. [Google Scholar] [CrossRef]
- Schaafsma, A.; Limay-Rios, V.; Baute, T.; Smith, J.; Xue, Y. Neonicotinoid insecticide residues in surface water and soil associated with commercial maize (corn) fields in southwestern Ontario. PLoS ONE 2015, 10, e0118139. [Google Scholar] [CrossRef] [PubMed]
- Mulla, S.I.; Ameen, F.; Talwar, M.P.; Eqani, S.A.M.A.S.; Bharagava, R.N.; Saxena, G.; Tallur, P.N.; Ninnekar, H.Z. Organophosphate pesticides: Impact on environment, toxicity, and their degradation. In Bioremediation of Industrial Waste for Environmental Safety; Springer: Berlin/Heidelberg, Germany, 2020; pp. 265–290. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. Int. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Meher, H.C.; Gajbhiye, V.T.; Singh, G.; Kamra, A.; Chawla, G. Persistence and nematicidal efficacy of carbosulfan, cadusafos, phorate, and triazophos in soil and uptake by chickpea and tomato crops under tropical conditions. J. Agric. Food Chem. 2010, 58, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. Int. 2015, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Acephate—Wikipedia. Available online: https://en.wikipedia.org/wiki/Acephate (accessed on 12 October 2023).
- Sipler, D.; Czyzewski, M.A. Impacts of Neonicotinoids on Non-Target Species and Ecosystems; New Jersey Audubon: Bernardsville, NJ, USA, 2018; pp. 1–14. Available online: https://njaudubon.org/wp-content/uploads/2019/02/NJA-Neonic-Impacts-to-Non-Target-Species-and-Ecosystems_Fall-2018.pdf (accessed on 28 June 2023).
- Da Costa Stuart, A.K.; Stuart, R.M.; Pimentel, I.C. Effect of agrochemicals on endophytic fungi community associated with crops of organic and conventional soybean (Glycine Max L. Merril). Agric. Nat. Resour. 2018, 52, 388–392. [Google Scholar] [CrossRef]
- Marcos, F.C.C.; Iório, R.d.P.F.; da Silveira, A.P.D.; Ribeiro, R.V.; Machado, E.C.; Lagôa, A.M.M.d.A. Endophytic bacteria affect sugarcane physiology without changing plant growth. Bragantia 2015, 75, 1–9. [Google Scholar] [CrossRef]
- Johnsen, K.; Jacobsen, C.S.; Torsvik, V.; Sørensen, J. Pesticide effects on bacterial diversity in agricultural soils—A Review. Biol. Fertil. Soils 2001, 33, 443–453. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of agrochemicals on soil microbiota and management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Onwona-Kwakye, M.; Plants-Paris, K.; Keita, K.; Lee, J.; Brink, P.J.V.d.; Hogarh, J.N.; Darkoh, C. Pesticides decrease bacterial diversity and abundance of irrigated rice fields. Microorganisms 2020, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Brühl, C.A.; Zaller, J.G. Biodiversity Decline as a Consequence of an inappropriate environmental risk assessment of pesticides. Front. Environ. Sci. 2019, 7, 464007. [Google Scholar] [CrossRef]
- Gunstone, T.; Cornelisse, T.; Klein, K.; Dubey, A.; Donley, N. Pesticides and soil invertebrates: A hazard assessment. Front. Environ. Sci. 2021, 9, 643847. [Google Scholar] [CrossRef]
- Prior, R.; Mittelbach, M.; Begerow, D. Impact of three different fungicides on fungal epi- and endophytic communities of common bean (Phaseolus vulgaris) and broad bean (Vicia faba). J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2017, 52, 376–386. [Google Scholar] [CrossRef]
- Muturi, E.J.; Donthu, R.K.; Fields, C.J.; Moise, I.K.; Kim, C.H. Effect of pesticides on microbial communities in container aquatic habitats. Sci. Rep. 2017, 7, 44565. [Google Scholar] [CrossRef]
- Campisano, A.; Antonielli, L.; Pancher, M.; Yousaf, S.; Pindo, M.; Pertot, I. Bacterial endophytic communities in the grapevine depend on pest management. PLoS ONE 2014, 9, e112763. [Google Scholar] [CrossRef]
- Win, P.M.; Matsumura, E.; Fukuda, K. Effects of pesticides on the diversity of endophytic fungi in tea plants. Microb. Ecol. 2021, 82, 62–72. [Google Scholar] [CrossRef]
- Moulas, C.; Petsoulas, C.; Rousidou, K.; Perruchon, C.; Karas, P.; Karpouzas, D.G. Effects of systemic pesticides imidacloprid and metalaxyl on the phyllosphere of pepper plants. Biomed Res. Int. 2013, 2013, 969750. [Google Scholar] [CrossRef] [PubMed]
- Ricupero, M.; Desneux, N.; Zappalà, L.; Biondi, A. Target and non-target impact of systemic insecticides on a polyphagous aphid pest and its parasitoid. Chemosphere 2020, 247, 125728. [Google Scholar] [CrossRef]
- Ju, F.; Zhang, T. Bacterial assembly and temporal dynamics in activated sludge of a full-scale municipal wastewater treatment plant. ISME J. 2014, 9, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Takagi, K.; Ito, K.; Kataoka, R. Changes in endophytic bacterial communities during different growth stages of Cucumber (Cucumis sativus L.). World J. Microbiol. Biotechnol. 2019, 35, 104. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 2020, 11, 610065. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, T.; Qian, H. Pesticide interference and additional effects on plant microbiomes. Sci. Total Environ. 2023, 888, 164149. [Google Scholar] [CrossRef]
- Li, Y.; Long, L.; Yan, H.; Ge, J.; Cheng, J.; Ren, L.; Yu, X. Comparison of uptake, translocation and accumulation of several neonicotinoids in komatsuna (Brassica rapa Var. perviridis) from Contaminated Soils. Chemosphere 2018, 200, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, L.; Yan, H.; Zhang, M.; Ge, J.; Yu, X. Uptake, translocation and accumulation of imidacloprid in six leafy vegetables at three growth stages. Ecotoxicol. Environ. Saf. 2018, 164, 690–695. [Google Scholar] [CrossRef]
- Fairbrother, A.; Purdy, J.; Anderson, T.; Fell, R. Risks of neonicotinoid insecticides to honeybees. Environ. Toxicol. Chem. 2014, 33, 719–731. [Google Scholar] [CrossRef]
- Yu, B.; Chen, Z.; Lu, X.; Huang, Y.; Zhou, Y.; Zhang, Q.; Wang, D.; Li, J. Effects on soil microbial community after exposure to neonicotinoid insecticides thiamethoxam and dinotefuran. Sci. Total Environ. 2020, 725, 138328. [Google Scholar] [CrossRef]
- Zaller, J.G.; Brühl, C.A. Editorial: Non-target effects of pesticides on organisms inhabiting agroecosystems. Front. Environ. Sci. 2019, 7, 449950. [Google Scholar] [CrossRef]
- Expósito, R.G.; Postma, J.; Raaijmakers, J.M.; De Bruijn, I. Diversity and activity of lysobacter species from disease suppressive soils. Front. Microbiol. 2015, 6, 166241. [Google Scholar] [CrossRef]
- Nozari, R.M.; Ortolan, F.; Astarita, L.V.; Santarém, E.R. Streptomyces Spp. enhance vegetative growth of maize plants under saline stress. Braz. J. Microbiol. 2021, 52, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.; Zhou, H.; Li, X.; Tan, Z. Adaptation mechanisms of Rhodococcus Sp. CNS16 under different temperature gradients: Physiological and transcriptome. Chemosphere 2020, 238, 124571. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Chaudhary, H.J.; Damalas, C.A. Microbial detoxification of dimethoate through mediated hydrolysis by Brucella Sp. PS4: Molecular profiling and plant growth-promoting traits. Environ. Sci. Pollut. Res. Int. 2022, 29, 2420–2431. [Google Scholar] [CrossRef]
- Pandey, G.; Dorrian, S.J.; Russell, R.J.; Oakeshott, J.G. Biotransformation of the neonicotinoid insecticides imidacloprid and thiamethoxam by Pseudomonas Sp. 1G. Biochem. Biophys. Res. Commun. 2009, 380, 710–714. [Google Scholar] [CrossRef]
- Yao, X.; Min, H. Isolation, Characterization and phylogenetic analysis of a bacterial strain capable of degrading Acetamiprid. J. Environ. Sci. 2006, 18, 141–146. [Google Scholar]
- Rana, S.; Jindal, V.; Mandal, K.; Kaur, G.; Gupta, V.K. Thiamethoxam degradation by Pseudomonas and Bacillus Strains isolated from agricultural soils. Environ. Monit. Assess. 2015, 187, 300. [Google Scholar] [CrossRef]
- Parte, S.G.; Kharat, A.S. Aerobic degradation of clothianidin to 2-chloro-methyl thiazole and methyl 3-(thiazole-yl) methyl guanidine produced by Pseudomonas stutzeri Smk. J. Environ. Public Health 2019, 2019, 4807913. [Google Scholar] [CrossRef]
- Phugare, S.S.; Jadhav, J.P. Biodegradation of acetamiprid by isolated bacterial strain Rhodococcus Sp. BCH2 and toxicological analysis of its metabolites in silkworm (Bombax mori). CLEAN Soil Air Water 2015, 43, 296–304. [Google Scholar] [CrossRef]
- Russell, R.J.; Scott, C.; Jackson, C.J.; Pandey, R.; Pandey, G.; Taylor, M.C.; Coppin, C.W.; Liu, J.W.; Oakeshott, J.G. The evolution of new enzyme function: Lessons from xenobiotic metabolizing bacteria versus insecticide-resistant insects. Evol. Appl. 2011, 4, 225. [Google Scholar] [CrossRef] [PubMed]
- Amano, Y. Classification of cultivated soils in japan. Jap. Agri. Res. Quar. 1985, 18, 275–283. [Google Scholar]
- Komatsuna—Wikipedia. Available online: https://en.wikipedia.org/wiki/Komatsuna (accessed on 12 October 2023).
- Acikgoz, F.E.; Adiloglu, A.; Daglioglu, F.; Adiloglu, S.; Celikyurt, G.; Karakas, O. The effect of increasing doses of nitrogen (n) application for some nutrient elements, vitamin C and protein contents of Komatsuna (Brassica rapa Var. perviridis) Plant. Bulg. J. Agric. Sci. 2014, 20, 321–324. [Google Scholar]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the illumina hiseq and miseq platforms. ISME J. Adv. Online Publ. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590. [Google Scholar] [CrossRef]





| (A) | ||||
|---|---|---|---|---|
| Treatments | 2nd Week | 4th Week | ||
| Culturable | Non-Culturable | Culturable | Non-Culturable | |
| C | 9 | 41 | 14 | 217 |
| N1 | 5 | 16 | 7 | 21 |
| N2 | 7 | 19 | 11 | 32 |
| N3 | 6 | 41 | 4 | 169 |
| N4 | 7 | 31 | 6 | 42 |
| (B) | ||||
| Treatments | 2nd Week | 4th Week | ||
| Culturable | Non-Culturable | Culturable | Non-Culturable | |
| C | 9 | 50 | 15 | 144 |
| T1 | 7 | 31 | 6 | 42 |
| T2 | 6 | 19 | 6 | 15 |
| T3 | 5 | 16 | 4 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salam, M.T.B.; Kataoka, R. Changes in the Endophytic Bacterial Community of Brassica rapa after Application of Systemic Insecticides. Int. J. Mol. Sci. 2023, 24, 15306. https://doi.org/10.3390/ijms242015306
Salam MTB, Kataoka R. Changes in the Endophytic Bacterial Community of Brassica rapa after Application of Systemic Insecticides. International Journal of Molecular Sciences. 2023; 24(20):15306. https://doi.org/10.3390/ijms242015306
Chicago/Turabian StyleSalam, Md. Tareq Bin, and Ryota Kataoka. 2023. "Changes in the Endophytic Bacterial Community of Brassica rapa after Application of Systemic Insecticides" International Journal of Molecular Sciences 24, no. 20: 15306. https://doi.org/10.3390/ijms242015306
APA StyleSalam, M. T. B., & Kataoka, R. (2023). Changes in the Endophytic Bacterial Community of Brassica rapa after Application of Systemic Insecticides. International Journal of Molecular Sciences, 24(20), 15306. https://doi.org/10.3390/ijms242015306






