Structural Characterization of the Metalized Radical Cations of Adenosine ([Ade+Li-H]•+ and [Ade+Na-H]•+) by Infrared Multiphoton Dissociation Spectroscopy and Theoretical Studies
Abstract
:1. Introduction
2. Results
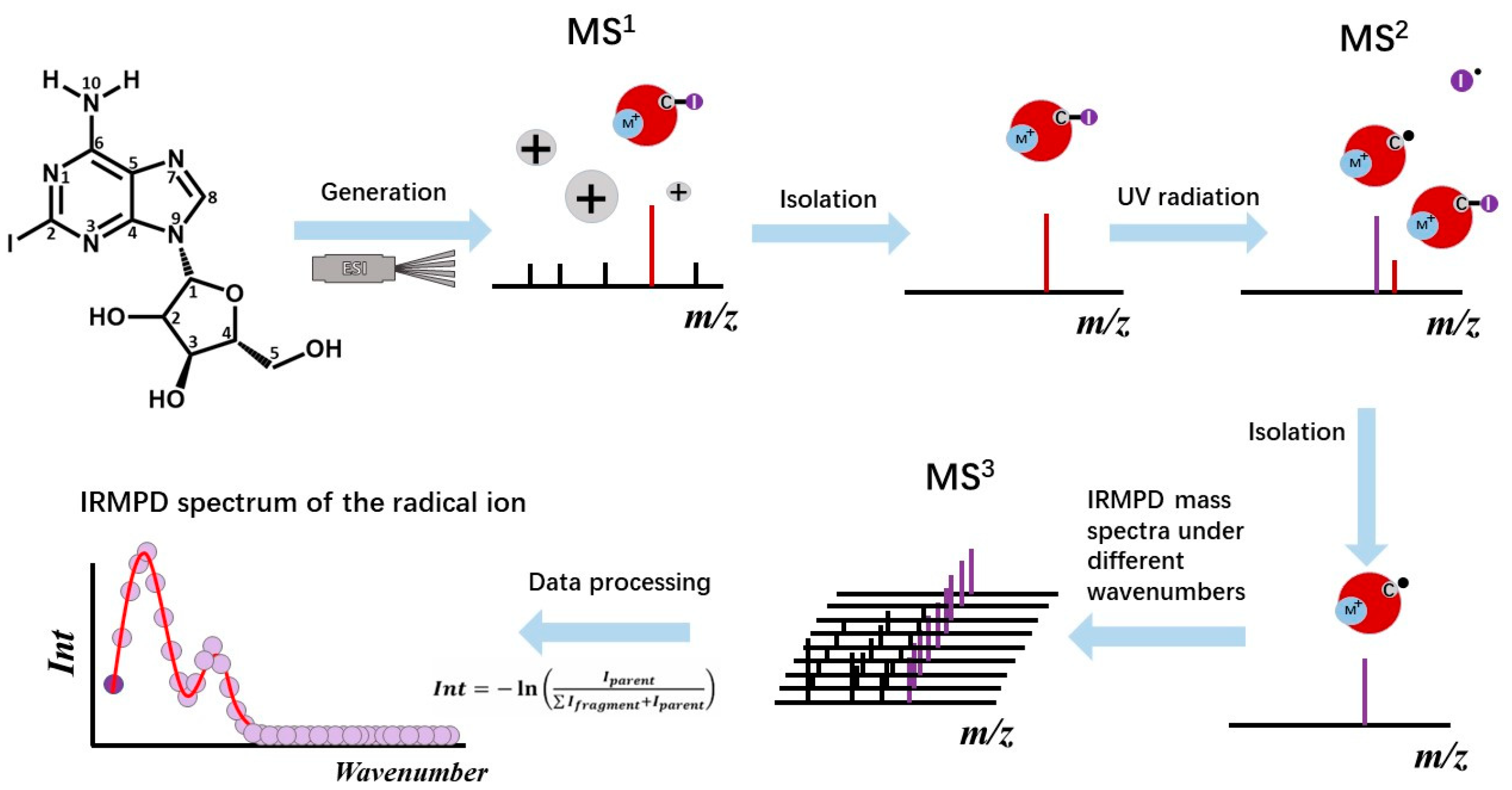
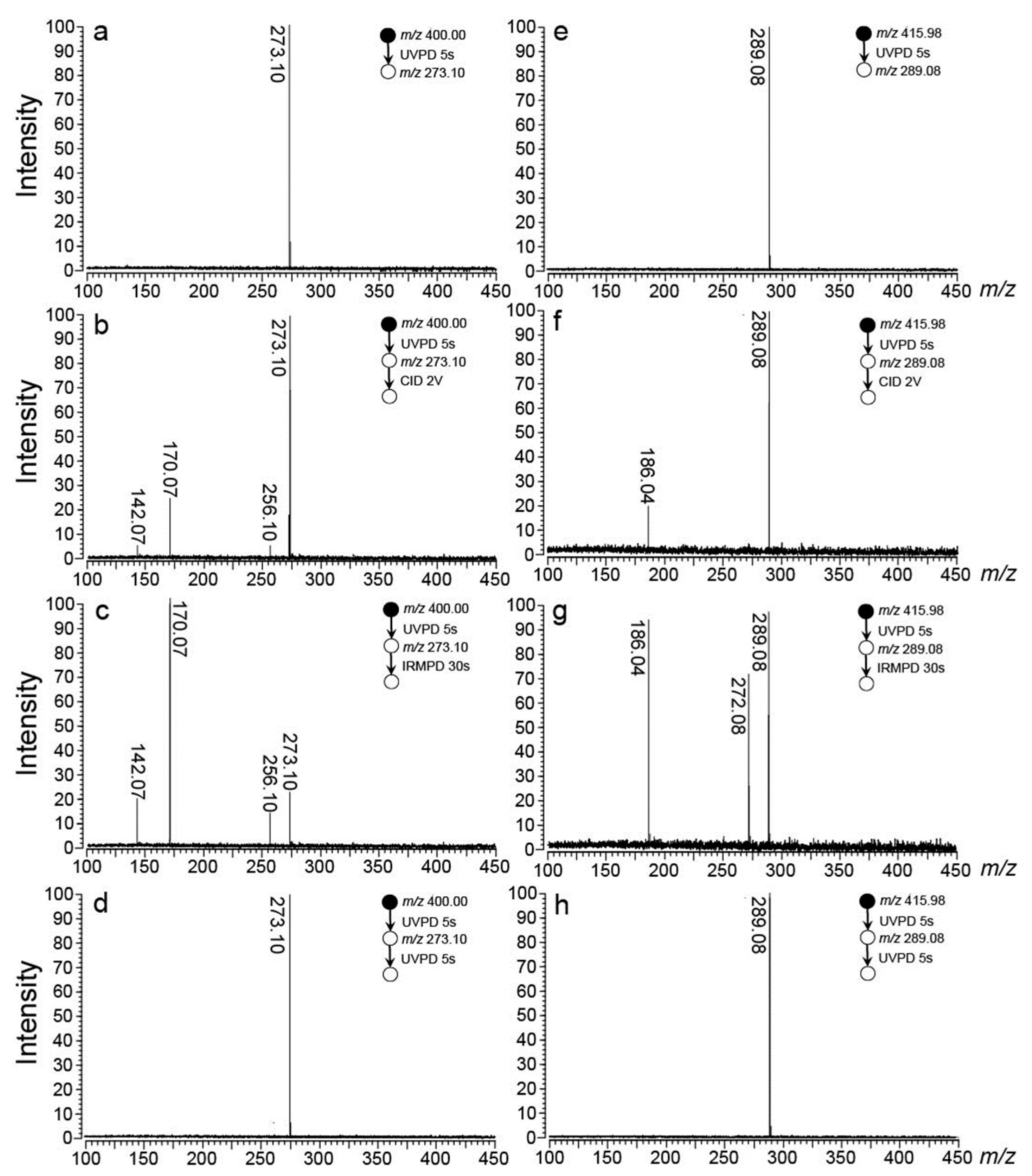
2.1. Generation of the Radical Cations
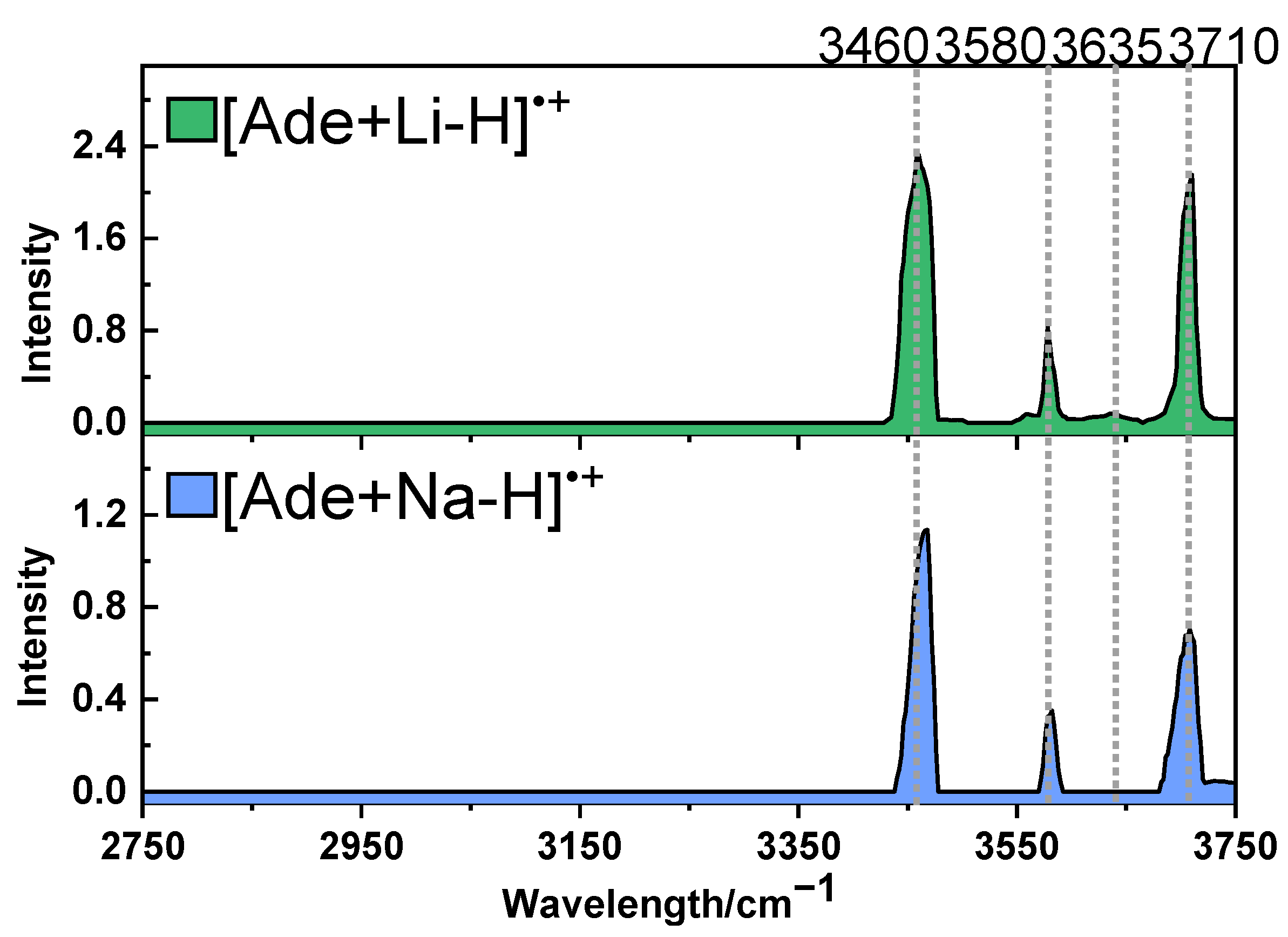
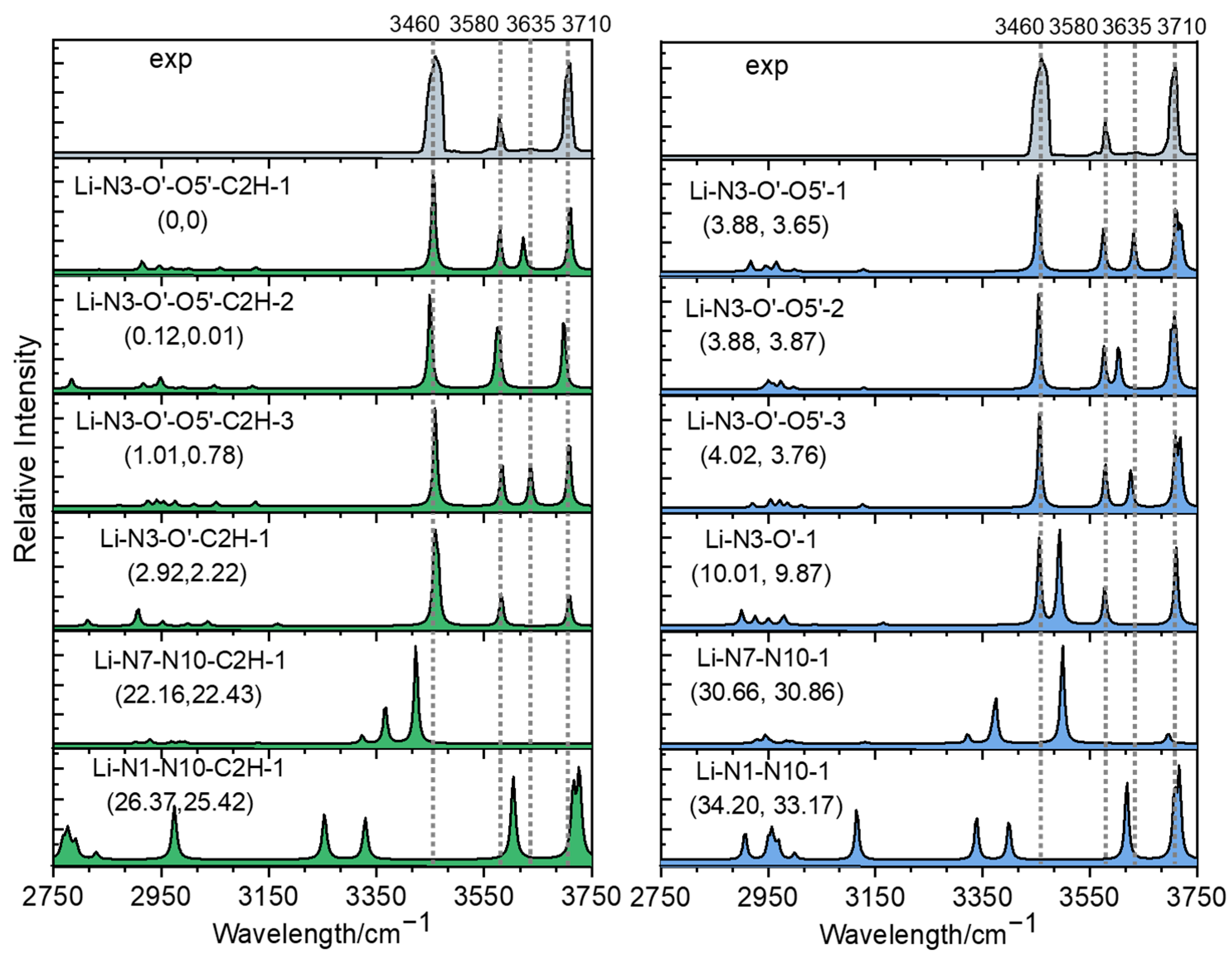
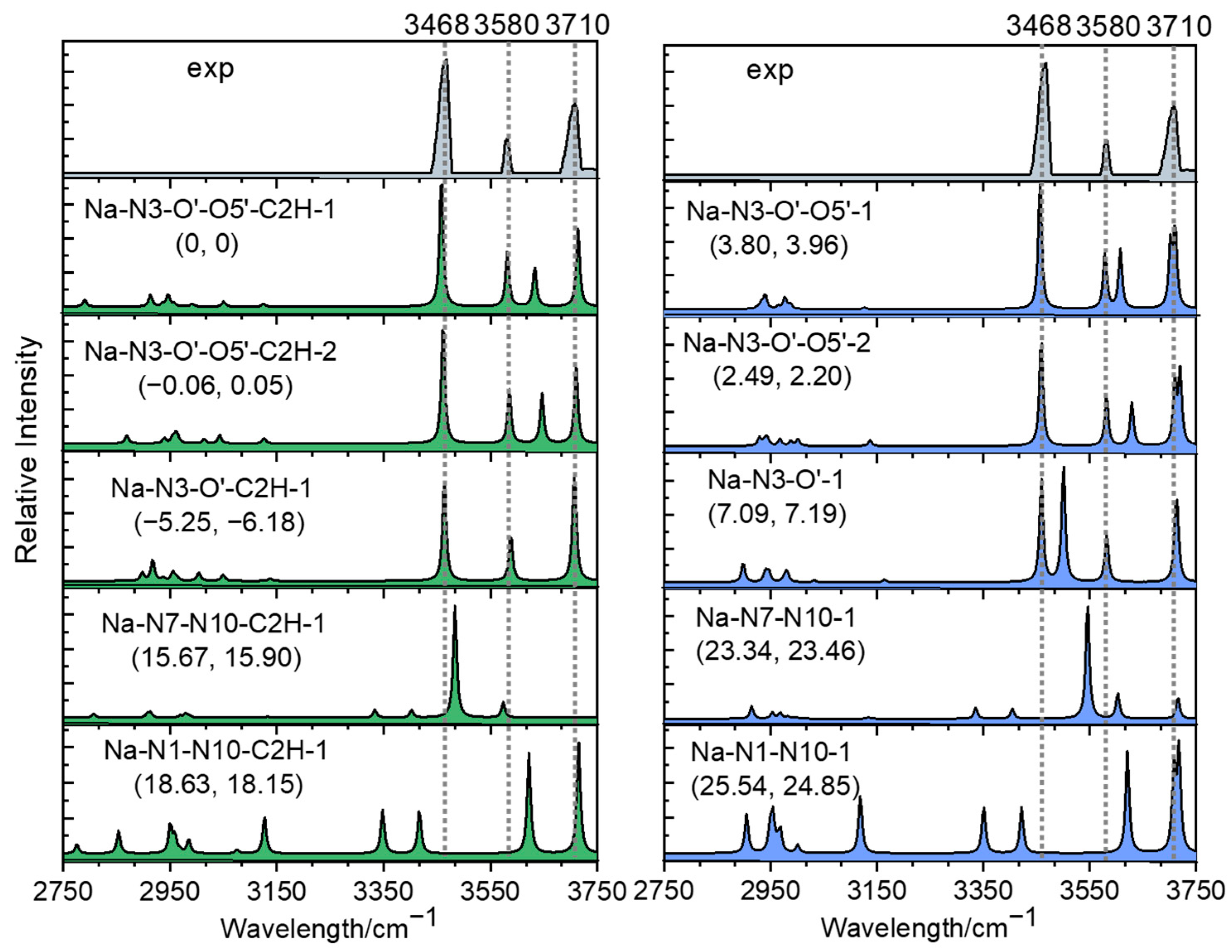
2.2. Photodissociation Spectroscopic Study of Metalized Adenosine Radical Cations
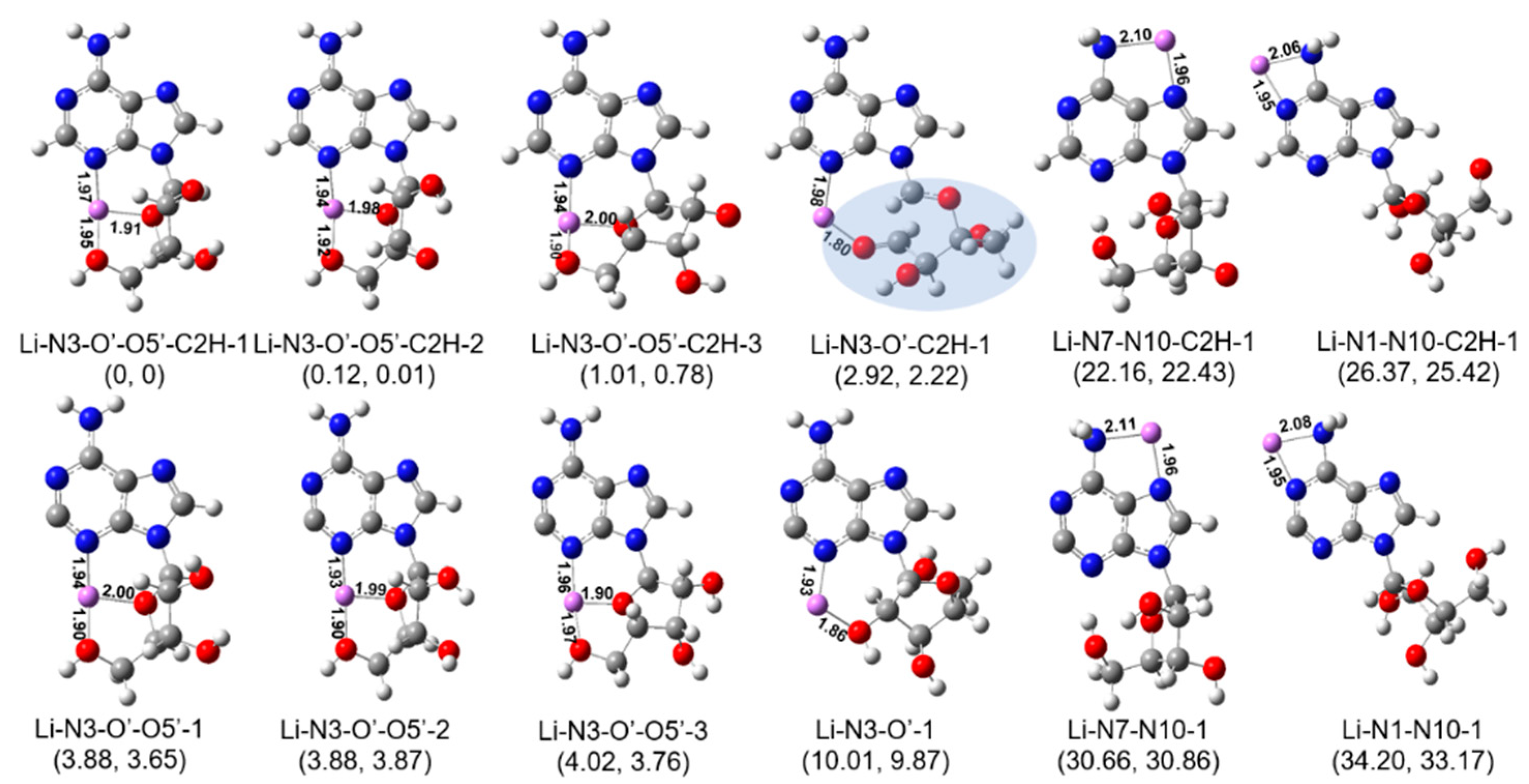
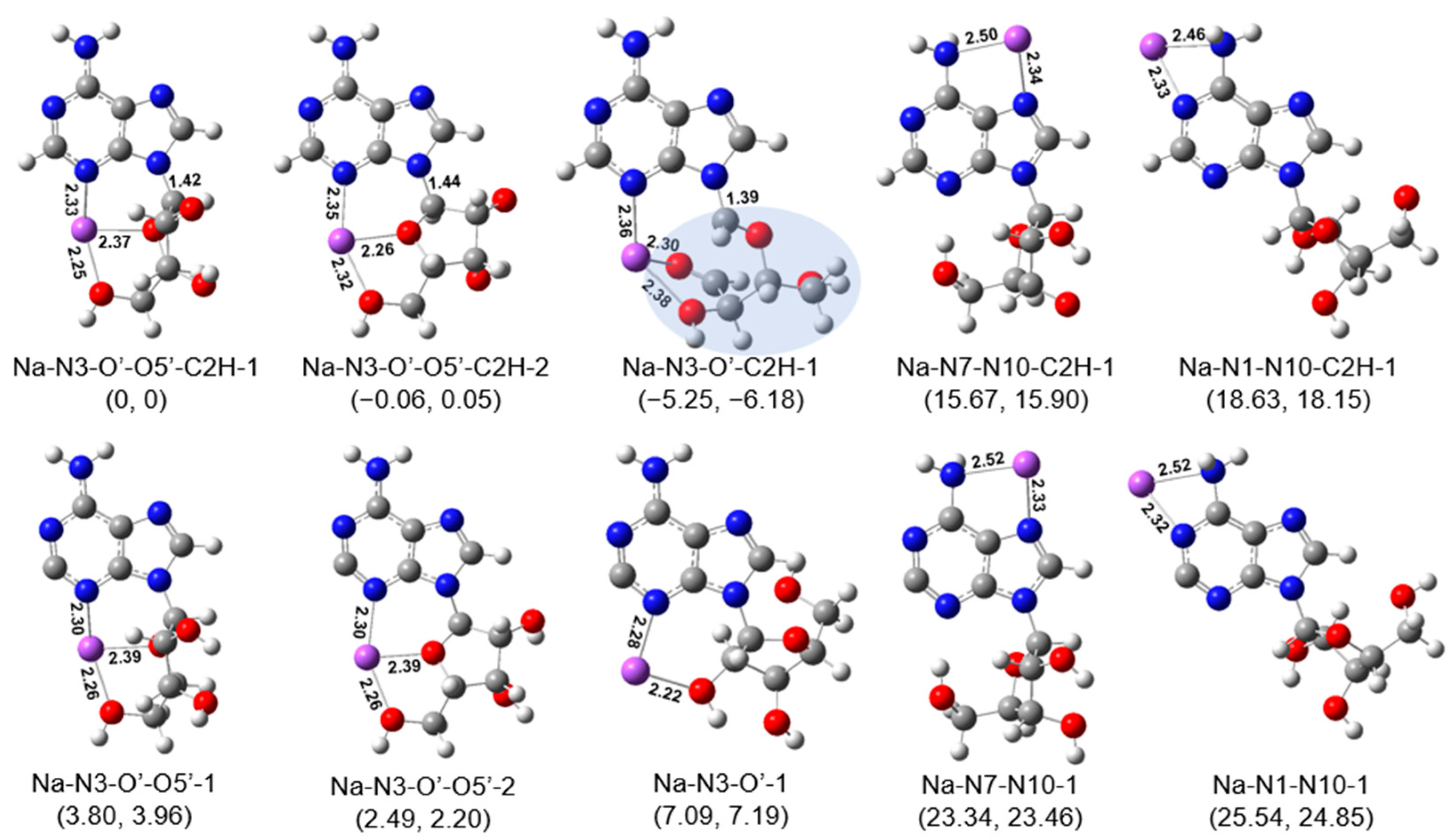
2.3. Structures of [Ade+Li-H]•+ and [Ade+Na-H]•+
3. Discussion
4. Materials and Methods
4.1. Mass Spectrometry and Photodissociation
4.2. Computational Details
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, L.W.; Greenberg, M.M. DNA Damage Emanating from a Neutral Purine Radical Reveals the Sequence Dependent Convergence of the Direct and Indirect Effects of γ-Radiolysis. J. Am. Chem. Soc. 2017, 139, 17751–17754. [Google Scholar] [CrossRef]
- Lundahl, M.N.; Sarksian, R.; Yang, H.; Jodts, R.J.; Pagnier, A.; Smith, D.F.; Mosquera, M.A.; Donk, W.A.; Hoffman, B.M.; Broderick, W.E.; et al. Mechanism of Radical S-Adenosyl-L-methionine Adenosylation: Radical Intermediates and the Catalytic Competence of the 5′-Deoxyadenosyl Radical. J. Am. Chem. Soc. 2022, 144, 5087–5098. [Google Scholar] [CrossRef]
- Zheng, L.W.; Greenberg, M.M. Traceless Tandem Lesion Formation in DNA from a Nitrogen Centered Purine Radical. J. Am. Chem. Soc. 2018, 140, 6400–6407. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.; O’Hair, R.A.J.; McFadyen, W.D. Can radical cations of the constituents of nucleic acids be formed in the gas phase using ternary transition metal complexes? Rapid Commun. Mass Spectrom. 2005, 19, 1797–1805. [Google Scholar] [CrossRef]
- Lesslie, M.; Lawler, J.T.; Dang, A.; Korn, J.A.; Daniel, B.; Steinmetz, V.; Matre, P.; Turecek, F.; Ryzhov, V. Cytosine Radical Cations: A Gas-Phase Study Combining IRMPD Spectroscopy, UVPD Spectroscopy, Ion–Molecule Reactions, and Theoretical Calculations. ChemPhysChem 2017, 18, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Nguyen, H.T.H.; Ruiz, H.; Piacentino, E.; Ryzhov, V.; Turecek, F. Experimental Evidence for Noncanonical Thymine Cation Radicals in the Gas Phase. J. Phys. Chem. B 2018, 122, 86–97. [Google Scholar] [CrossRef]
- Huang, S.R.; Dang, A.; Turecek, F. Ground and Excited States of Gas-Phase DNA Nucleobase Cation Radicals: A UV−vis Photodisociation Action Spectroscopy and Computational Study of Adenine and 9-Methyladenine. J. Am. Soc. Mass Spectrom. 2020, 31, 1271–1281. [Google Scholar] [CrossRef]
- Zima, V.; Liu, Y.; Turecek, F. Radical Cascade Dissociation Pathways to Unusual Nucleobase Cation Radicals. J. Am. Soc. Mass Spectrom. 2022, 33, 1038–1047. [Google Scholar] [CrossRef]
- Liu, Y.; Korn, J.A.; Dang, A.; Turecek, F. Hydrogen-Rich Cation Radicals of DNA Dinucleotides: Generation and Structure Elucidation by UV−Vis Action Spectroscopy. J. Phys. Chem. B 2018, 122, 9665–9680. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dang, A.; Urban, J.; Turecek, F. Charge-Tagged DNA Radicals in the Gas Phase Characterized by UV/Vis Photodissociation Action Spectroscopy. Angew. Chem. Int. Ed. 2020, 59, 7772–7777. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.C.; Nováková, G.; Marek, A.; Turecek, F. Charge-Tagged Nucleosides in the Gas Phase: UV−Vis Action Spectroscopy and Structures of Cytidine Cations, Dications, and Cation Radicals. J. Phys. Chem. A 2021, 125, 6096–6108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, C.C.; Leonen, C.J.A.; Chatterjee, C.; Novakova, G.; Marek, A.; Turecek, F. Tackling a Curious Case: Generation of Charge-Tagged Guanosine Radicals by Gas-Phase Electron Transfer and Their Characterization by UV−vis Photodissociation Action Spectroscopy and Theory. J. Am. Soc. Mass Spectrom. 2021, 32, 772–785. [Google Scholar] [CrossRef] [PubMed]
- Feketeova, L.; Chan, B.; Khairallah, G.N.; Steinmetz, V.; Maitre, P.; Radom, L.; O’Hair, R.A.J. Watson−Crick Base Pair Radical Cation as a Model for Oxidative Damage in DNA. J. Phys. Chem. Lett. 2017, 8, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Wolken, J.K.; Syrstad, E.A.; Vivekananda, S.; Turecek, F. Uracil Radicals in the Gas Phase: Specific Generation and Energetics. J. Am. Chem. Soc. 2001, 123, 5804–5805. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.X.; Cuadrado-Peinado, M.L.; Polsek, M.; Turecek, F. Specific Generation of 1-Methylcytosine Radicals in the Gas Phase. Angew. Chem. 2005, 117, 6866–6869. [Google Scholar] [CrossRef]
- Hong, I.S.; Carter, K.N.; Sato, K.; Greenberg, M.M. Characterization and Mechanism of Formation of Tandem Lesions in DNA by a Nucleobase Peroxyl Radical. J. Am. Chem. Soc. 2007, 129, 4089–4098. [Google Scholar] [CrossRef]
- Chen, X.H.; Syrstad, E.A.; Nguyen, M.T.; Gerbaux, P.; Turecek, F. Adenine Radicals in the Gas Phase: An Experimental and Computational Study of Hydrogen Atom Adducts to Adenine. J. Phys. Chem. A 2005, 109, 8121–8132. [Google Scholar] [CrossRef]
- Eichhorn, G.L. Metal Ions as Stabilizers or Destabilizers of the Deoxyribonucleic Acid Structure. Nature 1962, 194, 474–475. [Google Scholar] [CrossRef]
- Kim, N.S.; LeBreton, P.R. UV Photoelectron and Ab Initio Quantum Mechanical Evaluation of Nucleotide Ionization Potentials in Water−Counterion Environments: π Polarization Effects on DNA Alkylation by Carcinogenic Methylating Agents. J. Am. Chem. Soc. 1996, 118, 3694–3707. [Google Scholar] [CrossRef]
- Kim, N.S.; LeBreton, P.R. Ultraviolet Photoelectron Spectral Corrections Applied to the Quantum Mechanical Evaluation ofNucleotide Ionization Potentials in Water-Counterion Environments: The Valence Electronic Structure of Anionic 2′-Deoxyadenosine 5′-Phosphate. Int. J. Quantum Chem. 1997, 3, 1–16. [Google Scholar]
- Chiavarino, B.; Crestoni, M.E.; Fornarini, S.; Scuderi, D.; Salpin, J.-Y. Interaction of Cisplatin with 5′-dGMP: A Combined IRMPD and Theoretical Study. Inorg. Chem. 2015, 54, 3513–3522. [Google Scholar] [CrossRef]
- Chiavarino, B.; Crestoni, M.E.; Fornarini, S.; Scuderi, D.; Salpin, J.-Y. Undervalued N3 Coordination Revealed in the CisplatinComplex with 2′-Deoxyadenosine-5′-Monophosphate by A Combined IRMPD and Theoretical Study. Inorg. Chem. 2017, 56, 8793–8801. [Google Scholar] [CrossRef]
- Ciavardini, A.; Dalla Cort, A.; Fornarini, A.; Scuderi, S.; Giardini, D.; Forte, A.; Bodo, G.; Piccirillo, E. Adenosine monophosphate recognition by zinc–salophen complexes: IRMPD spectroscopy and quantum modeling study. J. Mol. Spectrosc. 2017, 335, 108–116. [Google Scholar] [CrossRef]
- Hamlow, L.; Nei, Y.w.; Wu, R.; Gao, J.; Steill, J.; Berden, G.; Oomens, J.; Rodgers, M. Impact of Sodium Cationization on Gas-Phase Conformations of DNA and RNA Cytidine Mononucleotides. J. Am. Soc. Mass Spectrom. 2019, 30, 1758–1767. [Google Scholar] [CrossRef]
- Salpin, J.Y.; Gamiette, L.; Tortajada, J.; Besson, T.; Maître, P. Structure of Pb2+/dCMP and Pb2+/CMP Complexes as Characterized by Tandem Mass Spectrometry and IRMPD Spectroscopy. Int. J. Mass Spectrom. 2011, 304, 154–164. [Google Scholar] [CrossRef]
- Salpin, J.Y.; MacAleese, L.; Chirot, F.; Dugourd, P. Structure of the Pb2+−Deprotonated dGMP Complex in the Gas Phase: A Combined MS-MS/IRMPD Spectroscopy/Ion Mobility Study. Phys. Chem. Chem. Phys. 2014, 16, 14127–14138. [Google Scholar] [CrossRef]
- Salpin, J.Y.; Guillaumont, S.; Ortiz, D.; Tortajada, J.; Maître, P. Direct Evidence for Tautomerization of the Uracil Moiety within the Pb2+/Uridine-5′-Monophosphate Complex: A Combined Tandem Mass Spectrometry and IRMPD Study. Inorg. Chem. 2011, 50, 7769–7778. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.M.; Dessent, C.E. Effect of Cation Complexation on the Structure of A Conformationally Flexible Multiply Charged Anion: Stabilization of Excess Charge in the Na+.Adenosine 5′-Triphosphate Dianion Ion-Pair Complex. J. Phys. Chem. A 2009, 113, 2683–2692. [Google Scholar] [CrossRef]
- Yuan, Q.; Chomicz-Mańka, L.; Makurat, S.; Cao, W.; Rak, J.; Wang, X.-B. Photoelectron Spectroscopy and Theoretical Inves-tigations of Gaseous Doubly Deprotonated 2′-Deoxynucleoside 5′-Monophosphate Dianions. J. Phys. Chem. Lett. 2021, 12, 9463–9469. [Google Scholar] [CrossRef] [PubMed]
- Makurat, S.; Yuan, Q.; Czub, J.; Chomicz-Mańka, L.; Cao, W.; Wang, X.-B.; Rak, J. Guanosine Dianions Hydrated by One to Four Water Molecules. J. Phys. Chem. Lett. 2022, 13, 3230–3236. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.Q.; Feng, W.W.; Cao, W.J.; Zhou, Y.C.; Cheng, L.J.; Wang, X.B. Sodium Cationization Enables Exotic Deprotonation Sites on Gaseous Mononucleotides. J. Phys. Chem. Lett. 2022, 13, 9975–9982. [Google Scholar] [CrossRef] [PubMed]
- Padash, R.; Ramazani, S. Investigation of stability of adenine and its tautomers in RNA and DNA, and their interaction with Na+, K+, Mg2+, Ca2+ and Zn2+. J. Mol. Struct. 2020, 1222, 128698. [Google Scholar] [CrossRef]
- Stasyuk, O.A.; Szatyłowicz, H.; Krygowski, T.M. Effect of H-bonding and complexation with metal ions on the π-electron structure of adenine tautomers. Org. Biomol. Chem. 2014, 12, 456. [Google Scholar] [CrossRef]
- Yang, B.; Wu, R.R.; Polfer, N.C.; Berden, G.; Oomens, J.; Rodgers, M.T. IRMPD Action Spectroscopy of Alkali Metal Cation-Cytosine Complexes: Effects of Alkali Metal Cation Size on Gas Phase Conformation. J. Am. Soc. Mass Spectrom. 2013, 24, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hamlow, L.A.; He, C.C.; Strobehn, S.F.; Lee, J.K.; Gao, J.; Berden, G.; Oomens, J.; Rodgers, M.T. Influence of Sodium Cationization versus Protonation on the Gas-Phase Conformations and Glycosidic Bond Stabilities of 2′-Deoxyadenosine and Adenosine. J. Phys. Chem. B 2016, 120, 8892–8904. [Google Scholar] [CrossRef] [PubMed]
- Ranka, K.; Zhao, N.; Yu, L.; Stanton, J.F.; Polfer, N.C. Radical Rearrangement Chemistry in Ultraviolet Photodissociation of Iodotyrosine Systems: Insights from Metastable Dissociation, Infrared Ion Spectroscopy, and Reaction Pathway Calculations. J. Am. Soc. Mass Spectrom. 2018, 29, 1791Y1801. [Google Scholar] [CrossRef]
- Zhang, K.L.; Shi, Y.Y.; Du, M.Y.; Xu, Y.C.; Wang, Y.; Kong, X.L. Versatile double-beam confocal laser system combined with a fourier transform ion cyclotron resonance mass spectrometer for photodissociation mass spectrometry and spectroscopy. Anal. Chem. 2021, 93, 9056–9063. [Google Scholar] [CrossRef]
- Yang, T.; Kaiser, R.I.; Troy, T.P.; Xu, B.; Kostko, O.; Ahmed, M.; Mebel, A.M.; Zagidullin, M.V.; Azyazov, V.N. HACAs Heritage: A Free-Radical Pathway to Phenanthrene in Circumstellar Envelopes of Asymptotic Giant Branch Stars. Angew. Chem. 2017, 129, 1–6. [Google Scholar]
- Shiels, O.J.; Kelly, P.D.; Bright, C.C.; Poad, B.L.; Blanksby, S.J.; Da Silva, G.; Trevitt, A.J. Reactivity trends in the gas-phase addition of acetylene to the N-protonated aryl radical cations of pyridine, aniline, and benzonitrile. J. Am. Soc. Mass Spectrom. 2021, 32, 537–547. [Google Scholar] [CrossRef]
- Chen, L.; Ma, Z.; Fournier, J.A. Origins of the diffuse shared proton vibrational signatures in proton-coupled electron transfer model dyad complexes. J.Chem. Phys. 2022, 157, 154308. [Google Scholar] [CrossRef]
- Bell, M.R.; Cruzeiro, V.W.D.; Cismesia, A.P.; Tesler, L.F.; Roitberg, A.E.; Polfer, N.C. Probing the structures of solvent-complexed ions formed in electrospray ionization using cryogenic infrared photodissociation spectroscopy. J. Phys. Chem. A 2018, 122, 7427–7436. [Google Scholar] [CrossRef] [PubMed]
- Cody, R.B.; Hein, R.E.; Goodman, S.D.; Marshall, A.G. Stored waveform inverse Fourier transform excitation for obtaining increased parent ion selectivity in collisionally activated dissociation: Preliminary results. Rapid Commun. Mass Spectrom. 1987, 1, 99–102. [Google Scholar] [CrossRef]
- Gauthier, J.W.; Trautman, T.R.; Jacobson, D.B. Sustained off-resonance irradiation for collision-activated dissociation involving Fourier transform mass spectrometry. Collision-activated dissociation technique that emulates infrared multiphoton dissociation. Anal. Chim. Acta 1991, 246, 211–225. [Google Scholar] [CrossRef]
- Laskin, J.; Futrell, J.H. Activation of large ions in FT-ICR mass spectrometry. Mass Spectrom. Rev. 2005, 24, 135–167. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G. Gaussian09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, M.; Jiao, L.; Xu, S.; Du, M.; Hou, Y.; Kong, X. Structural Characterization of the Metalized Radical Cations of Adenosine ([Ade+Li-H]•+ and [Ade+Na-H]•+) by Infrared Multiphoton Dissociation Spectroscopy and Theoretical Studies. Int. J. Mol. Sci. 2023, 24, 15385. https://doi.org/10.3390/ijms242015385
Kou M, Jiao L, Xu S, Du M, Hou Y, Kong X. Structural Characterization of the Metalized Radical Cations of Adenosine ([Ade+Li-H]•+ and [Ade+Na-H]•+) by Infrared Multiphoton Dissociation Spectroscopy and Theoretical Studies. International Journal of Molecular Sciences. 2023; 24(20):15385. https://doi.org/10.3390/ijms242015385
Chicago/Turabian StyleKou, Min, Luyang Jiao, Shiyin Xu, Mengying Du, Yameng Hou, and Xianglei Kong. 2023. "Structural Characterization of the Metalized Radical Cations of Adenosine ([Ade+Li-H]•+ and [Ade+Na-H]•+) by Infrared Multiphoton Dissociation Spectroscopy and Theoretical Studies" International Journal of Molecular Sciences 24, no. 20: 15385. https://doi.org/10.3390/ijms242015385







