Abstract
Pomegranate has shown a favorable effect on gingivitis/periodontitis, but the mechanisms involved are poorly understood. The aim of this study was to test the effect of pomegranate peel extract (PoPEx) on gingiva-derived mesenchymal stromal cells (GMSCs) under physiological and inflammatory conditions. GMSC lines from healthy (H) and periodontitis (P) gingiva (n = 3 of each) were established. The lines were treated with two non-toxic concentrations of PoPEX (low—10; high—40 µg/mL), with or without additional lipopolysaccharide (LPS) stimulation. Twenty-four genes in GMSCs involved in different functions were examined using real-time polymerase chain reaction (RT-PCR). PoPEx (mostly at higher concentrations) inhibited the basal expression of IL-6, MCP-1, GRO-α, RANTES, IP-10, HIF-1α, SDF-1, and HGF but increased the expression of IL-8, TLR3, TGF-β, TGF-β/LAP ratio, IDO-1, and IGFB4 genes in H-GMSCs. PoPEx increased IL-6, RANTES, MMP3, and BMP2 but inhibited TLR2 and GRO-α gene expression in P-GMSCs. LPS upregulated genes for proinflammatory cytokines and chemokines, tissue regeneration/repair (MMP3, IGFBP4, HGF), and immunomodulation (IP-10, RANTES, IDO-1, TLR3, COX-2), more strongly in P-GMSCs. PoPEx also potentiated most genes’ expression in LPS-stimulated P-GMSCs, including upregulation of osteoblastic genes (RUNX2, BMP2, COL1A1, and OPG), simultaneously inhibiting cell proliferation. In conclusion, the modulatory effects of PoPEx on gene expression in GMSCs are complex and dependent on applied concentrations, GMSC type, and LPS stimulation. Generally, the effect is more pronounced in inflammation-simulating conditions.
1. Introduction
Chronic periodontitis is a frequent form of periodontal disease and the most common oral health problem, characterized by inflammation and infection of the tissues surrounding the teeth. It is a progressive pathological process that affects the supporting structures of the teeth, including the gingiva, periodontal ligaments, and alveolar bone [1]. Its has been estimated to have a 5–15% prevalence among the general adult population [2]. Periodontitis occurs when the bacteria in dental plaque build up on the teeth and gingiva, leading to an immune response from the host. Over time, this immune response, which was ineffective in removing pathogens, can cause damage to the gingiva and supporting structures, leading to the formation of periodontal pockets, gingival recession, and bone loss [3]. Several factors can contribute to the development of chronic periodontitis, including poor oral hygiene, smoking, genetic predisposition, stress, certain systemic diseases (especially diabetes), hormonal changes, and certain drugs [3,4,5].
Several species of bacteria are associated with the development and progression of chronic periodontitis. Some of the most common bacteria implicated in this condition include Porphyromonas gingivalis, Fusobacterium nucleatum, Tannerella forsythia, Treponema denticola, Aggregatibacter actinomycetemcomitans, Filifactor alocis, and Prevotella intermedia [3,6]. In this context, oral-health-associated commensals protect the development and/or progression of periodontitis by inhibiting the growth of periodontitis-associated pathogens [3,7].
Except for bacterial products such as proteolytic enzymes, toxins, and lipopolysaccharide (LPS), immune cells are important players in chronic periodontitis, including keratinocytes, neutrophil granulocytes, mast cells, monocyte/macrophages, dendritic cells, and T and B cells [8,9,10]. The immune cells are involved in the inflammatory response, tissue destruction, and the restriction of inflammatory/destructive processes in the periodontium [11,12].
One cell type whose role in the pathogenesis of chronic periodontitis is not elucidated is the gingiva-derived mesenchymal stromal cell (GMSC). GMSCs are a type of mesenchymal stem cells that can be isolated from gingival tissue. They share many similarities with other dental MSCs, such as fibroblastoid morphology, adherence to plastic substrates, differentiation potential to other types of mesenchymal cells, and the expression of characteristic markers, including those found on pericytes [13,14]. However, GMSCs possess some characteristics different from the rest of dental MSCs, such as easy isolation, absence of spontaneous differentiation, additional neurogenic and epithelial differentiation potential due to gingival origin from the neural crest, and morphological, phenotypical, and telomerase stability after long-term cultures [14,15]. Some potential roles of GMSCs during periodontitis include hard and soft tissue repair, immunomodulatory properties, angiogenesis, and antibacterial effects [14,16]. However, it remains to be answered whether, at a certain stage of periodontitis development, GMSC could promote an inflammatory response that is needed to eradicate bacteria. In this context, the response of GMSCs to LPS is important because this component of the outer membrane of Gram-negative bacteria is known to elicit a strong immune response and trigger inflammation in the periodontal tissues [17].
The primary goal of periodontal therapy is to remove pathogenic biofilms and suppress inflammation. This strategy involves active anti-infective treatment, often combined with surgery to eliminate residual pockets [3,18]. Anti-infective treatment includes the application of systemic antibiotics, antiseptic mouthwashes, local drug delivery of antiseptics and antibiotics, and immunomodulating agents. However, plaque control remains the primary preventive measure [19]. In this context, searching for natural products (phytochemicals) seems to be a good approach because many bacteria causing periodontitis show antibiotic resistance. Plant natural products extracted from Curcuma zedoaria, Calendula officinalis, Acacia arabica, Azadirachta indica, Curcuma longa, Cymbopogam, Camellia sinensis, Ocimum sanctum, or Aloe vera have been used to treat many oral diseases [20,21]. Except for their antiseptic and antifungal properties, these herbal extracts possess antioxidant, anti-inflammatory, and wound-healing properties [21]. In a recent systemic review, Chatzopoulos et al. have shown that herbal products used as adjuncts to scaling and root planing or supragingival debridement led to superior clinical outcomes in comparison with placebo or no adjuncts [21]. Two papers showed similar beneficial effects of Calendula officinalis mouthwash [22] or Aloe vera gel [23]. To prevent gingivitis, toothpastes containing naturally occurring ingredients, including herbal essential oils, are recommended due to their safety, biocompatibility, and oral-health-promoting ability [24].
Different components from Punica granatum (pomegranate), such as peel, seed, and juice, are very rich in various phytochemicals, including ellagitannins, gallotanins, flavonoids, organic acids, and other polyphenols. These compounds possess antioxidant and immunomodulatory properties and have been associated with anti-inflammatory, anticancer, antidegenerative, skin-regenerative, neuroprotective, and cardiovascular health benefits. In addition, pomegranate extract has been shown to possess bactericidal, antifungal, antiviral, and antihelminthic effects [25,26,27]. Numerous studies have shown the beneficial effect of pomegranate extract in various oral diseases and gingivitis/periodontitis [20]. For example, a 10% pomegranate gel applied topically efficiently reduced recurrent aphthous stomatitis and improved ulcer healing. A similar 80% gel had anesthetic effects in the oral cavity. Another pomegranate gel efficiently treated candidiasis-associated denture stomatitis [20,28,29,30]. The pomegranate gels showed a potent anti-gingivitis effect, including acute ulcerative gingivitis and a significant reduction in plaque scores when used as an adjunct nonsurgical therapy [31,32,33]. The positive effects could be explained by the potent antimicrobial activity of the components from the pomegranate extract, their protective effect on oxidative stress, and their ability to suppress inflammation [20].
Considering the facts presented so far, including the important role of GMSCs in physiological and pathological conditions, the question arises regarding how pomegranate peel extract (PoPEx) affects GMSCs because such data are missing in the literature. In this context, a paper showed that kiwifruit extract enhanced the proliferation and migration of human gingival fibroblasts and promoted angiogenesis [34]. In addition, a pomegranate extract and punicallagin exerted antioxidant properties on human gingival fibroblasts but inhibited their proliferation at higher concentrations [35]. We hypothesized that PoPEx differently modulates gene expression in GMSCs established from healthy (H) and periodontitis-affected gingiva (P) based on our previous findings that many functional properties of these lines are different inflammation [15]. Therefore, the main goal of this study was to investigate and compare the effect of different concentrations of PoPEx on the proliferation of H-and P-GMSCs and the expression of genes in these lines involved in the processes of inflammation, immunomodulation, tissue damage, and remodeling, proliferation, senescence, and osteoblastogenesis, under physiological and pathological conditions. To further mimic the processes that occur during periodontitis, the cell lines were additionally stimulated with LPS.
2. Results
2.1. Establishment and Basic Characterization of GMSC Lines
Gingival samples were obtained from three healthy donors and three donors with periodontitis. The histological analysis confirmed the differences between these groups based on the presence or absence of abundant cellular infiltrate composed of polymorphonuclear and mononuclear cells (lymphocytes, plasma cells, and macrophages) in gingival tissue sections. These changes were not present in healthy gingiva (Supplementary Figure S1).
The lines exhibited typical fibroblastoid morphology, adherence to plastic, and differentiation capability to osteoblasts, chondroblasts, and adipocytes (Table 1 and Supplementary Figure S2). They expressed several markers characteristic of MSCs (Table 1 and Supplementary Figure S3). P-GMSCs showed a higher capability to differentiate towards osteoblasts compared to H-GMSCs. The differences between H- and P-GMSC lines to differentiate into chondroblasts (moderate potential) and adipocytes (low potential) were insignificant. CD90, CD73, CD44, CD73, CD105, and CD166 were expressed on more than 95% of both H-GMSC and P-GMSC lines, CD146, was present on 25–50% of GMSCs, whereas the lowest positivity (10–20%) was seen with antibodies to STRO-1, SSEA4, and pericyte antigens (PDGF-R and NG2). Less than 2% of both H-GMSCs and P-GMSCs expressed hematopoietic cell markers (CD45 and CD34), a myelomonocytic marker (CD14), and T/B-cell markers (CD3/CD19). Although the proportions of cells expressing CD146 and markers of stem cells/pericytes were slightly higher in P-GMSC, the differences were not statistically significant.

Table 1.
Phenotypic characteristics of GMSC lines and their differentiation capabilities.
One of these monoclonal antibodies (mAbs) (CD146) was used to stain gingival sections. Figure 1a shows positivity associated with small blood vessels. To better analyze these structures, confocal microscopy was performed. The staining of tissue sections with anti-CD146 mAb and anti-CD31 mAb (an endothelial marker) identified CD146 + CD31- pericytes (Figure 1b).
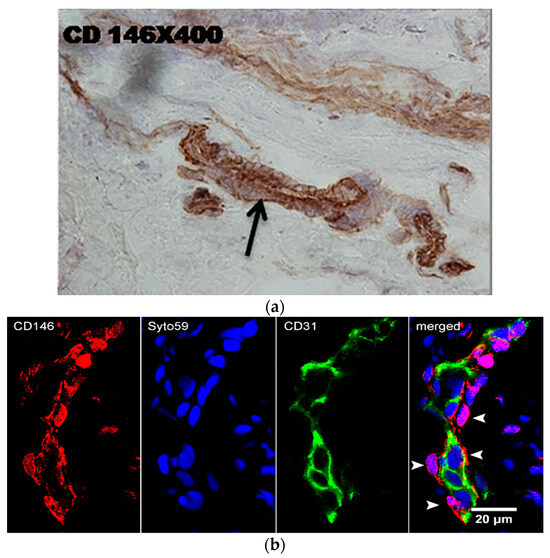
Figure 1.
Immunostaining of gingival tissue sections with anti-CD146 mAb (immunoperoxidase) (a) and anti-CD146-AlexaFluor 466 (red) and anti-CD31-AlexaFluor 433 (green) processed for confocal microscopy (b). Nuclei are stained in blue by Syto59. Note single CD146+ pericytes (arrowheads).
Cumulatively, our results showed that these GMSC lines are very similar to the lines that we had previously established [15] and, as such, were used for the current experiments.
2.2. Cytotoxicity and Anti-Proliferative Activity of GMSC Lines
At first, we studied the cytotoxicity of PoPEx on GMSC lines. The results presented in Figure 2A show that both H- and P-GMSCs responded similarly to PoPEx, which decreased cell viability from 60–100 µg/mL in a dose-dependent manner.
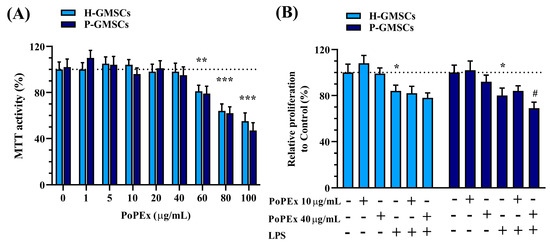
Figure 2.
The effect of PoPEx on the viability (MTT activity) (A) and proliferation (B) on H- and P-GMSCs in culture. Values are given as mean ± SD (n = 3). * p < 0.05; ** p < 0.01; *** p < 0.005 compared to corresponding controls (non-treated H- or P-GMSCs). # p < 0.05 compared to LPS-stimulated P-GMSCs.
Based on these findings, we used lower (10 µg/mL) and higher non-cytotoxic concentrations (40 µg/mL) of PoPEx for further experiments. Figure 2B shows that the treatment with LPS significantly inhibited cell proliferation of both types of cell lines (p < 0.05) without inducing cytotoxicity. Higher concentrations of PoPEx had an additional inhibitory effect on the proliferation of LPS-stimulated P-GMSCs (p < 0.05).
2.3. Effect of PoPEx on the Expression of Cytokine/Chemokine Genes Associated with Inflammation in Control and LPS-Stimulated GMSCs
Control and LPS-stimulated H- and P-GMSCs were incubated with low (10 µg/mL) or high (40 µg/mL) concentrations of PoPEx for 24 h, as described in the Materials and Methods Section. PoPEx showed significant modulation of the most investigated genes. As a rule, higher concentrations had a stronger modulatory effect. When the effect of lower concentrations was pronounced, these results were emphasized.
The expression of cytokine/chemokine genes related to inflammation, including Interleukin-6 (IL-6), IL-8, monocyte chemoattractant protein (MCP)-1, and growth-related oncogene (GRO)-α, was evaluated using qPCR (Figure 3).
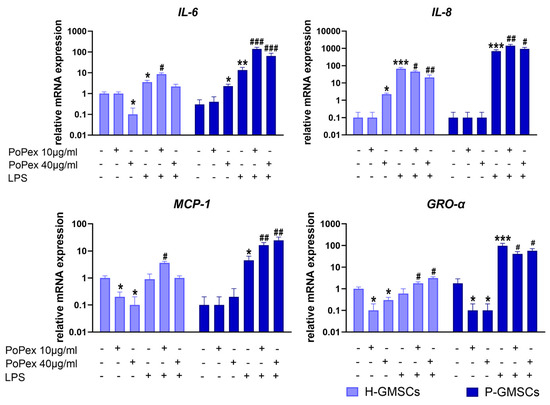
Figure 3.
The effect of PoPEx on the expression of IL-6, IL-8, MCP-1, and GRO-α mRNA in H- and P-GMSCs. Values are given as mean ± SD (n = 3). * p < 0.05; ** p < 0.01; *** p < 0.005 compared to corresponding controls (non-treated H- or P-GMSCs). # p < 0.05; ## p < 0.01; ### p < 0.005 compared to corresponding LPS-stimulated GMSCs.
The expression of IL-6 mRNA was slightly down- and upregulated by higher concentrations of PoPEx in H- and P-GMSCs, respectively (p < 0.05). LPS stimulated IL-6 expression about three times in H-GMSCs (p < 0.05) and about 30 times in P-GMSCs (p < 0.01), compared to the basal level, and its expression was additionally augmented by PoPEx. Lower concentrations had a much stronger upregulating effect, especially in LPS-stimulated P-GMSC cultures (p < 0.005).
IL-8 gene expression was almost non-detectable in both lines at the baseline level, and its expression was increased about 70 (H-GMSCs) and 700 times (P-GMSCs) after LPS stimulation (p < 0.005). PoPEx, which had a slight stimulatory effect on H-GMSCs (p < 0.05), and significantly augmented and inhibited IL-8 mRNA expression in LPS-stimulated P- and H-GMSC lines, respectively (p < 0.01).
PoPEx inhibited basal expression of MCP-1 in H-GMSCs (p < 0.05). In contrast, LPS stimulated MCP-1 mRNA expression in P-GMSCs (p < 0.05). PoPEx augmented MCP-1 expression in LPS-stimulated cultures, and the upregulating effect was stronger in P-GMSCs (p < 0.001) than in H-GMSCs (p < 0.05).
PoPEx inhibited basal gene expression of GRO-α in both lines (p < 0.05). LPS upregulated GRO-α in P-GMSCs about 50 times (p < 0.005) but had no significant effect in H-GMSCs. PoPEx had an opposite dose-dependent effect in LPS-stimulated cultures (stimulatory in H-GMSCs, inhibitory in P-GMSCs) (p < 0.05).
2.4. Effect of PoPEx on the Expression of Toll-like Receptors, Nuclear Factor Kappa-B 1, and Cyclooxygenase-2 Genes in Control and LPS-Stimulated GMSCs
The group of genes that includes genes such as Toll-Like Receptors (TLR) 2, 3, and 4, transcription molecule Nuclear Factor Kappa B 1 (NFKB1), and enzyme Cyclooxygenase (COX)-2 is involved in the inflammatory pathway. The effects of PoPEx on their expression in control and LPS-stimulated GMSCs are presented in Figure 4.
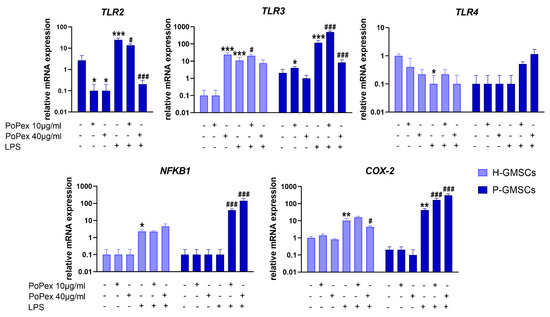
Figure 4.
The effect of PoPEx on the expression of TLR2, TLR3, TLR4, NFKB1, and COX-2 mRNA in H- and P-GMSCs. Values are given as mean ± SD (n = 3). * p < 0.05; ** p < 0.01; *** p < 0.005 compared to corresponding controls (non-treated H- or P-GMSCs). # p < 0.05; ### p < 0.005 compared to corresponding LPS-stimulated GMSCs.
TLR2 gene expression was non-detectable in control, LPS-stimulated or PoPEx-treated H-GMSCs. The expression of TLR2 mRNA in LPS-stimulated P-GMSCs was statistically significantly increased (about ten times) compared to the control P-GMSCs (p < 0.005). PoPEx completely downregulated the TLR2 mRNA expression by both non-treated and LPS-treated P-GMSCs, in a dose-dependent manner (p < 0.05 and p < 0.005, respectively).
The expression of TLR3 mRNA by H-GMSCs was almost non-detectable in contrast to P-GMSCs. However, PoPEx more strongly upregulated TLR3 expression in H-GMSCs (p < 0.005) compared to P-GMSCs (p < 0.05). The stimulatory effect of LPS on P-GMSCs was about ten times higher than H-GMSCs (p < 0.005). A lower concentration of PoPEx increased TLR3 in LPS-stimulated P-GMSCs (p < 0.005), in contrast to higher concentrations which were inhibitory (p < 0.05). A similar trend of modulation, but less strong, was observed with PoPEx on LPS-stimulated H-GMSCs (p < 0.05).
LPS inhibited basal expression of TLR4 mRNA expression in H-GMSCs (p < 0.05). TLR4 was not expressed in P-GMSCs and was not significantly modulated by LPS. PoPEx treatment did not significantly modulate TLR4 in either control or LPS-stimulated GMSC lines.
The expression of the NFKB1 gene was almost non-detectable in both H- and P-GMSCs and was not modulated by PoPEx. LPS slightly upregulated NFKB1 mRNA in H-GMSCs (p < 0.05) but not in P-GMSCs. PoPEx did not significantly affect LPS-stimulated H-GMSCs, but augmented NFKB1 expression in LPS-stimulated P-GMSCs, in a dose-dependent manner, by about 40 and 150 times, respectively (p < 0.005).
PoPEx did not significantly change COX-2 mRNA in both lines. LPS stimulated COX-2 expression about 10 times in H-GMSCs and about 40 times in P-GMSCs (p < 0.01). Lower concentrations of PoPEx upregulated COX-2 expression in LPS-stimulated P-GMSC lines (p < 0.005). In contrast, higher concentrations downregulated COX-2 in LPS-stimulated H-GMSCs (p < 0.05) but additionally upregulated its expression in LPS-stimulated P-GMSCs (p < 0.005).
2.5. Effect of PoPEx on the Expression of Genes Associated with Immunomodulation in Control and LPS-Stimulated GMSCs
Five genes associated with immunomodulation have been studied, and the results are presented in Figure 5.
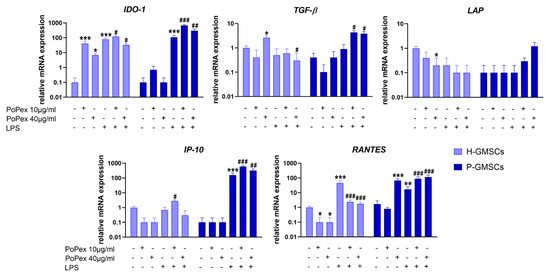
Figure 5.
The effect of PoPEx on the expression of IDO-1, TGF-β, LAP, IP-10, and RANTES mRNA in H- and P-GMSCs. Values are given as mean ± SD (n = 3). * p < 0.05; ** p < 0.01; *** p < 0.005 compared to corresponding controls (non-treated H- or P-GMSCs). # p < 0.05; ## p < 0.01; ### p < 0.005 compared to corresponding LPS-stimulated GMSCs.
Indoleamine 2, 3-Dioxygenase 1 (IDO-1) mRNA was almost non-detectable in both H- and P-GMSCs but was significantly increased upon LPS stimulation (p < 0.005). Both concentrations of PoPEX additionally upregulated IDO-1 in LPS-stimulated P-GMSCs (p < 0.005 and p < 0.01, respectively), as did lower concentrations in LPS-stimulated H-GMSCs (p < 0.05). However, higher concentrations of PoPEx inhibited IDO-1 in LPS-stimulated H-GMSC cultures (p < 0.05).
PoPEx stimulated transforming growth factor (TGF)-β mRNA expression in control H-GMSCs. LPS did not significantly modulate TGF-β mRNA expression in both lines. PoPEx upregulated TGF-β mRNA in LPS-stimulated P-GMSCs (p < 0.05) but decreased its expression in LPS-stimulated H-GMSCs (p < 0.05). Latency-associated peptide (LAP) was almost non-detectable in P-GMSCs. PoPEx downregulated LAP expression in H-GMSCs (p < 0.05). LPS did not significantly change LAP expression in both lines, and PoPEx did not additionally modulate its expression.
Interferon (IFN)-γ-induced protein 10 kDa (IP-10) mRNA was non-detectable in P-GMSCs. PoPEx completely suppressed its expression in both lines. LPS significantly upregulated IP-10 in P-GMSCs about 150 times (p < 0.005) but had no effect in H-GMSCs. PoPEx had little augmenting effect in LPS-stimulated H-GMSCs (p < 0.05), in contrast to the additional upregulation of IP-10 in LPS-treated P-GMSCs (p < 0.005).
Regulated upon activation, normal T-cell expressed and secreted (RANTES) mRNA expression was completely downregulated in H-GMSCs by PoPEx (p < 0.05). However, it was significantly increased by higher extract concentrations about 60 times in P-GMSCs (p < 0.005). LPS upregulated RANTES in H-GMSCs more strongly (p < 0.005) in comparison with P-GMSCs (p < 0.01). PoPEX upregulated RANTES in LPS-stimulated P-GMSCs, in a dose-dependent manner (p < 0.005), and almost completely inhibited its expression in LPS-stimulated H-GMSCs (p < 0.005).
2.6. Effect of PoPEx on the Expression of Genes Associated with Tissue Regeneration/Repair in Control and LPS-Stimulated GMSCs
Six genes associated with tissue regeneration/repair in GMSCs were studied, and the results are presented in Figure 6.
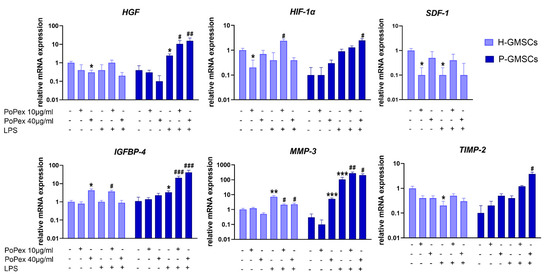
Figure 6.
The effect of PoPEx on the expression of HGF, HIF-α, SDF-1, IGFB4, MMP-3, and TIMP-2 mRNA in H- and P-GMSCs. Values are given as mean ± SD (n = 3). * p < 0.05; ** p < 0.01; *** p < 0.005 compared to corresponding controls (non-treated H- or P-GMSCs). # p < 0.05; ## p < 0.01; ### p < 0.005 compared to corresponding LPS-stimulated GMSCs.
PoPEx inhibited the basal expression of stromal cell-derived factor (SDF)-1 and hepatocyte growth factor (HGF) mRNA in H-GMSCs (p < 0.05). Both genes were almost non-detectable in P-GMSCs, nor did PoPEx significantly modulate their expression. LPS inhibited SDF-1 in H-GMSC and augmented HGF in P-GMSCs (p < 0.05). PoPEx did not modulate SDF-1 expression in both LPS-stimulated GMSC lines but additionally upregulated HGF mRNA expression in LPS-stimulated P-GMSCs, in a dose-dependent manner for up to three to six times (p < 0.05 and p < 0.01, respectively).
Hypoxia-inducible factor (HIF)-1α mRNA was hardly detectable in P-GMSCs, and LPS did not significantly modify its expression. Lower concentrations of PoPEx inhibited basal expression of HIF-1α in control H-GMSCs but potentiated its expression in LPS-stimulated P-GMSCs (p < 0.05). Similarly, higher concentrations of PoPEx upregulated HIF-1α mRNA in LPS-stimulated P-GMSCs.
Higher concentrations of PoPEx upregulated insulin-like growth factor binding protein 4 (IGFBP4) mRNA expression in H-GMSCs, as did LPS in P-GMSCs (p < 0.05). PoPEx augmented IGFBP4 expression in LPS-stimulated P-GMSCs, in a dose-dependent manner (p < 0.005). A much lower stimulatory effect was seen with lower concentrations of the extract in LPS-stimulated H-GMSCs (p < 0.05).
Matrix metalloproteinase (MMP)-3 mRNA was identified at baseline levels in both GMSC lines. Its inhibitor, tissue inhibitor of metalloproteinase (TIMP)-2, was almost undetected in P-GMSCs. Higher concentrations of PoPEx stimulated MMP-3 expression in P-GMSCs (p < 0.05). LPS inhibited TIMP-2 mRNA in H-GMSCs (p < 0.05) but significantly increased MMP-3 expression about seven times in H-GMSCs and more than 100 times in P-GMSCs (p < 0.005). PoPEx treatment downregulated the LPS-induced expression of MMP-3 in H-GMSCs (p < 0.05) but potentiated its expression in P-GMSCs (p < 0.01 and p < 0.05, respectively). The extract had only a slight stimulatory effect on TIMP-2 in LPS-stimulated P-GMSCs (p < 0.05). As a result, the MMP-3/TIMP-2 ratio was decreased from about 260 in LPS-stimulated P-GMSCs to about 70 after treatment of these cultures with PoPEx (40 µg/mL).
2.7. Effect of PoPEx on the Expression of Genes Associated with Osteoblastic Differentiation in Control and LPS-Stimulated GMSCs
Three genes involved in the early phase of osteoblastogenesis and a gene inhibiting osteoclastogenesis were studied in GMSC lines, and the results are presented in Figure 7.
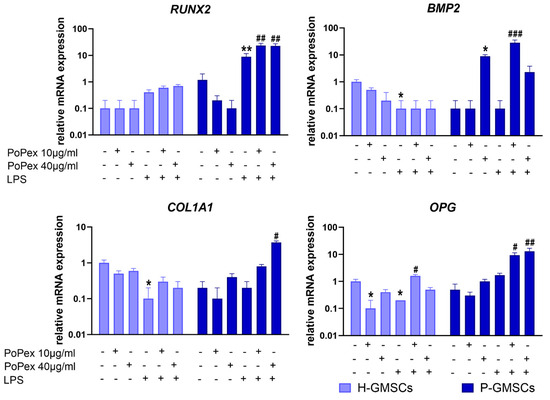
Figure 7.
The effect of PoPEx on the expression of RUNX2, BMP2, COL1A1, and OPG mRNA in H- and P-GMSCs. Values are given as mean ± SD (n = 3). * p < 0.05; ** p < 0.01; compared to corresponding controls (non-treated H- or P-GMSCs). # p < 0.05; ## p < 0.01; ### p < 0.005 compared to corresponding LPS-stimulated GMSCs.
Runt-related transcription factor 2 (RUNX2) mRNA was almost non-detectable in H-GMSCs or significantly modulated either by PoPEx or LPS treatment. LPS significantly upregulated (about 8 times) RUNX2 mRNA expression in P-GMSCs (p < 0.01), and the effect was significantly augmented by PoPEx (about 2.5 times, compared to LPS treatment) (p < 0.01).
LPS inhibited the basal expression of bone morphogenetic protein 2 (BMP2) mRNA in H-GMSCs (p < 0.05). Higher concentrations of PoPEx upregulated BMP2 mRNA expression in P-GMSCs (p < 0.05), as did lower concentrations of the extract in LPS-stimulated P-GMSCs (p < 0.005).
LPS inhibited the basal expression of Collagen, Type I, Alpha 1 (COL1A1) in H-GMSC lines (p < 0.05), in contrast to PoPEx, which was ineffective. PoPEx and LPS did not modulate the basal expression of COL1A1 in P-GMSCs when applied separately. However, higher concentrations of PoPEx significantly increased its expression in LPS-stimulated P-GMSCs (p < 0.05).
Lower concentrations of PoPEx and LPS applied individually inhibited the basal expression of osteoprotegerin (OPG) mRNA in H-GMSCs (p < 0.05). However, lower concentrations of PoPEx increased its expression in LPS-stimulated H-GMSCs. In P-GMSC cultures, PoPEx increased OPG mRNA expression in LPS-stimulated P-GMSC cultures (p < 0.05 and p < 0.01, respectively).
3. Discussion
In this study, we examined the impact of PoPEx on the cytotoxicity, proliferative capacity, and expression of 24 genes associated with various functions of GMSCs. Two GMSC lines were established from healthy gingiva and periodontitis-affected gingiva using the methodology we previously employed [15]. Additionally, one line from each gingival tissue was used from our previous experiment’s cell bank. Both H-GMSCs and P-GMSCs met the criteria necessary for a cell population to possess MSC characteristics, including a fibroblast-like appearance, adherence to substrates, colony-forming ability, characteristic phenotypic profile, and the capacity for differentiation into three mesenchymal cell lineages [36].
A subpopulation of our lines retained the expression of stem cell markers (STRO-1 and SSEA4) [37] and pericyte markers (PDGFR and NG2) even after prolonged passaging (up to eight passages). Through the use of high-affinity antibodies targeting the CD146 molecule (expressed on endothelial cells and pericytes) in combination with an antibody against the CD31 molecule (an endothelial marker) labeled with fluorescent markers suitable for confocal microscopy, we demonstrated in this study that CD146+ GMSCs originate from pericytes rather than endothelial cells. The network of small blood vessels was particularly prominent in the lamina propria of the gingiva affected by periodontal disease. Our findings also support the hypothesis that pericytes are the predominant—if not the sole—in vivo source of tissue in MSCs [38].
Similarly to the findings of our previous study [15] and studies conducted by other researchers [14], we demonstrated that the differentiation potential of GMSCs into adipocytes and chondroblasts is relatively low, while it is high towards differentiation into osteoblasts. The higher differentiation potential of P-GMSCs into osteoblasts compared to H-GMSCs is in line with the results of other authors [39]. However, it contradicts some other published data [40]. The reason for this disparity may be attributed to differences in tissue selection and quality, isolation, and propagation procedures of MSCs, as well as general conditions of their cultivation.
The cytotoxicity of PoPEx in this study was similar to the cytotoxicity observed in human peripheral blood mononuclear cells (PBMC) that we showed previously [27]. However, our results contradict previous results about the relative resistance of normal cells to cytotoxicity by PoPEx or its polyphenols, in contrast to the sensitivity of cancer cells [41]. The toxic effect of different plant derivatives in cell cultures is a well-known phenomenon, and according to publications, it critically depends upon the dose and cells (cell lines) used [42]. Therefore, testing cytotoxicity before using biologically active plant (food) extracts is necessary to find optimal concentrations for in vitro studies. Although we showed that lower and higher concentrations showed different, sometimes opposite effects, generally, higher concentrations had stronger modulatory activity.
The reason for investigating the effect of PoPEx in GMSCs culture has already been emphasized in the Introduction. So far, pomegranate extract has been studied in cases of gingivitis/periodontitis, showing positive effects [20,31,33]. On the other hand, our previous results demonstrated that GMSCs derived from periodontitis-affected gingiva retain various characteristics of the proinflammatory microenvironment from which they were isolated [15]. Therefore, comparing the effects of PoPEx on GMSCs from healthy and diseased gingiva can significantly contribute to understanding the mechanisms of action of biologically active components from the extract while shedding light on the role of GMSCs in physiological and pathological conditions. The design of this study also included LPS due to its proven role, as an endotoxin from periodontal microorganisms, in stimulating inflammatory processes in periodontitis, including its multiple effects on MSCs [43]. Thus, the stimulation of H-GMSCs with LPS imitates the response of these cells in the initial phase of periodontitis. On the other hand, the stimulation of P-GMSCs with LPS may represent the response of GMSCs during the exacerbation of chronic periodontitis.
Bearing in mind that the biological effects of dietary polyphenols are dose-dependent [44,45], we tested two different concentrations (lower one—10 µg/mL—which was the lowest effective concentration in our preliminary experiments; higher one—40 µg/mL—which is a maximal non-cytotoxic concentration). However, to our surprise, PoPEx exerted different effects on particular gene expression depending on the type of GMSCs and the applied concentration of the extract. Generally, PoPEx (mostly at higher concentrations) inhibited the basal expression of most proinflammatory genes (IL-6, MCP-1, GRO-α), genes involved in immunomodulation (RANTES, IP-10), and genes involved in tissue regeneration/repair (HIF-1α, SDF-1, HGF) in H-GMSCs. However, PoPEx increased the expression of IL-8, TLR3, TGF-β, TGF-β/LAP ratio, IDO-1, and IGFB4.
IL-6 is the dominant proinflammatory mediator in chronic periodontitis, affecting various functions of both specific and nonspecific immune cells. However, under certain circumstances, it can have anti-inflammatory properties [46]. GRO-α and IL-8 are potent chemoattractants for neutrophils [47]. MCP-1 is a chemoattractant for monocytes/ macrophages, while RANTES is a T-cell chemokine [48]. IP-10 (CXCL10) chemokine, which is produced in response to interferon (IFN)-γ, induces chemotaxis, especially inflammatory T helper 1 (Th1) cells into inflamed gingival tissue, stimulates apoptosis and cell proliferation (upon binding to CXCR3A), but inhibited angiogenesis or cell proliferation (upon binding to CXCR3B) [49,50]. These results suggest that under physiological conditions PoPEx tends to suppress most genes in GMSCs involved in inflammation, which agrees with many results published on other cells [51,52,53].
In contrast, upregulation of TLR-3, TGF-β, TGF-β/LAP ratio, and IDO-1 genes suggest that PoPEx enhanced immunosuppressive properties of H-GMSCs, a new phenomenon not published up to now. Increased expression of TLR3 mRNA, a dsRNA ligand, is associated with a greater ability to direct MSCs toward the immunosuppressive MSC2 phenotype [54]. On the other hand, IDO-1, the enzyme catalyzing the degradation of L-tryptophan into L-kynurenine [55], represents a key target of immunosuppressive mechanisms. TGF-β is one of the main immunosuppressive cytokines [56]. However, it controls proliferation, cell differentiation, apoptosis, angiogenesis, and different immune-mediated mechanisms. Therefore, the role of secreted TGF-β, which is also a product of MSCs [57], is very complex. In this context, the secreted TGF-β is bound to a latent complex consisting of LAP and latent TGF-β-binding protein (LTBP). LAP maintains the latency of TGF-β, while LTBP converts the latent form of TGF-β into the active form [58]. Therefore, an increase in the TGF-β/LAP ratio by PoPEx in H-GMSCs suggests that biologically active components from the extract promote TGF-β activity and thus additionally increase the immunomodulatory properties of these cells. GMSCs, like other MSCs, are also key players in tissue proliferation and regeneration, including regeneration/remodeling of inflamed periodontal tissue [14,16,59].
HIF-1α induces the transcription of many genes involved in increasing oxygen delivery in hypoxic tissues, accumulation of reactive oxygen species (ROS), MSC migration, inflammation, and tissue repair [60]. It upregulates SDF-1 (CXCL12 chemokine). SDF-1 promotes the recruitment and proliferation of MSCs, increases their osteogenic differentiation [61], stimulates angiogenesis [62], and down-modulates inflammation [63]. HGF is a multifunctional paracrine biomolecule that plays a role in tissue regeneration [64] and cell proliferation [65]. It has been shown that MSCs secrete HGF in the presence of inflammatory stimuli [66]. The inhibition of HIF-1, SDF-1, and HGF genes by PoPEx in H-GMSCs aligns with the concept of the anti-inflammatory effect and suppressive action of this extract on the processes of cell proliferation and regeneration of healthy gingival tissue. IGFBP4, an inhibitor of IGF, plays an important role in many cellular processes in MSCs, including cell growth, differentiation, bone metabolism, and cellular senescence [67,68]. In this context, increased expression of the IGFBP4 gene in PoPEx-treated H-GMSCs may be relevant for inhibition of GMSC proliferation, differentiation, and survival [67,69], and/or modulation of T regulatory cells (Tregs), key players in immunomodulation [70].
In contrast to H-GMSCs, PoPEx upregulated IL-6, RANTES, and MMP3 in P-GMSCs, did not modify IL-8, MCP-1, and most genes involved in immunomodulation and tissue repair, but inhibited TLR2 and GRO-α. These results suggest that GMSCs established from an inflammatory microenvironment (periodontitis) respond differently to PoPEx. In fact, P-GMSCs somehow retain their functional characteristics in vivo. It can be postulated that in the inflammatory microenvironment during periodontitis, their proinflammatory role is desirable as a defense mechanism against periopathogenic microorganisms. The effect of PoPEx could be additionally supportive in that way. However, it is not clear the significance of increased expression of MMP-3. MMP-3, as a member of the MMP family, is involved in the degradation of extracellular matrix (ECM) components. The natural inhibitors of MMP are TIMPs, and their mutual balance is crucial in maintaining the integrity of the ECM [71]. Since MMP-3 is involved in different physiological and pathological processes, including accumulation of inflammatory cells, stimulation of neo-angiogenesis, and supporting osteoclast differentiation simultaneously with inhibition of osteoblastogenesis and MSC proliferation, PoPEx could support all these mechanisms in P-GMSCs which are relevant for the inflammation control in chronic periodontitis.
The effect of LPS has been investigated on different types of dental MSCs, with the least research conducted on GMSCs [43,72]. Our results showed an inhibitory effect of LPS on the proliferation of both types of GMSCs. Even when applied in small concentrations, LPS significantly stimulates the expression of genes involved in inflammatory processes (IL-6, IL-8, GRO-α, COX-2), immunomodulation (IDO-1, RANTES, TLR3), and extracellular matrix degradation (MMP-3, MMP-3/TIMP2 ratio). The expression of certain genes was differently modulated in the cell lines: increased expression of MCP-1, TLR2, IGFB4, and HGF genes only in P-GMSCs; inhibited expression of TLR4, SDF1, and TIMP2, and increased expression of NFKB1 genes only in H-GMSCs. These findings suggest that LPS stimulates the proinflammatory functions of GMSCs, manifested by upregulation of proinflammatory cytokines/chemokines, similarly as demonstrated in other publications [73,74,75,76]. However, the modulatory potential of LPS also depended on whether GMSCs were isolated/established from healthy or inflamed gingiva.
Increased expression of NFKB1 and COX-2 aligns with the findings of cytokines/chemokines genes. NFKB1 encodes a 105 kD Rel protein-specific transcription inhibitor, which is processed by a proteasome to produce a 50 kD protein, a DNA binding subunit of the NF-kB protein complex. NF-kB is a transcription regulator that is activated by different signals originating from intra- and extra-cellular sources, ROS, bacterial or viral products, and many other stimuli. Upon activation, NF-kB is translocated into the nucleus, where it stimulates the expression of genes involved in different biological functions, including the production of proinflammatory cytokines/chemokines and cytokines involved in the immune response [71,77]. COX-2, a key enzyme mediating prostaglandin synthesis (PGE2) and its gene, is an early response gene for many proinflammatory stimuli [78].
Treatment of MSCs with LPS was followed by increased expression of some genes involved in their immunosuppressive functions, such as IDO-1, TLR3, and RANTES. As already mentioned, signaling through TLR3 stimulates differentiation of immunosuppressive MSC2 type, and the process was additionally enhanced by LPS-induced downregulation of TLR4 [54], similarly as we obtained in our study. An increased COX-2 gene expression by LPS and subsequent PGE2 production contributes to the immunosuppressive capability of GMSCs. Namely, it is known that MSC-produced PGE2 exerts numerous immunosuppressive effects on dendritic cells, macrophages, and T cells while maintaining the basic functions of MSCs, such as proliferation, migration, and differentiation [79]. Therefore, LPS preserves/increases the immunosuppressive properties of GMSCs which is opposite to its proinflammatory role. In addition, LPS can stimulate extracellular matrix degradation by increasing MMP-3 mRNA expression and MMP-3/TIMP2 ratio in GMSCs. This finding is in line with the fact that bacterial endotoxin is an important factor in the pathogenesis of chronic periodontitis [80].
Regarding the impact of LPS on MSCs’ osteoblastogenesis, it has been observed that higher concentrations of LPS have an inhibitory effect on the expression of numerous genes involved in these processes, such as RUNX2, BMP2, Alkaline Phosphatase (ALP), COL1A1, and others. On the other hand, lower concentrations (less than 1 µg/mL) have a stimulatory effect [43,81]. We used lower LPS concentrations in our study because they are more physiologically relevant for modeling the course of chronic periodontitis. Since our aim was not to study osteoblastogenesis, a long culture process, we concentrated on early osteoblastic genes (RUNX2, BMP2, and COL1A1). Our results showed that PoPEx had no significant effect on the expression of these genes in unstimulated GMSCs, except for an upregulation of BMP2 in P-GMSCs. LPS increased the expression of RUNX2 in P-GMSCs and decreased the expression of BMP2 in both lines. In addition, LPS decreased the expression of COL1A1 and OPG in H-GMSCs. These results support the concept that H- and P-GMSCs had different basal characteristics, that even lower concentrations of LPS downregulated osteoblastogenesis in GMSCs from healthy gingiva and that LPS stimulated osteoblastogenesis in GMSCs established from inflamed gingiva. This conclusion is based on the findings that RUNX2 is the earliest osteogenic transcription factor [82]. Our study also showed that PoPEx significantly stimulates LPS-induced osteoblastogenesis in P-GMSCs by upregulating RUNX2, BMP2, and COL1A1, in contrast to H-GMSCs, where such an effect was not visible. In addition, OPG was upregulated. It is known that OPG is a soluble decoy receptor for the receptor activator of nuclear factor-kappa B ligand (RANKL). By blocking RANKL, OPG inhibits osteoclast formation and activity and stimulates osteoblast differentiation [83].
This was not the only key difference in the effect of PoPEx on the LPS-treated GMSCs between the H and P lines. PoPEx potentiated immunosuppressive properties of LPS-stimulated P-GMSCs, as judged by the upregulation of IDO, TGF-β, TLR3, and COX-2 genes compared to LPS treatment alone. However, in H-GMSCs, higher concentrations of PoPEx inhibited IDO-1, TGF-β, and COX-2 gene expression. Although explaining these differences requires additional investigation, it can be assumed that PoPEx can enhance the immune response in the early stage of periodontitis (model of LPS treatment of H-GMSCs). In contrast, during the exacerbation of chronic periodontitis (model of LPS treatment of P-GMSCs), PoPEx enhanced the immunosuppressive mechanisms, which could be beneficial for the resolution of inflammation and restriction of overactivated immune responses. Although PoPEx increased the expression of both MMP-3 and TIMP-2 in LPS-stimulated P-GMSCs, the MMP-3/TIMP-2 ratio was lower than the index in only LPS-stimulated P-GMSC cultures, suggesting that PoPEx tends to restrict tissue destruction in chronic periodontitis. At the same time, stimulation of genes encoding proinflammatory cytokines and chemokines, including IP-10 and RANTES, allows the unhindered development or even enhancement of host defense mechanisms at both stages of periodontitis. The story regarding other genes such as IGFB4, HGF, and HIF-1α, which were upregulated in LPS-stimulated P-GMSCs and were differently modulated in LPS-stimulated H-GMSCs is more complicated and needs additional investigations, bearing in mind the complexity of functions that the genes control.
In conclusion, our results suggest that PoPEx differently modulated the expression of genes in GMSCs under basal conditions and inflammatory microenvironment, mimicked by the treatment of GMSCs by LPS. The differences also existed whether GMSCs were established from healthy or periodontitis-affected gingival tissues. PoPEx treatment of unstimulated H-GMSCs was followed by inhibition of most genes associated with inflammation and enhancement of genes involved in immunosuppression. In contrast, PoPEx treatment was the most effective in the upregulation of genes for proinflammatory cytokines and chemokines, additional upregulation of genes associated with the suppression of the immune responses, genes involved in tissue regeneration and repair, and early genes involved in the stimulation of osteogenesis in LPS-stimulated P-GMSCs. The study has limitations in that gene expression dynamics were not investigated, the gene expression was not correlated with its products, and other functions of PoPEx-treated GMSCs were not investigated especially their differentiation possibilities. However, these results are starting points to understand better the particular role of individual components from the extract, especially punicalagin and ellagic acid, which are mostly investigated in different biological systems [84,85] but little on MSCs. In addition, the obtained results may further elucidate the complex role of GMSCs in health and inflammatory diseases and better understand the possible application of PoPEx and its constituents, in the form of mouthwash or oral/gingival gel for preventing gingivitis and periodontitis and treating chronic periodontitis as an adjunct therapeutic modality.
4. Materials and Methods
4.1. Tissue Donors and General Study Design
This was a collaborative study conducted at the Medical Faculty University of Banja Luka, Bosnia and Herzegovina, Institute for the Application of Nuclear Energy Research (INEP), University of Belgrade, Serbia (Laboratory part of the study), and Clinic for Dentistry (Department for Oral Surgery), and Military Medical Academy (MMA), Belgrade, Serbia (Clinical part of the study). Gingival tissue samples were collected from three donors with chronic periodontitis and three donors with healthy gingiva after written informed consent was obtained from the donors. Clinically healthy gingival samples were collected from subjects (male) who had no history of periodontal disease and smoking, aged 28, 36, and 40 years, respectively.
The periodontitis group patients (male) were 38, 45, and 48 years old. Periodontitis was diagnosed according to the American Academy of Periodontology (AAP) Classification of Disease. Staging and gradation of the disease were performed according to the Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions [1]. All patients were classified as stage II-based. They had clinical attachment loss (CAL) of 4 mm at the site of the most significant loss, and the mean maximum probing depth was 4.33 mm. All patients were classified as periodontitis grade A (slow rate of progression). The subjects from both groups were non-smokers. The chronic periodontitis patients had no diabetes, malignant diseases, or systemic autoimmune diseases and did not receive antibiotics, vitamin supplements, or immunosuppressive drugs for two months before tissue sampling. Other data from the medical history of study participants were not recorded. Periodontitis gingival specimens were obtained during the flap debridement procedure, whereas healthy gingival tissues were taken during tooth extraction for orthodontic purposes.
A part of the gingival tissue from the donors was subjected to classical pathohistological processing. The paraffin sections were stained with hematoxylin and eosin (H&E) (Sigma-Aldrich, Darmstadt, Germany) and analyzed under a light microscope (Olympus, Hamburg, Germany).
4.2. Establishment of GMSC Lines
GMSC lines were established by the procedure that we have already published [15]. Gingival tissues were washed in phosphate-buffered saline (PBS). After that, the epithelial cell layer was removed by scalpel, minced with fine scissors, and digested with collagenase type II (5 µg/mL) and DNAse (40 IU/mL) in serum-free α-MEM for two hours at 37 °C and 5% CO2 in a cell incubator. All components were from Sigma-Aldrich, Darmstadt, Germany. The softened tissue was then gently pressed through a 30 µm nylon mesh, rinsed with α-MEM medium, and centrifuged at 1800 rpm for 10 min. The cell suspension was placed in 24-well cell culture dishes (Sarstedt, Numbrecht, Germany) at a density of 2000 per cm2 and cultured in a complete MSC medium. The medium contained α-MEM, 10% fetal calf serum (FCS), 100 IU/mL penicillin, 50 μg/mL streptomycin, 2.5 μg/mL amphotericin B (all from Thermo Fisher Scientific, Dreieich, Germany), 1% sodium pyruvate, and 100 µM L-ascorbate-2-phosphate (both from Sigma-Aldrich, Darmstadt, Germany). After three days, non-adherent cells were removed by washing the wells with α-MEM medium, followed by replacing the complete culture medium twice a week. When cell layers reached confluency, the detachment from the plastic was performed by treating the cells with 0.02% trypsin/0.02% Na EDTA (Sigma-Aldrich, Darmstadt, Germany) in PBS. The harvested cells were plated in 6-well cell culture dishes at a 5000 per 1 cm2 density in the complete culture medium without amphotericin B. The cells, which are further classified as GMSCs, were used for the experiments after the 4th passage.
4.3. In Vitro Differentiation of GMSCs
To determine osteogenic differentiation (OD), H- and P-GMSCs were plated at a density of 6 × 104 cells on plastic coverslips inserted into six-well plates until they reached confluence. Then, GMSCs were cultured for 21 days in the complete α-MEM culture medium supplemented with 10% 10 nM dexamethasone (Galenika, Belgrade, Serbia), 10 mM glycerophosphate and 0.05 mM ascorbic acid (both from Sigma-Aldrich, Darmstadt, Germany). The osteogenic medium was changed twice a week. At the end of the cultivation period, coverslips were washed with PBS, fixed with 4% paraformaldehyde for 60 min at room temperature, washed twice with distilled water, and stained with 2% Alizarin Red (Sigma-Aldrich, Darmstadt, Germany) for 45 min. Finally, the coverslips were mounted on microscopic slides.
To induce adipogenic differentiation (AD), confluent monolayers of GMSCs were cultured for 21 days in the complete α-MEM medium supplemented with 0.5 µM dexamethasone (Galenika, Belgrade, Serbia), 0.5 µM isobutyl-methylxanthine (IBMX), (Sigma-Aldrich, Darmstadt, Germany) and with 50 µM indomethacin (R&D Systems, Minneapolis, MN, USA). The complete cultivation procedure was the same as that described for OD. Coverslips were washed with 60% isopropanol for 5 min and stained with 0.3% Oil Red O (Sigma-Aldrich, Darmstadt, Germany). At the end, the coverslips were washed with tap water stained with hematoxylin for 1 min and mounted on microscopic slides.
For chondrogenic differentiation (CD), GMSCs (5 × 105) were placed in Eppendorf tubes and pelleted in by centrifugation (1800 rpm) for 10 min. The cell pellet was cultivated in the complete α-MEM medium supplemented with TGF-β3 (10 ng/mL) (R&D Systems, Minneapolis, MN, USA), dexamethasone (100 nM) (Galenika, Belgrade, Serbia), and ascorbic acid (50 ng/mL) (Sigma-Aldrich, Darmstadt, Germany) for 21 days. After cultivation, the cell pellets were cryopreserved in an embedding medium (Bio-Optica, Milan, Italy), frozen at −80 °C. Cryostat sections (Leica Biosystems, Barcelona, Spain) were air-dried and fixed with 4% paraformaldehyde, and stained with Alcian blue (Sigma-Aldrich, Darmstadt, Germany). The stained sections were washed with distilled water and counterstained with 0.1% Nuclear Fast Red solution (Sigma-Aldrich, Darmstadt, Germany). Negative controls for all differentiation procedures were GMSCs cultured in the complete basal α-MEM medium.
The stained cells/sections were observed under a light optical microscope (Olympus, Hamburg, Germany). All images were analyzed offline in ImageJ 1.47u software (National Institutes of Health, Bethesda, MD, USA). Semiquantitative analysis was performed as follows: Index 0—no visible positivity; Index 1—mild positivity of individual cells and the presence of 1–2 smaller mineralized islets (OD) or 1–2 positive cells (AD and CD) in at least 1 of the 10 analyzed microscopic fields; Index 2—mild positivity of individual cells and the presence of up to 5 small and medium-sized mineralized islets (OD) or up to 5 positive cells (AD and CD) in at least 2 of the 10 analyzed microscopic fields; Index 3—presence of up to 10 mineralized islets of different sizes (OD) or up to 10 positive cells (AD and CD) in at least 5 of the 10 analyzed microscopic fields; Index 4—presence of mineralized nuclei of all sizes, some merged (OD) or most positive cells (AD and CD) on all 10 analyzed microscopic fields.
4.4. Flow Cytometry
Phenotypic analysis of H-GMSCs and P-GMSCs was performed using monoclonal antibodies (mAbs) and flow cytometry. The cells were stained by the following mAbs using dilutions recommended by the manufacturer. Anti-CD14-FITC (63D3), anti-STRO-1-FITC (STRO-1), anti-CD45-APC (HI30), anti-CD90-PE (5E10), anti-CD73-biotin (AD2), anti-SSEA-4-biotin (MC-813-70), anti-CD166-PE (3A6), purified anti-NG2 (MEL62), and anti-CD105-APC (43A3) were obtained from all from BioLegend, Basel, Switzerland. Anti-PDGFRβ-Alexa Fluor 546 (D-6), anti-CD146-Alexa Fluor 488 (P1H12) (both from Santa Cruz Biotechnology, Dallas, TX, USA), anti-CD39-FITC (eBioA1) (eBioscience, San Diego, CA, USA), anti-CD34-FITC (581) (Elabscience, Wuhan, China), and anti-CD44-APCCy7 (IM7) were obtained from Sigma-Aldrich, Darmstadt, Germany. Anti-CD45-APC (HI30), anti-CD19-FITC (HIB19), and anti-CD3-PE (UCHT1) were from BioLegend, Basel, Switzerland. Streptavidin-APC and streptavidin APCCy7 were from BioLegend, Basel, Switzerland, whereas anti-mouse IgG and rabbit anti-goat polyclonal antibody-Alexa Fluor 488 were from Sigma-Aldrich, Darmstadt, Germany, and Abcam, Cambridge, UK, respectively. Negative isotype controls (mAbs conjugated with corresponding fluorochromes) were purchased from BioLegend, Basel, Switzerland. The mAbs were diluted in 2% FCS/0.01% NaN3 in PBS and incubated with the cells for 30 min at 4 °C. The labeled cells were analyzed on a BD LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The doublets were excluded according to forward scatter (FSC)-A/FSC-H. More than 5000 gated cells were analyzed according to their specific FSC-A/side-scatter (SSC)-A properties. Signal overlaps between the channels were compensated before each experiment using single labeled cells, and non-specific fluorescence was determined by using the appropriate isotype controls. The acquired data were analyzed offline in the FlowJoVX program (BD Biosciences, Franklin Lakes, NJ, USA).
4.5. Immunohistochemistry and Confocal Microscopy
The gingival tissue was sectioned using a cryostat (Leica CM 1950, Wetzlar, Germany). Tissue sections (approximately 6 µm thick) were incubated with anti-CD146 mAb followed by peroxidase-conjugated anti-rabbit Ig. Both Ab were purchased from Abcam, Cambridge, UK. The reaction was visualized using diaminobenzidine (Sigma-Aldrich, Darmstadt, Germany). Immunohistochemical analysis was conducted using a light microscope (Olympus, Hamburg, Germany). For further identification of CD146+ cells, gingival tissue sections were stained with anti-CD146 AlexaFluor 488 (Santa Cruz Biotechnology, Dallas, TX, USA) and anti-CD31 AlexaFluor 433 (ThermoFisher Scientific, Dreieich, Germany) mAbs, followed by Syto59 nuclear stain (ThermoFisher). Analysis was performed using a confocal microscope (Zeiss LSM 510/Axiovert 200 M, Jena, Germany). DAPI (4′,6-diamidino-2-phenylindole) (ThermoFisher Scientific, Dreieich, Germany) was used to counterstain nuclei.
4.6. Preparation and Analysis of PoPEx
The detailed procedure for preparing and analyzing PoPEx was published in our previous paper [27]. The powdered pomegranate peel was prepared from pomegranate fruits collected at a natural locality in southern Bosnia and Herzegovina. The peel was extracted with 50% ethanol, using 1:10 as a solid-to-solvent ratio. After filtration and evaporation, the extract was analyzed spectrophotometrically by the Folin–Ciocalteu method, where gallic acid was used to prepare the calibration curve. The results were expressed as mg of gallic acid equivalents per gram of dry weight. The pomegranate peel was deposited in the Botanical Garden “Jevremovac” University of Belgrade (voucher specimen No. BEOU 17742). HPLC analysis was performed on Agilent 1200 RR HPLC (Agilent, Waldbronn, Germany), equipped with a DAD detector, using reverse-phase analytical column Zorbax SB-C18 (Agilent, Waldbronn, Germany). Detection was performed at 260 and 320 nm. The quantity of analyzed compounds (punicalagin, punicalin, gallic acid, and ellagic acid) was calculated using calibration curves of authentic standards (Supplementary Figure S4). The results are expressed as mg per gram of dry weight. Experiments were repeated three times. Their mean content was as follows: punicalagin 67.26 ± 0.81 mg/g, punicalin 31.91 ± 0.22 mg/g, ellagic acid 25.11 ± 0.06 mg/g, and gallic acid 9.75 ± 0.05 mg/g.
4.7. MTT and Proliferation Assays
GMSCs were cultivated in 96-well plates (5 × 104/well; triplicates), in either the complete α-MEM medium or the medium with different dilutions of PoPEx. After a 48 h incubation period, the solution of 3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl tetrazolium bromide (MTT) (Sigma-Aldrich) (100 μL/well, final concentration 100 μg/mL), was added to the wells. Wells containing only different concentrations of PoPEx in the complete α-MEM medium were used to test the interaction of MTT-developed color with the extract. The wells with MTT served as blank controls. The plates were incubated with MTT for 4 h in an incubator at 37 °C, and after that, the formazan crystals were dissolved with 0.1N HCl/10% SDS (sodium dodecyl sulfate) (100 μL/well) overnight. The developed color’s optical density (OD) was read at 570/650 nm (ELISA reader, Behring II, Marburg, Germany). The results were presented as the relative metabolic activity in PoPEx-treated cultures compared to the metabolic activity of control cultures without PoPEx, where OD was used as 100%. The relative metabolic activity was calculated as follows: metabolic activity (%) = (OD of cultures with PoPEx − OD with PoPEx without cells/OD of control cultures without PoPEx − OD of medium without cells) × 100.
In the proliferation assay, GMSCs (2.5 × 104/well; triplicates) were incubated with PoPEx (two different concentrations) with or without LPS (50 ng/mL) (Sigma) in the complete α-MEM medium for 3 days. Control cultures were GMSCs without PoPEx. After cultivation, cells were treated with 0.25% trypsin to detach the cells from the plastic. The released cells were pelleted by centrifugation, and after that, the number of cells in each well was calculated by a cytometer. Trypan blue was used to detect cell viability which was about 98%, in each well. The relative proliferation of cells (cell growth) was expressed based on the number of recovered cells compared to the number of cells in control cultures, used as 100%. The calculation was as follows: relative proliferation (%) = number of cells in experimental cultures/number of cells in control cultures × 100.
4.8. Real-Time Quantitative Polymerase Chain Reaction
GMSCs were cultivated in plastic flasks (bottom square 25 cm2) (Sarsted, Dordreht, Germany) until confluence (usually for two days). After that, each flask was treated with 10 µg/mL or 40 µg/mL of PoPEx, with or without LPS (50 ng/mL). GMSCs cultivated alone served as controls. After 24 h, the cells were detached from the plastic substrate with 0.25% trypsin, pelleted by centrifugation, and stored in Trizol reagent (Thermo Fisher Scientific, Dreieich, Germany) at −80 °C until analysis. Total RNA was extracted from cultured cells using the Total RNA Purification Mini Spin Kit (Genaxxon Bioscience GmbH, Ulm, Germany) following the manufacturer’s protocol. A high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific, Dreieich, Germany) was used to transcribe 0.1 µg of isolated RNA as a template. The synthesized cDNA was then subjected to Real-Time Quantitative Polymerase Chain Reaction (qPCR) using a SYBR Green PCR Master Mix (Thermo Fisher Scientific, Dreieich, Germany) in a 7500 real-time PCR machine (Applied Biosystems, Waltham, MA, USA). The conditions were: 10 min at 95 °C activation, 40 cycles of 15 s at 95 °C and 60 s at 60 °C. The results were normalized against β-actin for each sample and expressed as a relative target abundance (versus the non-treated sample of each cell line) using the 2−ΔΔCt method [86]. To compare differences in the expression of each marker between H-GMSCs and P-GMSCs upon PoPEx, LPS, or PoPEx-LPS treatment, mRNA expression of each marker was calculated for each cell line as a fold change of basic level expression used as 1. To compare variances in basic expression levels of analyzed markers between non-treated H-GMSCs and P-GMSCs, the expression of each marker on non-treated cells was calculated as fold change of marker expression in H-GMSCs of one donor used as 1. Primers used in the study are listed in Table 2. All primers were purchased from Thermo Fisher Scientific, Dreieich, Germany.

Table 2.
Sequences of the primer pairs used for the real-time PCR experiments.
4.9. Statistics
To assess differences between H- and P-GMSCs parameters or between experimental and appropriate control samples Kruskal–Wallis or Mann–Whitney tests were used. Differences in mRNA expression between untreated, LPS-stimulated, PoPEx-treated, and LPS + PoPEx-treated GMSCs were analyzed using a ratio-paired t-test or Wilcoxon test. Values at p < 0.05 or less were considered to be statistically significant. The statistical analysis and graphs were performed in GraphPad Prism version 8.0.0 (GraphPad Software, San Diego, CA, USA).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms242015407/s1.
Author Contributions
Conceptualization: M.Č. and R.Š.; Data curation: N.M., A.K., M.B., J.Đ., S.T. and D.R.; Formal analysis: M.B., M.R., J.Đ., S.T. and D.R.; Funding acquisition: M.Č. and R.Š.; Investigation: N.M., A.K., M.B., J.Đ., S.T., M.E., D.R., M.R., B.I. and M.Č.; Methodology: M.B., N.M., A.K., M.R., J.Đ., S.T., D.R. and M.Č.; Project administration: N.M. and M.B.; Software: S.T.; Supervision: M.Č. and R.Š.; Writing—original draft preparation: M.Č. and S.T. Writing—review and editing: M.Č. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ministry of Science, Technological Development and Innovation, Republic of Serbia, (Contract No. 451-03-47/2023-01/ 200019); University of Defense in Belgrade, Medical Faculty of the Military Medical Academy, Belgrade, Serbia (project MFVMA/03/20-22); University of East Sarajevo, Medical Faculty Foča, Foča, Bosnia and Herzegovina, (project UIS/MFF: I.1.20-22) and Medical Faculty Banja Luka, University of Banja Luka, Bosnia and Herzegovina.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of MMA (permission number: 02-07/12.01/2019), the Ethics Committee of Medical Faculty Foča (permission number: 01-2-4/06.10.2020) and the Ethics Committee of Medical Faculty Banja Luka (01-2-7/26.10.2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are included in this article.
Acknowledgments
The authors thank Milan Marković, Institute for the Application of Nuclear Energy, Belgrade Serbia for help with references arrangement, Dragana Vučević, Medical Faculty of the Military Medical Academy, Belgrade, Serbia for help with drawing the original graphical abstract, and Katarina Šavikin, Institute “Josip Pančić”, Belgrade, Serbia for providing us with PoPEx.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef]
- Dye, B.A. Global periodontal disease epidemiology. Periodontol. 2000 2012, 58, 10–25. [Google Scholar] [CrossRef]
- Kononen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Varma, S.V.; Varghese, S.; Nair, S.V. Prevalence of Chronic Periodontitis and Chronic Stress in the South Indian Population. Cureus 2023, 15, e33215. [Google Scholar] [CrossRef]
- da Silva, M.K.; de Carvalho, A.C.G.; Alves, E.H.P.; da Silva, F.R.P.; Pessoa, L.D.S.; Vasconcelos, D.F.P. Genetic Factors and the Risk of Periodontitis Development: Findings from a Systematic Review Composed of 13 Studies of Meta-Analysis with 71,531 Participants. Int. J. Dent. 2017, 2017, 1914073. [Google Scholar] [CrossRef]
- Kononen, E.; Muller, H.P. Microbiology of aggressive periodontitis. Periodontol. 2000 2014, 65, 46–78. [Google Scholar] [CrossRef]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S5–S11. [Google Scholar] [CrossRef]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000 2014, 64, 57–80. [Google Scholar]
- Sochalska, M.; Potempa, J. Manipulation of Neutrophils by Porphyromonas gingivalis in the Development of Periodontitis. Front. Cell. Infect. Microbiol. 2017, 7, 197. [Google Scholar]
- Campbell, L.; Millhouse, E.; Malcolm, J.; Culshaw, S. T cells, teeth and tissue destruction—What do T cells do in periodontal disease? Mol. Oral Microbiol. 2016, 31, 445–456. [Google Scholar] [CrossRef]
- Garlet, G.P.; Giannobile, W.V. Macrophages: The Bridge between Inflammation Resolution and Tissue Repair? J. Dent. Res. 2018, 97, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Rojas, C.; Rojas, L.; Cafferata, E.A.; Monasterio, G.; Vernal, R. Regulatory T Lymphocytes in Periodontitis: A Translational View. Mediat. Inflamm. 2018, 2018, 7806912. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, N.; Xie, H.; Jin, Y. Characterization of mesenchymal stem cells from human normal and hyperplastic gingiva. J. Cell. Physiol. 2011, 226, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, A.E.; Xu, Q.; Zhang, Q.; Le, A.D. Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine—A Comprehensive Review. Front. Immunol. 2021, 12, 667221. [Google Scholar] [PubMed]
- Bekic, M.; Radanovic, M.; Dokic, J.; Tomic, S.; Erakovic, M.; Radojevic, D.; Duka, M.; Markovic, D.; Markovic, M.; Ismaili, B.; et al. Mesenchymal Stromal Cells from Healthy and Inflamed Human Gingiva Respond Differently to Porphyromonas gingivalis. Int. J. Mol. Sci. 2022, 23, 3510. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wang, X.; Zhou, H.; Zhang, C.; Wang, Y.; Huang, J.; Liu, M.; Yang, P.; Song, A. Enhancement of periodontal tissue regeneration by conditioned media from gingiva-derived or periodontal ligament-derived mesenchymal stem cells: A comparative study in rats. Stem Cell Res. Ther. 2020, 11, 42. [Google Scholar] [CrossRef]
- Jain, S.; Darveau, R.P. Contribution of Porphyromonas gingivalis lipopolysaccharide to periodontitis. Periodontol. 2000 2010, 54, 53–70. [Google Scholar]
- Reynolds, M.A.; Kao, R.T.; Camargo, P.M.; Caton, J.G.; Clem, D.S.; Fiorellini, J.P.; Geisinger, M.L.; Mills, M.P.; Nares, S.; Nevins, M.L. Periodontal regeneration—Intrabony defects: A consensus report from the AAP Regeneration Workshop. J. Periodontol. 2015, 86 (Suppl. S2), S105–S107. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar]
- Prasad, D.; Kunnaiah, R. Punica granatum: A review on its potential role in treating periodontal disease. J. Indian Soc. Periodontol. 2014, 18, 428–432. [Google Scholar] [CrossRef]
- Chatzopoulos, G.S.; Karakostas, P.; Kavakloglou, S.; Assimopoulou, A.; Barmpalexis, P.; Tsalikis, L. Clinical Effectiveness of Herbal Oral Care Products in Periodontitis Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10061. [Google Scholar]
- Khairnar, M.S.; Pawar, B.; Marawar, P.P.; Mani, A. Evaluation of Calendula officinalis as an anti-plaque and anti-gingivitis agent. J. Indian Soc. Periodontol. 2013, 17, 741–747. [Google Scholar]
- Ashouri Moghaddam, A.; Radafshar, G.; Jahandideh, Y.; Kakaei, N. Clinical Evaluation of Effects of Local Application of Aloe vera Gel as an Adjunct to Scaling and Root Planning in Patients with Chronic Periodontitis. J. Dent. 2017, 18, 165–172. [Google Scholar]
- Mazur, M.; Ndokaj, A.; Bietolini, S.; Nisii, V.; Duś-Ilnicka, I.; Ottolenghi, L.; Green dentistry: Organic toothpaste formulations. A literature review. Dent. Med. Probl. 2022, 59, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Baradaran Rahimi, V.; Ghadiri, M.; Ramezani, M.; Askari, V.R. Antiinflammatory and anti-cancer activities of pomegranate and its constituent, ellagic acid: Evidence from cellular, animal, and clinical studies. Phytother. Res. 2020, 34, 685–720. [Google Scholar] [PubMed]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar]
- Colic, M.; Bekic, M.; Tomic, S.; Dokic, J.; Radojevic, D.; Savikin, K.; Miljus, N.; Markovic, M.; Skrbic, R. Immunomodulatory Properties of Pomegranate Peel Extract in a Model of Human Peripheral Blood Mononuclear Cell Culture. Pharmaceutics 2022, 14, 1140. [Google Scholar] [CrossRef]
- Ghalayani, P.; Zolfaghary, B.; Farhad, A.R.; Tavangar, A.; Soleymani, B. The efficacy of Punica granatum extract in the management of recurrent aphthous stomatitis. J. Res. Pharm. Pract. 2013, 2, 88–92. [Google Scholar]
- Hekmatian, E.; Shadmehr, E.; Asghari, G. Effect of pomegranate peel extract lozenge on gag reflex in dental patients. J. Isfahan Dent. Sch. 2011, 7, 235–241. [Google Scholar]
- Vasconcelos, L.C.; Sampaio, M.C.; Sampaio, F.C.; Higino, J.S. Use of Punica granatum as an antifungal agent against candidosis associated with denture stomatitis. Mycoses 2003, 46, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Somu, C.A.; Ravindra, S.; Ajith, S.; Ahamed, M.G. Efficacy of a herbal extract gel in the treatment of gingivitis: A clinical study. J. Ayurveda Integr. Med. 2012, 3, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Eltay, E.G.; Gismalla, B.G.; Mukhtar, M.M.; Awadelkarim, M.O.A. Punica granatum peel extract as adjunct irrigation to nonsurgical treatment of chronic gingivitis. Complement. Ther. Clin. Pract. 2021, 43, 101383. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbini, G.T.; Shoukry, N.M. In vitro Effect of Pomegranate Peel Extract on Trichomonas tenax. Life Sci. J. 2012, 9, 791–797. [Google Scholar]
- Rahman, S.; Karibasappa, S.N.; Mehta, D.S. Evaluation of the wound-healing potential of the kiwifruit extract by assessing its effects on human gingival fibroblasts and angiogenesis. Dent. Med. Probl. 2023, 60, 71–77. [Google Scholar] [CrossRef]
- Celiksoy, V.; Moses, R.L.; Sloan, A.J.; Moseley, R.; Heard, C.M. Evaluation of the In Vitro Oral Wound Healing Effects of Pomegranate (Punica granatum) Rind Extract and Punicalagin, in Combination with Zn (II). Biomolecules 2020, 10, 1234. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Dorfer, C.E. Gingival Mesenchymal Stem/Progenitor Cells: A Unique Tissue Engineering Gem. Stem Cells Int. 2016, 2016, 7154327. [Google Scholar]
- Glenn, J.D.; Whartenby, K.A. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 2014, 6, 526–539. [Google Scholar] [CrossRef]
- Tomasello, L.; Mauceri, R.; Coppola, A.; Pitrone, M.; Pizzo, G.; Campisi, G.; Pizzolanti, G.; Giordano, C. Mesenchymal stem cells derived from inflamed dental pulpal and gingival tissue: A potential application for bone formation. Stem Cell Res. Ther. 2017, 8, 179. [Google Scholar] [PubMed]
- Li, N.; Liu, N.; Zhou, J.; Tang, L.; Ding, B.; Duan, Y.; Jin, Y. Inflammatory environment induces gingival tissue-specific mesenchymal stem cells to differentiate towards a pro-fibrotic phenotype. Biol. Cell 2013, 105, 261–275. [Google Scholar] [CrossRef]
- Lu, L.; Zhou, X.; Wang, J.; Zheng, S.G.; Horwitz, D.A. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-beta and retinoic acid. PLoS ONE 2010, 5, e15150. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.S.; Patel, S. In vitro antimitotic and cytotoxic potential of plant extracts: A comparative study of Mucuna pruriens, Asteracantha longifolia and Sphaeranthus indicus. Future J. Pharm. Sci. 2020, 6, 115. [Google Scholar] [CrossRef]
- Andrukhov, O. Toll-Like Receptors and Dental Mesenchymal Stromal Cells. Front. Oral Health 2021, 2, 648901. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Vicente, M.; Barrajon-Catalan, E.; Herranz-Lopez, M.; Segura-Carretero, A.; Joven, J.; Encinar, J.A.; Micol, V. Plant-Derived Polyphenols in Human Health: Biological Activity, Metabolites and Putative Molecular Targets. Curr. Drug Metab. 2018, 19, 351–369. [Google Scholar] [CrossRef]
- Teplova, V.; Isakova, E.; Klein, O.; Dergachova, D.; Gessler, N.; Deryabina, Y.I. Natural polyphenols: Biological activity, pharmacological potential, means of metabolic engineering. Appl. Biochem. Microbiol. 2018, 54, 221–237. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Fujiwara, K.; Matsukawa, A.; Ohkawara, S.; Takagi, K.; Yoshinaga, M. Functional distinction between CXC chemokines, interleukin-8 (IL-8), and growth related oncogene (GRO)alpha in neutrophil infiltration. Lab. Investig. 2002, 82, 15–23. [Google Scholar] [CrossRef]
- Rath-Deschner, B.; Memmert, S.; Damanaki, A.; Nokhbehsaim, M.; Eick, S.; Cirelli, J.A.; Gotz, W.; Deschner, J.; Jager, A.; Nogueira, A.V.B. CXCL1, CCL2, and CCL5 modulation by microbial and biomechanical signals in periodontal cells and tissues-in vitro and in vivo studies. Clin. Oral Investig. 2020, 24, 3661–3670. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef]
- Kabashima, H.; Yoneda, M.; Nagata, K.; Hirofuji, T.; Maeda, K. The presence of chemokine (MCP-1, MIP-1alpha, MIP-1beta, IP-10, RANTES)-positive cells and chemokine receptor (CCR5, CXCR3)-positive cells in inflamed human gingival tissues. Cytokine 2002, 20, 70–77. [Google Scholar] [CrossRef]
- Danesi, F.; Ferguson, L.R. Could Pomegranate Juice Help in the Control of Inflammatory Diseases? Nutrients 2017, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; El-Gendi, H.; El-Fakharany, E.M.; El-Ziney, M.G.; El-Yazbi, A.F.; Al Meqbaali, F.T.; Ibrahim, W.H. Antioxidant, Anti-Inflammatory, Antimicrobial, and Anticancer Activities of Pomegranate Juice Concentrate. Nutrients 2023, 15, 2709. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiovanni, F.; Mukhopadhya, A.; Lacetera, N.; Ryan, M.T.; Romani, A.; Bernini, R.; Sweeney, T. Anti-Inflammatory Effects of Pomegranate Peel Extracts on In Vitro Human Intestinal Caco-2 Cells and Ex Vivo Porcine Colonic Tissue Explants. Nutrients 2019, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Delarosa, O.; Dalemans, W.; Lombardo, E. Toll-like receptors as modulators of mesenchymal stem cells. Front. Immunol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-beta Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- de Araujo Farias, V.; Carrillo-Galvez, A.B.; Martin, F.; Anderson, P. TGF-beta and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev. 2018, 43, 25–37. [Google Scholar] [CrossRef]
- Robertson, I.B.; Rifkin, D.B. Regulation of the Bioavailability of TGF-beta and TGF-beta-Related Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021907. [Google Scholar] [CrossRef]
- Ge, S.; Mrozik, K.M.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Isolation and characterization of mesenchymal stem cell-like cells from healthy and inflamed gingival tissue: Potential use for clinical therapy. Regen. Med. 2012, 7, 819–832. [Google Scholar] [CrossRef]
- Su, W.; Shi, J.; Zhao, Y.; Yan, F.; Lei, L.; Li, H. Porphyromonas gingivalis triggers inflammatory responses in periodontal ligament cells by succinate-succinate dehydrogenase-HIF-1alpha axis. Biochem. Biophys. Res. Commun. 2020, 522, 184–190. [Google Scholar] [CrossRef]
- Wang, R.; Liu, W.; Du, M.; Yang, C.; Li, X.; Yang, P. The differential effect of basic fibroblast growth factor and stromal cell-derived factor-1 pretreatment on bone morrow mesenchymal stem cells osteogenic differentiation potency. Mol. Med. Rep. 2018, 17, 3715–3721. [Google Scholar] [CrossRef]
- Ratanavaraporn, J.; Furuya, H.; Kohara, H.; Tabata, Y. Synergistic effects of the dual release of stromal cell-derived factor-1 and bone morphogenetic protein-2 from hydrogels on bone regeneration. Biomaterials 2011, 32, 2797–2811. [Google Scholar] [CrossRef]
- Thevenot, P.T.; Nair, A.M.; Shen, J.; Lotfi, P.; Ko, C.-Y.; Tang, L. The effect of incorporation of SDF-1α into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials 2010, 31, 3997–4008. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Han, T.; Xiang, X.; Wang, Y.; Fang, H.; Niu, Y.; Shen, C. The role of hepatocyte growth factor in mesenchymal stem cell-induced recovery in spinal cord injured rats. Stem Cell Res. Ther. 2020, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Nakamura, T. Roles of HGF as a pleiotropic factor in organ regeneration. Exs 1993, 65, 225–249. [Google Scholar] [PubMed]
- Dohi, M.; Hasegawa, T.; Yamamoto, K.; Marshall, B.C. Hepatocyte growth factor attenuates collagen accumulation in a murine model of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2000, 162, 2302–2307. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; Miao, X.; Wu, Y.; Yuan, J.; Ding, M.; Li, J.; Shi, Z. Age-Related Insulin-Like Growth Factor Binding Protein-4 Overexpression Inhibits Osteogenic Differentiation of Rat Mesenchymal Stem Cells. Cell Physiol. Biochem. 2017, 42, 640–650. [Google Scholar] [CrossRef]
- Li, H.; Yu, S.; Hao, F.; Sun, X.; Zhao, J.; Xu, Q.; Duan, D. Insulin-like growth factor binding protein 4 inhibits proliferation of bone marrow mesenchymal stem cells and enhances growth of neurospheres derived from the stem cells. Cell Biochem. Funct. 2018, 36, 331–341. [Google Scholar] [CrossRef]
- Schneider, M.R.; Wolf, E.; Hoeflich, A.; Lahm, H. IGF-binding protein-5: Flexible player in the IGF system and effector on its own. J. Endocrinol. 2002, 172, 423–440. [Google Scholar] [CrossRef]
- Miyagawa, I.; Nakayamada, S.; Nakano, K.; Yamagata, K.; Sakata, K.; Yamaoka, K.; Tanaka, Y. Induction of Regulatory T Cells and Its Regulation with Insulin-like Growth Factor/Insulin-like Growth Factor Binding Protein-4 by Human Mesenchymal Stem Cells. J. Immunol. 2017, 199, 1616–1625. [Google Scholar] [CrossRef]
- Hannas, A.R.; Pereira, J.C.; Granjeiro, J.M.; Tjaderhane, L. The role of matrix metalloproteinases in the oral environment. Acta Odontol. Scand. 2007, 65, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Karlis, G.D.; Schoningh, E.; Jansen, I.D.C.; Schoenmaker, T.; Hogervorst, J.M.A.; van Veen, H.A.; Moonen, C.G.J.; Lagosz-Cwik, K.B.; Forouzanfar, T.; de Vries, T.J. Chronic Exposure of Gingival Fibroblasts to TLR2 or TLR4 Agonist Inhibits Osteoclastogenesis but Does Not Affect Osteogenesis. Front. Immunol. 2020, 11, 1693. [Google Scholar] [CrossRef] [PubMed]
- Herath, T.D.; Darveau, R.P.; Seneviratne, C.J.; Wang, C.Y.; Wang, Y.; Jin, L. Tetra- and penta-acylated lipid A structures of Porphyromonas gingivalis LPS differentially activate TLR4-mediated NF-kappaB signal transduction cascade and immuno-inflammatory response in human gingival fibroblasts. PLoS ONE 2013, 8, e58496. [Google Scholar] [CrossRef] [PubMed]
- Behm, C.; Blufstein, A.; Abhari, S.Y.; Koch, C.; Gahn, J.; Schaffer, C.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Response of Human Mesenchymal Stromal Cells from Periodontal Tissue to LPS Depends on the Purity but Not on the LPS Source. Mediat. Inflamm. 2020, 2020, 8704896. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, S.B.; Hakki, S.S.; Hakki, E.E.; Durak, Y.; Kantarci, A. Porphyromonas gingivalis Lipopolysaccharide Induces a Pro-inflammatory Human Gingival Fibroblast Phenotype. Inflammation 2017, 40, 144–153. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Nakae, H.; Matsuo, T. Increase of CCL20 expression by human gingival fibroblasts upon stimulation with cytokines and bacterial endotoxin. Clin. Exp. Immunol. 2005, 142, 285–291. [Google Scholar] [CrossRef]
- Somma, D.; Kok, F.O.; Kerrigan, D.; Wells, C.A.; Carmody, R.J. Defining the Role of Nuclear Factor (NF)-kappaB p105 Subunit in Human Macrophage by Transcriptomic Analysis of NFKB1 Knockout THP1 Cells. Front. Immunol. 2021, 12, 669906. [Google Scholar] [CrossRef]
- Ju, Z.; Li, M.; Xu, J.; Howell, D.C.; Li, Z.; Chen, F.E. Recent development on COX-2 inhibitors as promising anti-inflammatory agents: The past 10 years. Acta Pharm. Sin. B 2022, 12, 2790–2807. [Google Scholar] [CrossRef]
- Kulesza, A.; Paczek, L.; Burdzinska, A. The Role of COX-2 and PGE2 in the Regulation of Immunomodulation and Other Functions of Mesenchymal Stromal Cells. Biomedicines 2023, 11, 445. [Google Scholar] [CrossRef]
- Daly, C.G.; Seymour, G.J.; Kieser, J.B. Bacterial endotoxin: A role in chronic inflammatory periodontal disease? J. Oral Pathol. 1980, 9, 1–15. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, Y.; Jia, L.; Xu, X. Lipopolysaccharide from Escherichia coli stimulates osteogenic differentiation of human periodontal ligament stem cells through Wnt/beta-catenin-induced TAZ elevation. Mol. Oral Microbiol. 2019, 34. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Asano, T.; Muro, R.; Huynh, N.C.; Komatsu, N.; Okamoto, K.; Nakano, K.; Okamura, T.; Nitta, T.; Takayanagi, H. OPG Production Matters Where It Happened. Cell Rep. 2020, 32, 108124. [Google Scholar] [CrossRef] [PubMed]
- Rozadi, N.; Oktavia, S.; Fauziah, F. Pharmacological Activities of Punicalagin: A Review. J. Drug Deliv. Ther. 2022, 12, 148–155. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Velez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell. Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).