Photobiomodulation of Neurogenesis through the Enhancement of Stem Cell and Neural Progenitor Differentiation in the Central and Peripheral Nervous Systems
Abstract
:1. Introduction
2. Basic Mechanisms of PBM for Cell Differentiation
3. Promising Results
3.1. Effects of PBM in the CNS
3.1.1. Neurogenesis
3.1.2. Neuroprotective Effects of PBM
Glial Cells
Synaptogenesis
3.2. Effects of PBM on Peripheral Sensory Neural Structures
3.2.1. Peripheral Neural Diseases
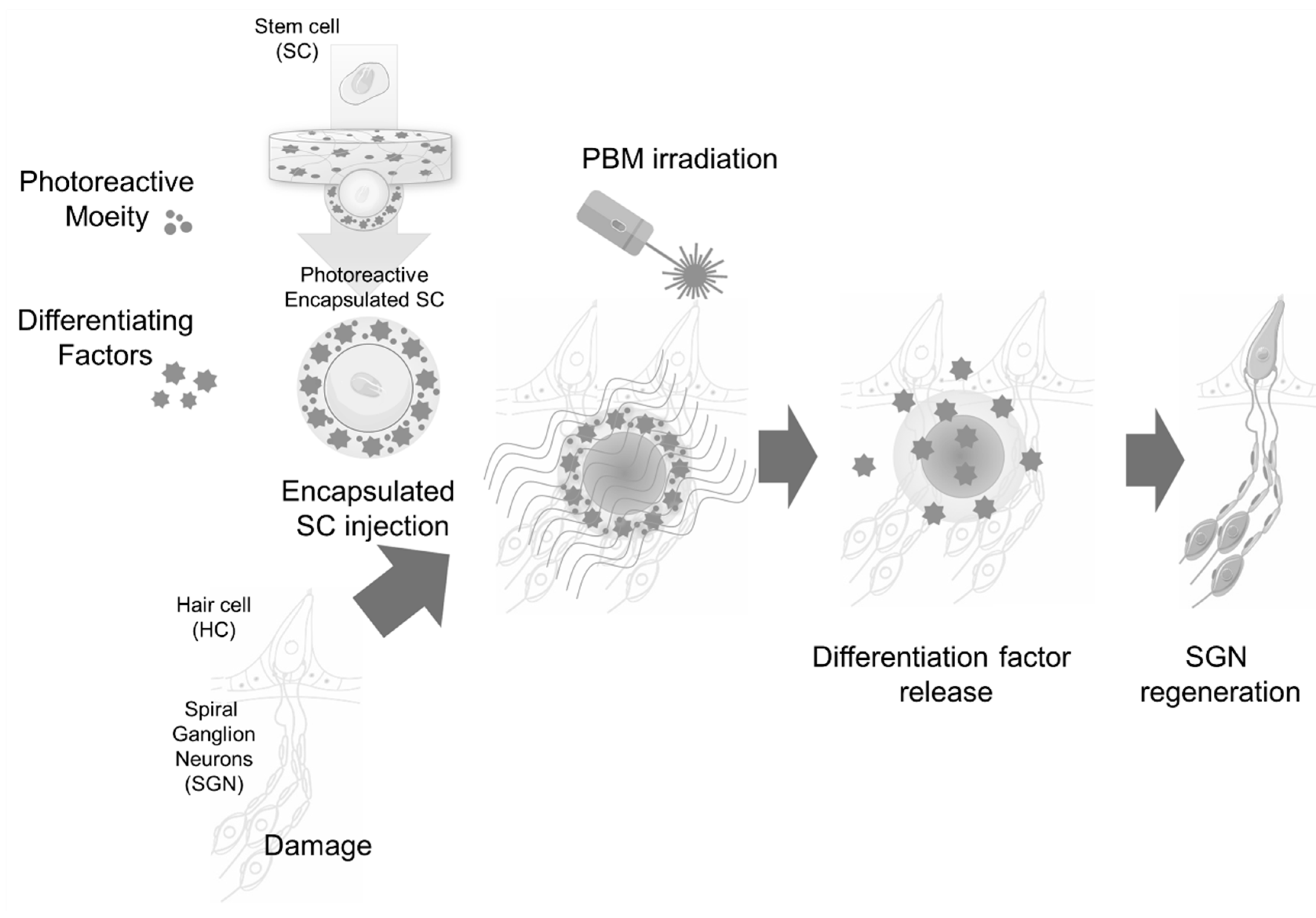
3.2.2. Hearing Loss, Stem Cells, and PBM
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, D.S.; Abeygunaratne, H.N. Experimental and Clinical Biomarkers for Progressive Evaluation of Neuropathology and Therapeutic Interventions for Acute and Chronic Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 11734. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef]
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of epilepsy, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 357–375. [Google Scholar] [CrossRef]
- Wang, R.; Nie, X.; Xu, S.; Zhang, M.; Dong, Z.; Yu, S. Interrelated Pathogenesis? Neuronal Intranuclear Inclusion Disease Combining with Hemiplegic Migraine. Headache 2020, 60, 382–395. [Google Scholar] [CrossRef]
- Stascheit, F.; Aigner, A.; Mergenthaler, P.; Hotter, B.; Hoffmann, S.; Lehnerer, S.; Meisel, C.; Meisel, A. Serum neurofilament light chain in myasthenia gravis subgroups: An exploratory cohort and case-Control study. Front. Neurol. 2022, 13, 1056322. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, A.V.; Mohan, A.G.; Covache-Busuioc, R.A.; Costin, H.P.; Glavan, L.A.; Corlatescu, A.D.; Saceleanu, V.M. Unraveling Molecular and Genetic Insights into Neurodegenerative Diseases: Advances in Understanding Alzheimer’s, Parkinson’s, and Huntington’s Diseases and Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2023, 24, 10809. [Google Scholar] [CrossRef]
- Brenner, D.; Ludolph, A.C. Gene-specific treatment of neurological diseases-current state and perspectives. Nervenarzt 2020, 91, 285–286. [Google Scholar] [CrossRef]
- Russo, M.V.; McGavern, D.B. Immune Surveillance of the CNS following Infection and Injury. Trends Immunol. 2015, 36, 637–650. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Jorg, S.; Grohme, D.A.; Erzler, M.; Binsfeld, M.; Haghikia, A.; Muller, D.N.; Linker, R.A.; Kleinewietfeld, M. Environmental factors in autoimmune diseases and their role in multiple sclerosis. Cell. Mol. Life Sci. 2016, 73, 4611–4622. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Z.; Lu, L.; Liu, Y. Microfluidic Manipulation for Biomedical Applications in the Central and Peripheral Nervous Systems. Pharmaceutics 2023, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Wulf, M.J.; Tom, V.J. Consequences of spinal cord injury on the sympathetic nervous system. Front. Cell. Neurosci. 2023, 17, 999253. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, L.G.; Russell, D.S.; Woolard, K.D.; Pardo, I.D.; Godfrey, V.; Jortner, B.S.; Butt, M.T.; Bolon, B. Comparative Pathology of the Peripheral Nervous System. Vet. Pathol. 2021, 58, 10–33. [Google Scholar] [CrossRef]
- Krinke, G.J.; Herrmann, A.; Korner, A.; Landes, C.; Sauner, F. Experience with examination of the spinal cord and peripheral nervous system (PNS) in mice: A brief overview. Exp. Toxicol. Pathol. 2014, 66, 277–280. [Google Scholar] [CrossRef]
- Ray, S.; Singhvi, A. Charging Up the Periphery: Glial Ionic Regulation in Sensory Perception. Front. Cell Dev. Biol. 2021, 9, 687732. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Correction: Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 18, 259. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef]
- Derakhshan, R.; Ahmadi, H.; Bayat, M.; Mehboudi, L.; Pourhashemi, E.; Amini, A.; Vatandoust, D.; Aghamiri, S.; Asadi, R.; Sabet, B. The Combined Effects of a Methacrylate Powder Dressing (Altrazeal Powder) and Photobiomodulation Therapy on the Healing of a Severe Diabetic Foot Ulcer in a Diabetic Patient: A Case Report. J. Lasers Med. Sci. 2022, 13, e38. [Google Scholar] [CrossRef]
- Santiago, R.; Gomes, S.; Ozsarfati, J.; Zitney, M. Photobiomodulation for modulation of neuropathic pain and improvement of scar tissue. Scars Burn. Heal. 2022, 8, 20595131221134052. [Google Scholar] [CrossRef]
- Reis, C.H.B.; Buchaim, D.V.; Ortiz, A.C.; Fideles, S.O.M.; Dias, J.A.; Miglino, M.A.; Teixeira, D.B.; Pereira, E.; da Cunha, M.R.; Buchaim, R.L. Application of Fibrin Associated with Photobiomodulation as a Promising Strategy to Improve Regeneration in Tissue Engineering: A Systematic Review. Polymers 2022, 14, 3150. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.W.; Hong, C.H.; Shih, M.C.; Tam, K.W.; Huang, Y.H.; Kuan, Y.C. Low-Level Laser Therapy for Fibromyalgia: A Systematic Review and Meta-Analysis. Pain. Physician 2019, 22, 241–254. [Google Scholar] [PubMed]
- Wickenheisser, V.A.; Zywot, E.M.; Rabjohns, E.M.; Lee, H.H.; Lawrence, D.S.; Tarrant, T.K. Laser Light Therapy in Inflammatory, Musculoskeletal, and Autoimmune Disease. Curr. Allergy Asthma Rep. 2019, 19, 37. [Google Scholar] [CrossRef]
- Gonzalez-Munoz, A.; Cuevas-Cervera, M.; Perez-Montilla, J.J.; Aguilar-Nunez, D.; Hamed-Hamed, D.; Aguilar-Garcia, M.; Pruimboom, L.; Navarro-Ledesma, S. Efficacy of Photobiomodulation Therapy in the Treatment of Pain and Inflammation: A Literature Review. Healthcare 2023, 11, 938. [Google Scholar] [CrossRef] [PubMed]
- Ailioaie, L.M.; Litscher, G. Photobiomodulation and Sports: Results of a Narrative Review. Life 2021, 11, 1339. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.; Crous, A.; Abrahamse, H. Photobiomodulation: An Effective Approach to Enhance Proliferation and Differentiation of Adipose-Derived Stem Cells into Osteoblasts. Stem Cells Int. 2021, 2021, 8843179. [Google Scholar] [CrossRef]
- Migaud, M.; Batailler, M.; Segura, S.; Duittoz, A.; Franceschini, I.; Pillon, D. Emerging new sites for adult neurogenesis in the mammalian brain: A comparative study between the hypothalamus and the classical neurogenic zones. Eur. J. Neurosci. 2010, 32, 2042–2052. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Kazanis, I. Neurogenesis in the adult mammalian brain: How much do we need, how much do we have? Curr. Top. Behav. Neurosci. 2013, 15, 3–29. [Google Scholar] [CrossRef]
- Lledo, P.M.; Valley, M. Adult Olfactory Bulb Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Fu, W. Axon regeneration impediment: The role of paired immunoglobulin-like receptor B. Neural Regen. Res. 2015, 10, 1338–1342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cui, H.; Nowicki, M.; Miao, S.; Lee, S.J.; Masood, F.; Harris, B.T.; Zhang, L.G. Three-Dimensional-Bioprinted Dopamine-Based Matrix for Promoting Neural Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 8993–9001. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, M.A.; Krishnakumar, G.S.; Giusto, E.; Furlani, F.; Bassi, G.; Rossi, A.; Molinari, F.; Lista, F.; Montesi, M.; Panseri, S. Bioactive injectable hydrogels for on demand molecule/cell delivery and for tissue regeneration in the central nervous system. Acta Biomater. 2022, 140, 88–101. [Google Scholar] [CrossRef]
- Hasanzadeh, E.; Seifalian, A.; Mellati, A.; Saremi, J.; Asadpour, S.; Enderami, S.E.; Nekounam, H.; Mahmoodi, N. Injectable hydrogels in central nervous system: Unique and novel platforms for promoting extracellular matrix remodeling and tissue engineering. Mater. Today Bio 2023, 20, 100614. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, R.; Hu, J.; Sun, L.; Zhao, X.; Zhao, Y.; Han, D.; Hu, S. Photobiomodulation Promotes Hippocampal CA1 NSC Differentiation Toward Neurons and Facilitates Cognitive Function Recovery Involving NLRP3 Inflammasome Mitigation Following Global Cerebral Ischemia. Front. Cell. Neurosci. 2021, 15, 731855. [Google Scholar] [CrossRef]
- Liao, Z.; Zhou, X.; Li, S.; Jiang, W.; Li, T.; Wang, N.; Xiao, N. Activation of the AKT/GSK-3beta/beta-catenin pathway via photobiomodulation therapy promotes neural stem cell proliferation in neonatal rat models of hypoxic-ischemic brain damage. Ann. Transl. Med. 2022, 10, 55. [Google Scholar] [CrossRef]
- Xuan, W.; Vatansever, F.; Huang, L.; Hamblin, M.R. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J. Biomed. Opt. 2014, 19, 108003. [Google Scholar] [CrossRef]
- Wu, X.; Shen, Q.; Zhang, Z.; Zhang, D.; Gu, Y.; Xing, D. Photoactivation of TGFbeta/SMAD signaling pathway ameliorates adult hippocampal neurogenesis in Alzheimer’s disease model. Stem Cell Res. Ther. 2021, 12, 345. [Google Scholar] [CrossRef]
- Yang, L.; Dong, Y.; Wu, C.; Youngblood, H.; Li, Y.; Zong, X.; Li, L.; Xu, T.; Zhang, Q. Effects of prenatal photobiomodulation treatment on neonatal hypoxic ischemia in rat offspring. Theranostics 2021, 11, 1269–1294. [Google Scholar] [CrossRef]
- Salehpour, F.; Farajdokht, F.; Cassano, P.; Sadigh-Eteghad, S.; Erfani, M.; Hamblin, M.R.; Salimi, M.M.; Karimi, P.; Rasta, S.H.; Mahmoudi, J. Near-infrared photobiomodulation combined with coenzyme Q10 for depression in a mouse model of restraint stress: Reduction in oxidative stress, neuroinflammation, and apoptosis. Brain Res. Bull. 2019, 144, 213–222. [Google Scholar] [CrossRef]
- Yang, L.; Wu, C.; Parker, E.; Li, Y.; Dong, Y.; Tucker, L.; Brann, D.W.; Lin, H.W.; Zhang, Q. Non-invasive photobiomodulation treatment in an Alzheimer Disease-like transgenic rat model. Theranostics 2022, 12, 2205–2231. [Google Scholar] [CrossRef] [PubMed]
- Meynaghizadeh-Zargar, R.; Sadigh-Eteghad, S.; Mohaddes, G.; Salehpour, F.; Rasta, S.H. Effects of transcranial photobiomodulation and methylene blue on biochemical and behavioral profiles in mice stress model. Lasers Med. Sci. 2020, 35, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.M.; Chang, S.F.; Li, C.C.; Chang, H. Transcranial photobiomodulation (808 nm) attenuates pentylenetetrazole-induced seizures by suppressing hippocampal neuroinflammation, astrogliosis, and microgliosis in peripubertal rats. Neurophotonics 2022, 9, 015006. [Google Scholar] [CrossRef]
- Blivet, G.; Meunier, J.; Roman, F.J.; Touchon, J. Neuroprotective effect of a new photobiomodulation technique against Abeta(25–35) peptide-induced toxicity in mice: Novel hypothesis for therapeutic approach of Alzheimer’s disease suggested. Alzheimer’s Dement. 2018, 4, 54–63. [Google Scholar] [CrossRef]
- Hong, N.; Kang, G.W.; Park, J.O.; Chung, P.S.; Lee, M.Y.; Ahn, J.C. Photobiomodulation regulates adult neurogenesis in the hippocampus in a status epilepticus animal model. Sci. Rep. 2022, 12, 15246. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, L.; Ma, X.; Huang, Z.; Zong, X.; Citadin, C.T.; Lin, H.W.; Zhang, Q. Photobiomodulation treatment inhibits neurotoxic astrocytic polarization and protects neurons in in vitro and in vivo stroke models. Neurochem. Int. 2023, 162, 105464. [Google Scholar] [CrossRef]
- Yang, L.; Wu, C.; Tucker, L.; Dong, Y.; Li, Y.; Xu, P.; Zhang, Q. Photobiomodulation Therapy Attenuates Anxious-Depressive-Like Behavior in the TgF344 Rat Model. J. Alzheimer’s Dis. 2021, 83, 1415–1429. [Google Scholar] [CrossRef]
- Yang, L.; Tucker, D.; Dong, Y.; Wu, C.; Lu, Y.; Li, Y.; Zhang, J.; Liu, T.C.; Zhang, Q. Photobiomodulation therapy promotes neurogenesis by improving post-stroke local microenvironment and stimulating neuroprogenitor cells. Exp. Neurol. 2018, 299, 86–96. [Google Scholar] [CrossRef]
- Wang, R.; Dong, Y.; Lu, Y.; Zhang, W.; Brann, D.W.; Zhang, Q. Photobiomodulation for Global Cerebral Ischemia: Targeting Mitochondrial Dynamics and Functions. Mol. Neurobiol. 2019, 56, 1852–1869. [Google Scholar] [CrossRef]
- Vogel, D.D.S.; Ortiz-Villatoro, N.N.; Araujo, N.S.; Marques, M.J.G.; Aimbire, F.; Scorza, F.A.; Scorza, C.A.; Albertini, R. Transcranial low-level laser therapy in an in vivo model of stroke: Relevance to the brain infarct, microglia activation and neuroinflammation. J. Biophotonics 2021, 14, e202000500. [Google Scholar] [CrossRef]
- Zein, R.; Selting, W.; Hamblin, M.R. Review of light parameters and photobiomodulation efficacy: Dive into complexity. J. Biomed. Opt. 2018, 23, 120901. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.K.; Zhu, Z.J.; Liang, Z.W.; Li, P.H.; Ma, Y.G.; Ding, T.; Li, K.; Zuo, X.S.; Ju, C.; et al. Photobiomodulation provides neuroprotection through regulating mitochondrial fission imbalance in the subacute phase of spinal cord injury. Neural Regen. Res. 2023, 18, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Youngblood, H.; Wu, C.; Zhang, Q. Mitochondria as a target for neuroprotection: Role of methylene blue and photobiomodulation. Transl. Neurodegener. 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Kim, E.Y.; Kwon, Y.S.; Lee, M.Y.; Ahn, J.C. NF-kappaB-mediated anti-inflammatory effects of an organic light-emitting diode (OLED) device in lipopolysaccharide (LPS)-induced in vitro and in vivo inflammation models. Front. Immunol. 2022, 13, 1050908. [Google Scholar] [CrossRef]
- Hong, N.; Kim, H.J.; Kang, K.; Park, J.O.; Mun, S.; Kim, H.G.; Kang, B.H.; Chung, P.S.; Lee, M.Y.; Ahn, J.C. Photobiomodulation improves the synapses and cognitive function and ameliorates epileptic seizure by inhibiting downregulation of Nlgn3. Cell Biosci. 2023, 13, 8. [Google Scholar] [CrossRef]
- George, S.; Hamblin, M.R.; Abrahamse, H. Photobiomodulation-Induced Differentiation of Immortalized Adipose Stem Cells to Neuronal Cells. Lasers Surg. Med. 2020, 52, 1032–1040. [Google Scholar] [CrossRef]
- George, S.; Hamblin, M.R.; Abrahamse, H. Neuronal differentiation potential of primary and immortalized adipose stem cells by photobiomodulation. J. Photochem. Photobiol. 2022, 230, 112445. [Google Scholar] [CrossRef]
- Vaz, A.; Ribeiro, I.; Pinto, L. Frontiers in Neurogenesis. Cells 2022, 11, 3567. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Salehpour, F. Photobiomodulation of the Brain: Shining Light on Alzheimer’s and Other Neuropathological Diseases. J. Alzheimer’s Dis. 2021, 83, 1395–1397. [Google Scholar] [CrossRef]
- Su, M.; Nizamutdinov, D.; Liu, H.; Huang, J.H. Recent Mechanisms of Neurodegeneration and Photobiomodulation in the Context of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 9272. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photobiomodulation for Alzheimer’s Disease: Has the Light Dawned? Photonics 2019, 6, 77. [Google Scholar] [CrossRef]
- Yoon, S.R.; Hong, N.; Lee, M.Y.; Ahn, J.C. Photobiomodulation with a 660-Nanometer Light-Emitting Diode Promotes Cell Proliferation in Astrocyte Culture. Cells 2021, 10, 1664. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, Y.; Ma, X.; Feng, Y.; Zong, X.; Jordan, J.D.; Zhang, Q. Photobiomodulation attenuates oligodendrocyte dysfunction and prevents adverse neurological consequences in a rat model of early life adversity. Theranostics 2023, 13, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Cardoso, F.; Dos Santos, J.C.C.; Gonzalez-Lima, F.; Araujo, B.H.S.; Lopes-Martins, R.A.B.; Gomes da Silva, S. Effects of Chronic Photobiomodulation with Transcranial Near-Infrared Laser on Brain Metabolomics of Young and Aged Rats. Mol. Neurobiol. 2021, 58, 2256–2268. [Google Scholar] [CrossRef]
- Barohn, R.J.; Amato, A.A. Pattern-recognition approach to neuropathy and neuronopathy. Neurol. Clin. 2013, 31, 343–361. [Google Scholar] [CrossRef]
- Chung, T.; Prasad, K.; Lloyd, T.E. Peripheral neuropathy: Clinical and electrophysiological considerations. Neuroimaging Clin. N. Am. 2014, 24, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Nadol, J.B., Jr.; Hedley-Whyte, E.T.; Amr, S.S.; O’Malley, J.T.; Kamakura, T. Histopathology of the Inner Ear in Charcot-Marie-Tooth Syndrome Caused by a Missense Variant (p.Thr65Ala) in the MPZ Gene. Audiol. Neuro-Otol. 2018, 23, 326–334. [Google Scholar] [CrossRef]
- Eggermann, K.; Gess, B.; Hausler, M.; Weis, J.; Hahn, A.; Kurth, I. Hereditary Neuropathies. Dtsch. Arztebl. Int. 2018, 115, 91–97. [Google Scholar] [CrossRef]
- Creigh, P.D.; Mountain, J.; Sowden, J.E.; Eichinger, K.; Ravina, B.; Larkindale, J.; Herrmann, D.N. Measuring peripheral nerve involvement in Friedreich’s ataxia. Ann. Clin. Transl. Neurol. 2019, 6, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Lerat, J.; Magdelaine, C.; Roux, A.F.; Darnaud, L.; Beauvais-Dzugan, H.; Naud, S.; Richard, L.; Derouault, P.; Ghorab, K.; Magy, L.; et al. Hearing loss in inherited peripheral neuropathies: Molecular diagnosis by NGS in a French series. Mol. Genet. Genom. Med. 2019, 7, e839. [Google Scholar] [CrossRef] [PubMed]
- Mahmut, M.K.; Musch, M.; Han, P.; Abolmaali, N.; Hummel, T. The effect of olfactory training on olfactory bulb volumes in patients with idiopathic olfactory loss. Rhinology 2020, 58, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Power Guerra, N.; Gunder, N.; Hahner, A.; Menzel, S. Olfactory Function and Olfactory Disorders. Laryngo-Rhino-Otol. 2023, 102, S67–S92. [Google Scholar] [CrossRef]
- Jha, N.K.; Ojha, S.; Jha, S.K.; Dureja, H.; Singh, S.K.; Shukla, S.D.; Chellappan, D.K.; Gupta, G.; Bhardwaj, S.; Kumar, N.; et al. Evidence of Coronavirus (CoV) Pathogenesis and Emerging Pathogen SARS-CoV-2 in the Nervous System: A Review on Neurological Impairments and Manifestations. J. Mol. Neurosci. 2021, 71, 2192–2209. [Google Scholar] [CrossRef]
- Soria, E.D.; Candaras, M.M.; Truax, B.T. Impairment of taste in the Guillain-Barre syndrome. Clin. Neurol. Neurosurg. 1990, 92, 75–79. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Gilden, D.H. Ramsay Hunt syndrome. J. Neurol. Neurosurg. Psychiatry 2001, 71, 149–154. [Google Scholar] [CrossRef]
- Hildebrand, M.S.; Newton, S.S.; Gubbels, S.P.; Sheffield, A.M.; Kochhar, A.; de Silva, M.G.; Dahl, H.M.; Rose, S.D.; Behlke, M.A.; Smith, R.J. Advances in Molecular and Cellular Therapies for Hearing Loss. Mol. Ther. J. Am. Soc. Gene Ther. 2008, 16, 224–236. [Google Scholar] [CrossRef]
- Mainland, J.D.; Barlow, L.A.; Munger, S.D.; Millar, S.E.; Vergara, M.N.; Jiang, P.; Schwob, J.E.; Goldstein, B.J.; Boye, S.E.; Martens, J.R.; et al. Identifying Treatments for Taste and Smell Disorders: Gaps and Opportunities. Chem. Senses 2020, 45, 493–502. [Google Scholar] [CrossRef]
- Chang, S.Y.; Carpena, N.T.; Mun, S.; Jung, J.Y.; Chung, P.S.; Shim, H.; Han, K.; Ahn, J.C.; Lee, M.Y. Enhanced Inner-Ear Organoid Formation from Mouse Embryonic Stem Cells by Photobiomodulation. Mol. Ther. Methods Clin. Dev. 2020, 17, 556–567. [Google Scholar] [CrossRef]
- Chang, S.Y.; Lee, M.Y. Photobiomodulation with a wavelength > 800 nm induces morphological changes in stem cells within otic organoids and scala media of the cochlea. Lasers Med. Sci. 2021, 36, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Litovsky, R.Y.; Moua, K.; Godar, S.; Kan, A.; Misurelli, S.M.; Lee, D.J. Restoration of spatial hearing in adult cochlear implant users with single-sided deafness. Hear. Res. 2019, 372, 69–79. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, S.; Sun, Y. Mechanism and Prevention of Spiral Ganglion Neuron Degeneration in the Cochlea. Front. Cell. Neurosci. 2021, 15, 814891. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Nishimoto, E.; Xing, Y.; Brown, L.N.; Noble, K.V.; Barth, J.L.; LaRue, A.C.; Ando, K.; Schulte, B.A. Contributions of Mouse and Human Hematopoietic Cells to Remodeling of the Adult Auditory Nerve After Neuron Loss. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 2000–2011. [Google Scholar] [CrossRef]
- Matsuoka, A.J.; Morrissey, Z.D.; Zhang, C.; Homma, K.; Belmadani, A.; Miller, C.A.; Chadly, D.M.; Kobayashi, S.; Edelbrock, A.N.; Tanaka-Matakatsu, M.; et al. Directed Differentiation of Human Embryonic Stem Cells Toward Placode-Derived Spiral Ganglion-Like Sensory Neurons. Stem Cells Transl. Med. 2017, 6, 923–936. [Google Scholar] [CrossRef]
- Schomann, T.; Mezzanotte, L.; De Groot, J.; Rivolta, M.N.; Hendriks, S.H.; Frijns, J.H.M.; Huisman, M.A. Neuronal differentiation of hair-follicle-bulge-derived stem cells co-cultured with mouse cochlear modiolus explants. PLoS ONE 2017, 12, e0187183. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Zheng, L.; Zhou, T.; Zhang, C.; Li, H. Light manipulation for fabrication of hydrogels and their biological applications. Acta Biomater. 2022, 137, 20–43. [Google Scholar] [CrossRef]
- Xuan, W.; Huang, L.; Hamblin, M.R. Repeated transcranial low-level laser therapy for traumatic brain injury in mice: Biphasic dose response and long-term treatment outcome. J. Biophotonics 2016, 9, 1263–1272. [Google Scholar] [CrossRef]
- Cassano, P.; Caldieraro, M.A.; Norton, R.; Mischoulon, D.; Trinh, N.-H.; Nyer, M.; Dording, C.; Hamblin, M.R.; Campbell, B.; Iosifescu, D.V. Reported Side Effects, Weight and Blood Pressure, After Repeated Sessions of Transcranial Photobiomodulation. Photobiomodulation Photomed. Laser Surg. 2019, 37, 651–656. [Google Scholar] [CrossRef]
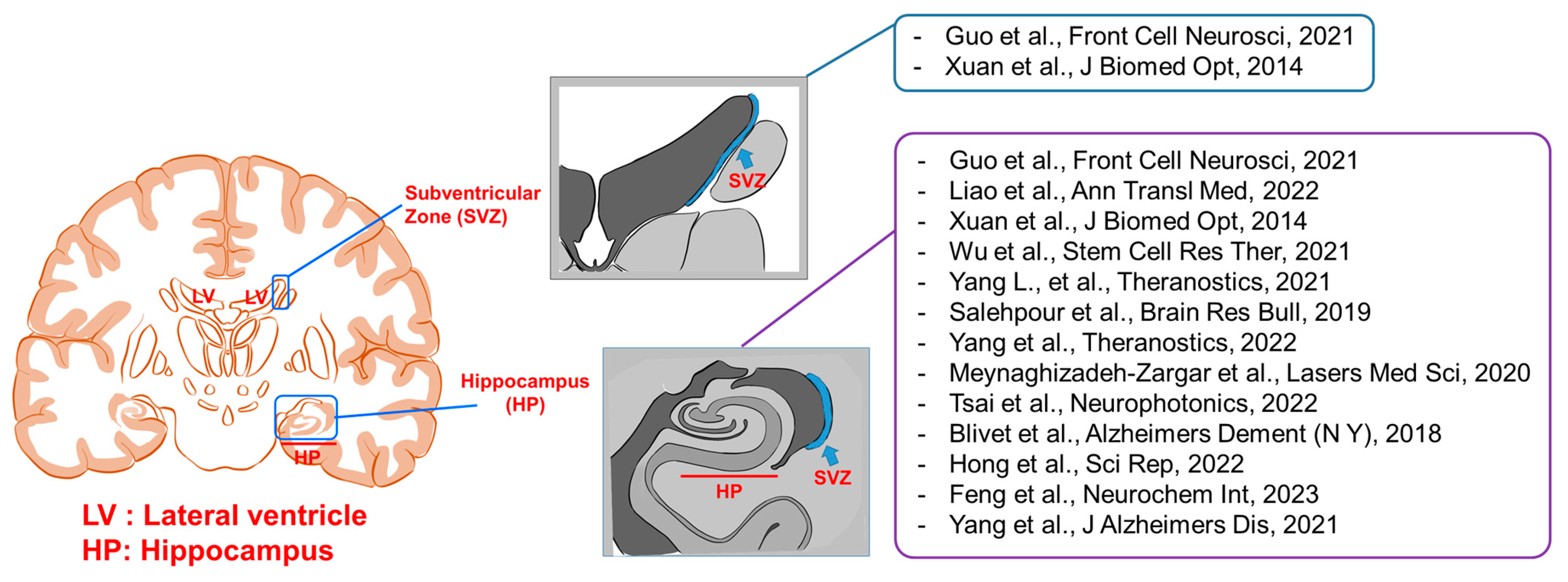
| Reference | Year | Light Source | Wavelength (nm) | Energy | Location | Animal Model | Target | Mechanism | Efficiency |
|---|---|---|---|---|---|---|---|---|---|
| [48] | 2018 | Diode laser | 808 | 120 s × 350 mW/cm2/day Total: 294 J for 7 days | Scalp | PT stroke rat | Neuroprogenitor cells in the peri-infarct cortical region |
|
|
| [35] | 2021 | Diode laser | 808 | 120 s × 20 mW/cm2 Total: 7.2 J/cm2 for 3 days | Cortical surface | Global cerebral ischemia rat | Hippocampal astrocyte and microglia cells |
|
|
| [36] | 2022 | Helium–neon laser | 660 | 600 s × 5 mW/cm2 Total: 42 J/cm2 for 14 days | Scalp and skull | HI brain damage rat | Hippocampal neural stem cells |
|
|
| [37] | 2014 | Laser | 810 | 720 s × 25 mW/cm2 Total: 54 J/cm2 for 3 days | Transcranial | CCI–TBI injury mouse | Hippocampal and SVZ neurons |
|
|
| [38] | 2021 | Semiconductor laser | 635 | 600 s × 0.1 mW/cm2 Total: 60 J/cm2 for 1 month | Scalp and skull | Amyloid precursor protein/presenilin 1 transgenic mouse | Hippocampal neural stem cells |
|
|
| Reference | Year | Light Source | Wavelength (nm) | Energy | Anatomical Location | Animal Model | Target Cells | Mechanism | Efficiency | |
|---|---|---|---|---|---|---|---|---|---|---|
| [49] | 2019 | Diode laser | 808 | 36 s × 8 mW/cm2 Total: 4 J/cm2 for 14 days | Scalp | Global cerebral ischemia rat | Hippocampal CA1 pyramidal neuron cells |
|
| |
| [39] | 2021 | Diode laser | 808 | 120 s × 8 mW/cm2 Total: 2.88 J/cm2/week for 3 weeks | Abdomen of pregnant rat from gestation day 1 to 21 | Neonatal HI rat | Myeloid cells and hippocampal astrocytes |
|
| |
| [40] | 2019 | GaAlAs laser | 810 | 5 s × 6.66 W/cm2 Total: 166.5 J/cm2 for 5 days | Transcranial Scalp | Sub-chronic restraint stress mouse | Prefrontal cortex and hippocampal neuron cells |
|
| |
| [41] | 2022 | Laser | 808 | 120 s × 350 mW/cm2 Total: 126 J/cm2/week for 16 months | Scalp | Transgenic TgF344-AD rat | Microglial cells and astrocytes in cortex and hippocampus |
|
| |
| [42] | 2020 | GaAlAs diode laser | 810 | 1.7 s × 4.75 W/cm2 Total: 5 J/cm2/week for 4 weeks | Transcranial | Unpredictable chronic mild stress mouse | Hippocampal cells |
|
| |
| [43] | 2022 | GaAlAs diode laser | 808 | 100 s × 1.333 W/cm2 Total: 133.3 J/cm2 | Transcranial | Pentylenetetrazole-induced SE rat | Hippocampal microglial cells and astrocytes |
|
| |
| [50] | 2021 | Diode laser | 780 | 120 s × 0.083 W/cm2 Total: 30 J/cm2/week for 60 days | Transcranial | Ischemic stroke rat | Brain tissue cells in the peri-lesional region |
|
| |
| [44] | 2018 | RGn500 (LED + laser) with static magnetic field | 625 850 | LED | 600 s × 28 mW/cm2 Total: 117.6 J/cm2 for 7 days | Transcranial and abdomen | Aβ25-35 peptide toxicity mouse | Hippocampal and frontal cortex cells |
|
|
| 850 | Laser | |||||||||
| [45] | 2022 | Diode laser | 830 | 750 s × 30 mW/cm2 Total: 180 J/cm2 | Scalp | SE mouse | Maturing granular cells in the hilus |
|
| |
| [46] | 2023 | Diode laser | 808 | 120 s × 350 mW/cm2 Total: 294 J/cm2 for 7 days | Skull | PT stroke rat | Neuronal dendrites and cortex astrocytes |
|
| |
| [47] | 2021 | Diode laser | 808 | 120 s × 350 mW/cm2 Total: 126 J/cm2/week for 8 months | Scalp | Transgenic TgF344-AD disease rat |
|
| ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-Y.; Lee, M.Y. Photobiomodulation of Neurogenesis through the Enhancement of Stem Cell and Neural Progenitor Differentiation in the Central and Peripheral Nervous Systems. Int. J. Mol. Sci. 2023, 24, 15427. https://doi.org/10.3390/ijms242015427
Chang S-Y, Lee MY. Photobiomodulation of Neurogenesis through the Enhancement of Stem Cell and Neural Progenitor Differentiation in the Central and Peripheral Nervous Systems. International Journal of Molecular Sciences. 2023; 24(20):15427. https://doi.org/10.3390/ijms242015427
Chicago/Turabian StyleChang, So-Young, and Min Young Lee. 2023. "Photobiomodulation of Neurogenesis through the Enhancement of Stem Cell and Neural Progenitor Differentiation in the Central and Peripheral Nervous Systems" International Journal of Molecular Sciences 24, no. 20: 15427. https://doi.org/10.3390/ijms242015427
APA StyleChang, S.-Y., & Lee, M. Y. (2023). Photobiomodulation of Neurogenesis through the Enhancement of Stem Cell and Neural Progenitor Differentiation in the Central and Peripheral Nervous Systems. International Journal of Molecular Sciences, 24(20), 15427. https://doi.org/10.3390/ijms242015427






