Metabolic and Transcriptomic Approaches of Chitosan and Water Stress on Polyphenolic and Terpenoid Components and Gene Expression in Salvia abrotanoides (Karl.) and S. yangii
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Variance Analysis of Phytochemical Traits
2.1.2. Total Phenolic Content of Roots and Leaves
2.1.3. Total Flavonoid Content
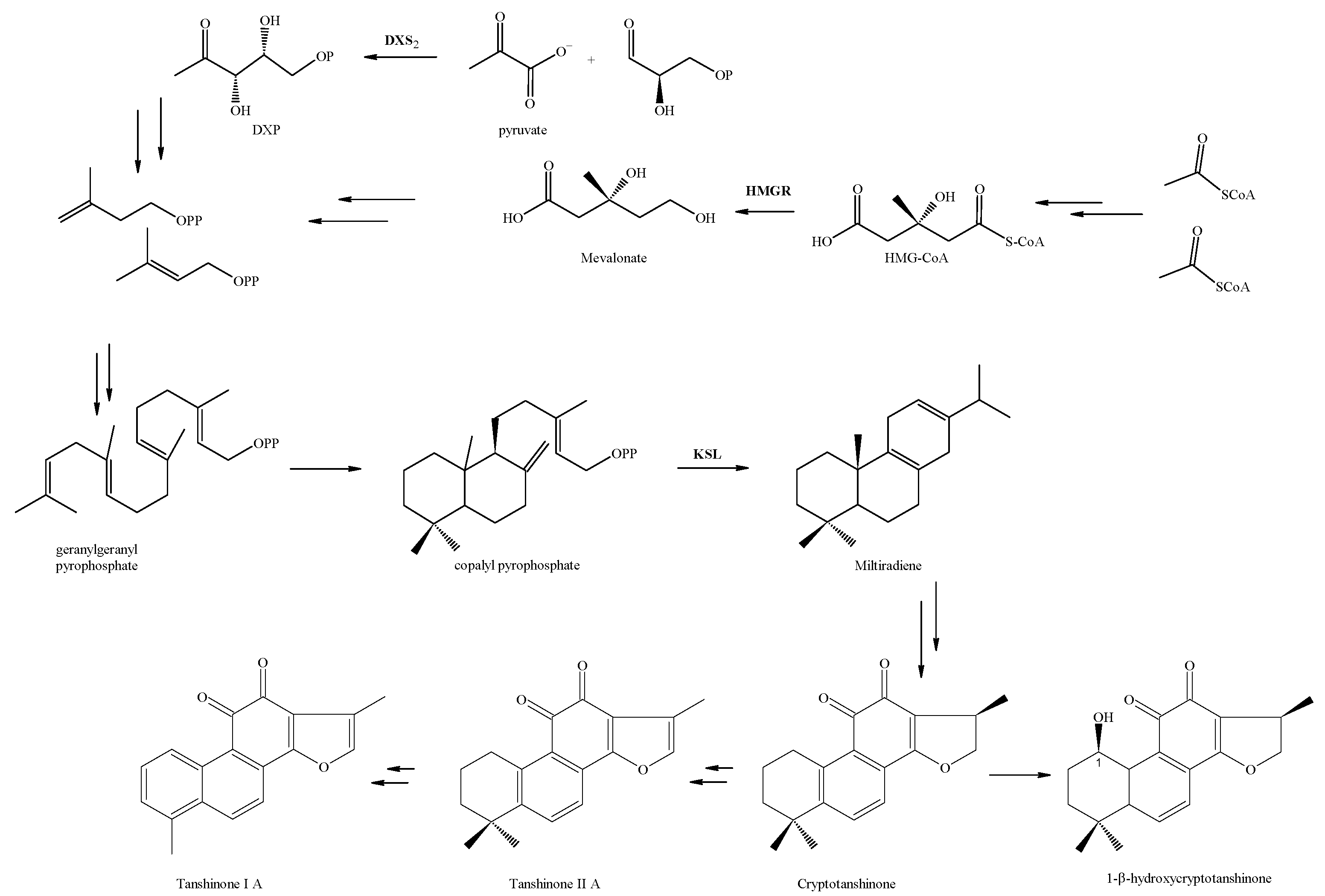
2.1.4. Total Tanshinone Content (TTC) and HPLC Analysis
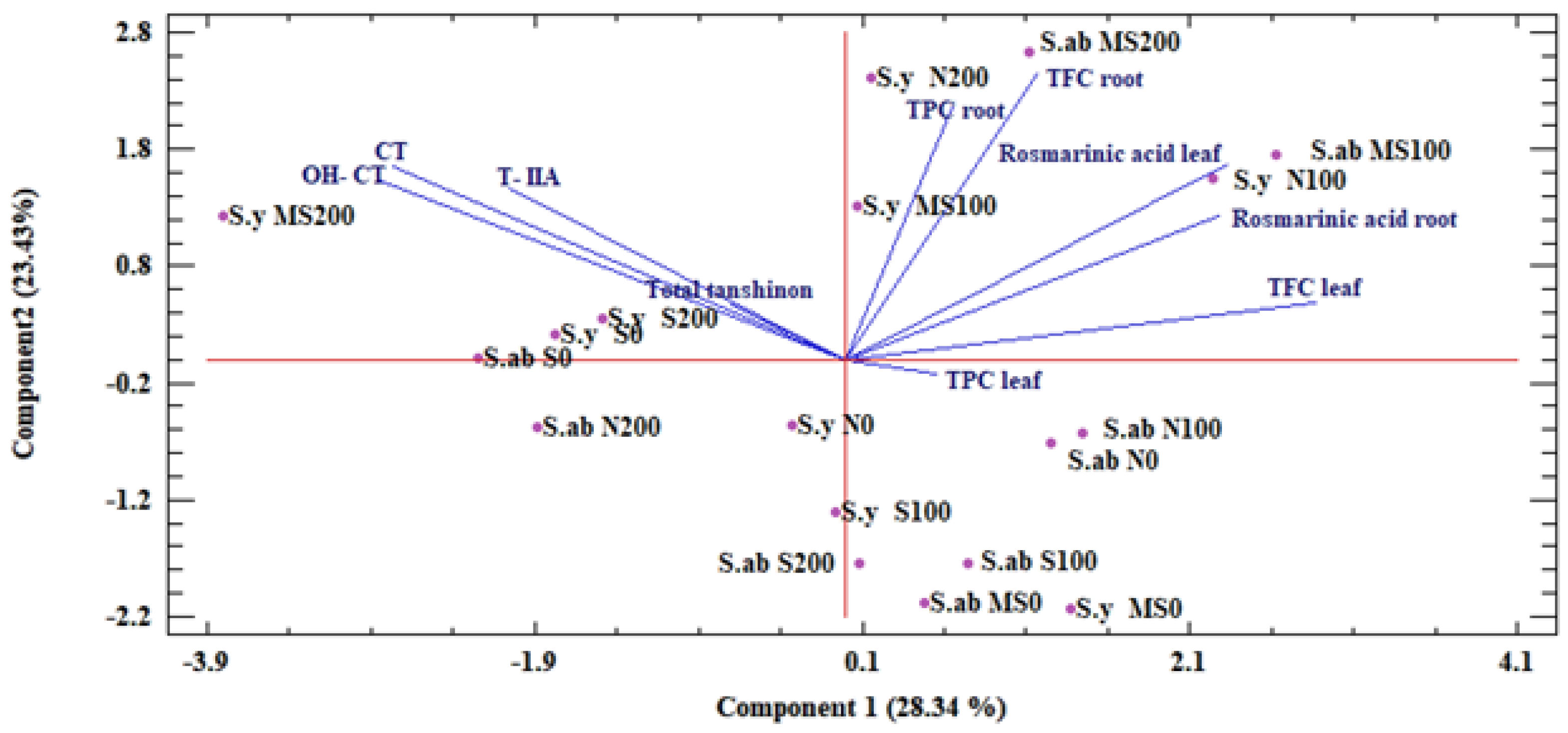
2.1.5. Principle Component Analysis (PCA)
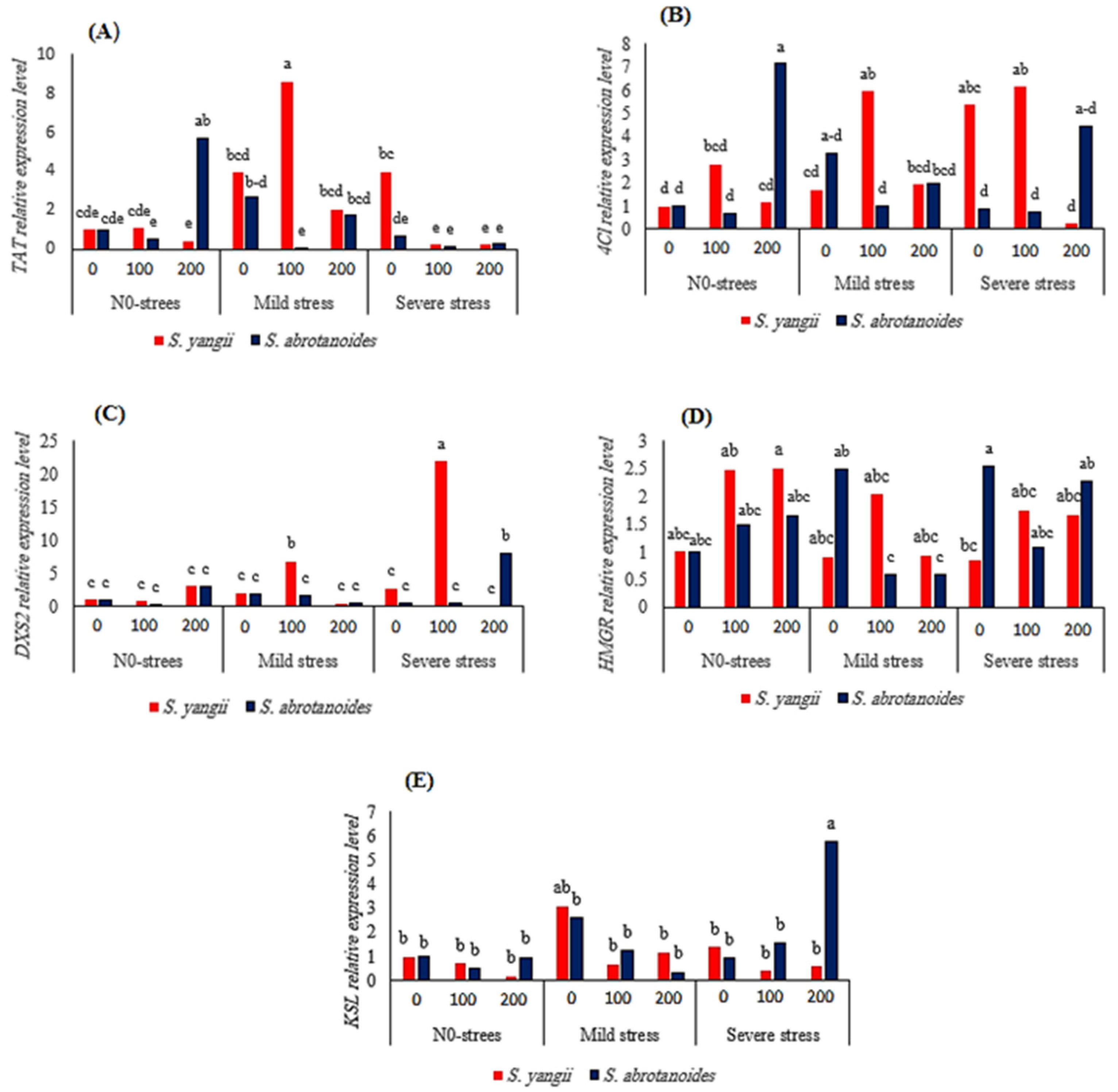
2.1.6. Gene Expression
2.1.7. Correlation Analysis
2.2. Discussion
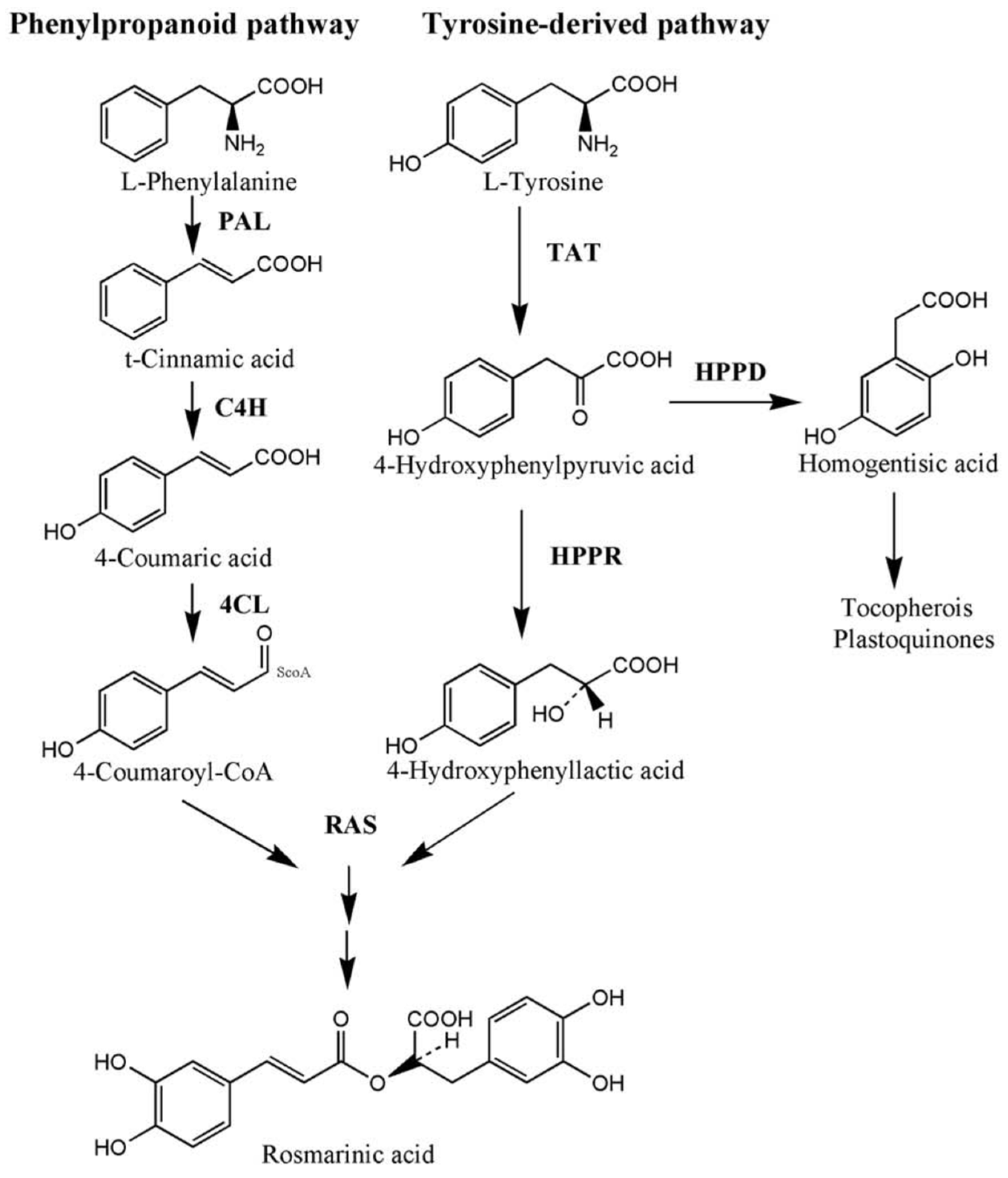
2.2.1. Total Phenolic Compounds and Total Flavonoid Contents
2.2.2. HPLC
2.2.3. Gene Expression
2.2.4. PCA Analysis
3. Materials and Methods
3.1. Ethics Statement
3.2. Plant Materials
3.3. Experimental Design and Treatments
3.4. Total Phenolic (TPC) and Flavonoid Contents (TFC) Assays
3.5. Total Tanshinone Content (TTC) and High-Performance Liquid Chromatography (HPLC) Analysis
3.6. Isolation of Total RNA, cDNA Synthesis, and Real-Time PCR
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bielecka, M.; Pencakowski, B.; Stafiniak, M.; Jakubowski, K.; Rahimmalek, M.; Gharibi, S.; Matkowski, A.; Ślusarczyk, S. Metabolomics and DNA-Based Authentication of Two Traditional Asian Medicinal and Aromatic Species of Salvia subg. Perovskia. Cells 2021, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Dinani, M.S.; Noushabadi, S.A.E.; Namdar, F.; Abharian, P.H.; Hejazi, S.H.; Sebghatollahi, Z. In Vitro Study of the Leishmanicidal Activity of Perovskia Abrotanoides Terpenoid-Rich Fractions against Leishmania Major (MRHO/IR/75/ER). Adv. Biomed. Res. 2023, 12, 67. [Google Scholar]
- Ślusarczyk, S.; Senol Deniz, F.S.; Abel, R.; Pecio, Ł.; Pérez-Sánchez, H.; Cerón-Carrasco, J.P.; Den-Haan, H.; Banerjee, P.; Preissner, R.; Krzyżak, E.; et al. Norditerpenoids with Selective Anti-Cholinesterase Activity from the Roots of Perovskia atriplicifolia Benth. Int. J. Mol. Sci. 2020, 21, 4475. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C.A.; Villena, G.K.; Rodríguez, E.F. Phytochemical profile and rosmarinic acid purification from two Peruvian Lepechinia Willd. species (Salviinae, Mentheae, Lamiaceae). Sci. Rep. 2021, 11, 7260. [Google Scholar] [CrossRef] [PubMed]
- Gharehbagh, H.J.; Ebrahimi, M.; Dabaghian, F.; Mojtabavi, S.; Hariri, R.; Saeedi, M.; Faramarzi, M.A.; Khanavi, M. Chemical composition, cholinesterase, and α-glucosidase inhibitory activity of the essential oils of some Iranian native Salvia species. BMC Complement. Med. Ther. 2023, 23, 184. [Google Scholar] [CrossRef]
- Pourhosseini, S.H.; Hadian, J.; Sonboli, A.; Nejad Ebrahimi, S.; Mirjalili, M.H. Genetic and chemical diversity in Perovskia abrotanoides Kar.(Lamiaceae) populations based on ISSR s markers and essential oils profile. Chem. Biodivers. 2018, 15, e1700508. [Google Scholar] [CrossRef]
- Pachura, N.; Zimmer, A.; Grzywna, K.; Figiel, A.; Szumny, A.; Łyczko, J. Chemical investigation on Salvia officinalis L. Affected by multiple drying techniques—The comprehensive analytical approach (HS-SPME, GC–MS, LC-MS/MS, GC-O and NMR). Food Chem. 2022, 397, 133802. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, Critical Pharmacological Components in Salvia miltiorrhiza. Front. Pharmacol. 2019, 10, 202. [Google Scholar] [CrossRef]
- Zaker, A.; Sykora, C.; Gössnitzer, F.; Abrishamchi, P.; Asili, J.; Hadi, S.; Wawrosch, C. Effects of some elicitors on tanshinone production in adventitious root cultures of Perovskia abrotanoides Karel. Ind. Crops Prod. 2015, 67, 97–102. [Google Scholar] [CrossRef]
- Afshari, M.; Rahimmalek, M.; Sabzalian, M.R.; Bielecka, M.; Matkowski, A.; Talebi, M. Changes in physiological, phytochemical traits and gene expression of two Perovskia species in response to water deficit. Sci. Hortic. 2022, 293, 110747. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S.; Tawfik, M.M.; Bakhoum, G.S. Role of chitosan and chitosan-based nanoparticles on drought tolerance in plants: Probabilities and prospects. In Role of Chitosan and Chitosan-Based Nanomaterials in Plant Sciences; Elsevier: Amsterdam, The Netherlands, 2022; pp. 475–501. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef]
- Moolphuerk, N.; Pattanagul, W. Pretreatment with different molecular weight chitosans encourages drought tolerance in rice (Oryza sativa L.) seedling. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 2072–2084. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Bistgani, Z.E.; Siadat, S.A.; Bakhshandeh, A.; Pirbalouti, A.G.; Hashemi, M. Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. Crop J. 2017, 5, 407–415. [Google Scholar] [CrossRef]
- Almeida, L.G.; Magalhães, P.C.; Karam, D.; da Silva, E.M.; Alvarenga, A.A. Chitosan application in the induction of water deficit tolerance in maize plants. Acta Sci. Agron. 2019, 42, e42463. [Google Scholar] [CrossRef]
- García-García, A.L.; Matos, A.R.; Feijão, E.; Cruz de Carvalho, R.; Boto, A.; Marques da Silva, J.; Jiménez-Arias, D. The use of chitosan oligosaccharide to improve artemisinin yield in well-watered and drought-stressed plants. Front. Plant Sci. 2023, 14, 1200898. [Google Scholar] [CrossRef]
- Ávila, R.G.; Magalhães, P.C.; Vitorino, L.C.; Bessa, L.A.; de Souza, K.R.D.; Queiroz, R.B.; Jakelaitis, A.; Teixeira, M.B. Chitosan Induces Sorghum Tolerance to Water Deficits by Positively Regulating Photosynthesis and the Production of Primary Metabolites, Osmoregulators, and Antioxidants. J. Soil Sci. Plant Nutr. 2023, 23, 1156–1172. [Google Scholar] [CrossRef]
- Khodadadi, F.; Ahmadi, F.S.; Talebi, M.; Moshtaghi, N.; Matkowski, A.; Szumny, A.; Rahimmalek, M. Essential oil composition, physiological and morphological variation in Salvia abrotanoides and S. yangii under drought stress and chitosan treatments. Ind. Crops Prod. 2022, 187, 115429. [Google Scholar] [CrossRef]
- Attaran Dowom, S.; Karimian, Z.; Mostafaei Dehnavi, M.; Samiei, L. Chitosan nanoparticles improve physiological and biochemical responses of Salvia abrotanoides (Kar.) under drought stress. BMC Plant Biol. 2022, 22, 364. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sun, H.; Xiao, H.; Cui, G.; Hillwig, M.L.; Jackson, A.; Wang, X.; Shen, Y.; Zhao, N.; Zhang, L.; et al. Combining metabolomics and transcriptomics to characterize tanshinone biosynthesis in Salvia miltiorrhiza. BMC Genom. 2014, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wang, M.; Li, J.; Lu, S. Transcriptomic analysis reveals potential genes involved in tanshinone biosynthesis in Salvia miltiorrhiza. Sci. Rep. 2019, 9, 14929. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ma, Y.; Tang, J.; He, Y.; Liu, Y.; Ma, X.; Shen, Y.; Cui, G.; Lin, H.; Rong, Q.; et al. The Biosynthetic Pathways of Tanshinones and Phenolic Acids in Salvia miltiorrhiza. Molecules 2015, 20, 16235–16254. [Google Scholar] [CrossRef]
- Vosoughi, N.; Gomarian, M.; Pirbalouti, A.G.; Khaghani, S.; Malekpoor, F. Essential oil composition and total phenolic, flavonoid contents, and antioxidant activity of sage (Salvia officinalis L.) extract under chitosan application and irrigation frequencies. Ind. Crops Prod. 2018, 117, 366–374. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, L.; Gao, S.; Saechao, S.; Di, P.; Chen, J. The c4h, tat, hppr and hppd Genes Prompted Engineering of Rosmarinic Acid Biosynthetic Pathway in Salvia miltiorrhiza Hairy Root Cultures. PLoS ONE 2011, 6, e29713. [Google Scholar] [CrossRef]
- Ali, E.F.; El-Shehawi, A.M.; Ibrahim, O.H.M.; Abdul-Hafeez, E.Y.; Moussa, M.M.; Hassan, F.A.S. A vital role of chitosan nanoparticles in improvisation the drought stress tolerance in Catharanthus roseus (L.) through biochemical and gene expression modulation. Plant Physiol. Biochem. 2021, 161, 166–175. [Google Scholar] [CrossRef]
- Alizadeh, R.; Kumleh, H.H.; Rezadoost, M.H. The simultaneous activity of cytosolic and mitochondrial antioxidant mechanisms in neutralizing the effect of drought stress in soybean. Plant Physiol. Rep. 2023, 28, 78–91. [Google Scholar] [CrossRef]
- Rajabbeigi, E.; Eichholz, I.; Beesk, N.; Ulrichs, C.; Kroh, L.W.; Rohn, S.; Huyskens-Keil, S. Interaction of drought stress and UV-B radiation-impact on biomass production and flavonoid metabolism in lettuce (Lactuca sativa L.). J. Appl. Bot. Food Qual. 2013, 86, 190–197. [Google Scholar]
- Salimgandomi, S.; Shabrangi, A. The effect of Chitosan on antioxidant activity and some secondary metabolites of Mentha piperita L. J. Pharm. Health Sci. 2016, 4, 135–142. [Google Scholar]
- Asghari, G.R.; Ghasemi, R.; Yosefi, M.; Mehdianezhad, N. Effect of hormones, salicylic acid, chitosan on phenolic compounds in Artemisia aucheri in vitro. J. Plant Process Funct. 2015, 3, 93–100. [Google Scholar]
- Amkha, S.; Rungcharoenthong, P. Effect of chitosan application on some secondary plant metabolites in chili. In Proceedings of the III Asian Horticultural Congress-AHC2020 1312, Bangkok, Thailand, 15–17 December 2020; pp. 243–248. [Google Scholar]
- Liu, H.; Wang, X.; Wang, D.; Zou, Z.; Liang, Z. Effect of drought stress on growth and accumulation of active constituents in Salvia miltiorrhiza Bunge. Ind. Crops Prod. 2011, 33, 84–88. [Google Scholar] [CrossRef]
- Dzung, N.A.; Khanh, V.T.P.; Dzung, T.T. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr. Polym. 2011, 84, 751–755. [Google Scholar] [CrossRef]
- Yang, D.; Du, X.; Liang, X.; Han, R.; Liang, Z.; Liu, Y.; Liu, F.; Zhao, J. Different roles of the mevalonate and methylerythritol phosphate pathways in cell growth and tanshinone production of Salvia miltiorrhiza hairy roots. PLoS ONE 2012, 7, e46797. [Google Scholar] [CrossRef]
- De Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Kwok, K.W.; Wu, J.Y. Enhancement of tanshinone production in Salvia miltiorrhiza Bunge (red or Chinese sage) hairy-root culture by hyperosmotic stress and yeast elicitor. Biotechnol. Appl. Biochem. 2007, 46, 191–196. [Google Scholar] [PubMed]
- Gerhardt, U.; Schroeter, A. Rosmarinic acid—A naturally occurring antioxidant in spices. Fleischwirtschaft 1983, 63, 1628–1630. [Google Scholar]
- Kamatou, G.P.P.; Viljoen, A.M.; Steenkamp, P. Antioxidant, antiinflammatory activities and HPLC analysis of South African Salvia species. Food Chem. 2010, 119, 684–688. [Google Scholar] [CrossRef]
- Janicsák, G.; Máthé, I.; Miklossy-Vari, V.; Blunden, G. Comparative studies of the rosmarinic and caffeic acid contents of Lamiaceae species. Biochem. Syst. Ecol. 1999, 27, 733–738. [Google Scholar] [CrossRef]
- Oreizi, E.; Rahiminejad, M.R.; Asghari, G. Influence of Environment on Glandular Trichomes and Composition of Essential Oil of Perovskia abrotanoides Karel. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e16432. [Google Scholar] [CrossRef]
- KH, R. Flora Iranica. Graz-Austria: Akademic Druck-u. Verlagsanstalt 1982, 150, 292–313. [Google Scholar]
- Jia, X.; Sun, C.; Li, G.; Li, G.; Chen, G. Effects of progressive drought stress on the physiology, antioxidative enzymes and secondary metabolites of Radix Astragali. Acta Physiol. Plant. 2015, 37, 1–14. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Afshari, M.; Sarfaraz, D.; Miroliaei, M. Using HPLC and multivariate analyses to investigate variations in the polyphenolic compounds as well as antioxidant and antiglycative activities of some Lamiaceae species native to Iran. Ind. Crops Prod. 2020, 154, 112640. [Google Scholar] [CrossRef]
- Rahimi, S.; Talebi, M.; Baninasab, B.; Gholami, M.; Zarei, M.; Shariatmadari, H. The role of plant growth-promoting rhizobacteria (PGPR) in improving iron acquisition by altering physiological and molecular responses in quince seedlings. Plant Physiol. Biochem. 2020, 155, 406–415. [Google Scholar] [CrossRef]
- Yousefian, S.; Lohrasebi, T.; Farhadpour, M.; Haghbeen, K. Effect of methyl jasmonate on phenolic acids accumulation and the expression profile of their biosynthesis-related genes in Mentha spicata hairy root cultures. Plant Cell Tissue Organ Cult. 2020, 142, 285–297. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
| Mean Squares | ||||||
|---|---|---|---|---|---|---|
| Source of Variation | DF 1 | Total Tanshinone Content (Root) | Total Phenolic Content (Leaf) | Total Flavonoid Content (Leaf) | Total Phenolic Content (Root) | Total Flavonoid Content (Root) |
| Species | 1 | 136.85 ** | 82.47 ns | 3.5 * | 357.77 ** | 0.62 ns |
| Stress | 2 | 141.66 ** | 51.05 ns | 0.95 ns | 48.12 ** | 1.2 * |
| Chitosan | 2 | 217.3 ** | 310.26 * | 5.86 ** | 6.18 ns | 1.14 * |
| Species × Stress 2 | 2 | 4.35 ns | 580.83 ** | 3.58 ns | 31.75 * | 2.5 ** |
| Species × Chitosan 3 | 2 | 91.75 ** | 168.86 ns | 0.69 ns | 24.20 * | 0.069 ns |
| Stress × Chitosan 4 | 4 | 118.44 ** | 615.3 ** | 3.88 ** | 43.41 ** | 1.51 ** |
| Species × Stress × Chitosan 5 | 4 | 38.56 ns | 241.03 * | 3.57 ** | 36.50 ** | 0.311 ns |
| Error | 36 | 16.84 | 81.97 | 0.502 | 7.04 | 0.348 |
| (A) | |||||
| Treatments | TTC 1 (mg/g DW) | TPC 2leaf (mg TAE/g DW) | TFC 3leaf (mg QE/gDW) | TPCroot (mg TAE/g DW) | TFCroot (mg QE/gDW) |
| No-stress | |||||
| 0 mg L−1 | 8.51 e | 35 e | 2.42 d | 10.54 bcd | 1.73 a |
| 100 mg L−1 | 18.02 b | 57.18 a | 5.04 a | 10.83 bcd | 1.98 a |
| 200 mg L−1 | 23.71 a | 43.61 cde | 3.34 bc | 13.25 ac | 2.018 a |
| Mild stress | |||||
| 0 mg L−1 | 8.34 e | 49.05 abc | 4.06 b | 12.65 abc | 0.6 c |
| 100 mg L−1 | 11.37 cde | 45.89 bcd | 4.13 b | 15.38 a | 2.09 a |
| 200 mg L−1 | 13.71 bcd | 44.26 cde | 3.11 cd | 14.45 a | 1.77 ab |
| Severe stress | |||||
| 0 mg L−1 | 15.47 bc | 36.85 de | 2.84 cd | 14.99 a | 1.69 ab |
| 100 mg L−1 | 10.13 de | 37.01 de | 3.43 bc | 8.65 d | 1.45 ab |
| 200 mg L−1 | 15.44 bc | 56.36 ab | 3.66 bc | 9.83 cd | 1.18 bc |
| (B) | |||||
| Species | Stress | TPCleaf (mg TAE/g DW) | TPCroot (mg TAE/g DW) | TFCroot (mg QE/gDW) | |
| S. abrotanoides | N0-stress | 37.48 c | 15.62 a | 2.28 a | |
| Mild stress | 48.10 ab | 15.73 a | 1.17 c | ||
| Severe stress | 45.78 abc | 13.22 ab | 1.71 bc | ||
| S. yangii | N0-stress | 53.05 a | 7.46 c | 1.54 bc | |
| Mild stress | 44.70 abc | 12.59 b | 1.81 ab | ||
| Severe stress | 41.03 bc | 9.08 c | 1.17 c | ||
| (C) | |||||
| Species | Chitosan | Total Tanshinone Content (mg/g DW) | Total Phenolic ContentRoot (mg TAE/g DW) | ||
| S. abrotanoides | 0 mg L−1 | 11.62 bcd | 16.54 a | ||
| 100 mg L−1 | 11.16 cd | 13.14 b | |||
| 200 mg L−1 | 14.01 bc | 14.89 ab | |||
| S. yangii | 0 mg L−1 | 9.93 d | 8.91 c | ||
| 100 mg L−1 | 15.18 b | 10.09 c | |||
| 200 mg L−1 | 21.23 a | 10.13 c | |||
| Species | S. abrotanoides | S. yangii | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Non-Stress | Mild Stress | Severe Stress | Non-Stress | Mild Stress | Severe Stress | ||||||||||||
| Compositions (mg/gDW) | 0 | 100 | 200 | 0 | 100 | 200 | 0 | 100 | 200 | 0 | 100 | 200 | 0 | 100 | 200 | 0 | 100 | 200 |
| Tanshinone-IIA | 0.448 | 0.480 | 1.123 | 0.368 | 0.807 | 0.832 | 1.040 | 0.401 | 0.277 | 0.496 | 0.700 | 0.977 | 0.455 | 0.509 | 1.022 | 1.757 | 0.553 | 0.975 |
| Hydroxy-Cryptotanshinone | 4.020 | 4.320 | 7.788 | 5.169 | 2.736 | 7.691 | 8.736 | 2.587 | 4.273 | 2.088 | 5.533 | 12.328 | 4.052 | 3.643 | 6.793 | 4.692 | 4.139 | 9.133 |
| Cryptotanshinone | 0.667 | 0.739 | 0.929 | 0.533 | 0.900 | 0.999 | 1.277 | 0.622 | 0.632 | 0.305 | 1.237 | 1.771 | 0.684 | 0.796 | 0.975 | 0.910 | 0.721 | 1.313 |
| Apigenin (Leaf) | 0.067 | 0.125 | 0.085 | 0.092 | 0.075 | 0.082 | 0.110 | 0.085 | 0.099 | 0.085 | 0.089 | 0.078 | 0.067 | 0.087 | 0.082 | 0.682 | 0.087 | 0.103 |
| Rosmarinic acid (Leaf) | 0.145 | 11.435 | 6.674 | 6.908 | 4.967 | 1.737 | 0.161 | 4.612 | 5.065 | 0.545 | 9.671 | 1.871 | 0.967 | 8.522 | 4.309 | 1.500 | 0.038 | 2.281 |
| Gallic acid (Leaf) | 0.455 | 0.059 | 0.037 | 0.055 | 0.062 | 0.046 | 0.006 | 0.458 | 0.058 | 0.059 | 0.066 | 0.083 | 0.052 | 0.049 | 0.066 | 0.012 | 0.255 | 0.034 |
| Caffeic acid (Leaf) | 0.080 | 0.227 | 0.188 | 0.208 | 0.252 | 0.098 | 0.110 | 0.103 | 0.246 | 0.105 | 0.155 | 0.192 | 0.121 | 0.210 | 0.218 | 0.074 | 0.082 | 0.318 |
| Chlorogenic acid (Leaf) | 0.020 | 0.328 | 0.149 | 0.169 | 0.201 | 0.103 | 0.071 | 0.034 | 0.136 | 0.089 | 0.060 | 0.112 | 0.084 | 0.099 | 0.115 | 0.071 | 0.169 | 0.166 |
| Apigenin (Root) | 0.068 | 0.070 | 0.065 | 0.057 | 0.048 | 0.065 | 0.060 | 0.064 | 0.062 | 0.057 | 0.075 | 0.068 | 0.076 | 0.082 | 0.068 | 0.056 | 0.085 | 0.055 |
| Rosmarinic acid (Root) | 3.812 | 3.051 | 5.787 | 4.259 | 3.863 | 1.154 | 1.747 | 2.254 | 0.536 | 1.952 | 1.956 | 1.402 | 1.467 | 4.004 | 3.384 | 2.108 | 1.348 | 1.075 |
| Gallic acid (Root) | 0.128 | 0.124 | 0.106 | 0.054 | 0.017 | 0.020 | 0.094 | 0.035 | 0.148 | 0.011 | 0.020 | 0.039 | 0.072 | 0.143 | 0.018 | 0.510 | 0.016 | 0.053 |
| Caffeic acid (Root) | 0.114 | 0.165 | 0.191 | 0.076 | 0.136 | 0.144 | 0.100 | 0.131 | 0.084 | 0.112 | 0.164 | 0.159 | 0.102 | 0.092 | 0.420 | 0.067 | 0.354 | 0.086 |
| Chlorogenic acid (Root) | 0.039 | 0.194 | 0.123 | 0.061 | 0.061 | 0.123 | 0.026 | 0.072 | 0.061 | 0.069 | 0.143 | 0.105 | 0.067 | 0.057 | 0.188 | 0.077 | 0.126 | 0.046 |
| Traits | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tanshinone-IIA | 1 | |||||||||
| 2 | Hydroxy-cryptotanshinone | 0.48 * | 1 | ||||||||
| 3 | Cryptotanshinone | 0.54 * | 0.86 ** | 1 | |||||||
| 4 | KSL 1 | −0.4 | −0.3 | −0.4 | 1 | ||||||
| 5 | 4CL 2 | 0.11 | −0.04 | 0.01 | 0.14 | 1 | |||||
| 6 | HMGR 3 | −0.13 | 0.02 | 0.12 | 0.12 | 0.2 | 1 | ||||
| 7 | DXS 4 | −0.17 | −0.18 | −0.15 | 0.07 | 0.62 ** | 0.21 | 1 | |||
| 8 | TAT 5 | 0.21 | 0.05 | 0.16 | −0.02 | 0.62 ** | 0.005 | 0.03 | 1 | ||
| 9 | Total phenolicleaf | −0.04 | 0.05 | 0.13 | 0.02 | −0.23 | 0.04 | −0.13 | −0.08 | 1 | |
| 10 | Total phenolicroot | 0.25 | 0.07 | 0.12 | −0.24 | −0.11 | −0.14 | −0.11 | 0.16 | −0.18 | 1 |
| Target Genes | Primers F/R (5′→3′) |
|---|---|
| KSL | AATTAGATACATGGAGCGAT |
| HMGR | ATTTTATACCGATGAACTGC |
| DXS2 | CTTGCCATCATTAATAGCC |
| TAT | TGCAATATTTATCGCCACT |
| 4Cl | GTTCCCCGACCGTTGCTTC |
| Elongation Factor | GCACCACCTGATCGTACCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodadadi, F.; Ahmadi, F.S.; Talebi, M.; Matkowski, A.; Szumny, A.; Afshari, M.; Rahimmalek, M. Metabolic and Transcriptomic Approaches of Chitosan and Water Stress on Polyphenolic and Terpenoid Components and Gene Expression in Salvia abrotanoides (Karl.) and S. yangii. Int. J. Mol. Sci. 2023, 24, 15426. https://doi.org/10.3390/ijms242015426
Khodadadi F, Ahmadi FS, Talebi M, Matkowski A, Szumny A, Afshari M, Rahimmalek M. Metabolic and Transcriptomic Approaches of Chitosan and Water Stress on Polyphenolic and Terpenoid Components and Gene Expression in Salvia abrotanoides (Karl.) and S. yangii. International Journal of Molecular Sciences. 2023; 24(20):15426. https://doi.org/10.3390/ijms242015426
Chicago/Turabian StyleKhodadadi, Farzaneh, Farajollah Shahriai Ahmadi, Majid Talebi, Adam Matkowski, Antoni Szumny, Mahvash Afshari, and Mehdi Rahimmalek. 2023. "Metabolic and Transcriptomic Approaches of Chitosan and Water Stress on Polyphenolic and Terpenoid Components and Gene Expression in Salvia abrotanoides (Karl.) and S. yangii" International Journal of Molecular Sciences 24, no. 20: 15426. https://doi.org/10.3390/ijms242015426






