Impact of Heavy Metals on Glioma Tumorigenesis
Abstract
:1. Introduction
2. Gliomas
Genetic Alterations in Gliomas
3. Heavy Metals
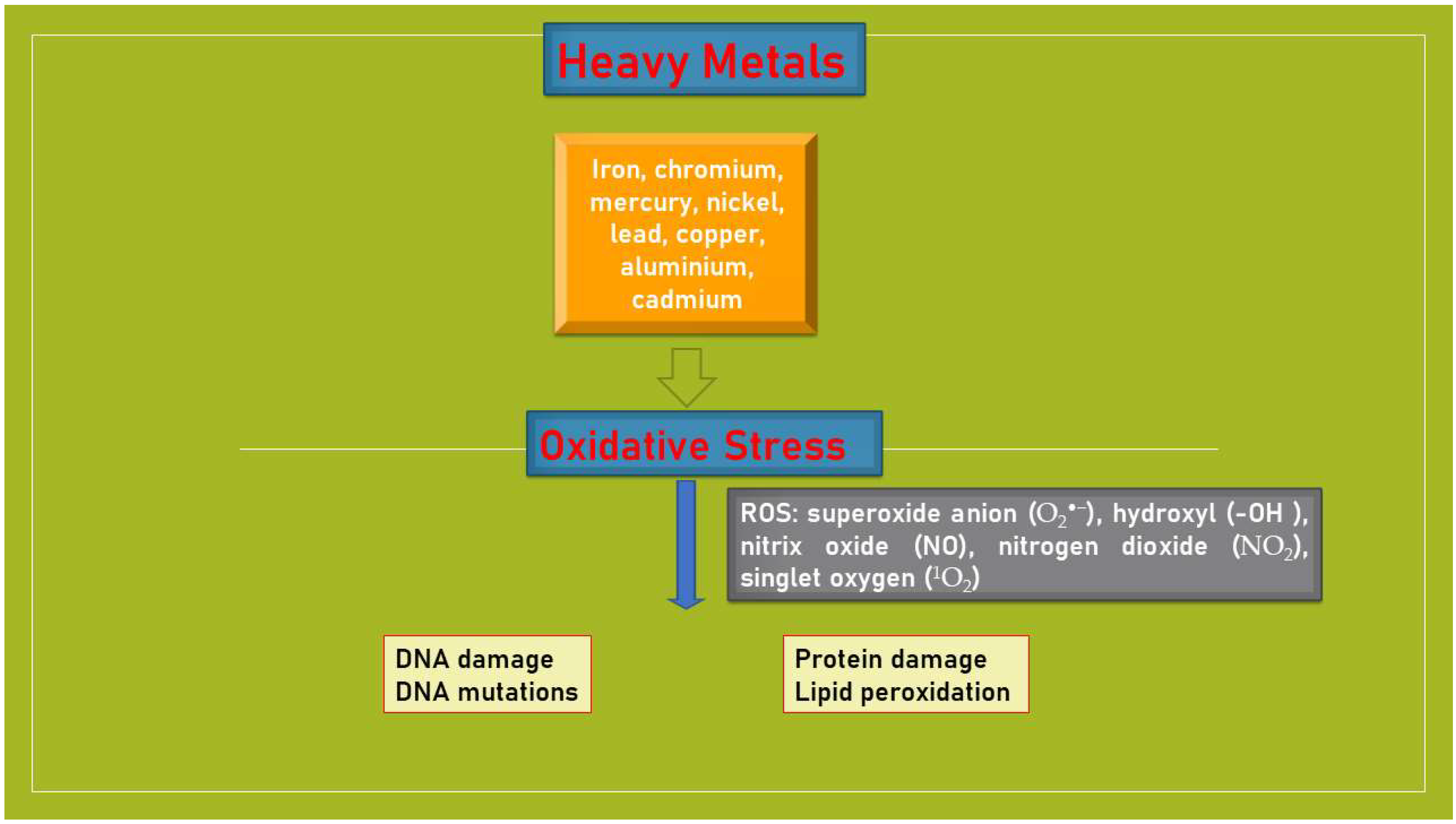
3.1. Heavy Metals and Oxidative Stress
3.2. Heavy Metals and Gliomas
Metal Nanoparticles
4. Heavy Metals and Other Brain Tumors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schottenfeld, D.; Beebe-Dimmer, J.L.; Buffler, P.A.; Omenn, G.S. Current Perspective on the Global and United States Cancer Burden Attributable to Lifestyle and Environmental Risk Factors. Annu. Rev. Public Health 2013, 34, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.M. Mechanisms of Chemical Carcinogenesis and Application to Human Cancer Risk Assessment. Toxicology 2001, 166, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Nohmi, T. Thresholds of Genotoxic and Non-Genotoxic Carcinogens. Toxicol. Res. 2018, 34, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.R.; Zaidi, S.R.; Batool, M.; Bhatti, A.A.; Durrani, A.I.; Mahmood, Z. Report: Central Nervous System (CNS) Toxicity Caused by Metal Poisoning: Brain as a Target Organ. Pak. J. Pharm. Sci. 2015, 28, 1417–1423. [Google Scholar] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2010–2014. Neuro Oncol. 2017, 19, v1–v88. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme-Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Fazzari, E.; Cardali, S.M.; Caffo, M. Applications of Nanoparticles in the Treatment of Gliomas. In Nanoparticle Drug Delivery Systems for Cancer Treatment, 1st ed.; Gali-Muhtasib, H., Chouaib, R., Eds.; Jenny Stanford Publishing: New York, NY, USA, 2020; pp. 182–216. [Google Scholar]
- Caruso, G.; Raudino, G.; Caffo, M. Patented Nanomedicines for the Treatment of Brain Tumors. Pharm. Pat. Anal. 2013, 2, 745–754. [Google Scholar] [CrossRef]
- Idbaih, A.; Silva, R.C.; Crinière, E.; Marie, Y.; Carpentier, C.; Boisselier, B.; Taillibert, S.; Rousseau, A.; Mokhtari, K.; Ducray, F.; et al. Genomic Changes in Progression of Low-Grade Gliomas. J. Neurooncol. 2008, 90, 133–140. [Google Scholar] [CrossRef]
- Jin, B.; Li, Y.; Robertson, K.D. DNA Methylation: Superior or Subordinate in the Epigenetic Hierarchy? Genes Cancer 2011, 2, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.C. Gliomagenesis: Genetic Alterations and Mouse Models. Nat. Rev. Genet. 2001, 2, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, K.; Kleihues, P. Genetic Pathways to Primary and Secondary Glioblastoma. Am. J. Pathol. 2007, 170, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Caffo, M.; Raudino, G.; Alafaci, C.; Salpietro, F.M.; Tomasello, F. Antisense Oligonucleotides as an Innovative Therapeutic Strategy in the Treatment of High-Grade Gliomas. Recent Pat. CNS Drug Discov. 2010, 5, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Hachem, L.D.; Mansouri, S.; Nassiri, F.; Laperriere, N.J.; Xia, D.; Lindeman, N.I.; Wen, P.Y.; Chakravarti, A.; Mehta, M.P.; et al. MGMT Promoter Methylation Status Testing to Guide Therapy for Glioblastoma: Refining the Approach Based on Emerging Evidence and Current Challenges. Neuro Oncol. 2019, 21, 167–178. [Google Scholar] [CrossRef]
- Lu, C.; Ward, P.S.; Kapoor, G.S.; Rohle, D.; Turcan, S.; Abdel-Wahab, O.; Edwards, C.R.; Khanin, R.; Figueroa, M.E.; Melnick, A.; et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 2012, 483, 474–478. [Google Scholar] [CrossRef]
- Kim, Y.Z. Altered Histone Modifications in Gliomas. Brain Tumor Res. Treat. 2014, 2, 7–21. [Google Scholar] [CrossRef]
- Häyry, V.; Tanner, M.; Blom, T.; Tynninen, O.; Roselli, A.; Ollikainen, M.; Sariola, H.; Wartiovaara, K.; Nupponen, N.N. Copy Number Alterations of the Polycomb Gene BMI1 in Gliomas. Acta Neuropathol. 2008, 116, 97–102. [Google Scholar] [CrossRef]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.-Y.; Jones, D.T.W.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.M.; Khuong-Quang, D.-A.; Tönjes, M.; et al. Driver Mutations in Histone H3.3 and Chromatin Remodelling Genes in Paediatric Glioblastoma. Nature 2012, 482, 226–231. [Google Scholar] [CrossRef]
- Caruso, G.; Caffo, M.; Raudino, G.; Raudino, F.; Venza, M.; Tomasello, F. Antisense Oligonucleotides in the Treatment of Malignant Gliomas. In From Nucleic Acids Sequences to Molecular Medicine, 1st ed.; Erdmann, V.A., Barciszewski, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 217–246. [Google Scholar]
- Chan, J.A.; Krichevsky, A.M.; Kosik, K.S. MicroRNA-21 is an Antiapoptotic Factor in Human Glioblastoma Cells. Cancer Res. 2005, 65, 6029–6033. [Google Scholar] [CrossRef]
- Gabriely, G.; Wurdinger, T.; Kesari, S.; Esau, C.C.; Burchard, J.; Linsley, P.S.; Krichevsky, A.M. MicroRNA 21 Promotes Glioma Invasion by Targeting Matrix Metalloproteinase Regulators. Mol. Cell Biol. 2008, 28, 5369–5380. [Google Scholar] [CrossRef] [PubMed]
- Aloizou, A.-M.; Pateraki, G.; Siokas, V.; Mentis, A.-F.A.; Liampas, I.; Lazopoulos, G.; Kovatsi, L.; Mitsias, P.D.; Bogdanos, D.P.; Paterakis, K.; et al. The Role of MiRNA-21 in Gliomas: Hope for a Novel Therapeutic Intervention? Toxicol. Rep. 2020, 7, 1514–1530. [Google Scholar] [CrossRef] [PubMed]
- Mulware, S.J. Comparative Trace Elemental Analysis in Cancerous and Noncancerous Human Tissues Using PIXE. J. Biophys. 2013, 2013, 192026. [Google Scholar] [CrossRef] [PubMed]
- Cilliers, K.; Muller, C.J.F.; Page, B.J. Trace Element Concentration Changes in Brain Tumors: A Review. Anat. Rec. 2020, 303, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy Metals and Living Systems: An Overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef]
- Arslan, M.; Demir, H.; Arslan, H.; Gokalp, A.S.; Demir, C. Trace Elements, Heavy Metals, and Other Biochemical Parameters in Malignant Glioma Patients. Asian Pac. J. Cancer Prev. 2011, 12, 447–451. [Google Scholar] [PubMed]
- Popov, B.; Gadjeva, V.; Valkanov, P.; Popova, S.; Tolekova, A. Lipid Peroxidation, Superoxide Dismutase and Catalase Activities in Brain Tumor Tissues. Arch. Physiol. Biochem. 2003, 111, 455–459. [Google Scholar] [CrossRef]
- Rinaldi, M.; Caffo, M.; Minutoli, L.; Marini, H.; Abbritti, R.V.; Squadrito, F.; Trichilo, V.; Valenti, A.; Barresi, V.; Altavilla, D.; et al. ROS and Brain Gliomas: An Overview of Potential and Innovative Therapeutic Ttrategies. Int. J. Mol. Sci. 2016, 17, 984. [Google Scholar] [CrossRef]
- Wang, L.; Wise, J.T.; Zhang, Z.; Shi, X. Progress and Prospects of Reactive Oxygen Species in Metal Carcinogenesis. Curr. Pharmacol. Rep. 2016, 2, 178–186. [Google Scholar] [CrossRef]
- Wise, J.T.; Wang, L.; Zhang, Z.; Shi, X. The 9th Conference on Metal Toxicity and Carcinogenesis: The Conference Overview. Toxicol. Appl. Pharmacol. 2017, 331, 1–5. [Google Scholar] [CrossRef]
- Xu, J.; Wise, J.T.F.; Wang, L.; Schumann, K.; Zhang, Z.; Shi, X. Dual Roles of Oxidative Stress in Metal Carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2017, 36, 345–376. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Bowen, P.E. DNA Damage, a Biomarker of Carcinogenesis: Its Measurement and Modulation by Diet and Environment. Crit. Rev. Food Sci. Nutr. 2007, 47, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.; Shen, S.; Chow, J.; Lin, C.; Shia, L.; Chen, Y. Contribution of Reactive Oxygen Species to Migration/Invasion of Human Glioblastoma Cells U87 via ERK-Dependent COX-2/PGE(2) Activation. Neurobiol. Dis. 2010, 37, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Caffo, M.; Caruso, G.; Barresi, V.; Pino, M.A.; Venza, M.; Alafaci, C.; Tomasello, F. Immunohistochemical Study of CD68 and CR3/43 in Astrocytic Gliomas. J. Analyt. Oncol. 2012, 1, 42–49. [Google Scholar] [CrossRef]
- Steenland, K.; Boffetta, P. Lead and Cancer in Humans: Where are we now? Am. J. Ind. Med. 2000, 38, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Cocco, P.; Heineman, E.F.; Dosemeci, M. Occupational Risk Factors for Cancer of the Central Nervous System (CNS) Among US Women. Am. J. Ind. Med. 1999, 36, 70–74. [Google Scholar] [CrossRef]
- Gaman, L.; Radoia, M.P.; Deliac, C.E.; Luzardo, O.P.; Zumbado, M.; Rodríguez-Hernándeze, A.; Stoiana, I.; Gilca, M.; Boada, L.D.; Henríquez-Hernández, L.A. Concentration of Heavy Metals and Rare Earth Elements in Patients with Brain Tumours: Analysis in Tumour Tissue, Non-Tumour Tissue, and Blood. Int. J. Environ. Health Res. 2021, 31, 741–754. [Google Scholar] [CrossRef]
- Nguyen, H.D. Prognostic Biomarker Prediction for Glioma Induced by Heavy Metals and their Mixtures: An in-Silico Study. Toxicol. Appl. Pharmacol. 2023, 15, 116356. [Google Scholar] [CrossRef]
- Xie, M.-Y.; Huang, G.-L.; Lin, Z.-Y.; Sun, X.-F.; Wu, C.-C.; Liu, Y.-W.; Liu, L.-Y.; Zeng, E.Y. Insufficient Evidence to Link Human Exposure to Heavy Metals with Biomarkers of Glioma. J. Hazard. Mater. 2023, 5, 130779. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.; Ye, J.; Mei, S.; Zhang, J. Identification of Iron Metabolism Related Genes as Prognostic Indicators for Lower-Grade Glioma. Front. Oncol. 2011, 11, 729103. [Google Scholar] [CrossRef]
- Pan, S.Y.; Ugnat, A.M.; Mao, Y. Occupational Risk Factors for Brain Cancer in Canada. J. Occup. Environ. Med. 2005, 47, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.-E.; Turner, M.C.; Lavoué, J.; Richard, H.; Figuerola, J.; Kincl, L.; Richardson, L.; Benke, G.; Blettner, M.; Fleming, S.; et al. Lifetime Occupational Exposure to Metals and Welding Fumes, and Risk of Glioma: A 7-Country Population-Based Case-Control Study. Environ. Health 2017, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Van Wijngaarden, E.; Dosemeci, M. Brain Cancer Mortality and Potential Occupational Exposure to Lead: Findings from the National Longitudinal Mortality Study, 1979–1989. Int. J. Cancer 2006, 119, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, H.G.; Sicard, D.; Torres, M.M. DNA Damage and Repair in Cells of Lead Exposed People. Am. J. Ind. Med. 2000, 38, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Cocco, P.; Dosemeci, M.; Heineman, E.F. Brain Cancer and Occupational Exposure to Lead. J. Occup. Environ. Med. 1998, 40, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Swaran, F.J.S. Arsenic-Induced Oxidative Stress and its Reversibility. Free Radic. Biol. Med. 2011, 51, 257–281. [Google Scholar] [CrossRef]
- Ishida, S.; Andreux, P.; Poitry-Yamate, C.; Auwerx, J.; Hanahan, D. Bioavailable Copper Modulates Oxidative Phosphorylation and Growth of Tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 19507–19512. [Google Scholar] [CrossRef]
- Arita, A.; Costa, M. Epigenetics in Metal Carcinogenesis: Nickel, Arsenic, Chromium and Cadmium. Metallomics 2009, 1, 222–228. [Google Scholar] [CrossRef]
- Verstraeten, S.V.; Nogueira, L.V.; Schreier, S.; Oteiza, P.I. Effect of Trivalent Metal Ions on Phase Separation and Membrane Lipid Packing: Role in Lipid Peroxidation. Arch. Biochem. Biophys. 1997, 338, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Caffo, M.; Alafaci, C.; Raudino, G.; Cafarella, D.; Lucerna, S.; Salpietro, F.M.; Tomasello, F. Could Nanoparticle Systems Have a Role in the Treatment of Cerebral Gliomas? Nanomedicine 2011, 7, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Marino, D.; Caffo, M. Nanoparticles and CNS Delivery of Therapeutic Agents in the Treatment of Primary Brain Tumors. J. Analyt. Oncol. 2014, 3, 105–112. [Google Scholar]
- Bystrzejewska-Piotrowska, G.; Golimowski, J.; Urban, P.L. Nanoparticles: Their Potential Toxicity, Waste and Environmental Management. Waste Manag. 2009, 29, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Medici, S.; Ledda, A.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M. Toxicity of Nanoparticles. Curr. Med. Chem. 2014, 21, 3837–3853. [Google Scholar] [CrossRef] [PubMed]
- De Prado Bert, P.; Mercader, E.M.H.; Pujol, J.; Sunyer, J.; Mortamais, M. The Effects of Air Pollution on the Brain: A Review of Studies Interfacing Environmental Epidemiology and Neuroimaging. Curr. Environ. Health Rep. 2018, 5, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Bantz, C.; Koshkina, O.; Lang, T.; Galla, H.J.; Kirkpatrick, C.J.; Stauber, R.H.; Maskos, M. The Surface Properties of NP Determine the Agglomeration State and the Size of the Particles Under Physiological Conditions. Beilstein J. Nanotechnol. 2014, 5, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hondroulis, E.; Liu, W.; Li, C. Biosensing Approaches for Rapid Genotoxicity and Cytotoxicity Assays Upon Nanomaterial Exposure. Small 2013, 9, 1821–1830. [Google Scholar] [CrossRef]
- Azad, M.B.; Chen, Y.; Gibson, S.B. Regulation of Autophagy by Reactive Oxygen Species (ROS): Implications for Cancer Progression and Treatment. Antioxid. Redox Signal. 2009, 11, 777–790. [Google Scholar] [CrossRef]
- Poli, G.; Leonarduzzi, G.; Biasi, F.; Chiarpotto, E. Oxidative Stress and Cell Signalling. Curr. Med. Chem. 2012, 11, 1163–1182. [Google Scholar] [CrossRef]
- Choi, A.O.; Brown, S.E.; Szyf, M.; Maysinger, D. Quantum Dot-Induced Epigenetic and Genotoxic Changes in Human Breast Cancer Cells. J. Mol. Med. 2008, 86, 291–302. [Google Scholar] [CrossRef]
- Caruso, G.; Ferrarotto, R.; Curcio, A.; Metro, L.; Pasqualetti, F.; Gaviani, P.; Barresi, V.; Angileri, F.F.; Caffo, M. Novel Advances in Treatment of Meningiomas: Prognostic and Therapeutic Implications. Cancers 2023, 15, 4521. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro Oncol. 2016, 18, v1–v75. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.S. Handbook of Neurosurgery, 9th ed.; Thieme: Stuttgart, Germany, 2019; ISBN 978-1-68420-137-2. [Google Scholar]
- Muller, W.; Iffland, R. Studies on Metals in Meningiomas by Atomic Absorption Spectometry. Acta Neuropathol. 1981, 55, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Nagaishi, M.; Yokoo, H.; Osawa, T.; Nobusawa, S.; Tanaka, Y.; Ikota, H.; Yoshimoto, Y.; Nakazato, Y. Cytoplasmic Iron Deposition is Associated with the Expression of Oxidative DNA Damage Marker in Meningiomas. Neuropathology 2013, 33, 526–532. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.K.; Thomas, T.L.; Stone, B.J.; Blot, W.J.; Malker, H.S.; Wiener, J.A.; Ericsson, J.L.; Malker, B.K. Occupational Risks for Meningiomas of the CNS in Sweden. J. Occup. Med. 1987, 29, 66–68. [Google Scholar] [PubMed]
- Preston-Martin, S.; Mack, W.; Henderson, B.E. Risk Factors for Gliomas and Meningiomas in Males in Los Angeles County. Cancer Res. 1989, 49, 6137–6143. [Google Scholar] [PubMed]
- Hu, J.; Little, J.; Xu, T.; Zhao, X.; Guo, L.; Jia, X.; Huang, G.; Bi, D.; Liu, R. Risk Factors for Meningioma in Adults: A Case-Control Study in Northeast China. Int. J. Cancer 1999, 83, 299–304. [Google Scholar] [CrossRef]
- Liao, L.M.; Friesen, M.C.; Xiang, Y.; Cai, H.; Koh, D.; Ji, B.; Yang, G.; Li, H.; Locke, S.J.; Rothman, N.; et al. Occupational Lead Exposure and Associations with Selected Cancers: The Shanghai Men’s and Women’s Health Study Cohorts. Environ. Health Perspect. 2016, 124, 97–103. [Google Scholar] [CrossRef]
- Sadetzki, S.; Chetrit, A.; Turner, M.C.; van Tongeren, M.; Benke, G.; Fifuerola, J.; Fleming, S.; Hours, M.; Kincl, L.; Krewski, D.; et al. Occupational Exposure to Metals and Risk of Meningioma: A Multinational Case-Control Study. J. Neurooncol. 2016, 130, 505–515. [Google Scholar] [CrossRef]
- Huang, X. Iron Overload and Its Association with Cancer Risk in Humans: Evidence for Iron as a Carcinogenic Metal. Mutat. Res. 2003, 533, 153–171. [Google Scholar] [CrossRef]
- Rajaraman, P.; Schwartz, B.S.; Rothman, N.; Yeager, M.; Fine, H.A.; Shapiro, W.R.; Selker, R.G.; Black, P.M.; Inskip, P.D. Delta-Aminolevulinic Acid Dehydratase Polymorphism and Risk of Brain Tumors in Adults. Environ. Health Perspect. 2005, 113, 1209–1211. [Google Scholar] [CrossRef]
- Meng, Y.; Tang, C.; Yu, J.; Meng, S.; Zhang, W. Exposure to Lead Increases the Risk of Meningioma and Brain Cancer: A Meta-Analysis. J. Trace Elem. Med. Biol. 2020, 60, 126474. [Google Scholar] [CrossRef] [PubMed]
- Pekic, S.; Stojanovic, M.; Popovic, V. Pituitary Tumors and the Risk of Other Malignancies: Is the Relationship Coincidental or Causal? Endocr. Oncol. 2022, 2, R1–R13. [Google Scholar] [CrossRef] [PubMed]
- Pepe, S.; Korbonits, M.; Iacovazzo, D. Germline and Mosaic Mutations Causing Pituitary Tumours: Genetic and Molecular Aspects. J. Endocrinol. 2019, 240, R21–R45. [Google Scholar] [CrossRef] [PubMed]
- Waalkes, M.P.; Anver, M.; Diwan, B.A. Carcinogenic Effects of Cadmium in the Noble (NBL/Cr) Rat: Induction of Pituitary, Testicular, and Injection Site Tumors and Intraepithelial Proliferative Lesions of the Dorsolateral Prostate. Toxicol. Sci. 1999, 52, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kulas, J.; Tucovic, D.; Zeljkovic, M.; Popovic, D.; Popov Aleksandrov, A.; Kataranovski, M.; Mirkov, I. Aryl Hydrocarbon Receptor is Involved in the Proinflammatory Cytokine Response to Cadmium. Biomed. Environ. Sci. 2021, 34, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Caffo, M.; Barresi, V.; Caruso, G.; Cutugno, M.; La Fata, G.; Venza, M.; Alafaci, C.; Tomasello, F. Innovative Therapeutic Strategies in the Treatment of Brain Metastases. Int. J. Mol. Sci. 2013, 14, 2135–2174. [Google Scholar] [CrossRef]
- Kruger, A.; Sanchez-Sweatman, O.H.; Martin, D.C.; Fata, J.E.; Ho, A.T.; Orr, F.W.; Ruther, U.; Khokha, R. Host TIMP-1 Overexpression Confers Resistance to Experimental Brain Metastasis of a Fibrosarcoma Cell Line. Oncogene 1998, 16, 2419–2423. [Google Scholar] [CrossRef]
- Haga, A.; Nagase, H.; Kito, H.; Sato, T. Enhanced Invasiveness of Tumour Cells After Host Exposure to Heavy Metals. Eur. J. Cancer 1996, 32A, 2342–2347. [Google Scholar] [CrossRef]
- Wei, T.; Jia, J.; Wada, Y.; Kapron, C.M.; Liu, J. Dose Dependent Effects of Cadmium on Tumor Angiogenesis. Oncotarget 2017, 8, 44944–44959. [Google Scholar] [CrossRef]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Roles of Dioxins and Heavy Metals in Cancer and Neurological Diseases Using ROS-Mediated Mechanisms. Free Radic. Biol. Med. 2010, 49, 1328–1341. [Google Scholar] [CrossRef]
- Green, S.E.; Luczak, M.W.; Morse, J.L.; DeLoughery, Z.; Zhitkovich, A. Uptake, P53 Pathway Activation, and Cytotoxic Responses for Co(II) and Ni(II) in Human Lung Cells: Implications for Carcinogenicity. Toxicol. Sci. 2013, 136, 467–477. [Google Scholar] [CrossRef] [PubMed]
| Heavy Metals | Atomic Number | Sources of Exposure |
|---|---|---|
| Iron (Fe) | 26 | Household appliances, utensils, food containers, motor vehicles, hulls, armaments, and in steel and cast-iron alloys |
| Chromium (Cr) | 24 | Enamels, paints, dyes, leather tanning, and fabric dyes |
| Mercury (Hg) | 80 | Detonators, pigments for antifouling paints (ship hulls), pesticides, for the preparation of soda in electrolytic cells, in the manufacture of physics apparatus (barometers and pressure gauges), batteries lamps, to make mirrors, and in dentistry |
| Nickel (Ni) | 28 | Rechargeable batteries, ship propellers, kitchen equipment, industrial chemical plant pipes, coins, coatings of iron, brass, and other metallic materials |
| Lead (Pb) | 82 | Construction, in the production of batteries, enamels for pottery, crystal glass, in the automotive sector, in bullets for firearms, and in the liquid state, as a coolant in some types of nuclear reactors |
| Copper (Cu) | 29 | Electric cables, pipes, furnishing finishes, sculptures, pictorial support, coins, musical instruments, dyes, pots, chemical solutions, fungicides, and dissipators |
| Aluminum (Al) | 13 | Transportation, packaging, construction, consumer durables, power lines, optics, and firearms or parts thereof—ammunition shells and shells |
| Cadmium (Cd) | 46 | Electrodes, coatings of metallic materials, solders, and pigments |
| Arsenic (As) | 33 | Insecticides, cosmetics, fireworks, photovoltaic panels, and dyes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, G.; Nanni, A.; Curcio, A.; Lombardi, G.; Somma, T.; Minutoli, L.; Caffo, M. Impact of Heavy Metals on Glioma Tumorigenesis. Int. J. Mol. Sci. 2023, 24, 15432. https://doi.org/10.3390/ijms242015432
Caruso G, Nanni A, Curcio A, Lombardi G, Somma T, Minutoli L, Caffo M. Impact of Heavy Metals on Glioma Tumorigenesis. International Journal of Molecular Sciences. 2023; 24(20):15432. https://doi.org/10.3390/ijms242015432
Chicago/Turabian StyleCaruso, Gerardo, Aristide Nanni, Antonello Curcio, Giuseppe Lombardi, Teresa Somma, Letteria Minutoli, and Maria Caffo. 2023. "Impact of Heavy Metals on Glioma Tumorigenesis" International Journal of Molecular Sciences 24, no. 20: 15432. https://doi.org/10.3390/ijms242015432





