Garden Cress Seed Oil Abrogates Testicular Oxidative Injury and NF-kB-Mediated Inflammation in Diabetic Mice
Abstract
1. Introduction
2. Results
2.1. Identification of the Bioactive Compounds in LSO by GC-MS Analysis
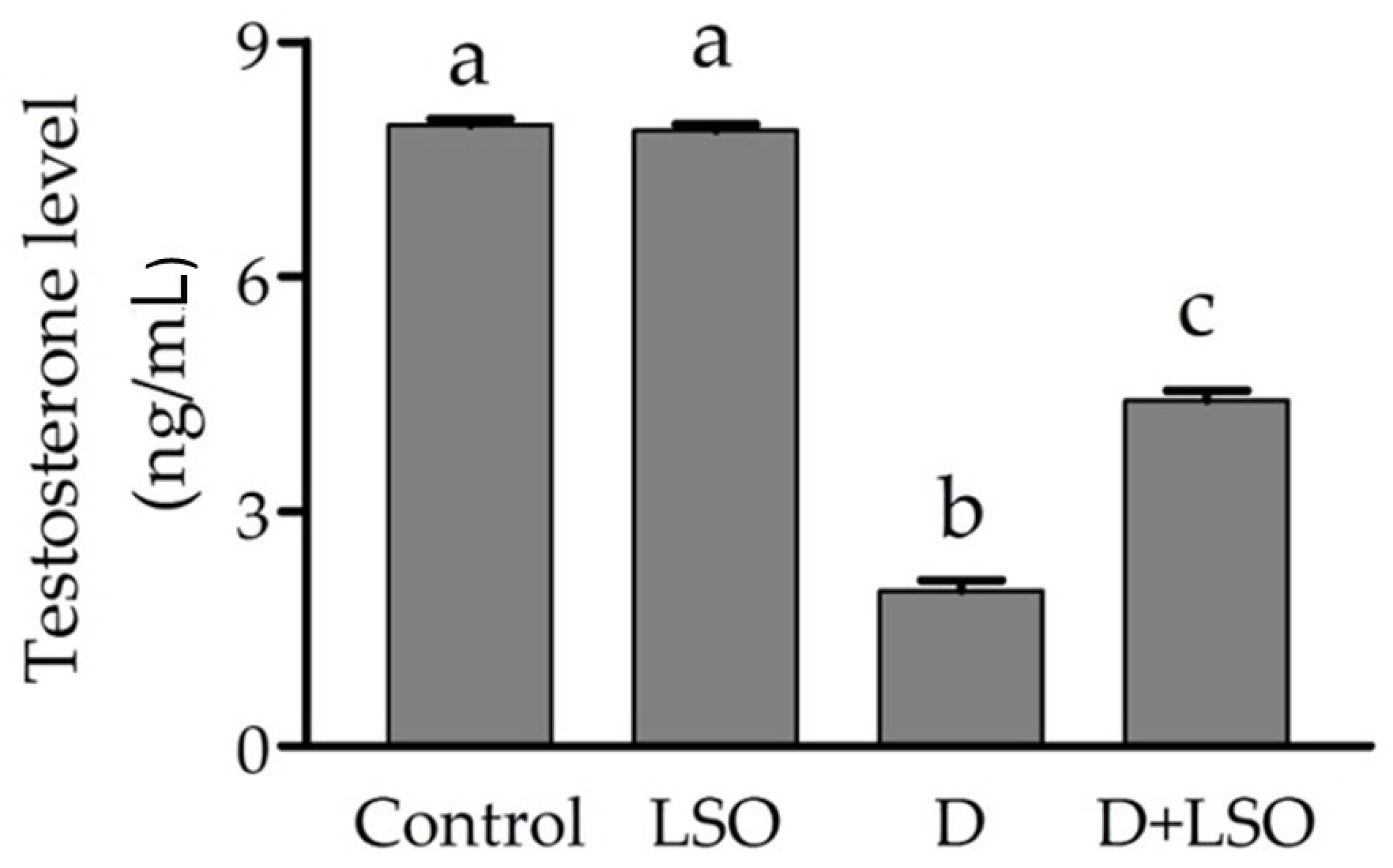
2.2. Serum Testosterone Level in Diabetic Mice Treated with/without LSO
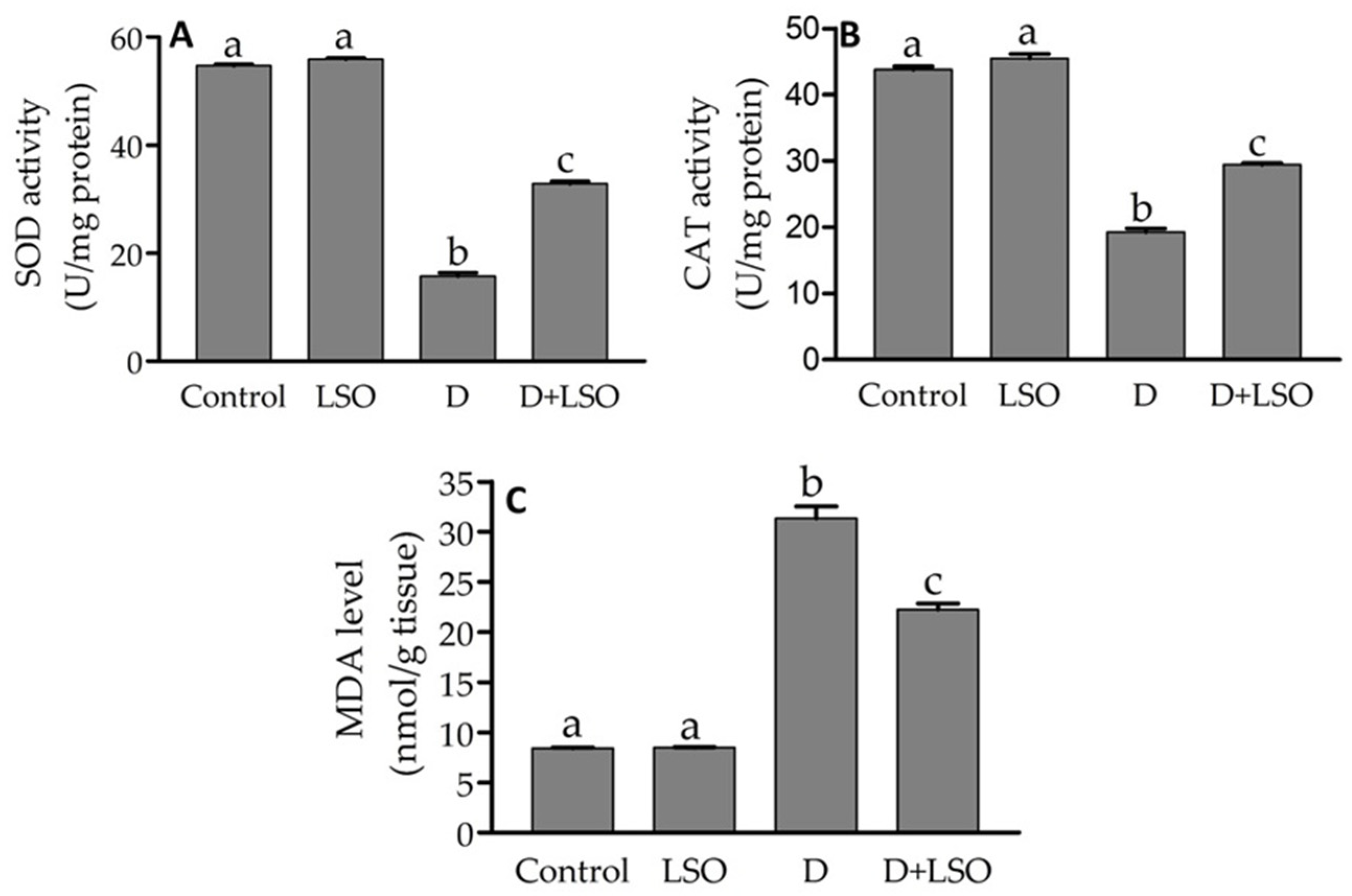
2.3. Redox Status in the Testes of Diabetic Mice Treated with/without LSO
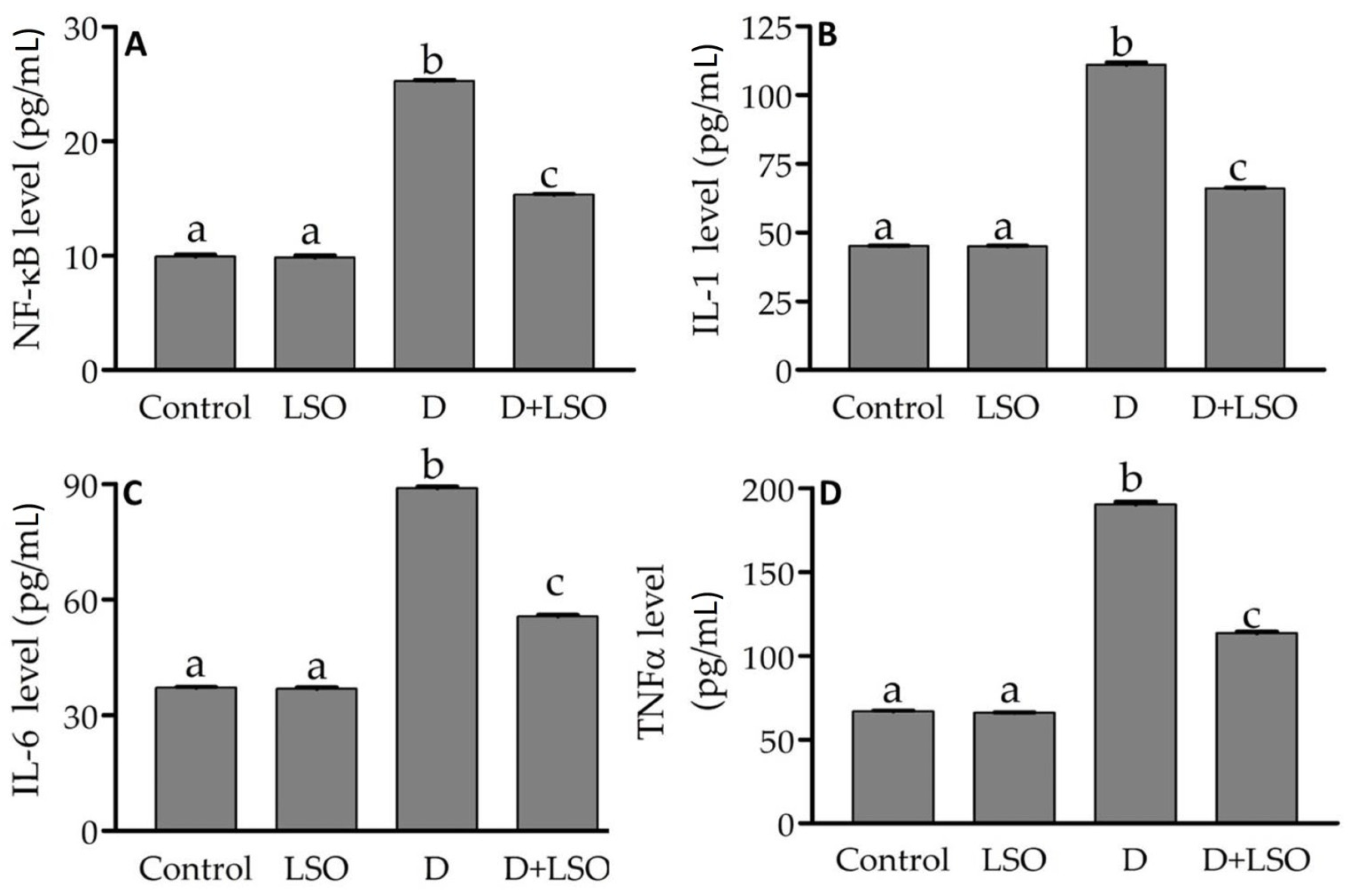
2.4. NF-kB and Pro-Inflammatory Cytokines in the Testes of Diabetic Mice Treated with/without LSO
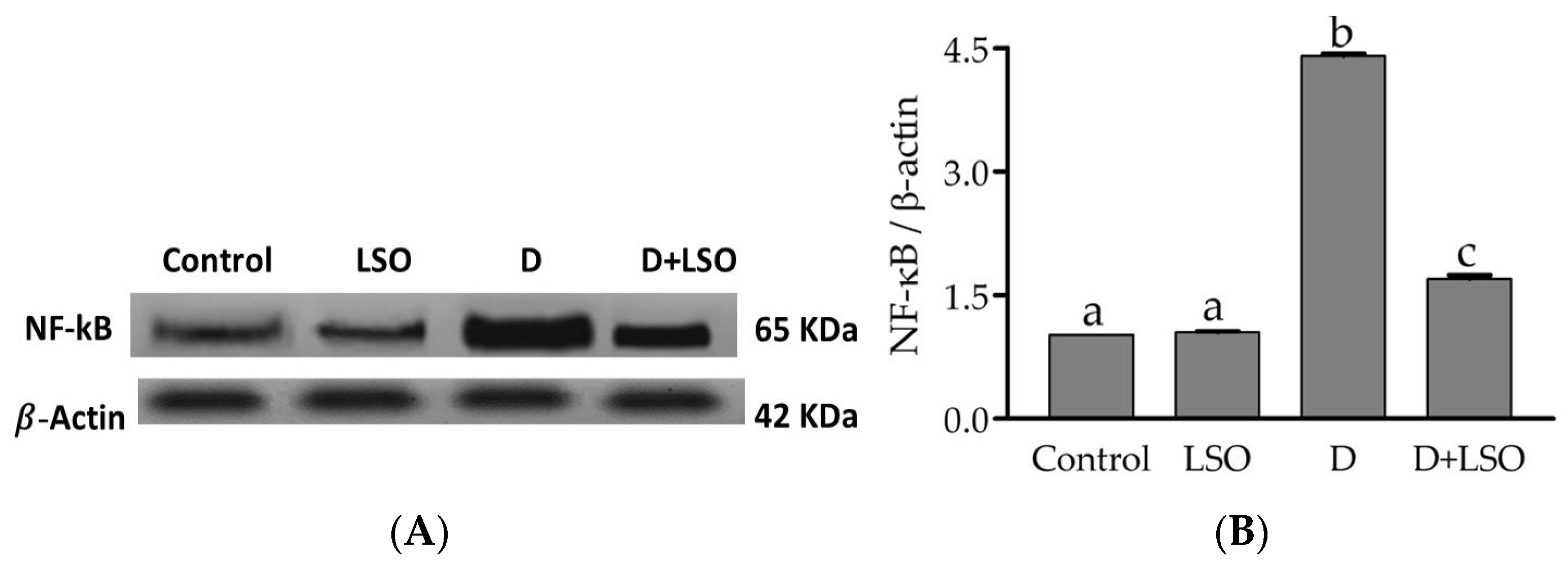
2.5. NF-kB p65 Subunit Expression in the Testes of Diabetic Mice Treated with/without LSO
2.6. Effect of LSO on Testicular Histoarchitecture in the Diabetic Mice Treated with/without LSO
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction of Fixed Oil
4.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of LSO
4.4. Animals
4.5. Induction of Diabetes
4.6. Experimental Design
- Control: The mice were injected intraperitoneally (i.p.) with citric acid–sodium citrate buffer solution.
- Diabetic (D): Diabetes was induced by a single intraperitoneal (i.p.) injection of streptozotocin (STZ; 50 mg/kg/day) for five consecutive days [115].
- Lepidium seed oil (LSO): The mice were administrated intragastrically with 0.5 mL/kg LSO twice a week for four weeks.
- D+LSO: The diabetic mice administrated with LSO as mentioned in the LSO-alone group.
4.7. Tissue Sampling
4.8. Assay of Serum Testosterone
4.9. Assessment of Redox Status
4.10. Determination of NF-kB Factor and Pro-Inflammatory Cytokines
4.11. Western Blotting
4.12. Histological Study on the Testes
4.13. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henninger, J.; Hammarstedt, A.; Rawshani, A.; Eliasson, B. Metabolic Predictors of Impaired Glucose Tolerance and Type 2 Diabetes in a Predisposed Population—A Prospective Cohort Study. BMC Endocr. Disord. 2015, 15, 51. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A. Vascular Complications of Diabetes. Circ. Res. 2016, 118, 1771–1785. [Google Scholar] [CrossRef]
- Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G.; Menini, S. Diabetic Complications and Oxidative Stress: A 20-Year Voyage Back in Time and Back to the Future. Antioxidants 2021, 10, 727. [Google Scholar] [CrossRef]
- Bereda, G. Complication of Diabetes Mellitus: Microvascular and Macrovascular Complications. Int. J. Diabetes 2022, 3, 123–128. [Google Scholar]
- Nellaiappan, K.; Preeti, K.; Khatri, D.K.; Singh, S.B. Diabetic Complications: An Update on Pathobiology and Therapeutic Strategies. Curr. Diabetes Rev. 2022, 18, e030821192146. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Mongioi, L.M.; Alamo, A.; Calogero, A.E. Diabetes Mellitus and Infertility: Different Pathophysiological Effects in Type 1 and Type 2 on Sperm Function. Front. Endocrinol. 2018, 9, 268. [Google Scholar] [CrossRef]
- Sansone, A.; Mollaioli, D.; Ciocca, G.; Limoncin, E.; Colonnello, E.; Jannini, E.A. Sexual Dysfunction in Men and Women with Diabetes: A Reflection of their Complications? Curr. Diabetes Rev. 2022, 18, e030821192147. [Google Scholar] [CrossRef]
- Tsampoukas, G.; Tharakan, T.; Narayan, Y.; Khan, F.; Cayetano, A.; Papatsoris, A.; Buchholz, N.; Minhas, S. Investigating the Therapeutic Options for Diabetes-Associated Male Infertility as Illustrated in Animal Experimental Models. Andrologia 2022, 54, e14521. [Google Scholar] [CrossRef]
- Andlib, N.; Sajad, M.; Kumar, R.; Thakur, S.C. Abnormalities in Sex Hormones and Sexual Dysfunction in Males with Diabetes Mellitus: A Mechanistic Insight. Acta Histochem. 2023, 125, 151974. [Google Scholar] [CrossRef]
- Sampannang, A.; Arun, S.; Burawat, J.; Sukhorum, W.; Iamsaard, S. Comparison of Male Reproductive Parameters in Mice with Type 1 and Type 2 Diabetes. Clin. Exp. Reprod. Med. 2020, 47, 20–33. [Google Scholar] [CrossRef]
- Bener, A.; Al-Ansari, A.A.; Zirie, M.; Al-Hamaq, A.O. Is Male Fertility Associated with Type 2 Diabetes Mellitus? Int. Urol. Nephrol. 2009, 41, 777–784. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Calogero, A.E.; Condorelli, R.; Lanzafame, F.; Giammusso, B.; Vicari, E. Andrological Characterization of the Patient with Diabetes Mellitus. Minerva Endocrinol. 2009, 34, 1–9. [Google Scholar] [PubMed]
- Lotti, F.; Maggi, M. Effects of Diabetes Mellitus on Sperm Quality and Fertility Outcomes: Clinical Evidence. Andrology 2023, 11, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Mantzoros, C.S. Leptin and the Hypothalamic-Pituitary Regulation of the Gonadotropin-Gonadal Axis. Pituitary 2001, 4, 87–92. [Google Scholar] [CrossRef]
- Pitteloud, N.; Hardin, M.; Dwyer, A.A.; Valassi, E.; Yialamas, M.; Elahi, D.; Hayes, F.J. Increasing Insulin Resistance Is Associated with a Decrease in Leydig Cell Testosterone Secretion in Men. J. Clin. Endocrinol. Metab. 2005, 90, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Serwaa, D.; Bello, F.A.; Osungbade, K.O.; Nkansah, C.; Osei-Boakye, F.; Appiah, S.K.; Antwi, M.H.; Danquah, M.; Buckman, T.A.; Owusu, E. Prevalence and Determinants of Low testosterone Levels in Men with Type 2 Diabetes Mellitus; a Case-Control Study in A District Hospital in Ghana. PLoS Glob. Public Health 2021, 1, e0000052. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tian, R.; Wang, X.; Sun, J.; Zhu, L.; An, X. The Occurrence of Hypogonadotropic Hypogonadism in Chinese Men with Type 2 Diabetes. Clin. Endocrinol. 2022, 96, 837–846. [Google Scholar] [CrossRef]
- Musa, E.; El-Bashir, J.M.; Sani-Bello, F.; Bakari, A.G. Clinical and Biochemical Correlates of Hypogonadism in Men with Type 2 Diabetes Mellitus. Pan Afr. Med. J. 2021, 38, 292. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, P.R.; Knoblovits, P. Male Gonadal Axis Function in Patients with Type 2 Diabetes. Horm. Mol. Biol. Clin. Investig. 2016, 26, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Vena, W.; Pizzocaro, A.; Vignozzi, L.; Sforza, A.; Maggi, M. Testosterone Therapy in Diabetes and Pre-Diabetes. Andrology 2023, 11, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Gianatti, E.J.; Grossmann, M. Testosterone Deficiency in Men with Type 2 Diabetes: Pathophysiology and Treatment. Diabet. Med. 2020, 37, 174–186. [Google Scholar] [CrossRef]
- Karimi, J.; Goodarzi, M.T.; Tavilani, H.; Khodadadi, I.; Amiri, I. Increased Receptor for Advanced Glycation End Products in Spermatozoa of Diabetic Men and Its Association with Sperm Nuclear DNA Fragmentation. Andrologia 2012, 44, 280–286. [Google Scholar] [CrossRef]
- Barkabi-Zanjani, S.; Ghorbanzadeh, V.; Aslani, M.; Ghalibafsabbaghi, A.; Chodari, L. Diabetes Mellitus and the Impairment of Male Reproductive Function: Possible Signaling Pathways. Diabetes Metab. Syndr. 2020, 14, 1307–1314. [Google Scholar] [CrossRef]
- Amaral, S.; Oliveira, P.J.; Ramalho-Santos, J. Diabetes and the Impairment of Reproductive Function: Possible Role of Mitochondria and Reactive Oxygen Species. Curr. Diabetes Rev. 2008, 4, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Agbaje, I.M.; Rogers, D.A.; McVicar, C.M.; McClure, N.; Atkinson, A.B.; Mallidis, C.; Lewis, S.E. Insulin Dependant Diabetes Mellitus: Implications for Male Reproductive Function. Hum. Reprod. 2007, 22, 1871–1877. [Google Scholar] [CrossRef]
- Calogero, A.E.; Duca, Y.; Condorelli, R.A.; La Vignera, S. Male Accessory Gland Inflammation, Infertility, and Sexual Dysfunctions: A Practical Approach to Diagnosis and Therapy. Andrology 2017, 5, 1064–1072. [Google Scholar] [CrossRef]
- Mostafa, T.; Abdel-Hamid, I.A. Ejaculatory Dysfunction in Men with Diabetes Mellitus. World J. Diabetes 2021, 12, 954–974. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; Duca, Y.; Favilla, V.; Morgia, G.; Cimino, S.; Di Mauro, M.; La Vignera, S. Prevalence of Male Accessory Gland Inflammations/Infections in Patients with Type 2 Diabetes Mellitus. J. Endocrinol. Invest. 2013, 36, 770–774. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic Regulation Is Important for Spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef]
- Mateus, I.; Feijo, M.; Espinola, L.M.; Vaz, C.V.; Correia, S.; Socorro, S. Glucose and Glutamine Handling in the Sertoli Cells of Transgenic Rats Overexpressing Regucalcin: Plasticity towards Lactate Production. Sci. Rep. 2018, 8, 10321. [Google Scholar] [CrossRef]
- Ding, G.L.; Liu, Y.; Liu, M.E.; Pan, J.X.; Guo, M.X.; Sheng, J.Z.; Huang, H.F. The Effects of Diabetes on Male Fertility and Epigenetic Regulation During Spermatogenesis. Asian J. Androl. 2015, 17, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Babel, R.A.; Dandekar, M.P. A Review on Cellular and Molecular Mechanisms Linked to the Development of Diabetes Complications. Curr. Diabetes Rev. 2021, 17, 457–473. [Google Scholar] [CrossRef]
- Maranta, F.; Cianfanelli, L.; Cianflone, D. Glycaemic Control and Vascular Complications in Diabetes Mellitus Type 2. Adv. Exp. Med. Biol. 2021, 1307, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. A Review of the Molecular Mechanisms of Hyperglycemia-Induced Free Radical Generation Leading to Oxidative Stress. J. Cell. Physiol. 2019, 234, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the Pathophysiology of Type 2 Diabetes and Related Complications: Current Therapeutics Strategies and Future Perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Singh, H.; Singh, R.; Singh, A.; Singh, H.; Singh, G.; Kaur, S.; Singh, B. Role of oxidative stress in diabetes-induced complications and their management with antioxidants. Arch. Physiol. Biochem. 2023, 1–26. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]
- Rato, L.; Oliveira, P.; Sousa, M.; Silva, B.; Alves, M. Chapter 2.6—Role of Reactive Oxygen Species in Diabetes-Induced Male Reproductive Dysfunction. In Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction; Henkel, R., Samanta, L., Agarwal, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 135–147. [Google Scholar]
- Abbasihormozi, S.H.; Babapour, V.; Kouhkan, A.; Niasari Naslji, A.; Afraz, K.; Zolfaghary, Z.; Shahverdi, A.H. Stress Hormone and Oxidative Stress Biomarkers Link Obesity and Diabetes with Reduced Fertility Potential. Cell J. 2019, 21, 307–313. [Google Scholar] [CrossRef]
- Leisegang, K. Oxidative Stress in Men with Obesity, Metabolic Syndrome and Type 2 Diabetes Mellitus: Mechanisms and Management of Reproductive Dysfunction. Adv. Exp. Med. Biol. 2022, 1358, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, A.; Argento, F.R.; Fini, E.; Coccia, M.E.; Taddei, N.; Becatti, M.; Fiorillo, C. The Impact of Oxidative Stress in Male Infertility. Front. Mol. Biosci. 2021, 8, 799294. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress That Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef]
- Hosen, M.B.; Islam, M.R.; Begum, F.; Kabir, Y.; Howlader, M.Z. Oxidative Stress Induced Sperm DNA Damage, a Possible Reason for Male Infertility. Iran. J. Reprod. Med. 2015, 13, 525–532. [Google Scholar] [PubMed]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef]
- Costa, J.; Braga, P.C.; Rebelo, I.; Oliveira, P.F.; Alves, M.G. Mitochondria Quality Control and Male Fertility. Biology 2023, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Hung, Y.J.; Huang, C.L.; Shieh, Y.S.; Chien, C.Y.; Chiang, C.F.; Liu, J.S.; Lu, C.H.; Hsieh, C.H.; Lin, C.M.; et al. Cilostazol Inhibits Hyperglucose-Induced Vascular Smooth Muscle Cell Dysfunction by Modulating the RAGE/ERK/NF-kB Signaling Pathways. J. Biomed. Sci. 2019, 26, 68. [Google Scholar] [CrossRef]
- Liu, X.; Lin, R.; Zhao, B.; Guan, R.; Li, T.; Jin, R. Correlation Between Oxidative Stress and the NF-kB Signaling Pathway in the Pulmonary Tissues of Obese Asthmatic Mice. Mol. Med. Rep. 2016, 13, 1127–1134. [Google Scholar] [CrossRef]
- Arkali, G.; Aksakal, M.; Kaya, S.O. Protective Effects of Carvacrol against Diabetes-Induced Reproductive Damage in Male Rats: Modulation of Nrf2/HO-1 Signalling Pathway and Inhibition of NF-kB-Mediated Testicular Apoptosis and Inflammation. Andrologia 2021, 53, e13899. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, F.; Tang, H.; Huang, C.; Xie, G.; Huang, T.; Li, Y.; Liu, C.; Wang, H.; Chen, B. Islet Transplantation Attenuating Testicular Injury in Type 1 Diabetic Rats Is Associated with Suppression of Oxidative Stress and Inflammation via Nrf-2/HO-1 and NF-kB Pathways. J. Diabetes Res. 2019, 2019, 8712492. [Google Scholar] [CrossRef]
- Hasan, H.; Bhushan, S.; Fijak, M.; Meinhardt, A. Mechanism of Inflammatory Associated Impairment of Sperm Function, Spermatogenesis and Steroidogenesis. Front. Endocrinol. 2022, 13, 897029. [Google Scholar] [CrossRef]
- Luo, Y.; Zhu, Y.; Basang, W.; Wang, X.; Li, C.; Zhou, X. Roles of Nitric Oxide in the Regulation of Reproduction: A Review. Front. Endocrinol. 2021, 12, 752410. [Google Scholar] [CrossRef]
- Han, X.X.; Jiang, Y.P.; Liu, N.; Wu, J.; Yang, J.M.; Li, Y.X.; Sun, M.; Sun, T.; Zheng, P.; Jian-Qiang, Y. Protective Effects of Astragalin on Spermatogenesis in Streptozotocin-Induced Diabetes in Male Mice by Improving Antioxidant Activity and Inhibiting Inflammation. Biomed. Pharmacother. 2019, 110, 561–570. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Fraczek, M.; Kurpisz, M. Inflammatory Mediators Exert Toxic Effects of Oxidative Stress on Human Spermatozoa. J. Androl. 2007, 28, 325–333. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Doble, M. Effect of Natural Products on Commercial Oral Antidiabetic Drugs in Enhancing 2-Deoxyglucose Uptake by 3T3-L1 Adipocytes. Ther. Adv. Endocrinol. Metab. 2011, 2, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Abdi, M.; Alizadeh, F.; Daneshi, E.; Abouzaripour, M.; Fathi, F.; Rahimi, K. Ameliorative Effect of Stevia rebaudiana Bertoni on Sperm Parameters, In Vitro Fertilization, and Early Embryo Development in a Streptozotocin-Induced Mouse Model of Diabetes. Zygote 2023, 31, 475–482. [Google Scholar] [CrossRef]
- Khazaei, M.R.; Gravandi, E.; Ghanbari, E.; Niromand, E.; Khazaei, M. Trifolium pratense Extract Increases Testosterone and Improves Sperm Characteristics and Antioxidant Status in Diabetic Rats. Biotech. Histochem. 2022, 97, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Ostovan, F.; Gol, A.; Javadi, A. Protective Properties of Rydingia persica in Reproductive Complications Induced by Diabetes in Male Rats: An Experimental Study. Int. J. Reprod. Biomed. 2022, 20, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Oroojan, A.A.; Ahangarpour, A.; Paknejad, B.; Zareian, P.; Hami, Z.; Abtahi, S.R. Effects of Myricitrin and Solid Lipid Nanoparticle-Containing Myricitrin on Reproductive System Disorders Induced by Diabetes in Male Mouse. World J. Mens Health 2021, 39, 147–157. [Google Scholar] [CrossRef]
- Gupta, R.; Choudhary, P. Botanical Description of Garden Cress (Lepidium sativum L.) Plant and Physical Characteristics of Its Seeds. J. Pharmacogn. Phytochem. 2020, 9, 2424–2428. [Google Scholar]
- Ramadan, M.F.; Oraby, H.F. Chapter 20—Lepidium sativum Seeds: Therapeutic Significance and Health-Promoting Potential. In Nuts and Seeds in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 273–289. [Google Scholar]
- Vazifeh, S.; Kananpour, P.; Khalilpour, M.; Eisalou, S.V.; Hamblin, M.R. Anti-Inflammatory and Immunomodulatory Properties of Lepidium sativum. Biomed. Res. Int. 2022, 2022, 3645038. [Google Scholar] [CrossRef]
- Ahmad, A.; Nabi, R.; Mishra, A.; Ahmad, I.Z. A Panoramic Review on Lepidium sativum L. Bioactives as Prospective Therapeutics. Drug Res. 2021, 71, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Kaiyrkulova, A.; Li, J.; Aisa, H.A. Chemical Constituents of Lepidium sativum Seeds. Chem. Nat. Compd. 2019, 55, 736–737. [Google Scholar] [CrossRef]
- Hekmatshoar, Y.; Ozkan, T.; Rahbar Saadat, Y. Evidence for Health-Promoting Properties of Lepidium sativum L.: An Updated Comprehensive Review. Turk. J. Pharm. Sci. 2022, 19, 714–723. [Google Scholar] [CrossRef]
- Painuli, S.; Quispe, C.; Herrera-Bravo, J.; Semwal, P.; Martorell, M.; Almarhoon, Z.M.; Seilkhan, A.; Ydyrys, A.; Rad, J.S.; Alshehri, M.M.; et al. Nutraceutical Profiling, Bioactive Composition, and Biological Applications of Lepidium sativum L. Oxid. Med. Cell. Longev. 2022, 2022, 2910411. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.B.; Dudhat, V.A.; Gadhvi, K.V. Lepidium sativum: A Potential Functional Food. J. Ayurvedic Herb. Med. 2021, 7, 140–149. [Google Scholar] [CrossRef]
- Bigoniya, P.; Shukla, A. Phytopharmacological Screening of Lepidium sativum Seeds Total Alkaloid: Hepatoprotective, Antidiabetic and In Vitro Antioxidant Activity Along with Identification by LC/MS/MS. PharmaNutrition 2014, 2, 90. [Google Scholar] [CrossRef]
- Sakran, M.; Selim, Y.; Zidan, N. A New Isoflavonoid From Seeds of Lepidium sativum L. and Its Protective Effect on Hepatotoxicity Induced by Paracetamol in Male Rats. Molecules 2014, 19, 15440–15451. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; Mahmoud, A.Z.; Farshori, N.N.; Alfaraj, R.; Al-Sheddi, E.S.; Alsarra, I.A. Chemical composition and antimicrobial, antioxidant, and anti-inflammatory activities of Lepidium sativum seed oil. Saudi J. Biol. Sci. 2019, 26, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Getahun, T.; Sharma, V.; Gupta, N. Chemical Composition, Antibacterial and Antioxidant Activities of oils Obtained by Different Extraction Methods from Lepidium sativum L. seeds. Ind. Crops Prod. 2020, 156, 112876. [Google Scholar] [CrossRef]
- Kumar, V.; Tomar, V.; Ranade, S.A.; Yadav, H.K.; Srivastava, M. Phytochemical, Antioxidant Investigaations and Fatty Acid Composition of Lepidium sativum Seeds. J. Environ. Biol. 2020, 41, 59–65. [Google Scholar] [CrossRef]
- Kamani, M.; Hosseini, E.S.; Kashani, H.H.; Atlasi, M.A.; Nikzad, H. Protective Effect of Lepidium sativum Seed Extract on Histopathology and Morphology of Epididymis in Diabetic Rat Model. Int. J. Morphol. 2017, 35, 603–610. [Google Scholar] [CrossRef]
- Kamani, M.; Mhabadi, J.A.; Atlasi, M.A.; Seyedi, F.; Kamani, E.; Nikzad, H. Protective Effect of Alcoholic Extract of Garden Cress Seeds on the Histopathological Changes of the Ventral Prostate in Streptozotocin Diabetic Rats. Int. J. Morphol. 2017, 35, 1118–1178. [Google Scholar] [CrossRef]
- Asl, F.R.; Khosravi, M.; Hajikhani, R.; Solati, J.; Fahimi, H. Complementary Effects of Coenzyme Q10 and Lepidium sativum Supplementation on the Reproductive Function of Mice: An Experimental Study. Int. J. Reprod. Biomed. 2021, 19, 607–618. [Google Scholar] [CrossRef]
- He, Z.; Yin, G.; Li, Q.Q.; Zeng, Q.; Duan, J. Diabetes Mellitus Causes Male Reproductive Dysfunction: A Review of the Evidence and Mechanisms. In Vivo 2021, 35, 2503–2511. [Google Scholar] [CrossRef]
- Umesha, S.S.; Naidu, K.A. Antioxidants and Antioxidant Enzymes Status of Rats Fed on n-3 PUFA Rich Garden Cress (Lepidium Sativum L.) Seed Oil and Its Blended Oils. J. Food Sci. Technol. 2015, 52, 1993–2002. [Google Scholar] [CrossRef]
- Ungurianu, A.; Zanfirescu, A.; Nitulescu, G.; Margina, D. Vitamin E Beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef]
- Ciarcia, G.; Bianchi, S.; Tomasello, B.; Acquaviva, R.; Malfa, G.A.; Naletova, I.; La Mantia, A.; Di Giacomo, C. Vitamin E and Non-communicable Diseases: A Review. Biomedicines 2022, 10, 2473. [Google Scholar] [CrossRef]
- Hossain, K.F.B.; Hosokawa, T.; Saito, T.; Kurasaki, M. Amelioration of Butylated Hydroxytoluene against Inorganic Mercury Induced Cytotoxicity and Mitochondrial Apoptosis in PC12 Cells via Antioxidant Effects. Food Chem. Toxicol. 2020, 146, 111819. [Google Scholar] [CrossRef] [PubMed]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the Chemistry Behind the Antioxidant Activities of Butylated Hydroxytoluene (BHT): A Review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Yehye, W.A.; Abdul Rahman, N.; Saad, O.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M.; Matlob, A.A. Rational Design and Synthesis of New, High Efficiency, Multipotent Schiff Base-1,2,4-triazole Antioxidants Bearing Butylated Hydroxytoluene Moieties. Molecules 2016, 21, 847. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.F.; Ajibade, S.O.; Lawal, A.Z.; Yusuf, S.A.; Babalola, J.B.; Mukadam, A.A.; Hamid, A.A. GC-MS Analysis, Antioxidant and Antimicrobial Properties of Eclipta prostrata Leaves. Int. J. Chem. Biochem. Sci. 2017, 11, 25–43. [Google Scholar]
- Ramya, B.; Malarvili, T.; Velavan, S. GC-MS Analysis of Bioactive Compounds in Bryonopsis laciniosa Fruit Extract. Int. J. Pharm. Sci. Res. 2015, 6, 3375–3379. [Google Scholar]
- Barupal, T.; Meena, M.; Sharma, K. Inhibitory Effects of Leaf Extract of Lawsonia inermis on Curvularia lunata and Characterization of Novel Inhibitory Compounds by GC-MS Analysis. Biotechnol. Rep. 2019, 23, e00335. [Google Scholar] [CrossRef]
- Black, H.S. A Synopsis of the Associations of Oxidative Stress, ROS, and Antioxidants with Diabetes Mellitus. Antioxidants 2022, 11, 2003. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight Into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Kermani, J.; Goodarzi, N.; Bakhtiari, M. An Experimental Study to Evaluate the Protective Effects of Solanum lycopersicum Seed Essential Oil on Diabetes-Induced Testicular Injuries. Medicina 2019, 55, 499. [Google Scholar] [CrossRef]
- Nasiri, K.; Akbari, A.; Nimrouzi, M.; Ruyvaran, M.; Mohamadian, A. Safflower Seed Oil Improves Steroidogenesis and Spermatogenesis in Rats with Type II Diabetes Mellitus by Modulating the Genes Expression Involved in Steroidogenesis, Inflammation and Oxidative Stress. J. Ethnopharmacol. 2021, 275, 114139. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Plowman, T.J.; Shah, M.H.; Fernandez, E.; Christensen, H.; Aiges, M.; Ramana, K.V. Role of Innate Immune and Inflammatory Responses in the Development of Secondary Diabetic Complications. Curr. Mol. Med. 2022, 23, 901–920. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhong, Z.; Karin, M. NF-kB: A Double-Edged Sword Controlling Inflammation. Biomedicines 2022, 10, 1250. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-κβ: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Yousef, H.; Khandoker, A.H.; Feng, S.F.; Helf, C.; Jelinek, H.F. Inflammation, Oxidative Stress and Mitochondrial Dysfunction in the Progression of Type II Diabetes Mellitus with Coexisting Hypertension. Front. Endocrinol. 2023, 14, 1173402. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.S.M.; Giribabu, N.; Yelumalai, S.; Shahzad, H.; Kilari, E.K.; Salleh, N. Myristic Acid Defends against Testicular Oxidative Stress, Inflammation, Apoptosis: Restoration of Spermatogenesis, Steroidogenesis in Diabetic Rats. Life Sci. 2021, 278, 119605. [Google Scholar] [CrossRef]
- Nna, V.U.; Abu Bakar, A.B.; Ahmad, A.; Eleazu, C.O.; Mohamed, M. Oxidative Stress, NF-kB-mediated Inflammation and Apoptosis in the Testes of Streptozotocin-Induced Diabetic Rats: Combined Protective Effects of Malaysian Propolis and Metformin. Antioxidants 2019, 8, 465. [Google Scholar] [CrossRef]
- Khosravi, Z.; Sedaghat, R.; Baluchnejadmojarad, T.; Roghani, M. Diosgenin Ameliorates Testicular Damage in Streptozotocin-Diabetic Rats Through Attenuation of Apoptosis, Oxidative Stress, and Inflammation. Int. Immunopharmacol. 2019, 70, 37–46. [Google Scholar] [CrossRef]
- Diwakar, B.T.; Lokesh, B.R.; Naidu, K.A. Modulatory Effect of α-Linolenic Acid-Rich Garden Cress (Lepidium sativum L.) Seed Oil on Inflammatory Mediators in Adult Albino Rats. Br. J. Nutr. 2011, 106, 530–539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reddy, K.V.; Maheswaraiah, A.; Naidu, K.A. Rice Bran Oil and N-3 Fatty Acid-Rich Garden Cress (Lepidium sativum) Seed Oil Attenuate Murine Model of Ulcerative Colitis. Int. J. Colorectal Dis. 2014, 29, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural Forms of Vitamin E: Metabolism, Antioxidant, and Anti-Inflammatory Activities and Their Role in Disease Prevention and Therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Di Vincenzo, A.; Tana, C.; El Hadi, H.; Pagano, C.; Vettor, R.; Rossato, M. Antioxidant, Anti-Inflammatory, and Metabolic Properties of Tocopherols and Tocotrienols: Clinical Implications for Vitamin E Supplementation in Diabetic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 5101. [Google Scholar] [CrossRef]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of Oxidative Stress, Infection and Inflammation in Male Infertility. Andrologia 2018, 50, e13126. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, C.; Hernández-Bello, R.; Morales-Montor, J. Regulation of Steroidogenesis in Reproductive, Adrenal and Neural Tissues by Cytokines. Open Neuroendocrinol. J. 2010, 3, 161–169. [Google Scholar]
- Syriou, V.; Papanikolaou, D.; Kozyraki, A.; Goulis, D.G. Cytokines and Male Infertility. Eur. Cytokine Netw. 2018, 29, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hales, D.B. Testicular Macrophage Modulation of Leydig Cell Steroidogenesis. J. Reprod. Immunol. 2002, 57, 3–18. [Google Scholar] [CrossRef]
- Chatoui, K.; Harhar, H.; El Kamli, T.; Tabyaoui, M. Chemical Composition and Antioxidant Capacity of Lepidium sativum Seeds from Four Regions of Morocco. Evid. Based Complement. Alternat. Med. 2020, 2020, 7302727. [Google Scholar] [CrossRef]
- Rezaei, N.; Mardanshahi, T.; Shafaroudi, M.M.; Abedian, S.; Mohammadi, H.; Zare, Z. Effects of L-Carnitine on the Follicle-Stimulating Hormone, Luteinizing Hormone, Testosterone, and Testicular Tissue Oxidative Stress Levels in Streptozotocin-Induced Diabetic Rats. J. Evid. Based Integr. Med. 2018, 23, 2515690X18796053. [Google Scholar] [CrossRef]
- Fajri, M.; Ahmadi, A.; Sadrkhanlou, R. Protective Effects of Equisetum arvense Methanolic Extract on Testicular Tissue Disorders in Streptozotocin-Induced Diabetic Murine Model. Vet. Res. Forum 2021, 12, 497–503. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists, INC.: Arlington, VA, USA, 1990. [Google Scholar]
- Liu, Y.; Yang, Z.; Kong, D.; Zhang, Y.; Yu, W.; Zha, W. Metformin Ameliorates Testicular Damage in Male Mice with Streptozotocin-Induced Type 1 Diabetes Through the PK2/PKR Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 5681701. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Johnsen, S.G. Testicular Biopsy Score Count—A Method for Registration of Spermatogenesis in Human Testes: Normal Values and Results in 335 Hypogonadal Males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef] [PubMed]



| Peak No. | Retention Time (RT) | Area% | Compound Name |
|---|---|---|---|
| 1 | 14.72 | 8.04 | Butylated Hydroxytoluene |
| 2 | 17.032 | 1.82 | Tridecane, 1-iodo- |
| 3 | 18.153 | 2.72 | 2-methyloctacosane |
| 4 | 19.142 | 3.91 | δ-Tocopherol |
| 5 | 20.123 | 4.81 | 10-Methylnonadecane |
| 6 | 25.975 | 2.67 | 2-methyltetracosane |
| 7 | 26.325 | 1.73 | Z-6-Pentadecen-1-ol acetate |
| 8 | 27.393 | 8.12 | 2-methylhexacosane |
| 9 | 28.701 | 62.1 | γ-Tocopherol |
| 10 | 29.041 | 4.08 | Tetracosane, 1-bromo- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Khudir, R.; Badr, G.M.; Abd El-Moaty, H.I.; Hamad, R.S.; Al Abdulsalam, N.K.; Abdelrahem, A.S.A.; Alqarni, S.; Alkuwayti, M.A.; Salam, S.A.; Abd El-Kareem, H.F. Garden Cress Seed Oil Abrogates Testicular Oxidative Injury and NF-kB-Mediated Inflammation in Diabetic Mice. Int. J. Mol. Sci. 2023, 24, 15478. https://doi.org/10.3390/ijms242015478
Abu-Khudir R, Badr GM, Abd El-Moaty HI, Hamad RS, Al Abdulsalam NK, Abdelrahem ASA, Alqarni S, Alkuwayti MA, Salam SA, Abd El-Kareem HF. Garden Cress Seed Oil Abrogates Testicular Oxidative Injury and NF-kB-Mediated Inflammation in Diabetic Mice. International Journal of Molecular Sciences. 2023; 24(20):15478. https://doi.org/10.3390/ijms242015478
Chicago/Turabian StyleAbu-Khudir, Rasha, Gehan M. Badr, Heba Ibrahim Abd El-Moaty, Rabab S. Hamad, Najla K. Al Abdulsalam, Aml Sayed Ali Abdelrahem, Saleha Alqarni, Mayyadah Abdullah Alkuwayti, Sherine Abdel Salam, and Hanaa F. Abd El-Kareem. 2023. "Garden Cress Seed Oil Abrogates Testicular Oxidative Injury and NF-kB-Mediated Inflammation in Diabetic Mice" International Journal of Molecular Sciences 24, no. 20: 15478. https://doi.org/10.3390/ijms242015478
APA StyleAbu-Khudir, R., Badr, G. M., Abd El-Moaty, H. I., Hamad, R. S., Al Abdulsalam, N. K., Abdelrahem, A. S. A., Alqarni, S., Alkuwayti, M. A., Salam, S. A., & Abd El-Kareem, H. F. (2023). Garden Cress Seed Oil Abrogates Testicular Oxidative Injury and NF-kB-Mediated Inflammation in Diabetic Mice. International Journal of Molecular Sciences, 24(20), 15478. https://doi.org/10.3390/ijms242015478






