Myocardial Fibrosis and Steatosis in Patients with Aortic Stenosis: Roles of Myostatin and Ceramides
Abstract
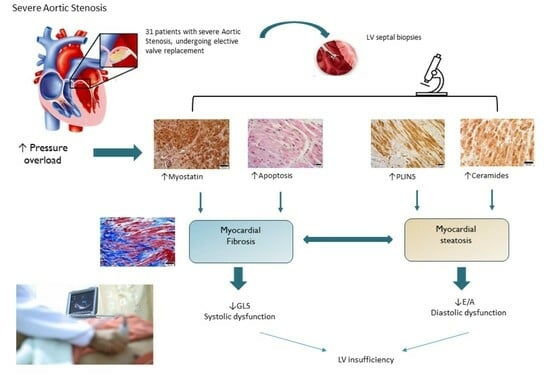
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Population of This Study
4.2. Bio-Clinical and Anthropometrical Evaluations
4.3. Echocardiographic Evaluation
4.4. Myocardium Collection
4.5. Immunohistochemistry (IHC) for Fat Deposition and Myostatin Assay
4.6. Immunohistochemistry for Ceramide Assay
4.7. Masson’s Trichrome Staining for Interstitial Fibrosis Assay
4.8. Apoptosis Assay
4.9. Image Capturing and Analyses
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bing, R.; Cavalcante, J.L.; Everett, R.J.; Clavel, M.A.; Newby, D.E.; Dweck, M.R. Imaging and Impact of Myocardial Fibrosis in Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Lorell, B.H.; Carabello, B.A. Left ventricular hypertrophy: Pathogenesis, detection, and prognosis. Circulation 2000, 102, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Huang, W.; Singh, G.K.; Toh, D.F.; Ewe, S.H.; Tang, H.C.; Loo, G.; Bryant, J.A.; Ang, B.; Tay, E.L.; et al. Echocardiographic Global Longitudinal Strain Is Associated With Myocardial Fibrosis and Predicts Outcomes in Aortic Stenosis. Front. Cardiovasc. Med. 2021, 8, 750016. [Google Scholar] [CrossRef] [PubMed]
- Castrichini, M.; Vitrella, G.; De Luca, A.; Altinier, A.; Korcova, R.; Pagura, L.; Radesich, C.; Sinagra, G. Clinical impact of myocardial fibrosis in severe aortic stenosis. Eur. Heart J. Suppl. 2021, 23, E147–E150. [Google Scholar] [CrossRef]
- Balčiūnaitė, G.; Besusparis, J.; Palionis, D.; Žurauskas, E.; Skorniakov, V.; Janušauskas, V.; Zorinas, A.; Zaremba, T.; Valevičienė, N.; Šerpytis, P.; et al. Exploring myocardial fibrosis in severe aortic stenosis: Echo, CMR and histology data from FIB-AS study. Int. J. Cardiovasc. Imaging 2022, 38, 1555–1568. [Google Scholar] [CrossRef]
- Scully, P.R.; Patel, K.P.; Klotz, E.; Augusto, J.B.; Thornton, G.D.; Saberwal, B.; Haberland, U.; Kennon, S.; Ozkor, M.; Mullen, M.; et al. Myocardial Fibrosis Quantified by Cardiac CT Predicts Outcome in Severe Aortic Stenosis After Transcatheter Intervention. JACC Cardiovasc. Imaging 2022, 15, 542–544. [Google Scholar] [CrossRef]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef]
- Weidemann, F.; Herrmann, S.; Störk, S.; Niemann, M.; Frantz, S.; Lange, V.; Beer, M.; Gattenlöhner, S.; Voelker, W.; Ertl, G.; et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009, 120, 577–584. [Google Scholar] [CrossRef]
- Biesemann, N.; Mendler, L.; Wietelmann, A.; Hermann, S.; Schäfers, M.; Krüger, M.; Boettger, T.; Borchardt, T.; Braun, T. Myostatin Regulates Energy Homeostasis in the Heart and Prevents Heart Failure. Circ. Res. 2014, 115, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Esposito, P.; Picciotto, D.; Battaglia, Y.; Costigliolo, F.; Viazzi, F.; Verzola, D. Myostatin: Basic biology to clinical application. Adv. Clin. Chem. 2022, 106, 181–234. [Google Scholar] [CrossRef] [PubMed]
- Bish, L.T.; George, I.; Maybaum, S.; Yang, J.; Chen, J.M.; Sweeney, H.L. Myostatin is elevated in congenital heart disease and after mechanical unloading. PLoS ONE 2011, 6, e23818. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Myokines and Heart Failure: Challenging Role in Adverse Cardiac Remodeling, Myopathy, and Clinical Outcomes. Dis. Markers 2021, 2021, 6644631. [Google Scholar] [CrossRef]
- Leader, C.J.; Moharram, M.; Coffey, S.; SaDmmut, I.A.; Wilkins, G.W.; Walker, R.J. Myocardial global longitudinal strain: An early indicator of cardiac interstitial fibrosis modified by spironolactone, in a unique hypertensive rat model. PLoS ONE 2019, 14, e0220837. [Google Scholar] [CrossRef] [PubMed]
- Spath, N.B.; Gomez, M.; Everett, R.J.; Semple, S.; Chin, C.W.L.; White, A.C.; Japp, A.G. Global Longitudinal Strain Analysis Using Cardiac MRI in Aortic Stenosis: Comparison with Left Ventricular Remodeling, Myocardial Fibrosis, and 2-year Clinical Outcomes. Radiol. Cardiothorac. Imaging 2019, 1, e190027. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.T.; Strudwick, M.; van der Geest, R.J.; Ng, A.C.C.; Gillinder, L.; Goo, S.Y.; Cowin, G.; Delgado, V.; Wang, W.Y.S.; Bax, J.J. Impact of Epicardial Adipose Tissue, Left Ventricular Myocardial Fat Content, and Interstitial Fibrosis on Myocardial Contractile Function. Circ. Cardiovasc. Imaging 2018, 11, e007372. [Google Scholar] [CrossRef]
- Mahmod, M.; Bull, S.; Suttie, J.J.; Pal, N.; Holloway, C.; Dass, S.; Myerson, S.G.; Schneider, J.E.; De Silva, R.; Petrou, M.; et al. Myocardial steatosis and left ventricular contractile dysfunction in patients with severe aortic stenosis. Circ. Cardiovasc. Imaging 2013, 6, 808–816. [Google Scholar] [CrossRef]
- Mazzali, G.; Fantin, F.; Zoico, E.; Sepe, A.; Bambace, C.; Faccioli, S.; Pedrotti, M.; Corzato, F.; Rizzatti, V.; Faggian, G.; et al. Heart Fat Infiltration In Subjects with and without Coronary Artery Disease. J. Clin. Endocrinol. Metab. 2015, 100, 3364–3371. [Google Scholar] [CrossRef]
- Capoulade, R.; Larose, É.; Mathieu, P.; Clavel, M.-A.; Dahou, A.; Arsenault, M.; Bédard, E.; Larue-Grondin, S.; Le Ven, F.; Dumesnil, J.G.; et al. Visceral Adiposity and Left Ventricular Mass and Function in Patients with Aortic Stenosis: The PROGRESSA Study. Can. J. Cardiol. 2014, 30, 1080–1087. [Google Scholar] [CrossRef]
- Klevstig, M.; Ståhlman, M.; Lundqvist, A.; Scharin Täng, M.; Fogelstrand, P.; Adiels, M.; Andersson, L.; Kolesnick, R.; Jeppsson, A.; Borén, J.; et al. Targeting acid sphingomyelinase reduces cardiac ceramide accumulation in the post-ischemic heart. J. Mol. Cell. Cardiol. 2016, 93, 69–72. [Google Scholar] [CrossRef]
- Ji, R.; Akashi, H.; Drosatos, K.; Liao, X.; Jiang, H.; Kennel, P.J.; Brunjes, D.L.; Castillero, E.; Zhang, X.; Deng, L.Y.; et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2017, 2, e82922. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Chan, L.; Bickel, P.E. The PAT family of lipid droplet proteins in heart and vascular cells. Curr. Hypertens. Rep. 2008, 10, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Lu, Y.Y.; Lin, Y.K.; Chen, Y.C.; Chen, Y.A.; Chung, C.C.; Lin, W.S.; Chen, S.A.; Chen, Y.J. Ceramide modulates electrophysiological characteristics and oxidative stress of pulmonary vein cardiomyocytes. Eur. J. Clin. Investig. 2022, 52, e13690. [Google Scholar] [CrossRef]
- Park, L.K.; Garr Barry, V.; Hong, J.; Heebink, J.; Sah, R.; Peterson, L.R. Links between ceramides and cardiac function. Curr. Opin. Lipidol. 2022, 33, 47–56. [Google Scholar] [CrossRef]
- Lindsey, J.B.; Marso, S.P. Steatosis and diastolic dysfunction: The skinny on myocardial fat. J. Am. Coll. Cardiol. 2008, 52, 1800–1802. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Nelson, M.D.; Szczepaniak, E.W.; Smith, L.; Mehta, P.K.; Thomson, L.E.; Berman, D.S.; Li, D.; Bairey Merz, C.N.; Szczepaniak, L.S. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H14–H19. [Google Scholar] [CrossRef] [PubMed]
- Varre, J.V.; Holland, W.L.; Summers, S.A. You aren’t IMMUNE to the ceramides that accumulate in cardiometabolic disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159125. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.R.; McPherron, A.C.; Winik, N.; Lee, S.J. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann. Neurol. 2002, 52, 832–836. [Google Scholar] [CrossRef]
- Sharma, M.; Kambadur, R.; Matthews, K.G.; Somers, W.G.; Devlin, G.P.; Conaglen, J.V.; Fowke, P.J.; Bass, J.J. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J. Cell. Physiol. 1999, 180, 1–9. [Google Scholar] [CrossRef]
- Shyu, K.G.; Lu, M.J.; Wang, B.W.; Sun, H.Y.; Chang, H. Myostatin expression in ventricular myocardium in a rat model of volume-overload heart failure. Eur. J. Clin. Investig. 2006, 36, 713–719. [Google Scholar] [CrossRef]
- Lim, S.; McMahon, C.D.; Matthews, K.G.; Devlin, G.P.; Elston, M.S.; Conaglen, J.V. Absence of Myostatin Improves Cardiac Function Following Myocardial Infarction. Heart Lung Circ. 2018, 27, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Paek, H.J.; Quan, B.H.; Choe, H.M.; Li, Z.Y.; Yin, X.J. Myostatin deficiency decreases cardiac extracellular matrix in pigs. Transgenic Res. 2022, 31, 553–565. [Google Scholar] [CrossRef]
- Shi, C.; Zijlstra, S.N.; de Wit, S.; Meijers, W.C.; Aboumsallem, J.P.; Silljé, H.H.W.; de Boer, R.A. Divergent effects of myostatin inhibition on cardiac and skeletal muscles in a mouse model of pressure overload. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H195–H201. [Google Scholar] [CrossRef]
- Zoico, E.; Corzato, F.; Bambace, C.; Rossi, A.P.; Micciolo, R.; Cinti, S.; Harris, T.B.; Zamboni, M. Myosteatosis and myofibrosis: Relationship with aging, inflammation and insulin resistance. Arch. Gerontol. Geriatr. 2013, 57, 411–416. [Google Scholar] [CrossRef]
- Zamboni, M.; Mazzali, G.; Brunelli, A.; Saatchi, T.; Urbani, S.; Giani, A.; Rossi, A.P.; Zoico, E.; Fantin, F. The Role of Crosstalk between Adipose Cells and Myocytes in the Pathogenesis of Sarcopenic Obesity in the Elderly. Cells 2022, 11, 3361. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.K.; Lee, J.K.; Hsu, J.C.; Su, M.M.; Wu, Y.F.; Lin, T.T.; Lan, C.W.; Hwang, J.J.; Lin, L.Y. Myocardial adipose deposition and the development of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 445–454. [Google Scholar] [CrossRef]
- Schiau, C.; Leucuța, D.C.; Dudea, S.M.; Manole, S. Myocardial Fibrosis as a Predictor of Ventricular Arrhythmias in Patients With Non-ischemic Cardiomyopathy. In Vivo 2021, 35, 1677–1685. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial Tissue Characterization and Fibrosis by Imaging. JACC Cardiovasc. Imaging 2020, 13, 1221–1234. [Google Scholar] [CrossRef]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Leslie, M. Straight from the heart. Science 2023, 379, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- Funai, K.; Summers, S.A.; Rutter, J. Reign in the membrane: How common lipids govern mitochondrial function. Curr. Opin. Cell Biol. 2020, 63, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Poss, A.M.; Summers, S.A. Too Much of a Good Thing? An Evolutionary Theory to Explain the Role of Ceramides in NAFLD. Front. Endocrinol. 2020, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Jiang, Z.; Tang, J.; Lin, C.P.; Zhang, H. Functions and origins of cardiac fat. FEBS J. 2023, 290, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, V.; Heuvingh, J.; du Roure, O.; Rouault, C.; Devulder, A.; Klein, C.; Lacasa, M.; Clément, E.; Lacasa, D.; Clément, K. Human adipocyte function is impacted by mechanical cues. J. Pathol. 2014, 233, 183–195. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2438–2488. [Google Scholar] [CrossRef]



| Variable | M + SD (Range) (n = 31: 12M, 19F) |
|---|---|
| Age (years) | 73.90 ± 5.94 (57–83) |
| Body mass index (BMI) (Kg/m2) | 27.37 ± 4.05 (17–70–38.39) |
| Waist circumference (cm) | 96.77 ± 14.42 (55–130) |
| Systolic blood pressure (mm Hg) | 133.64 ± 15.43 (105–175) |
| Diastolic blood pressure (mm Hg) | 69.19 ± 9.09 (55–80) |
| Total cholesterol (mg/dL) | 178.86 ± 40.39 (122–267) |
| HDL cholesterol (mg/dL) | 59.42 ± 17.72 (35–94) |
| LDL cholesterol (mg/dL) | 95.40 ± 37.51(42–196) |
| Triglycerides (mg/dL) | 114.76 ± 45.26 (56–242) |
| Glucose (mg/dL) | 110.58 ± 33.08 (81–217) |
| Hypertension (n; %) | 29 (93.5%) |
| Diabetes (n; %) | 10 (27%) |
| Statin therapy (n; %) | 14 (37.8%) |
| Oral glucose-lowering drug (n; %) | 9 (24.3%) |
| Insulin therapy (n; %) | 3 (8%) |
| Heart rate (bpm) | 71.7 ± 9.8 (52–91) |
| Stroke volume (mL) | 76.9 ± 19.6 (47.2–116.5) |
| Cardiac output (L/min) | 5.3 ± 1.5 (3.3–9) |
| Medium gradient (MG) (mm Hg) | 49.3 ± 12.3 (30–83) |
| Peak gradient (PG) (mm Hg) | 78.3 ± 18.9 (65–98) |
| E/A | 0.86 ± 0.44 (0.42 ± 2.77) |
| |Global longitudinal strain| (GLS) (%) | 14.80 ± 2.5 (9–19) |
| Ejection fraction (EF%) | 59.6 ± 8.4 |
| Interstitial Fibrosis (%) | Cardiomyocytes Apoptosis (n/10,000) | PLIN 5 (Area) | Ceramides (Area) | Myostatin (OD) | |
|---|---|---|---|---|---|
| Interstitial Fibrosis (%) | 1 | ||||
| Cardiomyocytes Apoptosis (n/10,000) | 0.454 p 0.007 | 1 | |||
| PLIN 5 (area) | 0.389 p 0.019 | 0.416 p 0.012 | 1 | ||
| Ceramides (area) | 0.235 NS | 0.067 NS | 0.241 NS | 1 | |
| Myostatin (OD) | 0.243 NS | −0.189 NS | 0.184 NS | 0.035 NS | 1 |
| |GLS| | EF | E/A | DTI Septal S’ | |
|---|---|---|---|---|
| Fibrosis | −0.266 p 0.086 | −0.148 NS | 0.194 NS | −0.409 p 0.05 |
| Apoptosis | −0.148 NS | −0.063 NS | 0.270 p 0.082 | 0.023 NS |
| PLIN5 | −0.177 NS | 0.029 NS | 0.444 p 0.009 | −0.156 NS |
| Ceramides | −0.126 NS | 0.153 NS | 0.172 NS | −0.253 NS |
| Myostatin | −0.336 p 0.040 | −0.298 p 0.062 | 0.067 NS | −0.186 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoico, E.; Giani, A.; Saatchi, T.; Rizzatti, V.; Mazzali, G.; Fantin, F.; Benfari, G.; Onorati, F.; Urbani, S.; Zamboni, M. Myocardial Fibrosis and Steatosis in Patients with Aortic Stenosis: Roles of Myostatin and Ceramides. Int. J. Mol. Sci. 2023, 24, 15508. https://doi.org/10.3390/ijms242115508
Zoico E, Giani A, Saatchi T, Rizzatti V, Mazzali G, Fantin F, Benfari G, Onorati F, Urbani S, Zamboni M. Myocardial Fibrosis and Steatosis in Patients with Aortic Stenosis: Roles of Myostatin and Ceramides. International Journal of Molecular Sciences. 2023; 24(21):15508. https://doi.org/10.3390/ijms242115508
Chicago/Turabian StyleZoico, Elena, Anna Giani, Tanaz Saatchi, Vanni Rizzatti, Gloria Mazzali, Francesco Fantin, Giovanni Benfari, Francesco Onorati, Silvia Urbani, and Mauro Zamboni. 2023. "Myocardial Fibrosis and Steatosis in Patients with Aortic Stenosis: Roles of Myostatin and Ceramides" International Journal of Molecular Sciences 24, no. 21: 15508. https://doi.org/10.3390/ijms242115508
APA StyleZoico, E., Giani, A., Saatchi, T., Rizzatti, V., Mazzali, G., Fantin, F., Benfari, G., Onorati, F., Urbani, S., & Zamboni, M. (2023). Myocardial Fibrosis and Steatosis in Patients with Aortic Stenosis: Roles of Myostatin and Ceramides. International Journal of Molecular Sciences, 24(21), 15508. https://doi.org/10.3390/ijms242115508