Design of Hollow Porous P-NiCo2O4@Co3O4 Nanoarray and Its Alkaline Aqueous Zinc-Ion Battery Performance
Abstract
:1. Introduction
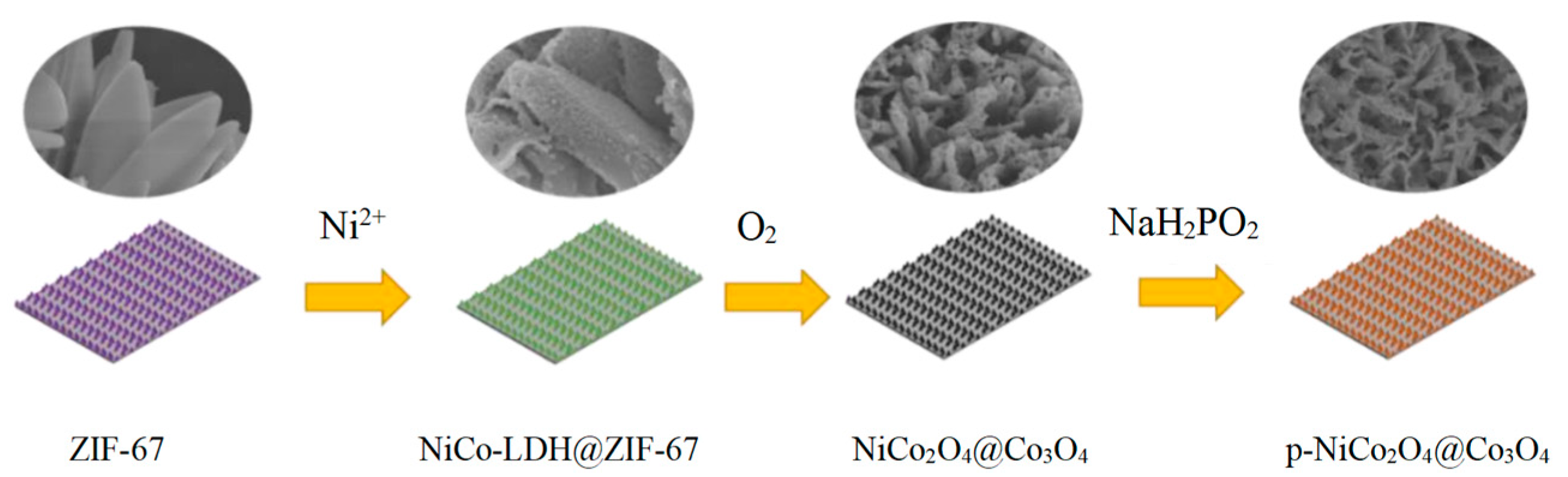
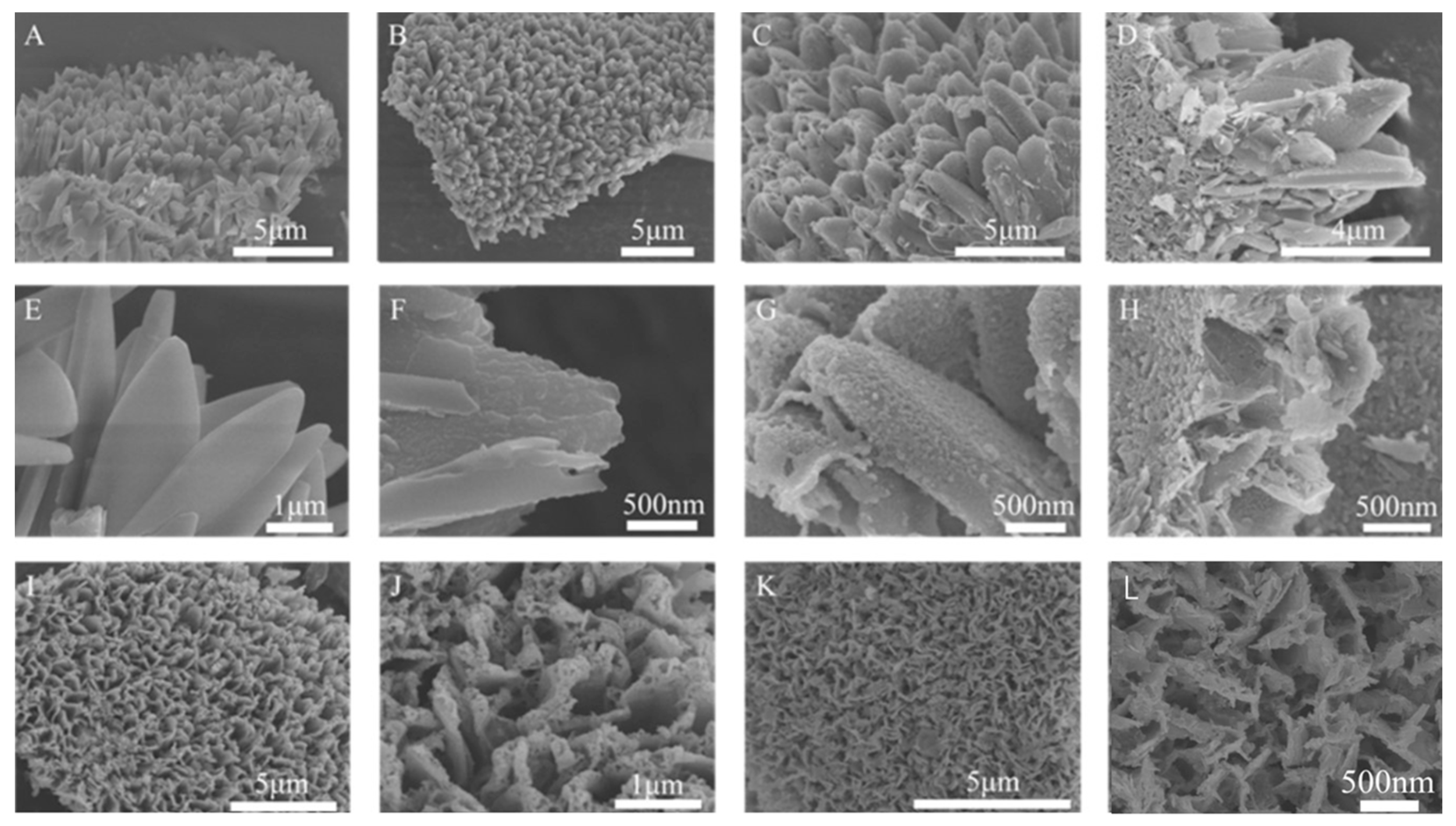
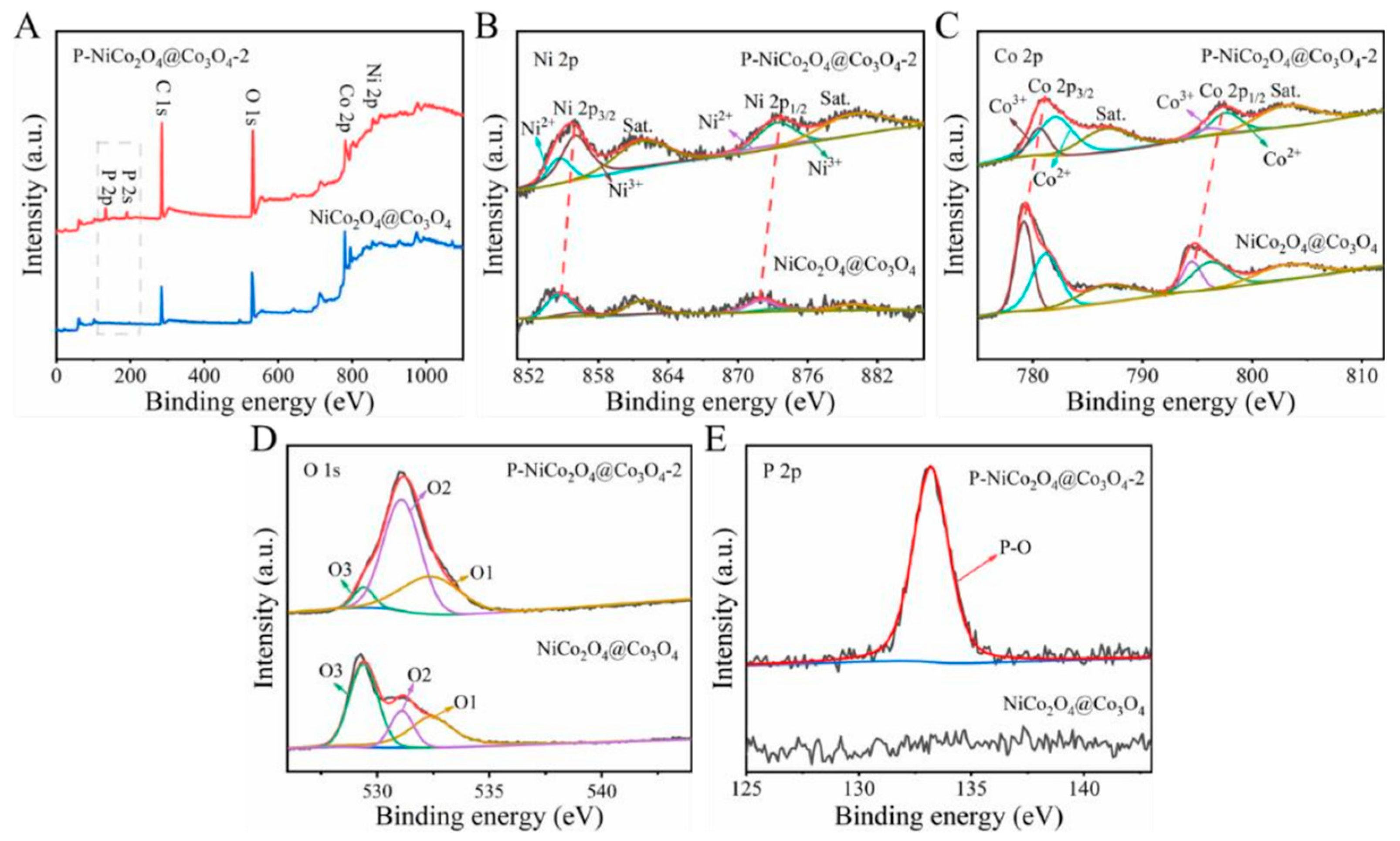
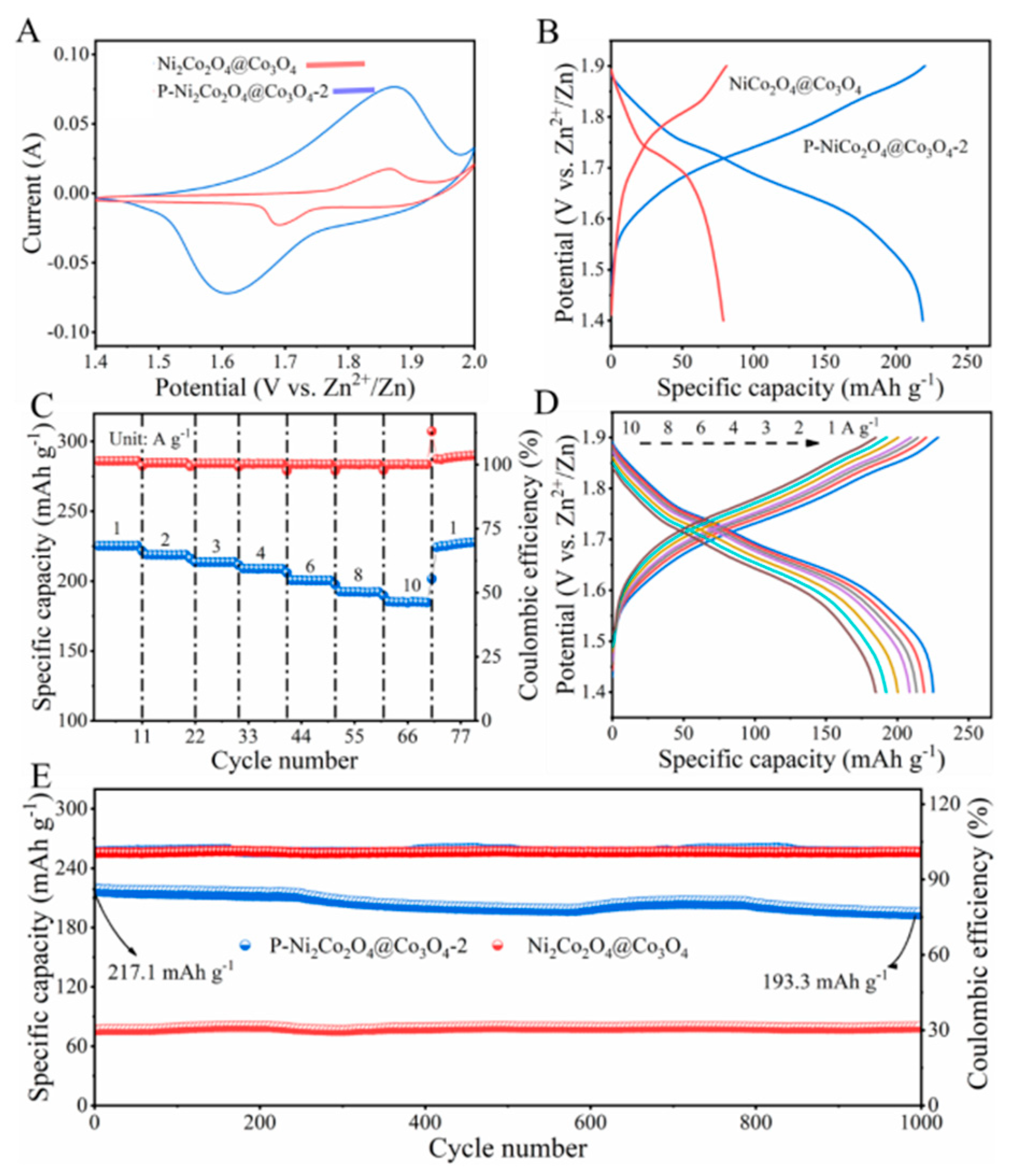
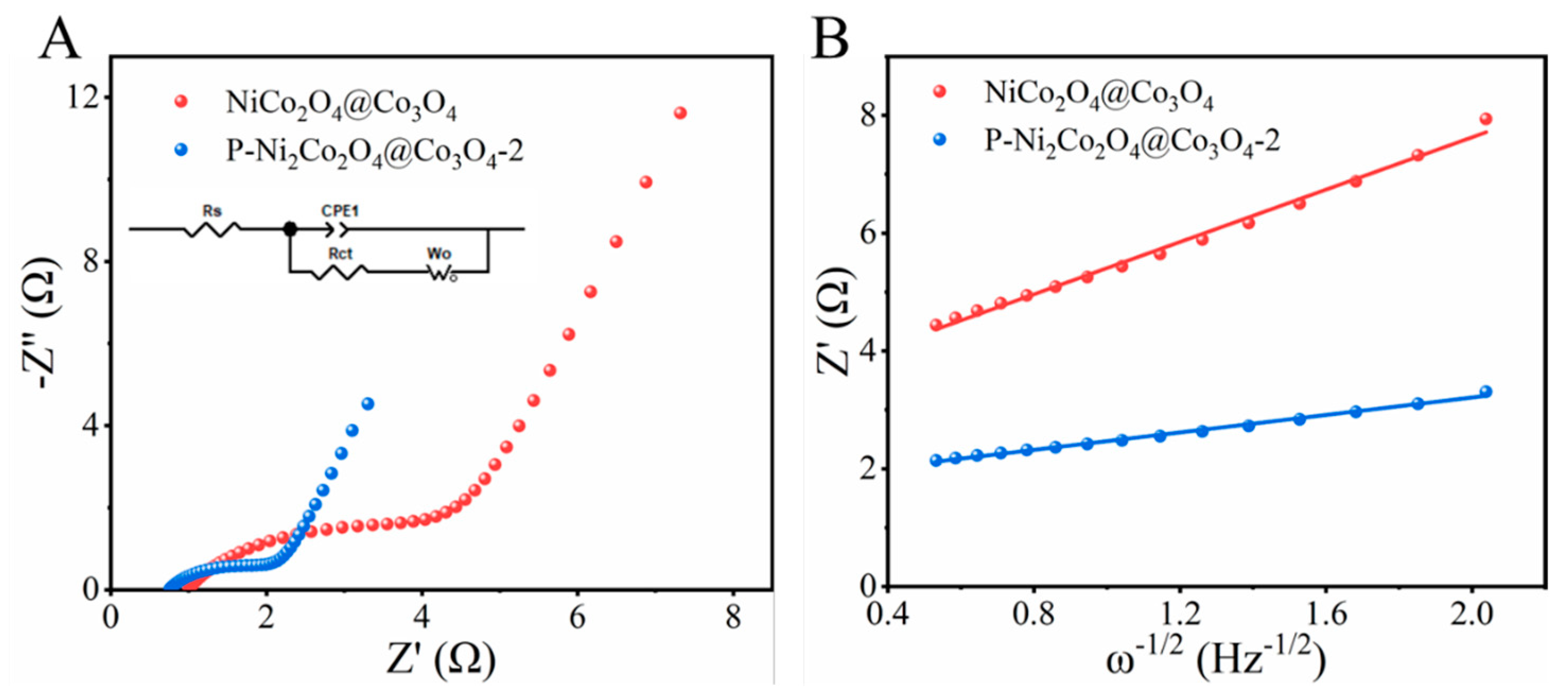
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of ZIF-67
3.2. Preparation of NiCo-LDH@ZIF-67
3.3. Preparation of P-NiCo2O4@Co3O4
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sambandam, B.; Mathew, V.; Kim, S.; Lee, S.; Kim, S.; Hwang, J.Y.; Fan, H.J.; Kim, J. An analysis of the electrochemical mechanism of manganese oxides in aqueous zinc batteries. Chem 2022, 8, 924–946. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Liu, R.; Huang, J.; Diao, Y.; Zhao, W.; Chen, H.-C. Heterogeneous Ni-Co phosphide/phosphate with a specific hollow sea-urchin-like structure for high-performance hybrid supercapacitor and alkaline zinc-metal battery applications. J. Colloid Interface Sci. 2023, 639, 263–273. [Google Scholar] [CrossRef]
- Jung, M.Y.; Cho, H.; Lee, C.; Yun, Y.J.; Balasingam, S.K.; Jun, Y. Synergetic effect of a battery-like nickel phosphide and a pseudocapacitive cobalt phosphide electrodes for enhanced energy storage. J. Energy Storage 2023, 66, 107321. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Wei, C.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Flexible and free-standing Ti3C2Tx Mxene@Zn paper for dendrite-free aqueous zinc metal batteries and nonaqueous lithium metal batteries. ACS Nano 2019, 13, 11676–11685. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xiong, P.; Maitra, U.; Langsdorf, D.; Yan, K.; Wang, C.; Janek, J.; Schröder, D.; Wang, G. Design strategies to enable the efficient use of sodium metal anodes in high-energy batteries. Adv. Mater. 2020, 32, 1903891. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Shi, J.; Liu, Y.; Zheng, S.; Zou, H.; Chen, Y.; Kuang, W.; Ding, K.; Chen, L.; et al. A 3D interconnected metal-organic framework-derived solid-state electrolyte for dendrite-free lithium metal battery. Energy Storage Mater. 2022, 47, 262–270. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Shen, F.; Ruan, Z.; Huang, Y.; Liu, Y.; Liu, Y.; Chen, L.; Lan, Y.-Q.; Zheng, Q. A MOF vertical array enables continuous ion transport pathways with high throughput. J. Mater. Chem. A 2023, 11, 14025–14033. [Google Scholar] [CrossRef]
- Chen, K.; Sun, Z.; Fang, R.; Shi, Y.; Cheng, H.-M.; Li, F. Metal–organic frameworks (MOFs)-derived nitrogendoped porous carbon anchored on graphene with multifunctional effects for lithium–sulfur batteries. Adv. Funct. Mater. 2018, 28, 1707592. [Google Scholar] [CrossRef]
- Tang, J.; Salunkhe, R.R.; Liu, J.; Torad, N.L.; Imura, M.; Furukawa, S.; Yamauchi, Y. Thermal conversion of core-shell metal-organic frameworks: A new method for selectively functionalized nanoporous hybrid carbon. J. Am. Chem. Soc. 2015, 137, 1572–1580. [Google Scholar] [CrossRef]
- Li, J.; Deng, Y.; Leng, L.; Liu, M.; Huang, L.; Tian, X.; Song, H.; Lu, X.; Liao, S. MOF-templated sword-like Co3O4@ NiCo2O4 sheet arrays on carbon cloth as highly efficient Li-O2 battery cathode. J. Power Sources 2020, 450, 227725. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Z.; Cui, Z.; Zhang, K.; Zou, R.; Yang, F.; Xu, K. Boosting the interface reaction activity and kinetics of cobalt molybdate by phosphating treatment for aqueous zinc-ion batteries with high energy density and long cycle life. J. Mater. Chem. A 2020, 8, 21044–21052. [Google Scholar] [CrossRef]
- Wang, F.; Ma, K.; Tian, W.; Dong, J.; Han, H.; Wang, H.; Deng, K.; Yue, H.; Zhang, Y.X.; Jiang, W.; et al. P-Doped NiMoO4 parallel arrays anchored on cobalt carbonate hydroxide with oxygen vacancies and mass transfer channels for supercapacitors and oxygen evolution. J. Mater. Chem. A 2019, 7, 19589–19596. [Google Scholar] [CrossRef]
- Liao, J.; Zou, P.; Su, S.; Nairan, A.; Wang, Y.; Wu, D.; Wong, C.P.; Kang, F.; Yang, C. Hierarchical nickel nanowire@ NiCo2S4 nanowhisker composite arrays with a test-tube-brush-like structure for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 15284–15293. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, H.; Zhang, H.; Jiang, H.; Li, C. Phosphorus-driven mesoporous Co3O4 nanosheets with tunable oxygen vacancies for the enhanced oxygen evolution reaction. Electrochim. Acta 2018, 259, 962–967. [Google Scholar] [CrossRef]
- Zhu, C.; Fu, S.; Du, D.; Lin, Y. Facilely tuning porous NiCo2O4 nanosheets with metal valence state alteration and abundant oxygen vacancies as robust electrocatalysts towards water splitting. Chem. Eur. J. 2016, 22, 4000–4007. [Google Scholar] [CrossRef]
- Guan, C.; Liu, X.; Ren, W.; Li, X.; Cheng, C.; Wang, J. Rational design of metal-organic framework derived hollow NiCo2O4 arrays for flexible supercapacitor and electrocatalysis. Adv. Energy Mater. 2017, 7, 1602391. [Google Scholar] [CrossRef]
- Peng, S.; Gong, F.; Li, L.; Yu, D.; Ji, D.; Zhang, T.; Hu, Z.; Zhang, Z.; Chou, S.; Du, Y.; et al. Necklace-like multishelled hollow spinel oxides with oxygen vacancies for efficient water electrolysis. J. Am. Chem. Soc. 2018, 140, 13644–13653. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Wang, Z.; Lin, T.; Zhu, X.; Luo, B.; Hu, H.; Xing, W.; Yan, Z.; Wang, L. Lithiation-induced vacancy engineering of Co3O4 with improved faradic reactivity for high-performance supercapacitor. Adv. Funct. Mater. 2020, 30, 2004172. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Wang, Y.; Zhao, Q.; Chen, L.; Wei, W.; Ma, J. Influence of anion substitution on 3d-architectured Ni-CoA (A=H, O, P) as efficient cathode materials towards rechargeable zn-based battery. Energy Storage Mater. 2021, 37, 336–344. [Google Scholar] [CrossRef]
- Lai, C.; Sun, Y.; Zhang, X.; Yang, H.; Lin, B. High-performance double ion-buffering reservoirs of asymmetric supercapacitors based on flower-like Co3O4-G>N-PEGm microspheres and 3D rGo-CNT>N-PEGm aerogels. Nanoscale 2018, 10, 17293–17303. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Sun, Y.; Zhang, X.; Yang, H.; Lin, B. Gradual “OH--incursion” outside-inside strategy in construction of 3D flower-like Co3O4-CNT>N-PEGm hierarchical microspheres for supercapacitors. Mater. Today Energy 2018, 9, 27–38. [Google Scholar] [CrossRef]
- Chu, W.; Shi, Z.; Hou, Y.; Ma, D.; Bai, X.; Gao, Y.; Yang, N. Trifunctional of phosphorus-doped NiCo2O4 nanowire materials for asymmetric supercapacitor, oxygen evolution reaction, and hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2020, 12, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Quan, J.; Loh, A.; Chai, J.; Chen, Y.; Tan, C.; Ge, X.; Hor, T.A.; Liu, Z.; Zhang, H.; et al. A robust hybrid Zn-battery with ultralong cycle life. Nano Lett. 2017, 17, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shen, Z.; Pan, Z.; Kou, Z.; Liu, X.; Zhang, H.; Gu, Q.; Guan, C.; Wang, J. Hierarchical micro-nano sheet arrays of nickel–cobalt double hydroxides for high-rate Ni–Zn batteries. Adv. Sci. 2019, 6, 1802002. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Lai, Z.; Han, Y.; Zhang, H.; Xie, S.; Lu, X. Oxygen-vacancy and surface modulation of ultrathin nickel cobaltite nanosheets as a high-energy cathode for advanced Zn-ion batteries. Adv. Mater. 2018, 30, 1802396. [Google Scholar] [CrossRef]
- Barbero, C.; Planes, G.A.; Miras, M.C. Redox coupled ion exchange in cobalt oxide films. Electrochem. Commun. 2001, 3, 113–116. [Google Scholar] [CrossRef]
- Wu, P.; Cheng, S.; Yao, M.; Yang, L.; Zhu, Y.; Liu, P.; Xing, O.; Zhou, J.; Wang, M.; Luo, H.; et al. A low-cost, self-standing NiCo2O4@CNT/CNT multilayer electrode for flexible asymmetric solid-state supercapacitors. Adv. Funct. Mater. 2017, 27, 1702160. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, X.; Liu, Y.; He, Y.; Gao, Y.; Liu, J. High rate capabilities of NiCo2O4-based hierarchical superstructures for rechargeable charge storage. J. Electrochem. Soc. 2014, 161, A1922. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, K.; Cen, Z.; Xu, K. Rational construction of multidimensional oxygendeficient Co3O4 nanosheet/nanowire arrays as high-performance electrodes for aqueous Zn-ion batteries and asymmetric supercapacitors. J. Alloys Compd. 2021, 879, 160439. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Zeng, S.; Zhou, L.; Xu, W.; Zheng, D.; Liu, J.; Zeng, Y.; Lu, X. An ultrathin defect-rich Co3O4 nanosheet cathode for high energy and durable aqueous zinc ion batteries. J. Mater. Chem. A 2019, 7, 21678–21683. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Li, H.; Zhang, Y.; Zeng, Y.; Tong, Y.; Zhang, P.; Lu, X. Flexible rechargeable Ni//Zn battery based on self supported NiCo2O4 nanosheets with high power density and good cycling stability. Green Energy Environ. 2018, 3, 56–62. [Google Scholar] [CrossRef]
- He, P.; Yan, M.; Zhang, G.; Sun, R.; Chen, L.; An, Q.; Mai, L. Layered VS2 nanosheet-based aqueous Zn-ion battery cathode. Adv. Energy Mater. 2017, 7, 1601920. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Cheng, S.; Jiang, K. A long-life aqueous Zn-ion battery based on Na3V2(PO4)2F3 cathode. Energy Storage Mater. 2018, 15, 14–21. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Mo, F.; Liang, G.; Liu, Z.; Tang, Z.; Ma, L.; Liu, J.; Shi, Z.; Zhi, C. MoS2 nanosheets with expanded interlayer spacing forrechargeable aqueous Zn-ion batteries. Energy Storage Mater. 2019, 19, 94–101. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Xu, J.; Li, J.; Wei, B.; Ding, Y.; Amorim, I.; Thomas, R.; Thalluri, S.M.; Liu, Y.; et al. High-performance flexible solid-state asymmetric supercapacitors based on bimetallic transition metal phosphide nanocrystals. ACS Nano 2019, 13, 10612–10621. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Jiang, S.; Xu, B.; Huang, C.; Hu, Y.; Qin, Y.; He, M.; Cao, H. Sea-urchin-like nickel–cobalt phosphide/phosphate composites as advanced battery materials for hybrid supercapacitors. J. Mater. Chem. A 2019, 7, 6241–6249. [Google Scholar] [CrossRef]
- Cheng, L.; Xu, M.; Zhang, Q.; Li, G.; Chen, J.; Lou, Y. NH4Fassisted and morphology-controlled fabrication of ZnCo2O4 nanostructures on ni-foam for enhanced energy storage devices. J. Alloys Compd. 2019, 781, 245–254. [Google Scholar] [CrossRef]



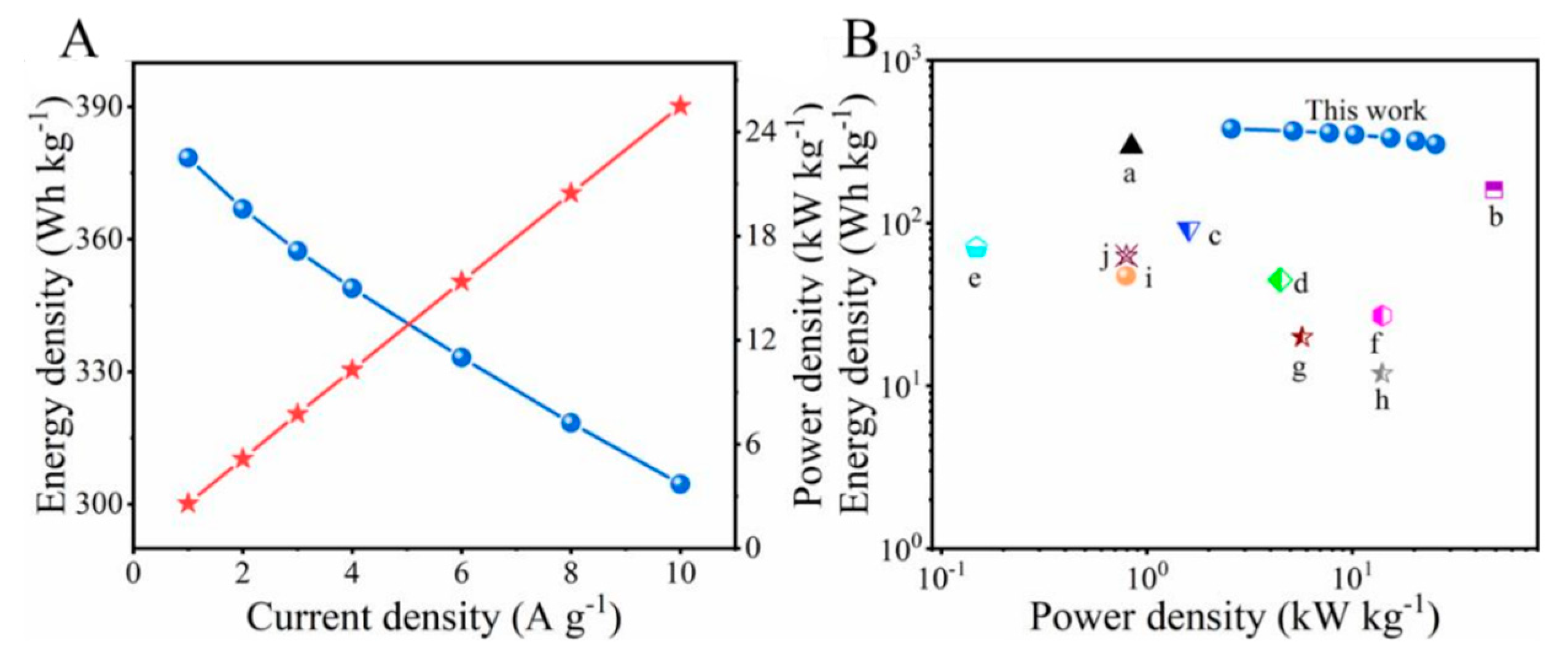

| Materials | Energy Density (Wh kg−1) | Power Density (kW kg−1) | Literature |
|---|---|---|---|
| a, R-Co3O4//Zn | 295.5 | 0.84 | [31] |
| b, NiCo2O4//Zn | 159.4 | 49 | [32] |
| c, VS2//Zn | 92 | 1.6 | [33] |
| d, NaV2(PO4)2F3//Zn | 44.7 | 4.47 | [34] |
| e, MoS2//Zn | 148.2 | 70.5 | [35] |
| f, Co0.1Ni0.9P//AC | 14 | 27 | [36] |
| g, Ni-CoP/POx//RGO | 5.7 | 19.9 | [37] |
| h, NiMoP@CoCH//a-MEGO | 14 | 11.9 | [13] |
| i, NiCo2S4//AC | 0.7935 | 47.29 | [14] |
| j, ZnCo2O4//AC | 0.7955 | 63 | [38] |
| This work | 304.5 | 25.5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Lv, C.; Wang, L.; Li, X.; Cheng, S.; Huo, Y. Design of Hollow Porous P-NiCo2O4@Co3O4 Nanoarray and Its Alkaline Aqueous Zinc-Ion Battery Performance. Int. J. Mol. Sci. 2023, 24, 15548. https://doi.org/10.3390/ijms242115548
Liang Z, Lv C, Wang L, Li X, Cheng S, Huo Y. Design of Hollow Porous P-NiCo2O4@Co3O4 Nanoarray and Its Alkaline Aqueous Zinc-Ion Battery Performance. International Journal of Molecular Sciences. 2023; 24(21):15548. https://doi.org/10.3390/ijms242115548
Chicago/Turabian StyleLiang, Zhe, Chenmeng Lv, Luyao Wang, Xiran Li, Shiwen Cheng, and Yuqiu Huo. 2023. "Design of Hollow Porous P-NiCo2O4@Co3O4 Nanoarray and Its Alkaline Aqueous Zinc-Ion Battery Performance" International Journal of Molecular Sciences 24, no. 21: 15548. https://doi.org/10.3390/ijms242115548
APA StyleLiang, Z., Lv, C., Wang, L., Li, X., Cheng, S., & Huo, Y. (2023). Design of Hollow Porous P-NiCo2O4@Co3O4 Nanoarray and Its Alkaline Aqueous Zinc-Ion Battery Performance. International Journal of Molecular Sciences, 24(21), 15548. https://doi.org/10.3390/ijms242115548






