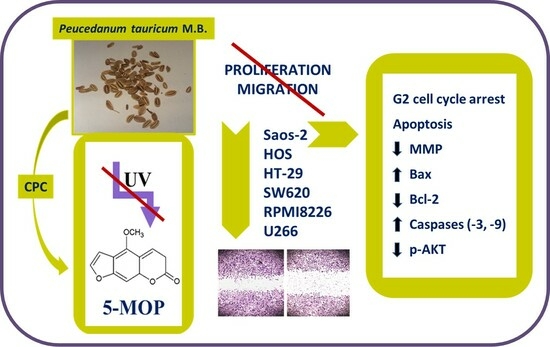
Evaluation of the Biological Effect of Non-UV-Activated Bergapten on Selected Human Tumor Cells and the Insight into the Molecular Mechanism of Its Action
Abstract
:1. Introduction
2. Results
2.1. CPC Isolation and LC–MS Identification of 5-MOP
2.2. Cytostatic and Cytotoxic Effect of 5-MOP
2.3. 5-MOP Decreases the Motility of Cultured Cancer Cells
2.4. 5-MOP Induces Alterations in Cell Cycle Distribution
2.5. 5-MOP Induces Apoptotic Cell Death in Saos-2 Cells
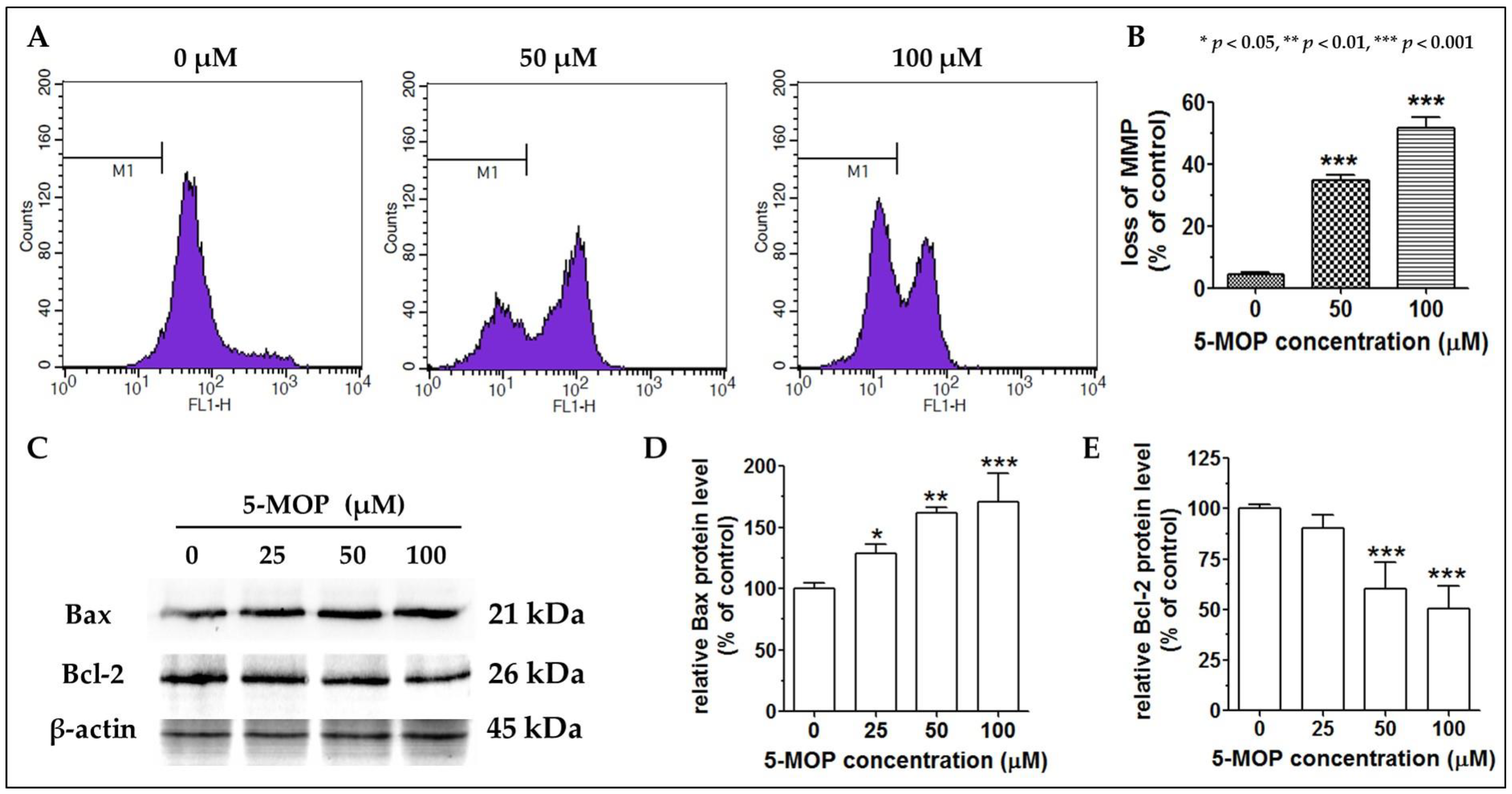
2.6. 5-MOP Affects the Mitochondrial Membrane Potential and Modulates Bax and Bcl-2 Protein Expression
2.7. Reducing the Level of AKT Phosphorylation Enhances the Pro-Apoptotic Effect of 5-MOP
3. Discussion
4. Materials and Methods
4.1. Solvents and Chemicals
4.2. Plant Material
4.3. Isolation, Purification, and Identification of 5-MOP
4.3.1. Extraction of the Plant Material
4.3.2. CPC Isolation of 5-MOP
4.3.3. LC–MS Identification of 5-MOP
4.4. Biological Studies
4.4.1. Cell Cultures
4.4.2. Proliferation Assay
4.4.3. Cytotoxicity Assay
4.4.4. Western Blot Method
4.4.5. Cell Cycle Analysis
4.4.6. Flow Cytometry
4.4.7. Measurement of the Mitochondrial Membrane Potential
4.4.8. ELISA Assay
4.4.9. Cell Migration Assessment
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jöhrer, K.; Cicek, S.S. Multiple Myeloma Inhibitory Activity of Plant Natural Products. Cancers 2021, 13, 2678. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Rega, D.; Costabile, V.; Duraturo, F.; Niglio, A.; Izzo, P.; Pace, U.; Delrio, P. The biological complexity of colorectal cancer: Insights into biomarkers for early detection and personalized care. Ther. Adv. Gastroenterol. 2016, 9, 861–886. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Bellavia, D.; Raimondi, L.; Carina, V.; Costa, V.; Fini, M.; Giavaresi, G. Multiple Effects of Resveratrol on Osteosarcoma Cell Lines. Pharmaceuticals 2022, 15, 342. [Google Scholar] [CrossRef]
- Raimondi, L.; De Luca, A.; Gallo, A.; Costa, V.; Russelli, G.; Cuscino, N.; Manno, M.; Raccosta, S.; Carina, V.; Bellavia, D.; et al. Osteosarcoma cell-derived exosomes affect tumor microenvironment by specific packaging of microRNAs. Carcinogenesis 2020, 41, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Dholwani, K.K.; Saluja, A.K.; Gupta, A.R.; Shah, D.R. A review on plant-derived natural products and their analogs with anti-tumor activity. Indian J. Pharmacol. 2008, 40, 49–58. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy. Front. Pharmacol. 2018, 9, 1534. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Sumorek-Wiadro, J.; Zając, A.; Maciejczyk, A.; Jakubowicz-Gil, J. Furanocoumarins in anticancer therapy—For and against. Fitoterapia 2020, 142, 104492. [Google Scholar] [CrossRef]
- Viola, G.; Fortunato, E.; Cecconet, L.; Disarò, S.; Basso, G. Induction of apoptosis in Jurkat cells by photoexcited psoralen derivatives: Implication of mitochondrial dysfunctions and caspases activation. Toxicol. Vitr. 2007, 21, 211–216. [Google Scholar] [CrossRef]
- Mi, C.; Ma, J.; Wang, K.S.; Zuo, H.X.; Wang, Z.; Li, M.Y.; Piao, L.X.; Xu, G.H.; Li, X.; Quan, Z.S.; et al. Imperatorin suppresses proliferation and angiogenesis of human colon cancer cell by targeting HIF-1α via the mTOR/p70S6K/4E-BP1 and MAPK pathways. J. Ethnopharmacol. 2017, 203, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Tiberio, P.; Cavadini, E.; Callari, M.; Daidone, M.G.; Appierto, V. AF1q: A novel mediator of basal and 4-HPR-induced apoptosis in ovarian cancer cells. PLoS ONE 2012, 7, e39968. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, M.; Sławińska-Brych, A.; Żurek, A.; Kandefer-Szerszeń, M.; Zdzisińska, B. 8-methoxypsoralen reduces AKT phosphorylation, induces intrinsic and extrinsic apoptotic pathways, and suppresses cell growth of SK-N-AS neuroblastoma and SW620 metastatic colon cancer cells. J. Ethnopharmacol. 2017, 207, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Luo, Y.; Zhao, X.; Luo, N.; Tong, A.; Liu, X.; Yuan, Z.; Wang, C.; Wei, Y. Caspase-9 mediates Puma activation in UCN-01-induced apoptosis. Cell Death Dis. 2014, 5, e1495. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.; Koury, J.; Thames, M.; Barlogie, B.; Epstein, J.; Siegel, D.S. Role of NF-κB in the rescue of multiple myeloma cells from glucocorticoid-induced apoptosis by bcl-2. Blood 1999, 93, 3044–3052. [Google Scholar] [CrossRef]
- Tian, Y.; Shi, R.; Gao, M.; Wang, H.; Du, Y.; Zhang, L.; Wang, O.; Zhang, M. Differentiation of Furanocoumarin Isomers with Ratio of Relative Abundance of Characteristic Fragment Ions and Application in Angelicae dahuricae Radix. Chromatographia 2017, 80, 1401–1410. [Google Scholar] [CrossRef]
- Bartnik, M. Efficient Separation of the Methoxyfuranocoumarins Peucedanin, 8-Methoxypeucedanin, and Bergapten by Centrifugal Partition Chromatography (CPC). Molecules 2023, 28, 1923. [Google Scholar] [CrossRef]
- Manoli, I.; Alesci, S.; Blackman, M.R.; Su, Y.A.; Rennert, O.M.; Chrousos, G.P. Mitochondria as key components of the stress response. Trends Endocr. Metabol. 2007, 18, 190–198. [Google Scholar] [CrossRef]
- Lee, H.-C.; Pen-Hui Yin, P.-H.; Lu, C.-Y.; Chi, C.-W.; Wei, Y.-H. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem. J. 2000, 348, 425–432. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.-E.-S. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614, Erratum in Front. Pharmacol. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.M.; Galson, D.L.; Roodman, G.D.; Ouyang, H. Resveratrol triggers the pro-apoptotic endoplasmic reticulum stress response and represses pro-survival XBP1 signaling in human multiple myeloma cells. Exp. Hematol. 2011, 39, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Khanna, P.; Chen, S.; Pei, X.Y.; Dent, P.; Grant, S. Statins synergistically potentiate 7-hydroxystaurosporine (UCN-01) lethality in human leukemia and myeloma cells by disrupting Ras farnesylation and activation. Blood 2007, 109, 4415–4423. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Wu, T.H.; Chen, S.F.; Chung, J.G. Effect of 5-methoxypsoralen (5-MOP) on cell apoptosis and cell cycle in human hepatocellular carcinoma cell line. Toxicol. Vitr. 2003, 17, 279–287. [Google Scholar] [CrossRef]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the Effectiveness of Natural Compound Mixtures in Cancer through Their Molecular Mode of Action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef]
- Deeni, Y.Y.; Ibbotson, S.H.; Woods, J.A.; Wolf, C.R.; Smith, G. Cytochrome P450 CYP1B1 interacts with 8-methoxypsoralen (8-MOP) and influences psoralen-ultraviolet A (PUVA) sensitivity. PLoS ONE 2013, 8, e75494. [Google Scholar] [CrossRef]
- Quetglas-Llabrés, M.M.; Quispe, C.; Herrera-Bravo, J.; Catarino, M.D.; Pereira, O.R.; Cardoso, S.M.; Dua, K.; Chellappan, D.K.; Pabreja, K.; Satija, S.; et al. Pharmacological Properties of Bergapten: Mechanistic and Therapeutic Aspects. Oxid. Med. Cell Longev. 2022, 2022, 8615242. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, L.; Liu, K.; Cao, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. Bergapten: A review of its pharmacology, pharmacokinetics, and toxicity. Phytother. Res. 2021, 35, 6131–6147. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, K.; Mei, W.; Wang, Y.; Yu, C.; Yu, B.; Deng, S.; Hu, J. Anti-inflammatory and proresolution activities of bergapten isolated from the roots of Ficus hirta in an in vivo zebrafish model. Biochem. Biophys. Res. Commun. 2018, 496, 763–769. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, A.; Kaur, J.; Bhatti, M.S.; Singh, P.; Bhatti, R. Bergapten inhibits chemically induced nociceptive behavior and inflammation in mice by decreasing the expression of spinal PARP, iNOS, COX-2 and inflammatory cytokines. Inflammopharmacology 2019, 27, 749–760. [Google Scholar] [CrossRef]
- Ibbotson, S. Drug and chemical induced photosensitivity from a clinical perspective. Photochem. Photobiol. Sci. 2018, 17, 1885–1903. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, M.; Sakanaka, M.; Taniguchi, M.; Baba, K.; Kimura, Y. Anti-tumor effects of various furanocoumarins isolated from the roots, seeds and fruits of Angelica and Cnidium species under ultraviolet A irradiation. J. Nat. Med. 2014, 68, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.S. PUVA Follow-Up Study. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: A 30-year prospective study. J. Am. Acad. Dermatol. 2012, 66, 553–562. [Google Scholar] [CrossRef]
- Dowdy, J.C.; Sayre, R.M. Melanoma Risk From Dietary Furocoumarins: How Much More Evidence Is Required? J. Clin. Oncol. 2016, 34, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Panno, M.L.; Giordano, F.; Palma, M.G.; Bartella, V.; Rago, V.; Maggiolini, M.; Sisci, D.; Lanzino, M.; De Amicis, F.; Andò, S. Evidence that bergapten, independently of its photoactivation, enhances p53 gene expression and induces apoptosis in human breast cancer cells. Curr. Cancer Drug Targets 2009, 9, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Küpeli Akkol, E.; Capasso, R. Anticancer Potential of Furanocoumarins: Mechanistic and Therapeutic Aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef]
- Fujioka, T.; Furumi, K.; Fujii, H.; Okabe, H.; Mihashi, K.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M. Antiproliferative constituents from Umbelliferae plants. V. A new furanocoumarin and falcarindiol furanocoumarin ethers from the root of Angelica japonica. Chem. Pharm. Bull. 1999, 47, 96–100. [Google Scholar] [CrossRef]
- Girennavar, B. Grapefruit-Drug Interactions: Isolation, Synthesis, and Biological Activities of Furanocoumarins and Their Variation Due to Pre- and Post-Harvest Factors. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2007; pp. 113–115. [Google Scholar]
- Um, Y.R.; Kong, C.; Lee, J.I.; Kim, Y.A.; Nam, T.J.; Seo, Y. Evaluation of chemical constituents from Glehnia littoralis for antiproliferative activity against HT-29 human colon cancer cells. Process Biochem. 2010, 45, 114–119. [Google Scholar] [CrossRef]
- Panno, M.L.; Giordano, F.; Mastroianni, F.; Palma, M.G.; Bartella, V.; Carpino, A.; Aquila, S.; Andò, S. Breast cancer cell survival signal is affected by bergapten combined with an ultraviolet irradiation. FEBS Lett. 2010, 584, 2321–2326. [Google Scholar] [CrossRef]
- Guo, H.; He, Y.; Bu, C.; Peng, Z. Antitumor and apoptotic effects of 5-methoxypsoralen in U87MG human glioma cells and its effect on cell cycle, autophagy and PI3K/Akt signaling pathway. Arch. Med. Sci. 2019, 15, 1530–1538. [Google Scholar] [CrossRef]
- Chiang, S.R.; Lin, C.S.; Lin, H.H.; Shieh, P.C.; Kao, S.H. Bergapten induces G1 arrest of non-small cell lung cancer cells, associated with the p53-mediated cascade. Mol. Med. Rep. 2019, 19, 1972–1978. [Google Scholar] [CrossRef]
- Liu, W.X.; Jia, F.L.; He, Y.Y.; Zhang, B.X. Protective effects of 5-methoxypsoralen against acetaminophen-induced hepatotoxicity in mice. World J. Gastroenterol. 2012, 18, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.; de Farias, M.T.; Teles, A.L.; Dos Santos Junior, M.C.; de Cerqueira, M.D.; Lima, R.M.; El-Bachá, R.S. 8-Methoxypsoralen is a competitive inhibitor of glutathione S-transferase P1-1. Front. Cell. Neurosci. 2014, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, S.P.; Bose, P.; Sunita, P.; Siddique, M.U.M.; Lapenna, A. Bergapten inhibits liver carcinogenesis by modulating LXR/PI3K/Akt and IDOL/LDLR pathways. Biomed. Pharmacother. 2018, 108, 297–308. [Google Scholar] [CrossRef]
- Lin, C.P.; Lin, C.S.; Lin, H.H.; Li, K.T.; Kao, S.H.; Tsao, S.M. Bergapten induces G1 arrest and pro-apoptotic cascade in colorectal cancer cells associating with p53/p21/PTEN axis. Environ. Toxicol. 2019, 34, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Via, L.D.; Gia, O.; Magno, S.M.; Santana, L.; Teijeira, M.; Uriarte, E. New tetracyclic analogues of photochemotherapeutic drugs 5-MOP and 8-MOP: Synthesis, DNA interaction, and antiproliferative activity. J. Med. Chem. 1999, 42, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef]
- De Amicis, F.; Aquila, S.; Morelli, C.; Guido, C.; Santoro, M.; Perrotta, I.; Mauro, L.; Giordano, F.; Nigro, A.; Andò, S.; et al. Bergapten drives autophagy through the up-regulation of PTEN expression in breast cancer cells. Mol. Cancer 2015, 14, 130. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef] [PubMed]
- West, K.A.; Castillo, S.S.; Dennis, P.A. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist. Updat. 2002, 5, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef]
- Zhang, Y.; Weng, Q.; Han, J.; Chen, J. Alantolactone suppresses human osteosarcoma through the PI3K/AKT signaling pathway. Mol. Med. Rep. 2020, 21, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.W.; Janeway, K.A. Emerging concepts for PI3K/mTOR inhibition as a potential treatment for osteosarcoma. F1000Research 2016, 5, F1000 Faculty Rev-1590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, L.B.; Peng, A.F.; Wang, T.F.; Long, X.H.; Gao, S.; Zhou, R.P.; Liu, Z.L. LY294002 inhibits the malignant phenotype of osteosarcoma cells by modulating the phosphatidylinositol 3-kinase/Akt/fatty acid synthase signaling pathway in vitro. Mol. Med. Rep. 2015, 11, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Sumorek-Wiadro, J.; Maciejczyk, A.; Langner, E.; Wertel, I.; Rzeski, W.; Jakubowicz-Gil, J. LY294002 and sorafenib as inhibitors of intracellular survival pathways in the elimination of human glioma cells by programmed cell death. Cell Tissue Res. 2021, 386, 17–28. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Lee, H.; Hwang-Bo, H.; Ji, S.Y.; Kim, M.Y.; Hong, S.H.; Jeong, J.W.; Han, M.H.; Choi, S.H.; et al. Induction of G2/MCell Cycle Arrest and Apoptosis by Genistein in Human Bladder Cancer T24 Cells through Inhibition of the ROS-Dependent PI3k/Akt Signal Transduction Pathway. Antioxidants 2019, 8, 327. [Google Scholar] [CrossRef]
- Liang, C.; Li, H.; Shen, C.; Lai, J.; Shi, Z.; Liu, B.; Tao, H.M. Genistein potentiates the anti-cancer effects of gemcitabine in human osteosarcoma via the downregulation of Akt and nuclear factor-_B pathway. Antican. Agents Med. Chem. 2012, 12, 554–563. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wan, Y.; Huo, B.; Li, B.; Jin, Y.; Hu, X. Extracts and components of Ficus carica leaves suppress survival, cell cycle, and migration of triple-negative breast cancer MDA-MB-231 cells. OncoTargets Ther. 2018, 11, 4377–4386. [Google Scholar] [CrossRef] [PubMed]
- Lauvrak, S.U.; Munthe, E.; Kresse, S.H.; Stratford, E.W.; Namløs, H.M.; Meza-Zepeda, L.A.; Myklebost, O. Functional characterization of osteosarcoma cell lines and identification of mRNAs and miRNAs associated with aggressive cancer phenotypes. Br. J. Cancer. 2013, 109, 2228–2236. [Google Scholar] [CrossRef]
- Zhu, X.L.; Liang, L.; Ding, Y.Q. Expression of FMNL2 and its relation to the metastatic potential of human colorectal cancer cells. Nan Fang Yi Ke Da Xue Xue Bao 2008, 28, 1775–1778. [Google Scholar]
- Jiang, S.; Zhou, F.; Zhang, Y.; Zhou, W.; Zhu, L.; Zhang, M.; Luo, J.; Ma, R.; Xu, X.; Zhu, J.; et al. Identification of tumorigenicity-associated genes in osteosarcoma cell lines based on bioinformatic analysis and experimental validation. J. Cancer. 2020, 11, 3623–3633. [Google Scholar] [CrossRef]
- Catalogue of Somatic Mutations in Cancer. Available online: https://cancer.sanger.ac.uk/cosmic (accessed on 17 October 2023).
- COSMIC Cell Line Gene Mutation Profiles. Available online: https://maayanlab.cloud/Harmonizome/dataset/COSMIC+Cell+Line+Gene+Mutation+Profiles (accessed on 17 October 2023).
- Piechowska, K.; Mizerska-Kowalska, M.; Zdzisińska, B.; Cytarska, J.; Baranowska-Łączkowska, A.; Jaroch, K.; Łuczykowski, K.; Płaziński, W.; Bojko, B.; Kruszewski, S.; et al. Tropinone-Derived Alkaloids as Potent Anticancer Agents: Synthesis, Tyrosinase Inhibition, Mechanism of Action, DFT Calculation, and Molecular Docking Studies. Int. J. Mol. Sci. 2020, 21, 9050. [Google Scholar] [CrossRef]
- Sławińska-Brych, A.; Zdzisińska, B.; Czerwonka, A.; Mizerska-Kowalska, M.; Dmoszyńska-Graniczka, M.; Stepulak, A.; Gagoś, M. Xanthohumol exhibits anti-myeloma activity in vitro through inhibition of cell proliferation, induction of apoptosis via the ERK and JNK-dependent mechanism, and suppression of sIL-6R and VEGF production. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129408. [Google Scholar] [CrossRef] [PubMed]
- Sławińska-Brych, A.; Król, S.K.; Dmoszyńska-Graniczka, M.; Zdzisińska, B.; Stepulak, A.; Gagoś, M. Xanthohumol inhibits cell cycle progression and proliferation of larynx cancer cells in vitro. Chem. Biol. Interact. 2015, 240, 110–118. [Google Scholar] [CrossRef]
- Sławińska-Brych, A.; Mizerska-Kowalska, M.; Król, S.K.; Stepulak, A.; Zdzisińska, B. Xanthohumol Impairs the PMA-Driven Invasive Behaviour of Lung Cancer Cell Line A549 and Exerts Anti-EMT Action. Cells 2021, 10, 1484. [Google Scholar] [CrossRef]
- Piaton, E.; Fabre, M.; Goubin-Versini, I.; Bretz-Grenier, M.F.; Courtade-Saïdi, M.; Vincent, S.; Belleannée, G.; Thivolet, F.; Boutonnat, J.; Debaque, H.; et al. Technical recommendations and best practice guidelines for May-Grünwald-Giemsa staining: Literature review and insights from the quality assurance. Ann. Pathol. 2015, 35, 294–305. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartnik, M.; Sławińska-Brych, A.; Mizerska-Kowalska, M.; Zdzisińska, B. Evaluation of the Biological Effect of Non-UV-Activated Bergapten on Selected Human Tumor Cells and the Insight into the Molecular Mechanism of Its Action. Int. J. Mol. Sci. 2023, 24, 15555. https://doi.org/10.3390/ijms242115555
Bartnik M, Sławińska-Brych A, Mizerska-Kowalska M, Zdzisińska B. Evaluation of the Biological Effect of Non-UV-Activated Bergapten on Selected Human Tumor Cells and the Insight into the Molecular Mechanism of Its Action. International Journal of Molecular Sciences. 2023; 24(21):15555. https://doi.org/10.3390/ijms242115555
Chicago/Turabian StyleBartnik, Magdalena, Adrianna Sławińska-Brych, Magdalena Mizerska-Kowalska, and Barbara Zdzisińska. 2023. "Evaluation of the Biological Effect of Non-UV-Activated Bergapten on Selected Human Tumor Cells and the Insight into the Molecular Mechanism of Its Action" International Journal of Molecular Sciences 24, no. 21: 15555. https://doi.org/10.3390/ijms242115555