Pyrrolizidine Alkaloids—Pros and Cons for Pharmaceutical and Medical Applications
Abstract
:1. Introduction
2. Chemical Structure and General Characteristics of PAs
3. Plants as a Source of PAs
4. Environmental and Food Safety
5. Functions of Microbial PAs in the Environment and Their Significance for Humans
| Type of Microorganism | Name of Species/Origin | PA | Refs. |
|---|---|---|---|
| Endophytic fungi | Epichloe sp. (Ascomycota: Clavicipitaceae), endophytes of grasses | lolines (saturated 1 -aminopyrrolizidines) | [8,44] |
| Penicillium sp. GD6, endophyte of Bruguiera gymnorhiza | penibruguieramine A | [43] | |
| Fungi | Pochonia suchlasporia var. suchlasporia TAMA 87 from soil | pochonicine | [55] |
| Aspergillus sclerotiicarbonarius (IBT 28362) | sclerolizine (oxidized pyrrolizidine) | [66] | |
| Bacteria | Actinosporangium sp. C36,145 (ATCC 31127) | bohemamine | [56] |
| Streptomyces UMA-044 from sediment collected in a catfish pond, Stoneville, Mississippi, USA | bohemamine 3 NP25302 | [57] | |
| Streptomyces sp. CNQ-583 from marine sediment | bohemamines 4, B, C 5-chlorobohemamine NP25302 | [58] | |
| Streptomyces spinoverrucosus from marine sediment | bohemamine A, B | [59] | |
| Streptomyces spinoverrucosus SNB-032 | bohemamines N; 1; 5-Cl; 5-Br dibohemamines A–C | [60] | |
| Streptomyces sp. CPCC 200497 | dibohamamine A, D–F | [61] | |
| Streptomyces sp. CB02009 | bohemamines 1–4 | [26] | |
| Streptomyces MF990-BF4 | clazamycins A, B | [64] | |
| Streptomyces puniceus subsp. doliceus NRRL 11160 | clazamycin B | [67] | |
| Streptomyces sp. MA37 from Ghanaian soil | legonmycins A, B | [7] | |
| Streptomyces sp. HK10297 | jenamidines A–C | [68] | |
| Streptomyces spinoverrucosus | spithioneines A, B | [59] | |
| Pseudomonas fluorescens HK 10770 from forest soil in Germany | pyreudiones A–D | [69] |
6. Various Susceptibility to PAs
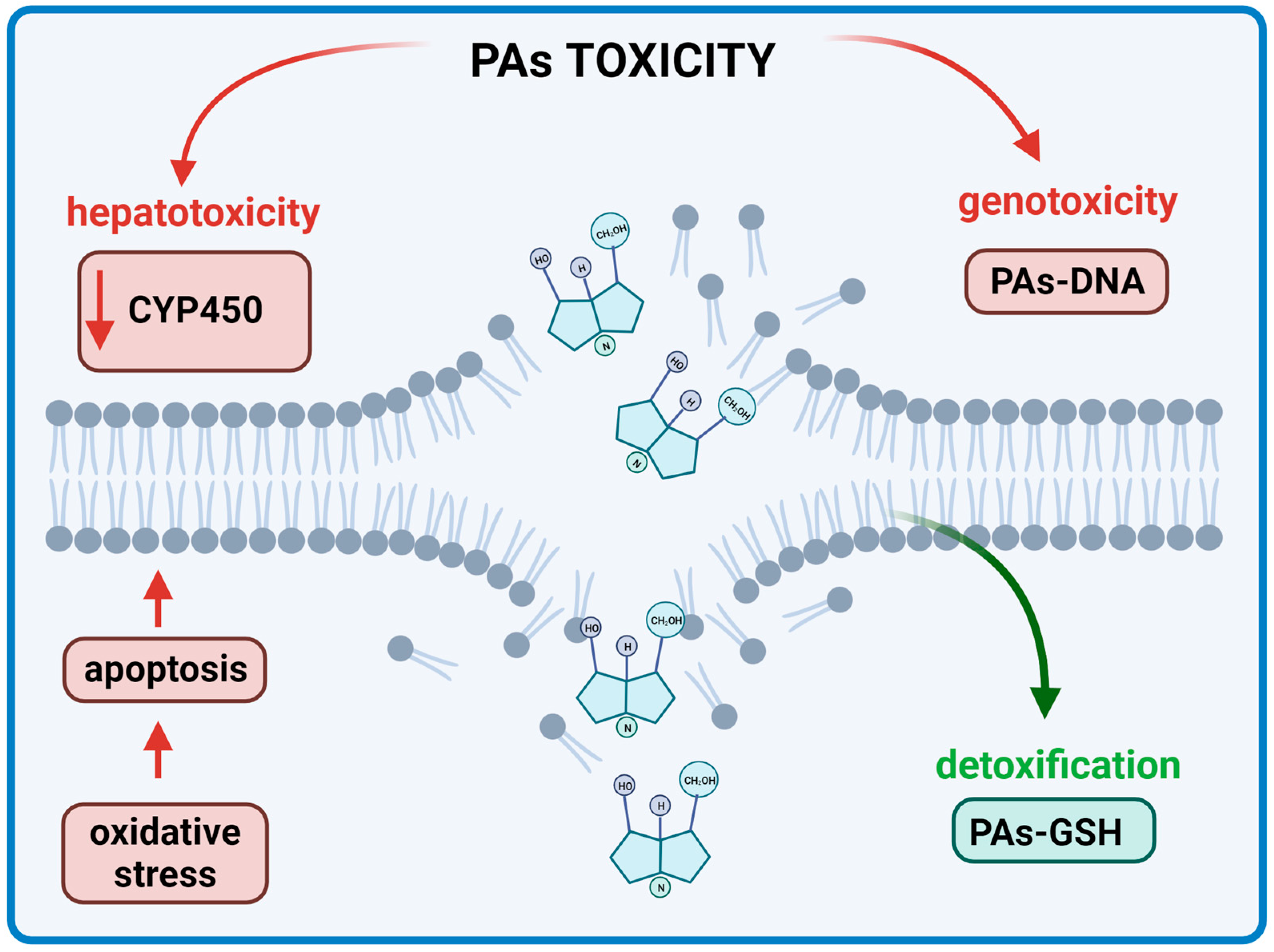
7. Hepatotoxicity
8. Pyrrolizidine Alkaloids and Their Positive Activities
8.1. Anticancer Properties
| Compound | Plant Source | Cell Line | Dose | Effect | Ref. |
|---|---|---|---|---|---|
| In Vitro | |||||
Indicine-N-oxide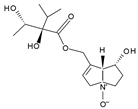 | Heliotropium indicum | B16 (mouse melanoma), walker 256 (carcinosarcoma in rats), P388, and L1210 (murine leukemia) | 46–100 μM | Inhibition of cell proliferation | [111] |
| 7-angeloyl heliotrine  | Heliotropium subulatum | Chinese hamster V79 cells | 100, 50, 30, 20, 10, 5, 3 and 1 µg/mL for 5 days | Cytotoxic effect at IC50 concentrations of 10 and 5 mg/mL | [115] |
Ligulachyroine A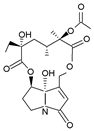 | Ligularia achyrotricha | human leukemia cell (HL-60) and human hepatoma cell (SMMC-7721) | The cells were treated with various concentrations for 48 h | A moderate cytotoxic effect at IC50 values of 12.17 µg/mL and 11.88 µg/mL, respectively | [116] |
| In Vivo | |||||
Pyrroline bis(carbamate)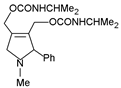 | synthetic analogue of indicine-N-oxide | P388 lymphocytic leukemia | 100, 50, 25, and 12.5 mg/kg by injection of a solution in distilled water. 5× daily doses were administered beginning 24 h after tumor inoculation | Antileukemic effect of 225% T/C at 100 mg/kg (the most active among 5 other analogues of indicine-N-oxide) | [110] |
| 7-angeloyl heliotrine  | Heliotropium subulatum | ICR albino mice implanted with Sarcoma 180 (1 × 10−6 cells/0.1 mL ascitic fluid) | Test group: 50–100 mg/kg/day of test crude extracts + 5–10 mg/kg/day of test compounds; control group: saline only | Maximum inhibition of 41.7% at 5 mg/kg/day (the highest inhibition among five other PAs) | [115] |
8.2. Antimicrobial Properties
8.3. Potential Applications of PAs and Future Perspectives
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine alkaloids: Biosynthesis, biological activities and occurrence in crop plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef]
- Lewis, W.J.M.; Shaw, D.M.; Robertson, J. Synthesis of legonmycins A and B, C(7a)-hydroxylated bacterial pyrrolizidines. Beilstein J. Org. Chem. 2021, 17, 334–342. [Google Scholar] [CrossRef]
- Kopp, T.; Abdel-Tawab, M.; Mizaikoff, B. Extracting and analyzing pyrrolizidine alkaloids in medicinal plants: A review. Toxins 2020, 12, 320. [Google Scholar] [CrossRef]
- Wei, X.; Ruan, W.; Vrieling, K. Current knowledge and perspectives of pyrrolizidine alkaloids in pharmacological applications: A mini-review. Molecules 2021, 26, 1970. [Google Scholar] [CrossRef]
- Wiedenfeld, H.; Edgar, J. Toxicity of pyrrolizidine alkaloids to humans and ruminants. Phytochem. Rev. 2011, 10, 137–151. [Google Scholar] [CrossRef]
- Robertson, J.; Stevens, K. Pyrrolizidine alkaloids: Occurrence, biology, and chemical synthesis. Nat. Prod. Rep. 2017, 34, 62–89. [Google Scholar] [CrossRef]
- Huang, S.; Tabudravu, J.; Elsayed, S.S.; Travert, J.; Peace, D.; Tong, M.H.; Kyeremeh, K.; Kelly, S.M.; Trembleau, L.; Ebel, R.; et al. Discovery of a single monooxygenase that catalyzes carbamate formation and ring contraction in the biosynthesis of the legonmycins. Angew. Chem. 2015, 127, 12888–12892. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, T.; Ming, Q.; Wu, L.; Rahman, K.; Qin, L. Alkaloids produced by endophytic fungi: A review. Nat. Prod. Commun. Vol. 2012, 7, 963–968. [Google Scholar] [CrossRef]
- El-Shazly, A.; Wink, M. Diversity of pyrrolizidine alkaloids in the Boraginaceae structures, distribution, and biological properties. Diversity 2014, 6, 188–282. [Google Scholar] [CrossRef]
- Moreira, R.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Pyrrolizidine alkaloids: Chemistry, pharmacology, toxicology and food safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef]
- Klapper, M.; Götze, S.; Barnett, R.; Willing, K.; Stallforth, P. Bacterial alkaloids prevent amoebal predation. Angew. Chem. 2016, 128, 9090–9093. [Google Scholar] [CrossRef]
- Smith, L.W.; Culvenor, C.C.J. Plant sources of hepatotoxic pyrrolizidine alcaloids. J. Nat. Prod. 1981, 44, 129–152. [Google Scholar] [CrossRef]
- Nobre, V.M.T.; Dantas, A.F.M.; Riet-Correa, F.; Barbosa Filho, J.M.; Tabosa, I.M.; Vasconcelos, J.S. Acute intoxication by Crotalaria retusa in sheep. Toxicon 2005, 45, 347–352. [Google Scholar] [CrossRef]
- Wiedenfeld, H. Plants containing pyrrolizidine alkaloids: Toxicity and problems. Food Addit. Contam.—Part A 2011, 28, 282–292. [Google Scholar] [CrossRef]
- Carvalho, S.; Macel, M.; Schlerf, M.; Moghaddam, F.E.; Mulder, P.P.J.; Skidmore, A.K.; van der Putten, W.H. Changes in plant defense chemistry (pyrrolizidine alkaloids) revealed through high-resolution spectroscopy. ISPRS J. Photogramm. Remote Sens. 2013, 80, 51–60. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Ma, J.; Lin, G. Metabolism-mediated cytotoxicity and genotoxicity of pyrrolizidine alkaloids. Arch. Toxicol. 2021, 95, 1917–1942. [Google Scholar] [CrossRef]
- Chen, T.; Mei, N.; Fu, P.P. Genotoxicity of pyrrolizidine alkaloids. J. Appl. Toxicol. 2010, 30, 183–196. [Google Scholar] [CrossRef]
- Molyneux, R.J.; Gardner, D.L.; Colegate, S.M.; Edgar, J.A. Pyrrolizidine alkaloid toxicity in livestock: A paradigm for human poisoning? Food Addit. Contam.—Part A 2011, 28, 293–307. [Google Scholar] [CrossRef]
- Fu, P.P. Pyrrolizidine alkaloids: Metabolic activation pathways leading to liver tumor initiation. Chem. Res. Toxicol. 2017, 30, 81–93. [Google Scholar] [CrossRef]
- He, X.; Xia, Q.; Zhu, L.; He, Y.; Bryant, M.S.; Lin, G.; Fu, P.P. Formation of DHP-DNA Adducts from Rat Liver Microsomal Metabolism of 1,2-Unsaturated Pyrrolizidine Alkaloid-Containing Plant Extracts and Dietary Supplements. Chem. Res. Toxicol. 2023, 36, 243–250. [Google Scholar] [CrossRef]
- Geburek, I.; Rutz, L.; Gao, L.; Kupper, J.H.; These, A.; Schrenk, D. Metabolic pattern of hepatotoxic pyrrolizidine alkaloids in liver cells. Chem. Res. Toxicol. 2021, 34, 1101–1113. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Lin, G.; Chou, M.W. Pyrrolizidine alkaloids—Genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab. Rev. 2004, 36, 1–55. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Yang, X.; Xiong, A.; Yang, L.; Wang, Z. Pyrrolizidine alkaloids: An update on their metabolism and hepatotoxicity mechanism. Liver Res. 2019, 3, 176–184. [Google Scholar] [CrossRef]
- Schimming, O.; Challinor, V.L.; Tobias, N.J.; Adihou, H.; Grün, P.; Pöschel, L.; Richter, C.; Schwalbe, H.; Bode, H.B. Structure, biosynthesis, and occurrence of bacterial pyrrolizidine alkaloids. Angew. Chem. 2015, 127, 12893–12896. [Google Scholar] [CrossRef]
- Grote, R.; Zeeck, A.; Stümpfel, J.; Zähner, H. Metabolic products of microorganisms, 256. Pyrrolams, new pyrrolizidinones produced by Streptomyces olivaceus. Liebigs Ann. Chem. 1990, 525–530. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Sun, R.; Qin, X.; Ju, J.; Zhang, C.; Duan, Y.; Duan, Y.; Duan, Y.; Huang, Y.; et al. Activation and characterization of bohemamine biosynthetic gene cluster from Streptomyces sp. CB02009. Org. Lett. 2020, 22, 4614–4619. [Google Scholar] [CrossRef]
- Plants of the World Online; Board of Trustees of the Royal Botanic Gardens Kew. Available online: https://powo.science.kew.org (accessed on 17 October 2023).
- Barny, L.A.; Tasca, J.A.; Sanchez, H.A.; Smith, C.R.; Koptur, S.; Livshultz, T.; Minbiole, K.P.C. Chemotaxonomic investigation of Apocynaceae for retronecine-type pyrrolizidine alkaloids using HPLC-MS/MS. Phytochemistry 2021, 185, 112662. [Google Scholar] [CrossRef]
- Prada, F.; Stashenko, E.E.; Martínez, J.R. LC/MS study of the diversity and distribution of pyrrolizidine alkaloids in Crotalaria species growing in Colombia. J. Sep. Sci. 2020, 43, 4322–4337. [Google Scholar] [CrossRef]
- Benamar, H.; Marouf, A.; Bennaceur, M. Analysis and chemotaxonomic significance of pyrrolizidine alkaloids from two Boraginaceae species growing in Algeria. Z. Naturforsch.—Sect. C J. Biosci. 2021, 76, 205–212. [Google Scholar] [CrossRef]
- Semerdjieva, I.; Petrova, G.; Yankova-Tsvetkova, E.; Doncheva, T.; Kostova, N.; Nikolova, R.; Zheljazkov, V.D. Genetic diversity, reproductive capacity and alkaloids content in three endemic Alkanna species. PLoS ONE 2020, 15, e0233516. [Google Scholar] [CrossRef]
- Ahmad, L.; He, Y.; Semotiuk, A.J.; Liu, Q.R.; Hao, J.C. Survey of pyrrolizidine alkaloids in the tribe Lithospermeae (Boraginaceae) from Pan-Himalaya and their chemotaxonomic significance. Biochem. Syst. Ecol. 2018, 81, 49–57. [Google Scholar] [CrossRef]
- Ahmad, L.; He, Y.; Semotiuk, A.; Liu, Q.; Jan, H. Pan-Himalaya ethnomedicine safety: Lithospermeae (Boraginaceae) herbal remedies containing toxic pyrrolizidine alkaloids. J. Complement. Med. Res. 2019, 10, 129. [Google Scholar] [CrossRef]
- IPNI—The International Plant Names Index. Available online: https://www.ipni.org (accessed on 17 October 2023).
- European Commission. Summary Report of the Standing Committee on Plants, Animals, Food and Feed Held in Brussels on 29 March 2019 (Section Novel Food and Toxicological Safety of the Food Chain); European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Mädge, I.; Gehling, M.; Schöne, C.; Winterhalter, P.; These, A. Pyrrolizidine alkaloid profiling of four Boraginaceae species from Northern Germany and implications for the analytical scope proposed for monitoring of maximum levels. Food Addit. Contam.—Part A 2020, 37, 1339–1358. [Google Scholar] [CrossRef]
- Stefova, E.; Cvetanoska, M.; Bogdanov, J.; Matevski, V.; Stanoeva, J.P. Assessment of distribution and diversity of pyrrolizidine alkaloids in the most prevalent Boraginaceae species in Macedonia. Chem. Biodivers. 2022, 19, e202200066. [Google Scholar] [CrossRef]
- Caty, S.N.; Alvarez-Buylla, A.; Byrd, G.D.; Vidoudez, C.; Roland, A.B.; Tapia, E.E.; Budnik, B.; Trauger, S.A.; Coloma, L.A.; O’Connell, L.A. Molecular physiology of chemical defenses in a poison frog. J. Exp. Biol. 2019, 222, jeb204149. [Google Scholar] [CrossRef]
- Jiao, W.; Zhu, L.; Shen, T.; Wang, L.; Li, Q.X.; Wang, C.; Wu, X.; Chen, H.; Hua, R. Simultaneous determination of 15 pyrrolizidine alkaloids and their N-oxides in weeds, soil, fresh tea leaves, and tea: Exploring the pollution source of pyrrolizidine alkaloids in tea. Food Chem. 2024, 434, 137305. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products (HMPC). EMA/HMPC/893108/2011 Rev. 1; European Medicines Agency: Amsterdam, The Netherlands, 2021; pp. 1–36.
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017, 15, e04908. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157.
- Zhou, Z.F.; Kurtán, T.; Yang, X.H.; Mándi, A.; Geng, M.Y.; Ye, B.P.; Taglialatela-Scafati, O.; Guo, Y.W. Penibruguieramine A, a novel pyrrolizidine alkaloid from the endophytic fungus Penicillium sp. GD6 associated with chinese mangrove Bruguiera gymnorrhiza. Org. Lett. 2014, 16, 1390–1393. [Google Scholar] [CrossRef]
- Pan, J.; Bhardwaj, M.; Faulkner, J.R.; Nagabhyru, P.; Charlton, N.D.; Higashi, R.M.; Miller, A.F.; Young, C.A.; Grossman, R.B.; Schardl, C.L. Ether bridge formation in loline alkaloid biosynthesis. Phytochemistry 2014, 98, 60–68. [Google Scholar] [CrossRef]
- Blankenship, J.D.; Spiering, M.J.; Wilkinson, H.H.; Fannin, F.F.; Bush, L.P.; Schardl, C.L. Production of loline alkaloids by the grass endophyte, Neotyphodium uncinatum, in defined media. Phytochemistry 2001, 58, 395–401. [Google Scholar] [CrossRef]
- Tofern, B.; Kaloga, M.; Witte, L.; Hartmann, T.; Eich, E. Occurrence of loline alkaloids in Argyreia mollis (Convolvulaceae). Phytochemistry 1999, 51, 1177–1180. [Google Scholar] [CrossRef]
- Siegel, M.R.; Latch, G.C.M.; Bush, L.P.; Fannin, F.F.; Rowan, D.D.; Tapper, B.A.; Bacon, C.W.; Johnson, M.C. Fungal endophyte-infected grasses: Alkaloid accumulation and aphid response. J. Chem. Ecol. 1990, 16, 3301–3315. [Google Scholar] [CrossRef]
- Riedell, W.E.; Kieckhefer, R.E.; Petroski, R.J.; Powell, R.G. Naturally-occurring and synthetic loline alkaloid derivatives: Insect feeding behavior modification and toxicity. J. Entomol. Sci. 1990, 26, 122–129. [Google Scholar] [CrossRef]
- Wilkinson, H.H.; Siegel, M.R.; Blankenship, J.D.; Mallory, A.C.; Bush, L.P.; Schardl, C.L. Contribution of fungal loline alkaloids to protection from aphids in a grass-endophyte mutualism. Am. Phytopathol. Soc. 2000, 13, 1027–1033. [Google Scholar] [CrossRef]
- Espinoza, J.; Chacón-Fuentes, M.; Quiroz, A.; Bardehle, L.; Escobar-Bahamondes, P.; Ungerfeld, E. Antifeedant effects and repellent activity of loline alkaloids from endophyte-infected tall fescue against horn flies, Haematobia irritans (Diptera: Muscidae). Molecules 2021, 26, 817. [Google Scholar] [CrossRef]
- Bush, L.P.; Wilkinson, H.H.; Schardl, C. Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol. 1997, 114, 1–7. [Google Scholar] [CrossRef]
- Bacetty, A.A.; Snook, M.E.; Glenn, A.E.; Noe, J.P.; Hill, N.; Culbreath, A.; Timper, P.; Nagabhyru, P.; Bacon, C.W. Toxicity of endophyte-infected tall fescue alkaloids and grass metabolites on Pratylenchus scribneri. Phytopathology 2009, 99, 1336–1345. [Google Scholar] [CrossRef]
- Bacetty, A.A.; Snook, M.E.; Glenn, A.E.; Noe, J.P.; Nagabhyru, P.; Bacon, C.W. Chemotaxis disruption in pratylenchus scribneri by tall fescue root extracts and alkaloids. J. Chem. Ecol. 2009, 35, 844–850. [Google Scholar] [CrossRef]
- Finch, S.C.; Munday, J.S.; Munday, R.; Kerby, J.W.F. Short-term toxicity studies of loline alkaloids in mice. Food Chem. Toxicol. 2016, 94, 243–249. [Google Scholar] [CrossRef]
- Usuki, H.; Toyo-oka, M.; Kanzaki, H.; Okuda, T.; Nitoda, T. Pochonicine, a polyhydroxylated pyrrolizidine alkaloid from fungus Pochonia suchlasporia var. suchlasporia TAMA 87 as a potent β-N-acetylglucosaminidase inhibitor. Bioorg. Med. Chem. 2009, 17, 7248–7253. [Google Scholar] [CrossRef]
- Nettleton, D.; Balitz, D.; Doyle, T.; Bradner, W.; Johnson, D. Antitumor agents from bohemic acid complex, III. The isolation of hiarcellomycin, musettamycin, rudolphohiycin, mimimycin, collinemycin, alcindoromycin, and bohemamine. J. Nat. Prod. 1977, 43, 242–258. [Google Scholar] [CrossRef]
- Zhang, Q.; Schrader, K.K.; Elsohlya, H.N.; Takamatsua, S. New cell-cell adhesion inhibitors from Streptomyces sp. UMA-044. J. Antibiot. 2003, 56, 673–681. [Google Scholar] [CrossRef]
- Bugni, T.S.; Woolery, M.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Bohemamines from a marine-derived Streptomyces sp. J. Nat. Prod. 2006, 69, 1626–1628. [Google Scholar] [CrossRef]
- Fu, P.; Macmillan, J.B. Spithioneines A and B, two new bohemamine derivatives possessing ergothioneine moiety from a marine-derived Streptomyces spinoverrucosus. Org. Lett. 2015, 17, 3046–3049. [Google Scholar] [CrossRef]
- Fu, P.; Legako, A.; La, S.; MacMillan, J.B. Discovery, characterization, and analogue synthesis of bohemamine dimers generated by non-enzymatic biosynthesis. Chem.—A Eur. J. 2016, 22, 3491–3495. [Google Scholar] [CrossRef]
- Jiang, B.; Zhao, W.; Li, S.; Liu, H.; Yu, L.; Zhang, Y.; He, H.; Wu, L. Cytotoxic dibohemamines D-F from a Streptomyces Species. J. Nat. Prod. 2017, 80, 2825–2829. [Google Scholar] [CrossRef]
- Jiang, B.; Zhao, W.; Li, S.; Liu, H.; Yu, L.; Niu, W.; He, H.; Wu, L. Quinohemanine, a quinoxalinone-bohemamine hybrid compound from Streptomyces sp. CPCC 200497. J. Antibiot. 2018, 71, 965–967. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, X.; Yin, S.; Wang, W.; Zhu, W.; Fu, P. Discovery of new bohemamines and synthesis of methylene-bridged chimeric derivatives through natural product chimera strategy. Chin. J. Chem. 2022, 40, 1413–1421. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Kondo, S.; Ikeda, T.; Ikeda, D.; Miura, K.; Hamada, M.; Takeuchi, T.; Umezawa, H. New antibiotics, clazamycins A and B. J. Antibiot. 1979, 32, 762–764. [Google Scholar] [CrossRef]
- Newman, J.L.; Thurston, D.E.; Mason, T.G. An investigation into the mechanism of action of clazamycin as an antibacterial agent towards Pseudomonas aeruginosa. J. Pharm. Pharmacol. 1990, 42, 45P. [Google Scholar] [CrossRef]
- Petersen, L.M.; Frisvad, J.C.; Knudsen, P.B.; Rohlfs, M.; Gotfredsen, C.H.; Larsen, T.O. Induced sclerotium formation exposes new bioactive metabolites from Aspergillus sclerotiicarbonarius. J. Antibiot. 2015, 68, 603–608. [Google Scholar] [CrossRef]
- Dolak, L.A.; Deboer, C. Clazamycin B is antibiotic 354. J. Antibiot. 1980, 33, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.-F.; Wunderlicha, D.; Thierickea, R.; Dahsea, H.-M.; Grableya, S.; Fengb, X.-Z.; Sattlera, I. Jenamidines A to C: Unusual alkaloids from Streptomyces sp. with specific antiproliferative properties obtained by chemical screening. J. Antibiot. 2003, 56, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Klapper, M.; Braga, D.; Lackner, G.; Herbst, R.; Stallforth, P. Bacterial alkaloid biosynthesis: Structural diversity via a minimalistic nonribosomal peptide synthetase. Cell Chem. Biol. 2018, 25, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Snider, B.B.; Duvall, J.R. Synthesis of jenamidines A1/A2. Org. Lett. 2005, 7, 4519–4522. [Google Scholar] [CrossRef] [PubMed]
- Huan, J.-Y.; Miranda, C.L.; Buhler, D.R.; Cheeke, P.R. Species differences in the hepatic microsomal enzyme metabolism of the pyrrolizidine alkaloids. Toxicol. Lett. 1998, 99, 127–137. [Google Scholar] [CrossRef]
- Dueker, S.R.; Lam, M.W.; Segall, H.J. Hydrolysis of pyrrolizidine alkaloids by guinea pig hepatic carboxylesterases’ hydrolysis of pyrrolizidine alkaloids by guinea pig hepatic carboxylesterases. Toxicol. Appl. Pharmacol. 1992, 117, 116–121. [Google Scholar] [CrossRef]
- Schoental, R. Toxicology and carcinogenic action of pyrrolizidine alkaloids. Cancer Res. 1968, 28, 2237–2246. [Google Scholar]
- He, X.; Xia, Q.; Shi, Q.; Fu, P.P. Effects of glutathione and cysteine on pyrrolizidine alkaloid-induced hepatotoxicity and DNA adduct formation in rat primary hepatocytes. J. Environ. Sci. Health Part C Toxicol. Carcinog. 2020, 38, 109–123. [Google Scholar] [CrossRef]
- Shang, H.; Huang, C.; Xiao, Z.; Yang, P.; Zhang, S.; Hou, X.; Zhang, L. Gut microbiota-derived tryptophan metabolites alleviate liver injury via AhR/Nrf2 activation in pyrrolizidine alkaloids-induced sinusoidal obstruction syndrome. Cell Biosci. 2023, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Chou, M.W.; Yan, J.; Young, J.F.; Chan, P.C.; Doerge, D.R. Toxicokinetics of riddelliine, a carcinogenic pyrrolizidine alkaloid, and metabolites in rats and mice. Toxicol. Appl. Pharmacol. 2002, 182, 98–104. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shi, M.; Wu, X.; Ma, J.; Tak-Pan Ng, K.; Xia, Q.; Zhu, L.; Pi-Cheng Fu, P.; Man, K.; Kwok-Wing Tsui, S.; et al. Mutational signature analysis reveals widespread contribution of pyrrolizidine alkaloid exposure to human liver cancer. Hepatology 2021, 74, 2021. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.; Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 2007, 76, 391–396. [Google Scholar] [PubMed]
- Michalets, E.L. Clinically significant cytochrome P-450 drug interactions-author’s reply. Pharmacotherapy 1998, 18, 892–893. [Google Scholar] [CrossRef]
- Zhou, S.F.; Liu, J.P.; Chowbay, B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab. Rev. 2009, 41, 89–295. [Google Scholar] [CrossRef]
- Rasenack, R.; Müller, C.; Kleinschmidt, M.; Rasenack, J.; Wiedenfeld, H. Veno-occlusive disease in a fetus caused by pyrrolizidine alkaloids of food origin. Fetal Diagn. Ther. 2003, 18, 223–225. [Google Scholar] [CrossRef]
- He, Y.; Ma, J.; Fan, X.; Ding, L.; Ding, X.; Zhang, Q.Y.; Lin, G. The key role of gut–liver axis in pyrrolizidine alkaloid-induced hepatotoxicity and enterotoxicity. Acta Pharm. Sin. B 2021, 11, 3820–3835. [Google Scholar] [CrossRef]
- Li, W.; Cheng, T.; Jiang, T.; Zhou, M.; Gong, B.; Zhao, G.; Li, J. Hepatic RNA adduction derived from metabolic activation of retrorsine in vitro and in vivo. Chem. Biol. Interact. 2022, 365, 110047. [Google Scholar] [CrossRef]
- Wang, J.Y.; Gao, H. Tusanqi and hepatic sinusoidal obstruction syndrome. J. Dig. Dis. 2014, 15, 105–107. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.; Yin, Y.; Wang, X.; Ye, X.; Jiang, K.; Zhang, Y.; Zhang, J.; Zhang, W.; Zhuge, Y.; et al. A TMT-based shotgun proteomics uncovers overexpression of thrombospondin 1 as a contributor in pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome. Arch. Toxicol. 2022, 96, 2003–2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiao, D.; Li, Y.; Xu, F. Risk factors for hepatic veno-occlusive disease caused by Gynura segetum: A retrospective study. BMC Gastroenterol. 2018, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, Z.; Yu, H.; Wang, Y.; Zou, Z.; Wei, H.; Liang, J.; Yang, D.; Liu, Y.; Zhang, J.; et al. Prognostic risk factors for patients with hepatic sinusoidal obstruction syndrome caused by pyrrolizidine alkaloids. Medine 2023, 102, E34698. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xiong, A.; Wang, X.; Yan, X.; Chen, Y.; Ye, X.; Wang, Z.; Ding, L.; Yang, L. Hyperoside attenuates pyrrolizidine alkaloids-induced liver injury by ameliorating TFEB-mediated mitochondrial dysfunction. Arch. Pharm. Res. 2023, 46, 694–712. [Google Scholar] [CrossRef]
- Hadi, N.S.A.; Bankoglu, E.E.; Stopper, H. Genotoxicity of pyrrolizidine alkaloids in metabolically inactive human cervical cancer HeLa cells co-cultured with human hepatoma HepG2 cells. Arch. Toxicol. 2023, 97, 295–306. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, J.; Song, Z.; Ye, Y.; Fu, P.P.; Lin, G. The role of formation of pyrrole–ATP synthase subunit beta adduct in pyrrolizidine alkaloid-induced hepatotoxicity. Arch. Toxicol. 2018, 92, 3403–3414. [Google Scholar] [CrossRef]
- Zheng, P.; Xu, Y.; Ren, Z.; Wang, Z.; Wang, S.; Xiong, J.; Zhang, H.; Jiang, H. Toxic prediction of pyrrolizidine alkaloids and structure-dependent induction of apoptosis in HepaRG cells. Oxid. Med. Cell. Longev. 2021, 8822304. [Google Scholar] [CrossRef]
- Yim, S.Y.; Lee, J.-S. An Overview of the genomic characterization of hepatocellular carcinoma. J. Hepatocell. Carcinoma 2021, 8, 1077–1088. [Google Scholar] [CrossRef]
- Gao, H.; Ruan, J.Q.; Chen, J.; Li, N.; Ke, C.Q.; Ye, Y.; Lin, G.; Wang, J.Y. Blood pyrrole-protein adducts as a diagnostic and prognostic index in pyrrolizidine alkaloid-hepatic sinusoidal obstruction syndrome. Drug Des. Dev. Ther. 2015, 9, 4861–4868. [Google Scholar] [CrossRef]
- Haas, M.; Wirachowski, K.; Thibol, L.; Küpper, J.H.; Schrenk, D.; Fahrer, J. Potency ranking of pyrrolizidine alkaloids in metabolically competent human liver cancer cells and primary human hepatocytes using a genotoxicity test battery. Arch. Toxicol. 2023, 97, 1413–1428. [Google Scholar] [CrossRef]
- Gao, L.; Rutz, L.; Schrenk, D. Structure-dependent hepato-cytotoxic potencies of selected pyrrolizidine alkaloids in primary rat hepatocyte culture. Food Chem. Toxicol. 2020, 135, 110923. [Google Scholar] [CrossRef] [PubMed]
- Buchmueller, J.; Kaltner, F.; Gottschalk, C.; Maares, M.; Braeuning, A.; Hessel-Pras, S. Structure-dependent toxicokinetics of selected pyrrolizidine alkaloids in vitro. Int. J. Mol. Sci. 2022, 23, 9214. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Olofinsan, K.; Abrahamse, H.; George, B.P. Therapeutic role of alkaloids and alkaloid derivatives in cancer management. Molecules 2023, 28, 5578. [Google Scholar] [CrossRef] [PubMed]
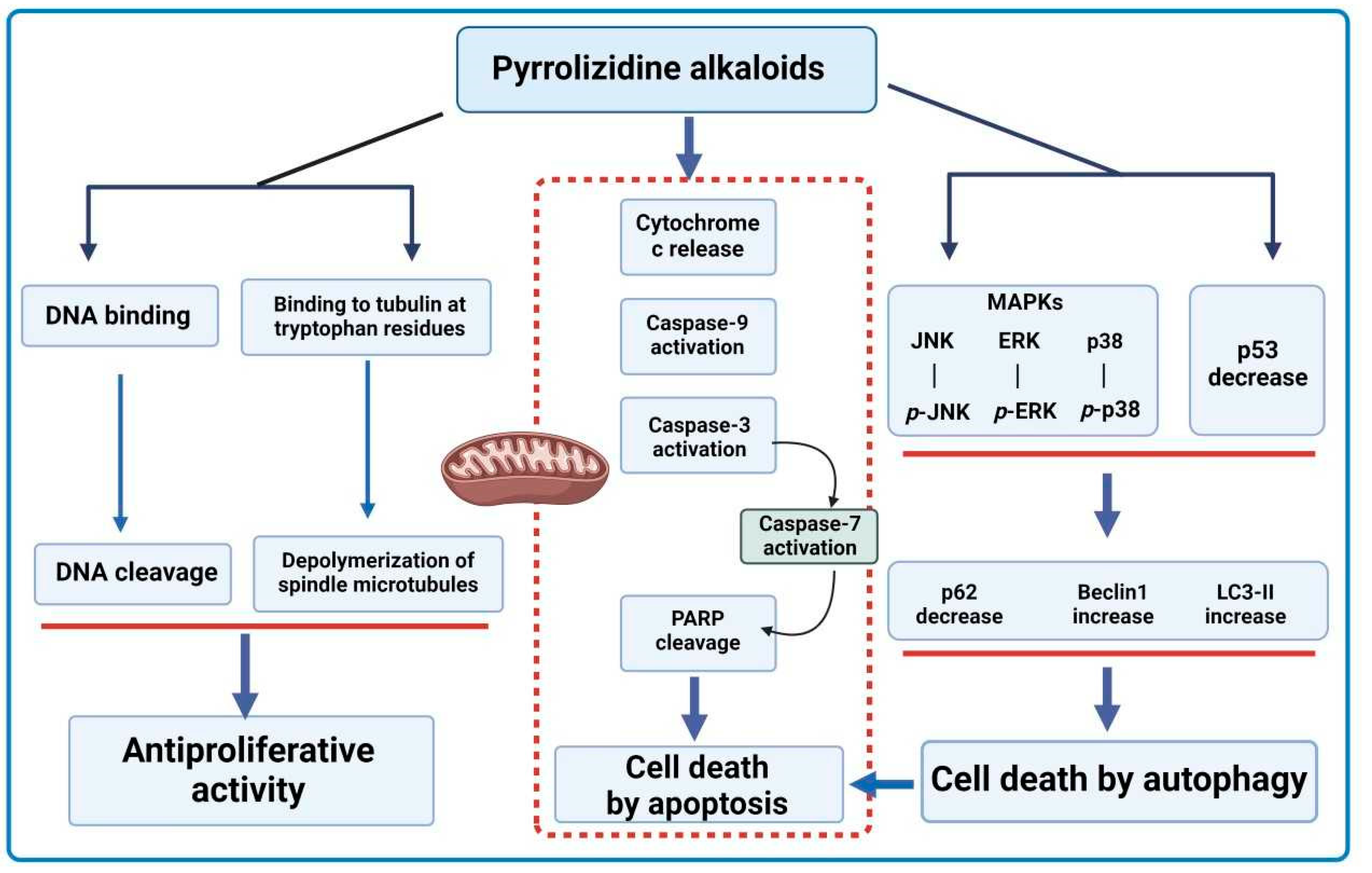
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, Y.; Hua, Z.C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, 1–17. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Ketelut-Carneiro, N.; Fitzgerald, K.A. Apoptosis, pyroptosis, and necroptosis—Oh my! The many ways a cell can die. J. Mol. Biol. 2022, 434, 167378. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Goel, N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. In Advances in Protein Chemistry and Structural Biology; Academic Press Inc.: Cambridge, MA, USA, 2021; Volume 125, pp. 73–120. [Google Scholar]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Aswandi, A.; Kholibrina, C.R. Ethnomedicinal properties of orchidaceae by local communities in Lake Toba region, North Sumatra, Indonesia. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bogor, Indonesia, 7–8 September 2021; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 914, pp. 1–7. [Google Scholar]
- Chen, L.; Huang, S.; Li, C.Y.; Gao, F.; Zhou, X.L. Pyrrolizidine alkaloids from Liparis nervosa with antitumor activity by modulation of autophagy and apoptosis. Phytochemistry 2018, 153, 147–155. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, S.M.; Shan, L.H.; Zhou, X.L. Antitumor activity of nervosine VII, and the crosstalk between apoptosis and autophagy in HCT116 human colorectal cancer cells. Chin. J. Nat. Med. 2020, 18, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Mondal, M.; Khanom, B.; Hossain, M.M.; Hossain, M.S.; Sureda, A.; Islam, M.T.; Martorell, M.; Kumar, M.; Sharifi-Rad, J.; et al. Heliotropium indicum L.: From farm to a source of bioactive compounds with therapeutic activity. Evid.-Based Complement. Altern. Med. 2021, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Dash, G.K.; Abdullah, M.S. A review on Heliotropium indicum L. (Boraginaceae). Int. J. Pharm. Sci. Res. 2013, 4, 1253. [Google Scholar]
- Anderson, W.K.; Milowsky, A.S. 3-Pyrroline N-oxide bis(carbamate) tumor inhibitors as analogues of indicine N-oxide. J. Med. Chem. 1987, 30, 2144–2147. [Google Scholar] [CrossRef] [PubMed]
- Appadurai, P.; Rathinasamy, K. Indicine N-oxide binds to tubulin at a distinct site and inhibits the assembly of microtubules: A mechanism for its cytotoxic activity. Toxicol. Lett. 2014, 225, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Culvenor, C.C.J. Tumor-inhibitory activity of pyrrolizidine alkaloids. J. Pharm. Sci. 1968, 57, 1112–1117. [Google Scholar] [CrossRef]
- Miser, J.; Smithson, W.A.; Krivit, W.; Hughes, C.; Davis, D. Phase II trial of indicine N-oxide in relapsed acute leukemia of childhood. Am. J. Clin. Oncol. 1992, 15, 135–140. [Google Scholar] [CrossRef]
- Letendre, L.; Smithson, W.A.; Gilchrist, G.S.; Burgert, E.O.; Hoagland, C.H.; Ames, M.M.; Powis, G.; Kovach, J.S. Activity of indicine N-oxide in refractory acute leukemia. Cancer 1981, 47, 437–441. [Google Scholar] [CrossRef]
- Singh, B.; Sahu, P.M.; Jain, S.C.; Singh, S. Antineoplastic and antiviral screening of pyrrolizidine alkaloids from Heliotropium subulatum. Pharm. Biol. 2002, 40, 581–586. [Google Scholar] [CrossRef]
- Hua, L.; Chen, J.; Gao, K. A new pyrrolizidine alkaloid and other constituents from the roots of Ligularia achyrotricha (Diels) Ling. Phytochem. Lett. 2012, 5, 541–544. [Google Scholar] [CrossRef]
- Kurimoto, M.; Chang, T.-c.; Nishiyama, Y.; Suzuki, T.; Dohmae, N.; Tanaka, K.; Yokoshima, S. Anticancer approach inspired by the hepatotoxic mechanism of pyrrolizidine alkaloids with glycosylated artificial metalloenzymes. Angew. Chem.—Int. Ed. 2022, 134, e202205541. [Google Scholar] [CrossRef]
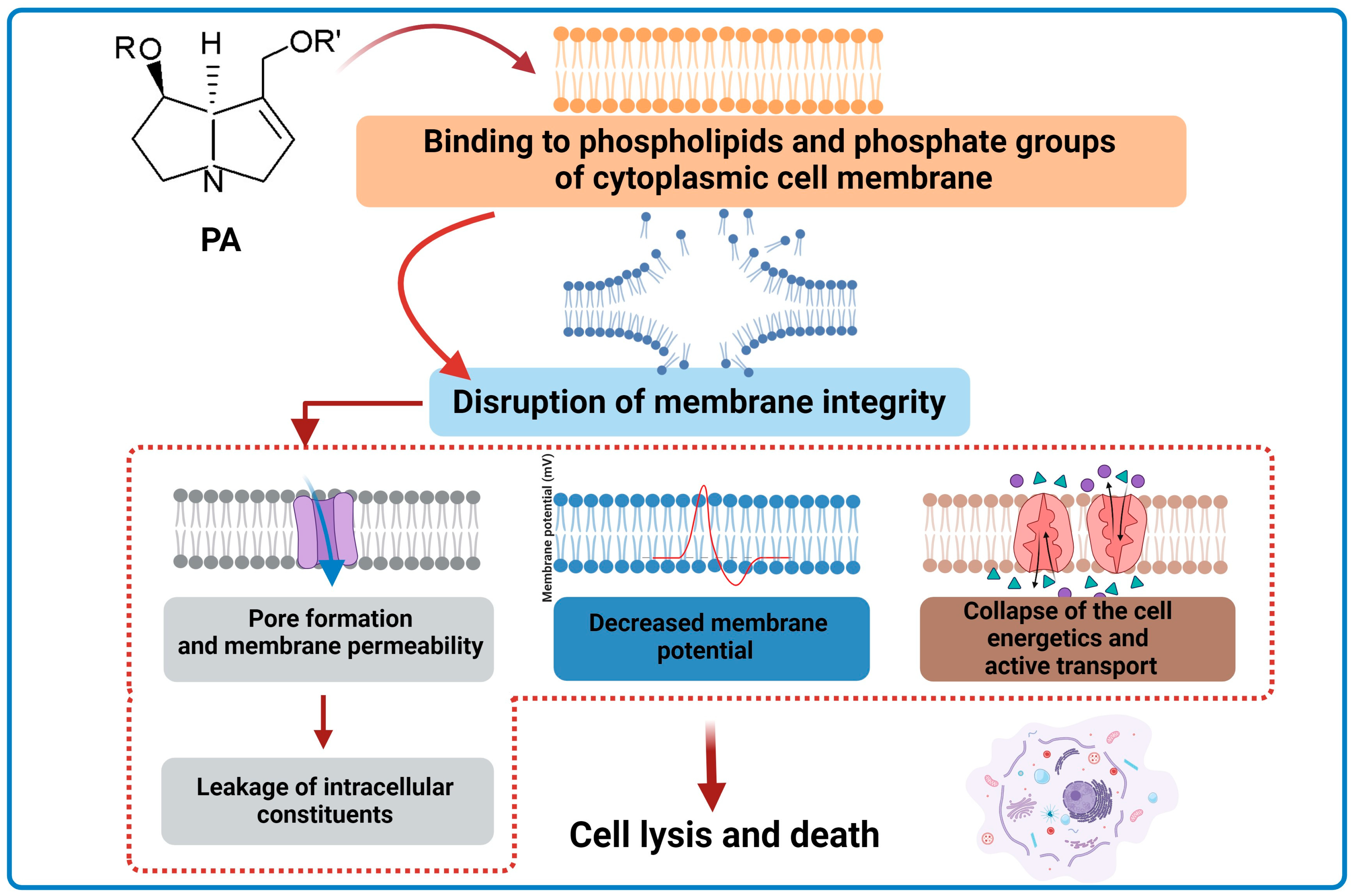
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Tan, S.N.; Cui, J.; Guo, N.; Wang, W.; Zu, Y.G.; Jin, S.; Xu, X.X.; Liu, Q.; Fu, Y.J. PA-1, a novel synthesized pyrrolizidine alkaloid, inhibits the growth of Escherichia coli and Staphylococcus aureus by damaging the cell membrane. J. Antibiot. 2014, 67, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.C.; Sharma, R. Antimicrobial activity of pyrrolizidine alkaloids from Heliotropium ellipticum. Chem. Pharm. Bull. 1987, 35, 3487–3489. [Google Scholar] [CrossRef]
- Hori, M.; Naito, K.; Sakata, N. Inhibition of DNA replication and membrane transport of some nutrients by clazamycin in Escherichia coli. J. Antibiot. 1984, 37, 260–266. [Google Scholar] [CrossRef]
- da Silva Negreiros Neto, T.; Gardner, D.; Hallwass, F.; Leite, A.J.M.; de Almeida, C.G.; Silva, L.N.; de Araújo Roque, A.; de Bitencourt, F.G.; Barbosa, E.G.; Tasca, T.; et al. Activity of pyrrolizidine alkaloids against biofilm formation and Trichomonas vaginalis. Biomed. Pharmacother. 2016, 83, 323–329. [Google Scholar] [CrossRef]
- Hol, W.H.G.; Van Veen, J.A. Pyrrolizidine alkaloids from Senecio jacobaea affect fungal growth. J. Chem. Ecol. 2002, 28, 1763–1772. [Google Scholar] [CrossRef]
- Cheng, S.; Sun, W.; Zhao, X.; Wang, P.; Zhang, W.; Zhang, S.; Chang, X.; Ye, Z. Simultaneous determination of 32 pyrrolizidine alkaloids in two traditional Chinese medicine preparations by UPLC-MS/MS. J. Anal. Methods Chem. 2022, 2022. [Google Scholar] [CrossRef]
- Rizzo, S.; Celano, R.; Piccinelli, A.L.; Serio, S.; Russo, M.; Rastrelli, L. An analytical platform for the screening and identification of pyrrolizidine alkaloids in food matrices with high risk of contamination. Food Chem. 2023, 406. [Google Scholar] [CrossRef]
- Mukhtar, M.; Saleem, M.; Nazir, M.; Riaz, N.; Shafiq, N.; Saleem, H.; Tauseef, S.; Khan, S.; Ehsan Mazhar, M.; Bakhsh Tareen, R.; et al. Identification of pyrrolizidine alkaloids and flavonoid glycosides through HR-LCMS/MS analysis, biological screening, DFT and molecular docking studies on Heliotropium dasycarpum Ledeb. Arab. J. Chem. 2023, 16. [Google Scholar] [CrossRef]
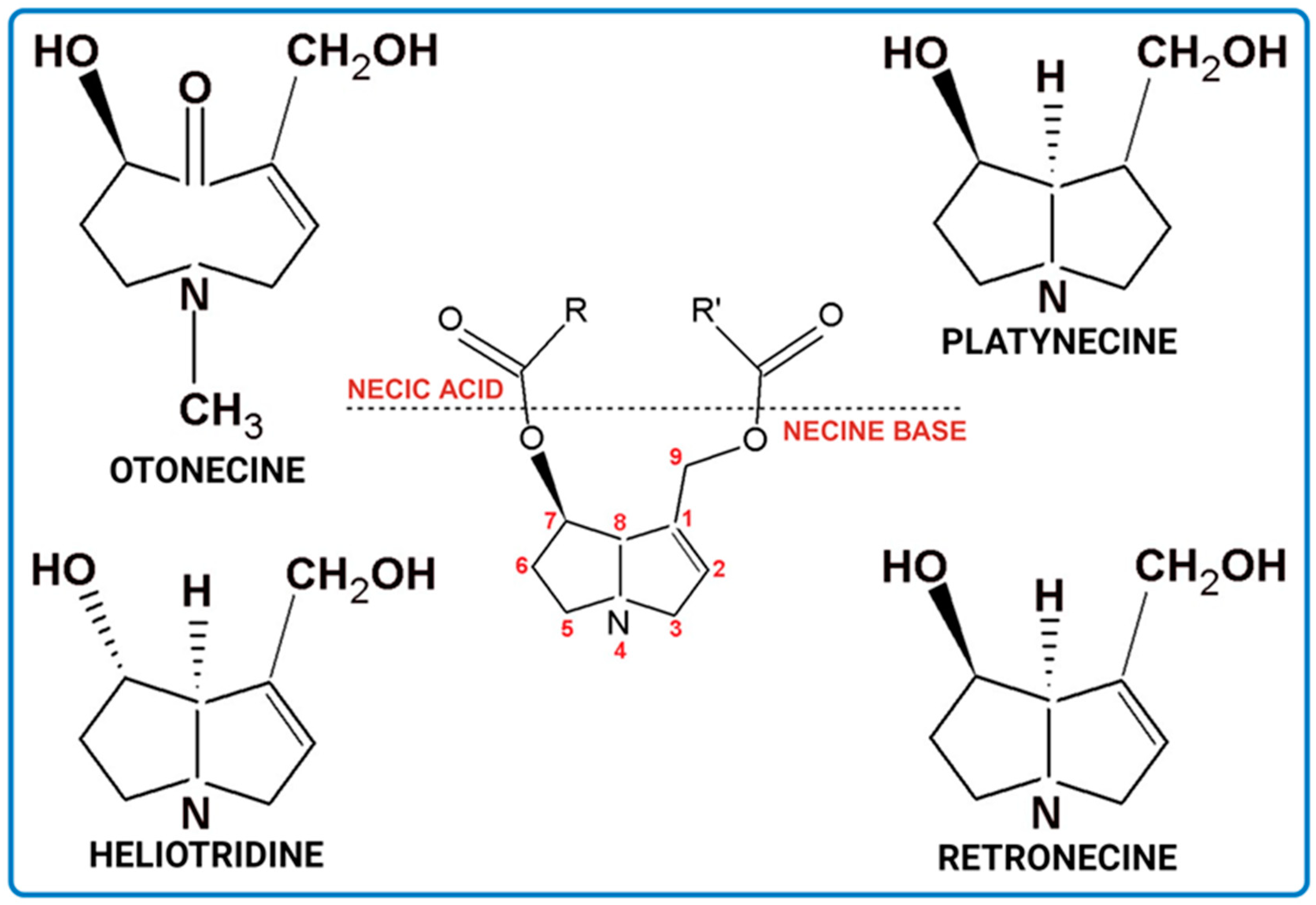

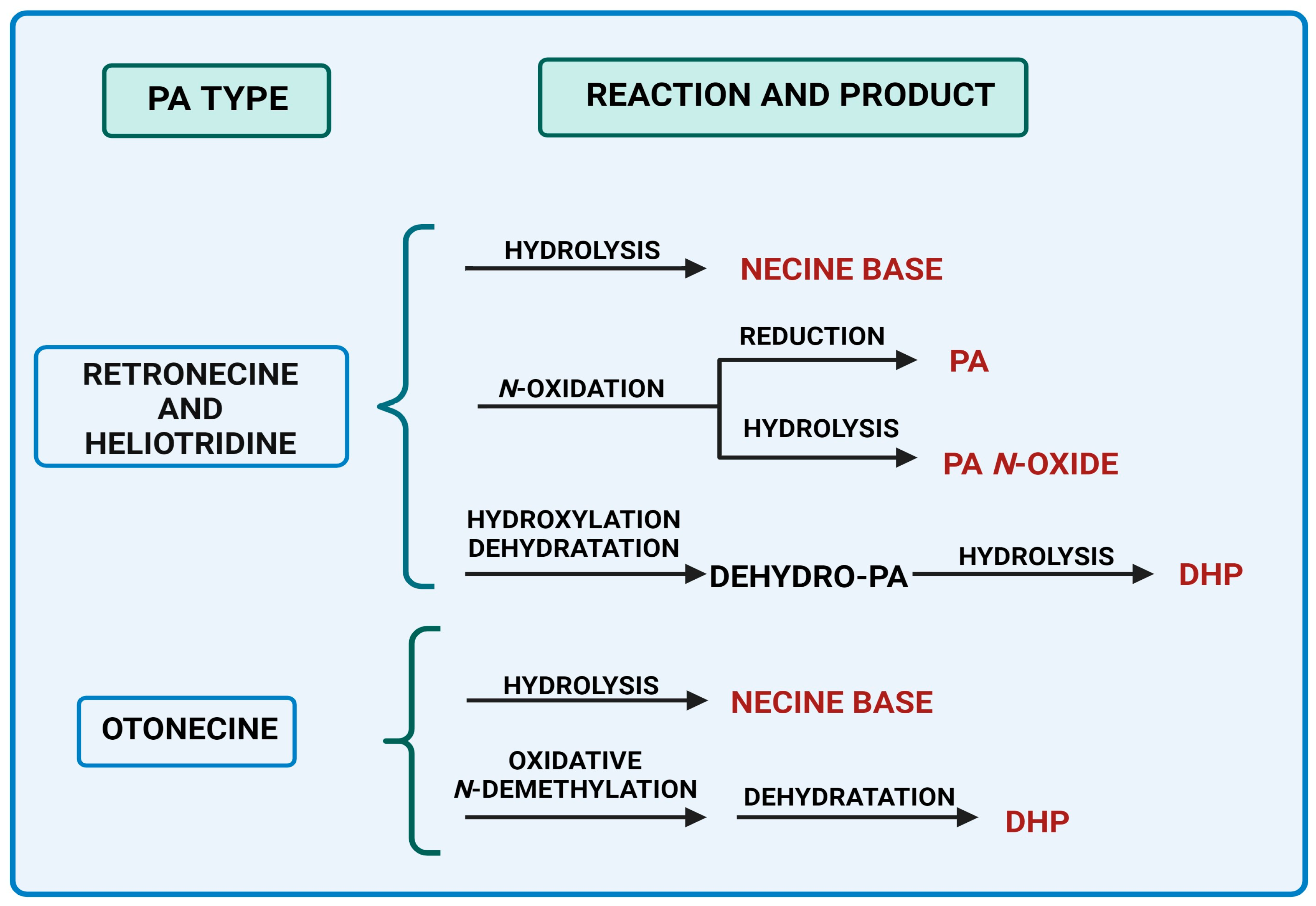
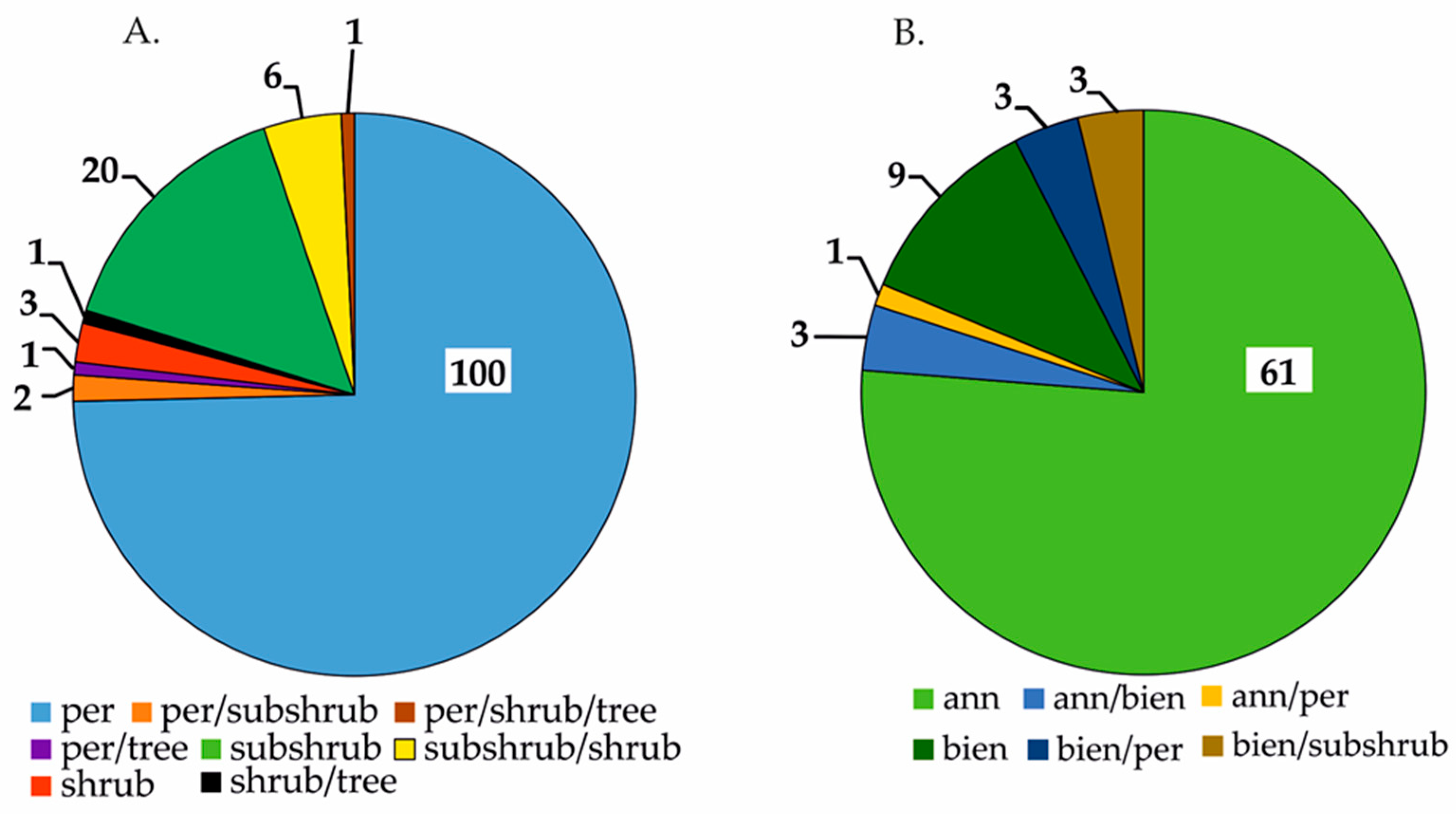
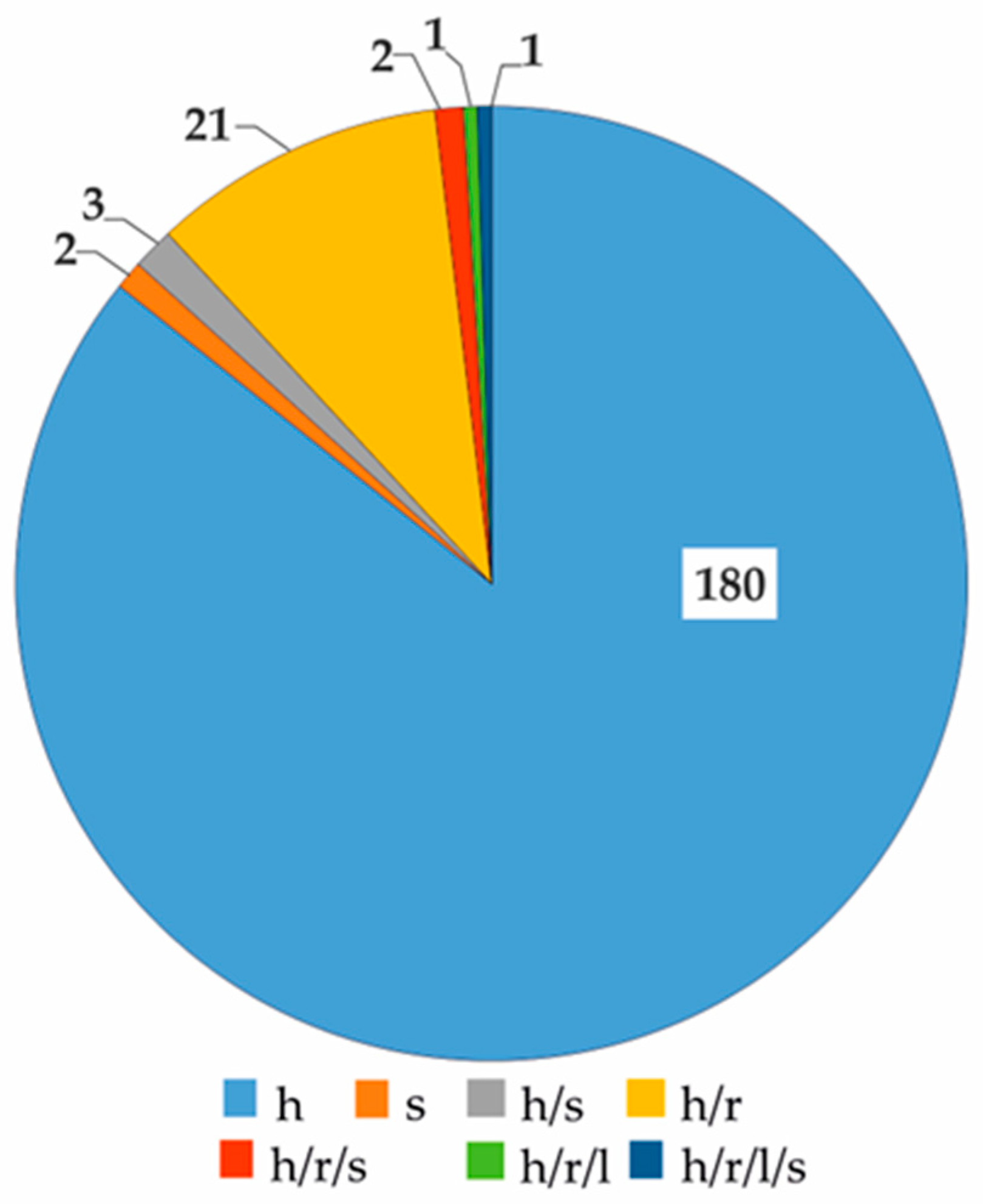
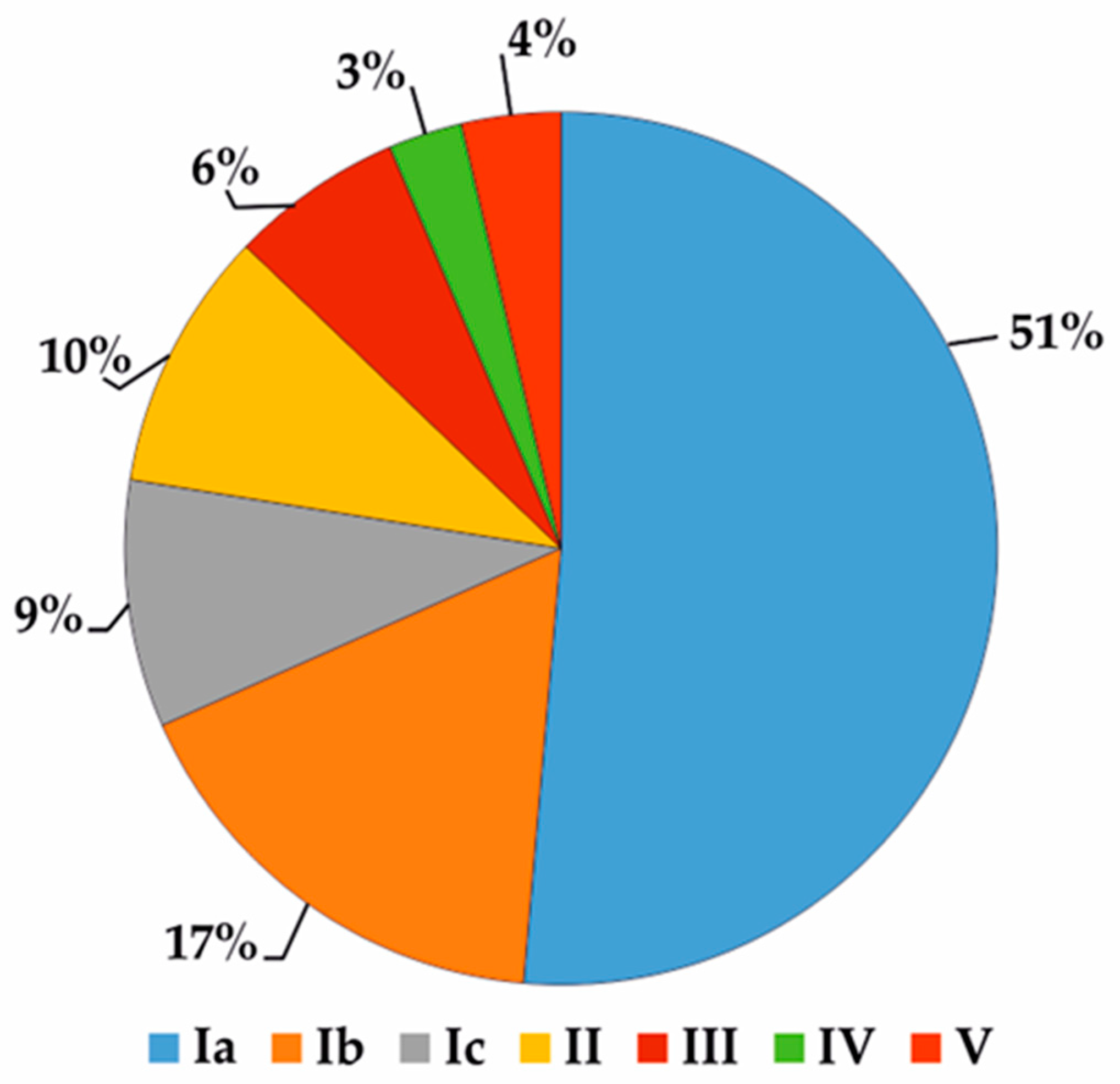
| Characteristics | Retronecine | Heliotridine | Otonecine | Platynecine |
|---|---|---|---|---|
| Ring | bicyclic | bicyclic | monocyclic | bicyclic |
| Unsaturated 1,2-double bond | + | + | + | − |
| Toxicity | + | + | + | − |
| Combination of Necine Base and Necic Acid Forming Type of PA | Examples of PA | Examples of PA Structures |
|---|---|---|
| senecionine | senecionine, platyphylline, rosmarinine, nemorensine |  |
| triangularine | triangularine, sarracine, macrophylline | 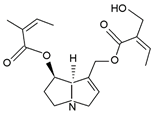 |
| lycopsamine | lycopsamine, uplandicine |  |
| monocrotaline | monocrotaline, aucherine | 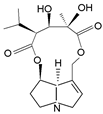 |
| phalaenopsine and ipanguline | nervosine VII, nervosine I, ipanguline B | 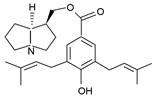 |
| triangularine and lycopsamine | echimidine, heliosupine, scorpioidine |  |
| simple | acetyllaburnine, 7-acetylretronecine |  |
| unusual | madurensine, labunamine, tussilagine |  |
| Basic PAs | Additional PAs |
|---|---|
| intermedine/lycopsamine | indicine |
| senecionine/senecivernine | echinatine |
| seneciphylline | rinderine |
| retrorsine | integerrimine |
| echimidine | heliosupine |
| lasiocarpine | spartioidine |
| europine | usaramine |
| heliotrine | with their N-oxide forms |
| with their N-oxide forms | |
| senkirkine |
| Products | Max Level of PA [µg/kg] | Exceptions | Max Level of PA [µg/kg] |
|---|---|---|---|
| Tea, flavored tea, and herbal infusions (liquid product) for infants and young children | 1 | − | − |
| Tea and flavored tea (dried product) | 150 |  | 75 |
| Herbal infusions (dried product) and ingredients used for herbal infusions (dried products) | 200 | ||
| but of rooibos, anise, lemon balm, chamomile, thyme, peppermint, lemon verbena and mixtures exclusively composed of them | 400 | ||
| Cumin | 400 | − | − |
| Food supplements containing botanical preparation including extracts | 400 | Pollen-based food supplements Pollen and pollen products | 500 |
| Dried herbs | 400 | Borage, lovage, marjoram, and oregano (dried product) and mixtures exclusively composed of them | 1000 |
| PA | Microorganism Source | Cell Line | Assay Type | Effect | Ref. |
|---|---|---|---|---|---|
| derivatives of bohemamines: a quinoxalinone-bohemamine hybrid compound quinohemanine (1), 1-methyl-2(H)-quinoxalin-2-one (2) | Streptomyces sp. CPCC 200497, soil isolate from China | liver cancer cell line HepG2 | as in sulforhodamine B (SRB) method | moderate cytotoxicity against HepG2 with IC50 65.9 and 52.5 μM for (1) and (2), respectively) | [62] |
| dibohemamine F | Streptomyces sp. CPCC 200497 | cancer cell lines of lungs A549 and liver HepG2 | as in SRB assay | cytotoxicity against cancer cell lines A549 and HepG2 with IC50 of 1.1 and 0.3 μM, respectively | [61] |
| dibohemamines D and E | Streptomyces sp. CPCC 200497 | cancer cell lines A549 and HepG2 of lungs and liver, respectively | as in SRB assay | moderate cytotoxicity against both cancer cells; for dibohemamines D and E IC50 from 7.3 for HepG2 to 39.2 for A549, respectively | [61] |
| dibohemamines B and C | Streptomyces spinoverrucosus marine-derived | non-small cell lung cancer cell line A549 | not mentioned | dibohemamines B and C extremely potent against A549 with IC50 values of 0.140 and 0.145 µm, respectively | [60] |
| dibohemamine C | Streptomyces spinoverrucosus marine-derived | non-small cell lung cancer cell line HCC1171 | not mentioned | IC50 value of 1.2 µm | [60] |
| Microorganism | PA | PA Dose | Observations | Antimicrobial Activity | Ref. |
|---|---|---|---|---|---|
| Bacteria | |||||
| Bacillus anthracis | heliotric acid | 2 mg/mL | Zone of inhibition 14.61 ± 0.443 | High | [115] |
| Bacillus subtilis | PA-1 | - | MIC: 0.0156 mg/mL | Moderate | [119] |
| Enterobacter cloacae | lasiocarpine | 2 mg/mL | Zone of inhibition: 10.00 ± 0.41 mm | High | [120] |
| Enterobacter cloacae | lasiocarpine-N-oxide | 2 mg/mL | Zone of inhibition: 9.00 ± 0.70 mm | High | [120] |
| Escherichia coli | lasiocarpine | 2 mg/mL | Zone of inhibition: 12.00 ± 0.34 mm | High | [120] |
| Escherichia coli | lasiocarpine-N-oxide | 2 mg/mL | Zone of inhibition: 9.00 ± 0.89 mm | High | [120] |
| Escherichia coli | clazamycin | - | MIC: 0.1 mg/mL | Low | [121] |
| Proteus vulgaris | PA-1 | - | MIC: 0.0313 mg/mL | Moderate | [119] |
| Pseudomonas aeruginosa | PA-1 | - | MIC: 0.0313 mg/mL | Moderate | [119] |
| Pseudomonas aeruginosa | clazamycin | - | MIC: 0.024–0.036 mg/mL | Low | [65] |
| Staphylococcus aureus | PA-1 | - | MIC: 0.0039 mg/mL, 2*MIC within 8 h | Very High | [119] |
| Staphylococcus epidermidis | usaramine | 1 mg/mL | Biofilm inhibition by 50% | Moderate | [122] |
| Staphylococcus epidermidis | PA-1 | - | MIC: 0.0078 mg/mL | High | [119] |
| Streptococcus pneumoniae | heliotric acid | 2 mg/mL | Zone of inhibition 13.64 ± 0.691 mm | High | [115] |
| Streptococcus pneumoniae | 7-angeloyl heliotrine | 2 mg/mL | Zone of inhibition 12.69 ± 0.317 | High | [115] |
| Fungi | |||||
| Aspergillus fumigatus | heliotric acid | 2 mg/mL | Zone of inhibition 10.59 ± 0.221 | High | [115] |
| Aspergillus niger | PA-1 | - | MIC: 0.125 mg/mL | Very low | [119] |
| Candida albicans | PA-1 | - | MIC: 0.0625 mg/mL | Low | [119] |
| Drechslera tetramera | heliotridine | 2 mg/mL | Zone of inhibition: 7.00 ± 0.51 mm | Moderate | [120] |
| Fusarium moniliforme | asiocarpine-N-oxide | 2 mg/mL | Zone of inhibition: 7.00 ± 0.54 mm | High | [120] |
| Penicillium chrysogenum | 7-angeloyl heliotrine | 2 mg/mL | Zone of inhibition 11.61 ± 0.268 | High | [115] |
| Rhizoctonia phaseoli | heliotric acid | 2 mg/mL | Zone of inhibition 11.51 ± 0.187 | High | [115] |
| PA | Potential Application | Refs. |
|---|---|---|
| Nervosin VII | human colorectal cancer cells | [106,107] |
| N-oxides of monocrotaline and heliotrine | a potential treatment for hepatomas– if significant hepatotoxicity targeted against tumor cells specifically | [112] |
| Indicine-N-oxide or its analogues | used as a microtubule-targeted anticancer drug | [111] |
| Retrorsine | antifungal agent against phytopathogenic fungi | [123] |
| Usaramine and monocrotaline | biomaterials surface coatings–usaramine antibiofilm activity and monocrotaline activity against Trichomonas vaginalis | [122] |
| PA-1 | pro-drug against Gram-positive bacteria | [119] |
| Synthetic DHP | potential anticancer treatment–targets cancer cells specifically | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayawickreme, K.; Świstak, D.; Ozimek, E.; Reszczyńska, E.; Rysiak, A.; Makuch-Kocka, A.; Hanaka, A. Pyrrolizidine Alkaloids—Pros and Cons for Pharmaceutical and Medical Applications. Int. J. Mol. Sci. 2023, 24, 16972. https://doi.org/10.3390/ijms242316972
Jayawickreme K, Świstak D, Ozimek E, Reszczyńska E, Rysiak A, Makuch-Kocka A, Hanaka A. Pyrrolizidine Alkaloids—Pros and Cons for Pharmaceutical and Medical Applications. International Journal of Molecular Sciences. 2023; 24(23):16972. https://doi.org/10.3390/ijms242316972
Chicago/Turabian StyleJayawickreme, Kavindi, Dawid Świstak, Ewa Ozimek, Emilia Reszczyńska, Anna Rysiak, Anna Makuch-Kocka, and Agnieszka Hanaka. 2023. "Pyrrolizidine Alkaloids—Pros and Cons for Pharmaceutical and Medical Applications" International Journal of Molecular Sciences 24, no. 23: 16972. https://doi.org/10.3390/ijms242316972
APA StyleJayawickreme, K., Świstak, D., Ozimek, E., Reszczyńska, E., Rysiak, A., Makuch-Kocka, A., & Hanaka, A. (2023). Pyrrolizidine Alkaloids—Pros and Cons for Pharmaceutical and Medical Applications. International Journal of Molecular Sciences, 24(23), 16972. https://doi.org/10.3390/ijms242316972








