Early Neuroprotective Effects of Bovine Lactoferrin Associated with Hypothermia after Neonatal Brain Hypoxia-Ischemia in Rats
Abstract
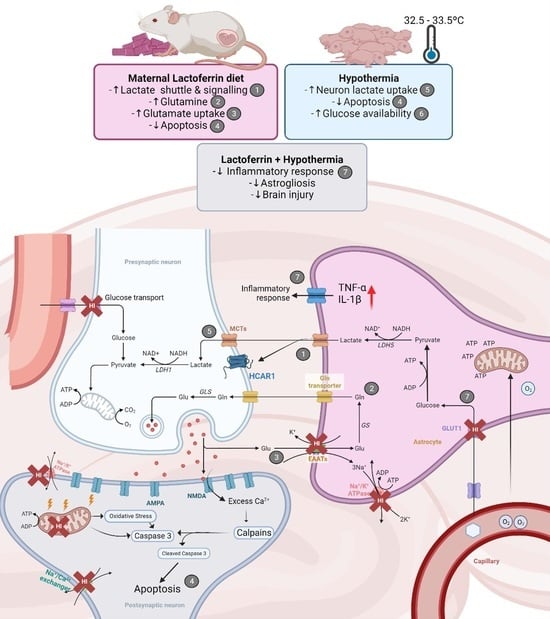
:1. Introduction
2. Results
2.1. Body Growth and Neurodevelopmental Reflexes
2.2. Brain Metabolic Profile Is Changed after HI with Partial HT and LF Effects
2.3. HT and LF Have Distinct but Complimentary Pathways for Neuroprotection following HI
2.4. Combination of LF and HT Decreases the T2W Hyperintense Signal 24 h after HI
2.5. HT Prevented CA1 Apoptosis with No Additive Effects of LF following HI
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Brain Hypoxia-Ischemia
4.3. Therapeutic Strategies
4.4. H-Magnetic Resonance Spectroscopy (MRS) and T2WI Lesion Volume Assessment
4.5. Neurodevelopmental Reflex Testing
4.6. Tissue Harvesting and Processing
4.7. RT-qPCR
4.8. Immunofluorescence
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fatemi, A.; Mary, A.W.; Michael, J.V. Hypoxic Ischemic Encephalopathy in the Term Infant. Clin. Perinatol. 2009, 36, 835–858. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, S.J.; Hagberg, H. Hypoxia-Ischemia in the Immature Brain. J. Exp. Biol. 2004, 207, 3149–3154. [Google Scholar] [CrossRef] [PubMed]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of Neonatal Encephalopathy and Hypoxic-Ischaemic Encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Lawn, J.E.; Lee, A.C.C.; Kinney, M.; Sibley, L.; Carlo, W.A.; Paul, V.K.; Pattinson, R.; Darmstadt, G.L. Two Million Intrapartum-Related Stillbirths and Neonatal Deaths: Where, Why, and What Can Be Done? Int. J. Gynaecol. Obstet. 2009, 107 (Suppl. S1), S5–S19. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Zhang, J.H. Translational Stroke Research Guideline Projections: The 20/20 Standards. Transl. Stroke Res. 2018, 9, 9–12. [Google Scholar] [CrossRef]
- Parikh, P.; Juul, S.E. Neuroprotective Strategies in Neonatal Brain Injury. J. Pediatr. 2018, 192, 22–32. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care; American Academy of Pediatrics: Elk Grove Village, IL, USA; American College of Obstetricians and Gynecologists: Washington, DC, USA, 2017. [Google Scholar]
- Singh-Mallah, G.; Nair, S.; Sandberg, M.; Mallard, C.; Hagberg, H. The Role of Mitochondrial and Endoplasmic Reticulum Reactive Oxygen Species Production in Models of Perinatal Brain Injury. Antioxid. Redox Signal. 2019, 31, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Wassink, G.; Davidson, J.O.; Dhillon, S.K.; Zhou, K.; Bennet, L.; Thoresen, M.; Gunn, A.J. Therapeutic Hypothermia in Neonatal Hypoxic-Ischemic Encephalopathy. Curr. Neurol. Neurosci. Rep. 2019, 19, 2. [Google Scholar] [CrossRef]
- Shipley, L.; Gale, C.; Sharkey, D. Trends in the Incidence and Management of Hypoxic-Ischaemic Encephalopathy in the Therapeutic Hypothermia Era: A National Population Study. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 529–534. [Google Scholar] [CrossRef]
- McQuillen, P.S.; Ferriero, D.M. Selective Vulnerability in the Developing Central Nervous System. Pediatr. Neurol. 2004, 30, 227–235. [Google Scholar] [CrossRef]
- Khwaja, O.; Volpe, J.J. Pathogenesis of Cerebral White Matter Injury of Prematurity. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F153–F161. [Google Scholar] [CrossRef] [PubMed]
- Sizonenko, S.V.; Camm, E.J.; Dayer, A.; Kiss, J.Z. Glial Responses to Neonatal Hypoxic-Ischemic Injury in the Rat Cerebral Cortex. Int. J. Dev. Neurosci. 2008, 26, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L.A.; Smith, G.N. Proinflammatory Cytokines: A Link between Chorioamnionitis and Fetal Brain Injury. J. Obstet. Gynaecol. Can. 2002, 24, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Neonatal Encephalitis and White Matter Injury: More than Just Inflammation? Ann. Neurol. 2008, 64, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, P.N.; Zanirati, G.; Venturin, G.T.; Schu, G.G.; Durán-Carabali, L.E.; Odorcyk, F.K.; Soares, A.V.; Laguna, G.d.O.; Netto, C.A.; Zimmer, E.R.; et al. Long-Term Changes in Metabolic Brain Network Drive Memory Impairments in Rats Following Neonatal Hypoxia-Ischemia. Neurobiol. Learn. Mem. 2020, 171, 107207. [Google Scholar] [CrossRef] [PubMed]
- Rumajogee, P.; Bregman, T.; Miller, S.P.; Yager, J.Y.; Fehlings, M.G. Rodent Hypoxia-Ischemia Models for Cerebral Palsy Research: A Systematic Review. Front. Neurol. 2016, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Northington, F.J.; Chavez-Valdez, R.; Martin, L.J. Neuronal Cell Death in Neonatal Hypoxia-Ischemia. Ann. Neurol. 2011, 69, 743–758. [Google Scholar] [CrossRef]
- Shankaran, S. Therapeutic Hypothermia for Neonatal Encephalopathy. Curr. Treat. Options Neurol. 2012, 14, 608–619. [Google Scholar] [CrossRef]
- Patel, S.D.; Pierce, L.; Ciardiello, A.J.; Vannucci, S.J. Neonatal Encephalopathy: Pre-Clinical Studies in Neuroprotection. Biochem. Soc. Trans. 2014, 42, 564–568. [Google Scholar] [CrossRef]
- Juul, S.E.; Ferriero, D.M. Pharmacologic Neuroprotective Strategies in Neonatal Brain Injury. Clin. Perinatol. 2014, 41, 119–131. [Google Scholar] [CrossRef]
- Davidson, J.O.; Wassink, G.; van den Heuij, L.G.; Bennet, L.; Gunn, A.J. Therapeutic Hypothermia for Neonatal Hypoxic–Ischemic Encephalopathy—Where to from Here? Front. Neurol. 2015, 6, 198. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Gonzalez, F.F. Erythropoietin: A Novel Therapy for Hypoxic-Ischaemic Encephalopathy? Dev. Med. Child Neurol. 2015, 57 (Suppl. S3), 34–39. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-Y.; Sung, Y.-H.; Wang, S.-M.; Lung, H.-L.; Chang, J.-H.; Hsu, C.-H.; Jim, W.-T.; Lee, C.-H.; Hung, H.-F. Short- and Long-Term Outcomes in Very Low Birth Weight Infants with Admission Hypothermia. PLoS ONE 2015, 10, e0131976. [Google Scholar] [CrossRef] [PubMed]
- Celik, Y.; Özgür, A.; Sungur, M.A.; Yıldırım, N.; Teke, S. Is Selective Head Cooling Combined with Whole-Body Cooling the Most Effective Hypothermia Method for Neonatal Hypoxic-Ischemic Encephalopathy? Ther. Hypothermia Temp. Manag. 2023, 13, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Yang, Z.-J.; Wang, B.; Carter, E.L.; Larson, A.C.; Martin, L.J.; Koehler, R.C. Early Antioxidant Treatment and Delayed Hypothermia after Hypoxia-Ischemia Have No Additive Neuroprotection in Newborn Pigs. Anesth. Analg. 2012, 115, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.; Lim, S.; Kang, E.; Furmanski, O.; Song, H.; Ryu, Y.K.; Mintz, C.D. Effects of Neonatal Hypoxic-Ischemic Injury and Hypothermic Neuroprotection on Neural Progenitor Cells in the Mouse Hippocampus. Dev. Neurosci. 2015, 37, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, F.; Li, Q.; He, X.; Ma, J. Mild Hypothermia Combined with Neural Stem Cell Transplantation for Hypoxic-Ischemic Encephalopathy: Neuroprotective Effects of Combined Therapy. Neural Regen. Res. 2014, 9, 1745–1752. [Google Scholar] [PubMed]
- Zhou, K.Q.; Davidson, J.O.; Bennet, L.; Gunn, A.J. Combination Treatments with Therapeutic Hypothermia for Hypoxic-Ischemic Neuroprotection. Dev. Med. Child Neurol. 2020, 62, 1131–1137. [Google Scholar] [CrossRef]
- Netto, C.A.; Sanches, E.F.; Odorcyk, F.; Duran-Carabali, L.E.; Sizonenko, S.V. Pregnancy as a Valuable Period for Preventing Hypoxia-Ischemia Brain Damage. Int. J. Dev. Neurosci. 2018, 70, 12–24. [Google Scholar] [CrossRef]
- Arteaga, O.; Revuelta, M.; Urigüen, L.; Álvarez, A.; Montalvo, H.; Hilario, E. Pretreatment with Resveratrol Prevents Neuronal Injury and Cognitive Deficits Induced by Perinatal Hypoxia-Ischemia in Rats. PLoS ONE 2015, 10, e0142424. [Google Scholar] [CrossRef]
- Cardinali, D.P. An Assessment of Melatonin’s Therapeutic Value in the Hypoxic-Ischemic Encephalopathy of the Newborn. Front. Synaptic Neurosci. 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Loren, D.J.; Seeram, N.P.; Schulman, R.N.; Holtzman, D.M. Maternal Dietary Supplementation with Pomegranate Juice Is Neuroprotective in an Animal Model of Neonatal Hypoxic-Ischemic Brain Injury. Pediatr. Res. 2005, 57, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.R.; Mozurkewich, E.; Liu, Y.; Barks, J. Docosahexaenoic Acid Pretreatment Confers Neuroprotection in a Rat Model of Perinatal Cerebral Hypoxia-Ischemia. Obstet. Anesth. Dig. 2010, 30, 227. [Google Scholar] [CrossRef]
- Berman, D.R.; Liu, Y.Q.; Barks, J.; Mozurkewich, E. Docosahexaenoic Acid Confers Neuroprotection in a Rat Model of Perinatal Hypoxia-Ischemia Potentiated by Escherichia Coli Lipopolysaccharide-Induced Systemic Inflammation. Am. J. Obstet. Gynecol. 2010, 202, 469.e1–469.e6. [Google Scholar] [CrossRef] [PubMed]
- Schirmbeck, G.H.; Sizonenko, S.; Sanches, E.F. Neuroprotective Role of Lactoferrin during Early Brain Development and Injury through Lifespan. Nutrients 2022, 14, 2923. [Google Scholar] [CrossRef] [PubMed]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a Multiple Bioactive Protein: An Overview. Biochim. Biophys. Acta 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.M.; Baker, E.N. Lactoferrin and Iron: Structural and Dynamic Aspects of Binding and Release. Biometals 2004, 17, 209–216. [Google Scholar] [CrossRef] [PubMed]
- de Ferrer, P.A.R.; Baroni, A.; Sambucetti, M.E.; López, N.E.; Ceriani Cernadas, J.M. Lactoferrin Levels in Term and Preterm Milk. J. Am. Coll. Nutr. 2000, 19, 370–373. [Google Scholar] [CrossRef]
- van de Looij, Y.; Ginet, V.; Chatagner, A.; Toulotte, A.; Somm, E.; Hüppi, P.S.; Sizonenko, S.V. Lactoferrin during Lactation Protects the Immature Hypoxic-Ischemic Rat Brain. Ann. Clin. Transl. Neurol. 2014, 1, 955–967. [Google Scholar] [CrossRef]
- Ginet, V.; van de Looij, Y.; Petrenko, V.; Toulotte, A.; Kiss, J.; Hüppi, P.S.; Sizonenko, S.V. Lactoferrin during Lactation Reduces Lipopolysaccharide-Induced Brain Injury. Biofactors 2016, 42, 323–336. [Google Scholar] [CrossRef]
- van de Looij, Y.; Larpin, C.; Cabungcal, J.-H.; Sanches, E.F.; Toulotte, A.; Do, K.Q.; Sizonenko, S.V. Nutritional Intervention for Developmental Brain Damage: Effects of Lactoferrin Supplementation in Hypocaloric Induced Intrauterine Growth Restriction Rat Pups. Front. Endocrinol. 2019, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Somm, E.; Larvaron, P.; van de Looij, Y.; Toulotte, A.; Chatagner, A.; Faure, M.; Métairon, S.; Mansourian, R.; Raymond, F.; Gruetter, R.; et al. Protective Effects of Maternal Nutritional Supplementation with Lactoferrin on Growth and Brain Metabolism. Pediatr. Res. 2013, 75, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Sanches, E.; van de Looij, Y.; Sow, S.; Toulotte, A.; da Silva, A.; Modernell, L.; Sizonenko, S. Dose-Dependent Neuroprotective Effects of Bovine Lactoferrin Following Neonatal Hypoxia-Ischemia in the Immature Rat Brain. Nutrients 2021, 13, 3880. [Google Scholar] [CrossRef] [PubMed]
- Sanches, E.F.; Arteni, N.; Nicola, F.; Aristimunha, D.; Netto, C.A. Sexual Dimorphism and Brain Lateralization Impact Behavioral and Histological Outcomes Following Hypoxia–Ischemia in P3 and P7 Rats. Neuroscience 2015, 290, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Sanches, E.F.; Van de Looij, Y.; Toulotte, A.; da Silva, A.R.; Romero, J.; Sizonenko, S.V. Brain Metabolism Alterations Induced by Pregnancy Swimming Decreases Neurological Impairments Following Neonatal Hypoxia-Ischemia in Very Immature Rats. Front. Neurol. 2018, 9, 480. [Google Scholar] [CrossRef]
- Verleysdonk, S.; Martin, H.; Willker, W.; Leibfritz, D.; Hamprecht, B. Rapid Uptake and Degradationof Glycine by Astroglial Cells in Culture:Synthesis and Releaseof Serine and Lactate. Glia 1999, 27, 239–248. [Google Scholar] [CrossRef]
- Patri, M. Synaptic Transmission and Amino Acid Neurotransmitters. In Neurochemical Basis of Brain Function and Dysfunction; IntechOpen: London, UK, 2019; ISBN 9781789859997. [Google Scholar]
- Wang, H.; Zheng, X.; Liu, B.; Xia, Y.; Xin, Z.; Deng, B.; He, L.; Deng, J.; Ren, W. Aspartate Metabolism Facilitates IL-1β Production in Inflammatory Macrophages. Front. Immunol. 2021, 12, 753092. [Google Scholar] [CrossRef]
- Roumes, H.; Pellerin, L.; Bouzier-Sore, A.-K. Astrocytes as Metabolic Suppliers to Support Neuronal Activity and Brain Functions. Essays Biochem. 2023, 67, 27–37. [Google Scholar]
- Sizonenko, S.V.; Kiss, J.Z.; Inder, T.; Gluckman, P.D.; Williams, C.E. Distinctive Neuropathologic Alterations in the Deep Layers of the Parietal Cortex after Moderate Ischemic-Hypoxic Injury in the P3 Immature Rat Brain. Pediatr. Res. 2005, 57, 865–872. [Google Scholar] [CrossRef]
- Zang, J.; Colucci, A.C.M.; Tassinari, I.D.; Nunes, R.R.; Andrade, M.K.G.; Spies, F.F.; de Oliveira, M.R.; Durán-Carabali, L.E.; Rigon, P.; Netto, C.A.; et al. Short-Term Effects of Therapeutic Hypothermia Following Hypoxia-Ischemia in Neonatal Male and Female Rats. Int. J. Dev. Neurosci. 2023, 83, 165–177. [Google Scholar] [CrossRef]
- Ho, D.; Sanches, E.F.; Sizonenko, S.V. Early Neurodevelopmental Reflex Impairments in a Rodent Model of Cerebral Palsy. Int. J. Dev. Neurosci. 2022, 82, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.; Abiola, S.; Kim, N.; Avaritt, O.; Flock, D.; Yu, J.; Northington, F.J.; Chavez-Valdez, R. Therapeutic Hypothermia Provides Variable Protection against Behavioral Deficits after Neonatal Hypoxia-Ischemia: A Potential Role for Brain-Derived Neurotrophic Factor. Dev. Neurosci. 2017, 39, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.L.; Won, S.; Geddes, R.I.; Sayeed, I.; Stein, D.G. Sex-Related Differences in Effects of Progesterone Following Neonatal Hypoxic Brain Injury. Behav. Brain Res. 2015, 286, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Tassinari, I.D.Á.; Andrade, M.K.G.; da Rosa, L.A.; Hoff, M.L.M. Lactate Administration Reduces Brain Injury and Ameliorates Behavioral Outcomes Following Neonatal Hypoxia–Ischemia. Neuroscience 2020, 448, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Finckenberg, P.; Martonen, E.; Ahlroos-Lehmus, A.; Pilvi, T.K.; Korpela, R.; Mervaala, E.M. Metabolic Effects of Lactoferrin during Energy Restriction and Weight Regain in Diet-Induced Obese Mice. J. Funct. Foods 2012, 4, 66–78. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.J.; Bassols, J.; Ricart, W.; Fernández-Real, J.M. Decreased Circulating Lactoferrin in Insulin Resistance and Altered Glucose Tolerance as a Possible Marker of Neutrophil Dysfunction in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 4036–4044. [Google Scholar] [CrossRef] [PubMed]
- Lana, D.; Ugolini, F.; Giovannini, M.G. An Overview on the Differential Interplay among Neurons-Astrocytes-Microglia in CA1 and CA3 Hippocampus in Hypoxia/Ischemia. Front. Cell. Neurosci. 2020, 14, 585833. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.J.; Hawley, J.A. Reassessing the Relationship between MRNA Levels and Protein Abundance in Exercised Skeletal Muscles. Nat. Rev. Mol. Cell Biol. 2022, 23, 773–774. [Google Scholar] [CrossRef]
- Van De Looij, Y.; Chatagner, A.; Hüppi, P.S.; Gruetter, R.; Sizonenko, S.V. Longitudinal MR Assessment of Hypoxic Ischemic Injury in the Immature Rat Brain. Magn. Reson. Med. 2011, 65, 305–312. [Google Scholar] [CrossRef]
- Cheong, J.L.Y.; Cady, E.B.; Penrice, J.; Wyatt, J.S.; Cox, I.J.; Robertson, N.J. Proton MR Spectroscopy in Neonates with Perinatal Cerebral Hypoxic-Ischemic Injury: Metabolite Peak-Area Ratios, Relaxation Times, and Absolute Concentrations. Am. J. Neuroradiol. 2006, 27, 1546–1554. [Google Scholar]
- Hagberg, H.; Mallard, C.; Rousset, C.I.; Thornton, C. Mitochondria: Hub of Injury Responses in the Developing Brain. Lancet Neurol. 2014, 13, 217–232. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.; Ferriero, D. Mechanisms of Hypoxic-Ischemic Injury in the Term Infant. Semin. Perinatol. 2004, 28, 425–432. [Google Scholar] [CrossRef] [PubMed]
- van de Looij, Y.; Dean, J.M.; Gunn, A.J.; Hüppi, P.S.; Sizonenko, S.V. Advanced Magnetic Resonance Spectroscopy and Imaging Techniques Applied to Brain Development and Animal Models of Perinatal Injury. Int. J. Dev. Neurosci. 2015, 45, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, J.; Shi, J.; Gong, Q.; Weng, X. Cerebral and Functional Adaptation with Chronic Hypoxia Exposure: A Multi-Modal MRI Study. Brain Res. 2010, 1348, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Roelants-Van Rijn, A.M.; Van Der Grond, J.; De Vries, L.S.; Groenendaal, F. Value Of1H-MRS Using Different Echo Times in Neonates with Cerebral Hypoxia-Ischemia. Pediatr. Res. 2001, 49, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate Metabolism and Recycling at the Excitatory Synapse in Health and Neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.; Jung, C.-G.; Zhou, C.; Abdullah, M.; Nakano, M.; Wakabayashi, H.; Abe, F.; Michikawa, M. Dietary Lactoferrin Supplementation Prevents Memory Impairment and Reduces Amyloid-β Generation in J20 Mice. J. Alzheimer’s Dis. 2020, 74, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-F.; Pang, Z.-Q.; Fan, Y.-G.; Zhang, Y.-H.; Meng, Y.-H.; Bai, C.-Y.; Jia, M.-Y.; Chen, Y.-H.; Wang, Z.-Y.; Guo, C. Astrocyte-Specific Loss of Lactoferrin Influences Neuronal Structure and Function by Interfering with Cholesterol Synthesis. Glia 2022, 70, 2392–2408. [Google Scholar] [CrossRef]
- Strasser, A.; Luksys, G.; Xin, L.; Pessiglione, M.; Gruetter, R.; Sandi, C. Glutamine-to-Glutamate Ratio in the Nucleus Accumbens Predicts Effort-Based Motivated Performance in Humans. Neuropsychopharmacology 2020, 45, 2048–2057. [Google Scholar] [CrossRef]
- de Heredia, F.P.; Wood, I.S.; Trayhurn, P. Hypoxia Stimulates Lactate Release and Modulates Monocarboxylate Transporter (MCT1, MCT2, and MCT4) Expression in Human Adipocytes. Pflug. Arch. 2010, 459, 509–518. [Google Scholar] [CrossRef]
- Hewett, S.J.; Jackman, N.A.; Claycomb, R.J. Interleukin-1β in Central Nervous System Injury and Repair. Eur. J. Neurodegener. Dis. 2012, 1, 195–211. [Google Scholar] [PubMed]
- Bernardino, L.; Xapelli, S.; Silva, A.P.; Jakobsen, B.; Poulsen, F.R.; Oliveira, C.R.; Vezzani, A.; Malva, J.O.; Zimmer, J. Modulator Effects of Interleukin-1beta and Tumor Necrosis Factor-Alpha on AMPA-Induced Excitotoxicity in Mouse Organotypic Hippocampal Slice Cultures. J. Neurosci. 2005, 25, 6734–6744. [Google Scholar] [CrossRef] [PubMed]
- Carlson, N.G.; Wieggel, W.A.; Chen, J.; Bacchi, A.; Rogers, S.W.; Gahring, L.C. Inflammatory Cytokines IL-1 Alpha, IL-1 Beta, IL-6, and TNF-Alpha Impart Neuroprotection to an Excitotoxin through Distinct Pathways. J. Immunol. 1999, 163, 3963–3968. [Google Scholar] [CrossRef] [PubMed]
- Strijbos, P.J.; Rothwell, N.J. Interleukin-1 Beta Attenuates Excitatory Amino Acid-Induced Neurodegeneration in Vitro: Involvement of Nerve Growth Factor. J. Neurosci. 1995, 15, 3468–3474. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wang, X.; Cheng, X.; Qiu, L.; Xu, F.; Simbruner, G.; Blomgren, K. Post-Ischemic Hypothermia-Induced Tissue Protection and Diminished Apoptosis after Neonatal Cerebral Hypoxia-Ischemia. Brain Res. 2004, 996, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on MRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Hasel, P.; Rose, I.V.L.; Sadick, J.S.; Kim, R.D.; Liddelow, S.A. Neuroinflammatory Astrocyte Subtypes in the Mouse Brain. Nat. Neurosci. 2021, 24, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Odorcyk, F.K.; Duran-Carabali, L.E.; Rocha, D.S.; Sanches, E.F.; Martini, A.P.; Venturin, G.T.; Greggio, S.; da Costa, J.C.; Kucharski, L.C.; Zimmer, E.R.; et al. Differential Glucose and Beta-Hydroxybutyrate Metabolism Confers an Intrinsic Neuroprotection to the Immature Brain in a Rat Model of Neonatal Hypoxia Ischemia. Exp. Neurol. 2020, 330, 113317. [Google Scholar] [CrossRef]
- Sabir, H.; Scull-Brown, E.; Liu, X.; Thoresen, M. Immediate Hypothermia Is Not Neuroprotective after Severe Hypoxia-Ischemia and Is Deleterious When Delayed by 12 Hours in Neonatal Rats. Stroke 2012, 43, 3364–3370. [Google Scholar] [CrossRef]
- Wood, T.; Osredkar, D.; Puchades, M.; Maes, E.; Falck, M.; Flatebø, T.; Walløe, L.; Sabir, H.; Thoresen, M. Treatment Temperature and Insult Severity Influence the Neuroprotective Effects of Therapeutic Hypothermia. Sci. Rep. 2016, 6, 23430. [Google Scholar] [CrossRef]
- Mlynárik, V.; Gambarota, G.; Frenkel, H.; Gruetter, R. Localized Short-Echo-Time Proton MR Spectroscopy with Full Signal-Intensity Acquisition. Magn. Reson. Med. 2006, 56, 965–970. [Google Scholar] [CrossRef]
- Gruetter, R.; Tkáč, I. Field Mapping without Reference Scan Using Asymmetric Echo-Planar Techniques. Magn. Reson. Med. 2000, 43, 319–323. [Google Scholar] [CrossRef]
- Provencher, S.W. Estimation of Metabolite Concentrations from Localized in Vivo Proton NMR Spectra. Magn. Reson. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef]
- Khazipov, R.; Zaynutdinova, D.; Ogievetsky, E.; Valeeva, G.; Mitrukhina, O.; Manent, J.-B.; Represa, A. Atlas of the Postnatal Rat Brain in Stereotaxic Coordinates. Front. Neuroanat. 2015, 9, 161. [Google Scholar] [CrossRef]
















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanches, E.; van de Looij, Y.; Ho, D.; Modernell, L.; da Silva, A.; Sizonenko, S. Early Neuroprotective Effects of Bovine Lactoferrin Associated with Hypothermia after Neonatal Brain Hypoxia-Ischemia in Rats. Int. J. Mol. Sci. 2023, 24, 15583. https://doi.org/10.3390/ijms242115583
Sanches E, van de Looij Y, Ho D, Modernell L, da Silva A, Sizonenko S. Early Neuroprotective Effects of Bovine Lactoferrin Associated with Hypothermia after Neonatal Brain Hypoxia-Ischemia in Rats. International Journal of Molecular Sciences. 2023; 24(21):15583. https://doi.org/10.3390/ijms242115583
Chicago/Turabian StyleSanches, Eduardo, Yohan van de Looij, Dini Ho, Laura Modernell, Analina da Silva, and Stéphane Sizonenko. 2023. "Early Neuroprotective Effects of Bovine Lactoferrin Associated with Hypothermia after Neonatal Brain Hypoxia-Ischemia in Rats" International Journal of Molecular Sciences 24, no. 21: 15583. https://doi.org/10.3390/ijms242115583
APA StyleSanches, E., van de Looij, Y., Ho, D., Modernell, L., da Silva, A., & Sizonenko, S. (2023). Early Neuroprotective Effects of Bovine Lactoferrin Associated with Hypothermia after Neonatal Brain Hypoxia-Ischemia in Rats. International Journal of Molecular Sciences, 24(21), 15583. https://doi.org/10.3390/ijms242115583