The Preventive Effects of Salubrinal against Pyrethroid-Induced Disruption of Adult Hippocampal Neurogenesis in Mice
Abstract
:1. Introduction
2. Results
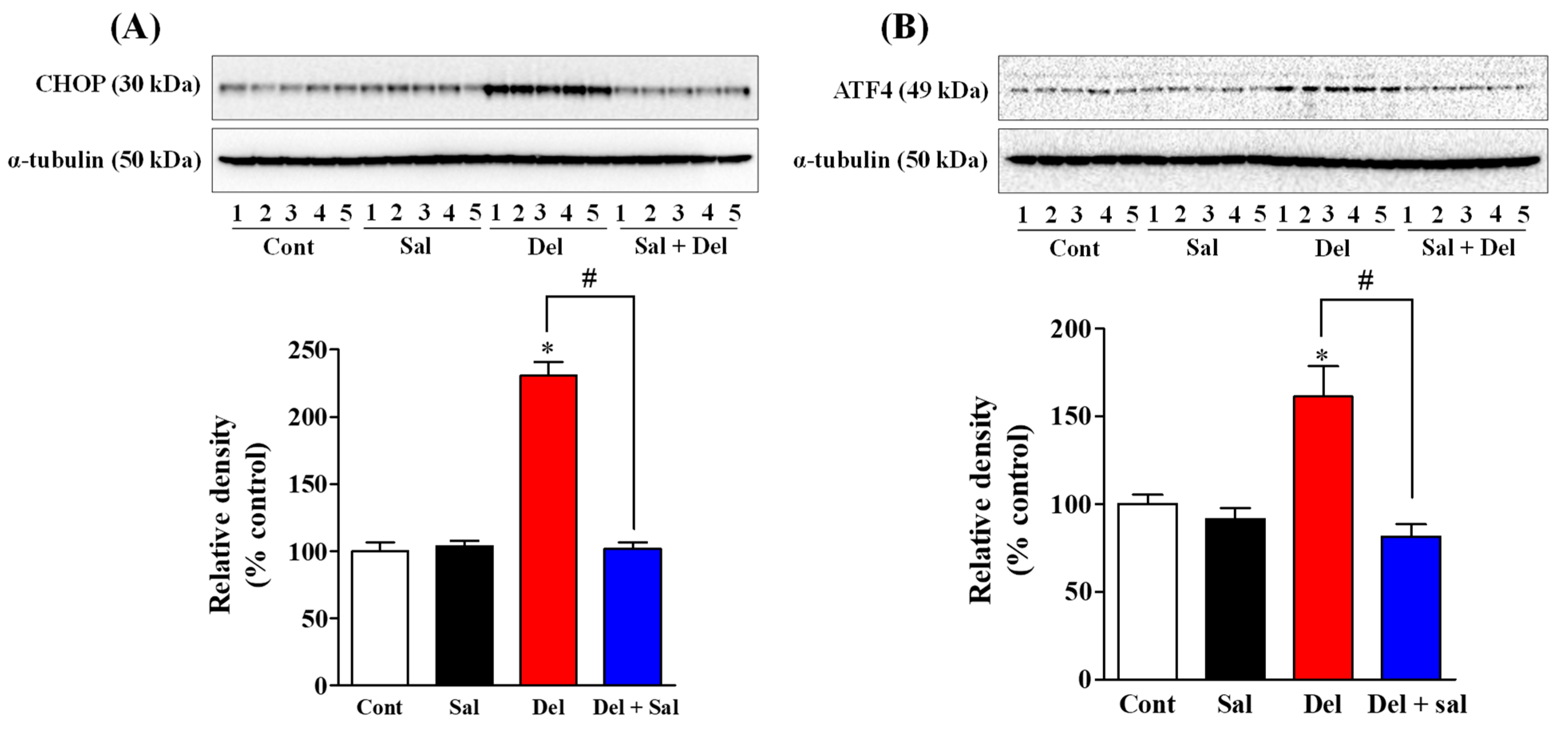
2.1. Salubrinal Administration Effectively Prevents the Activation of the ER Stress in the Hippocampus of Adult Mice Induced by Exposure to Deltamethrin
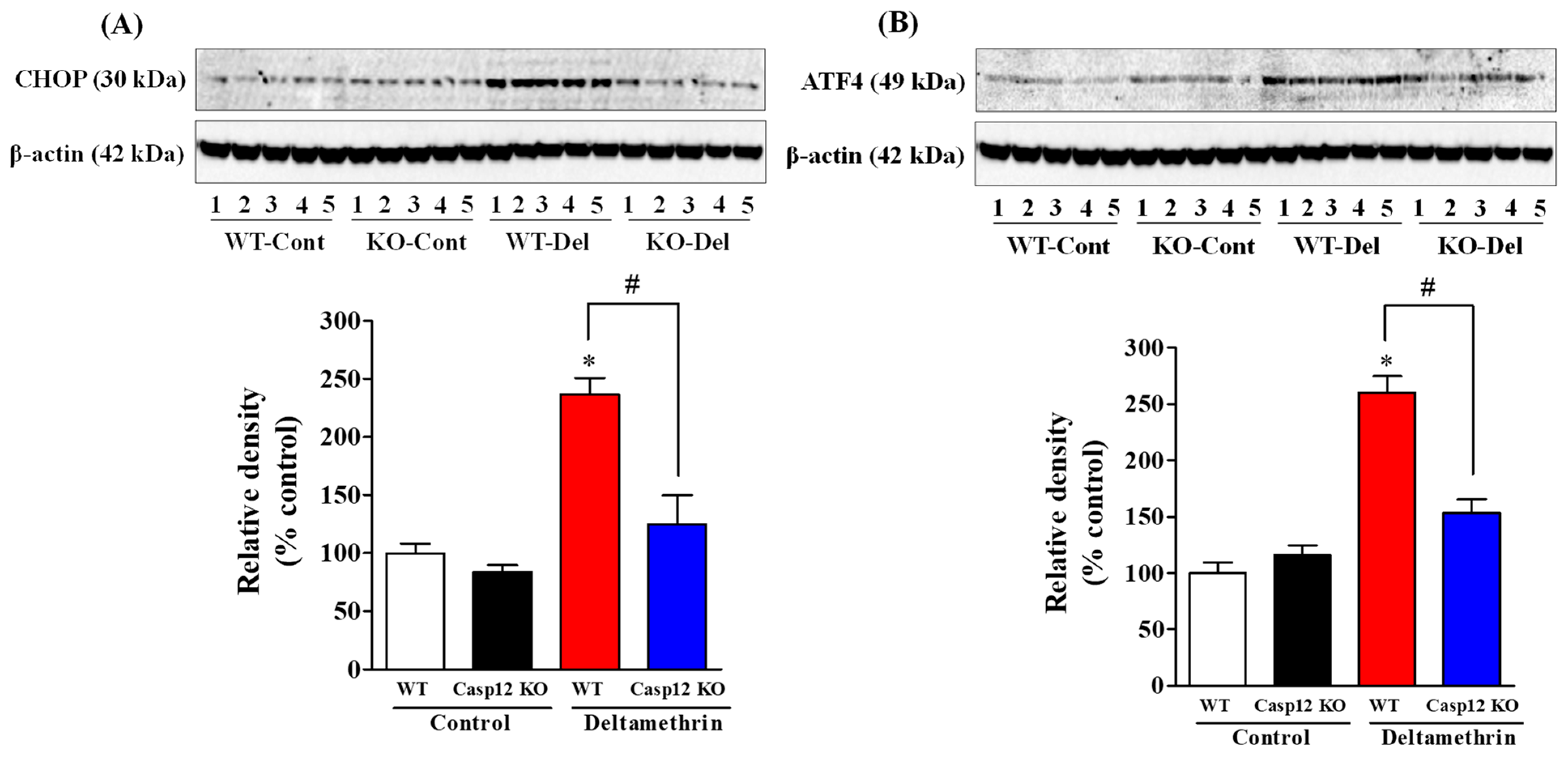
2.2. Caspase-12 KO Mice Are Protected from Deltamethrin-Induced ER Stress
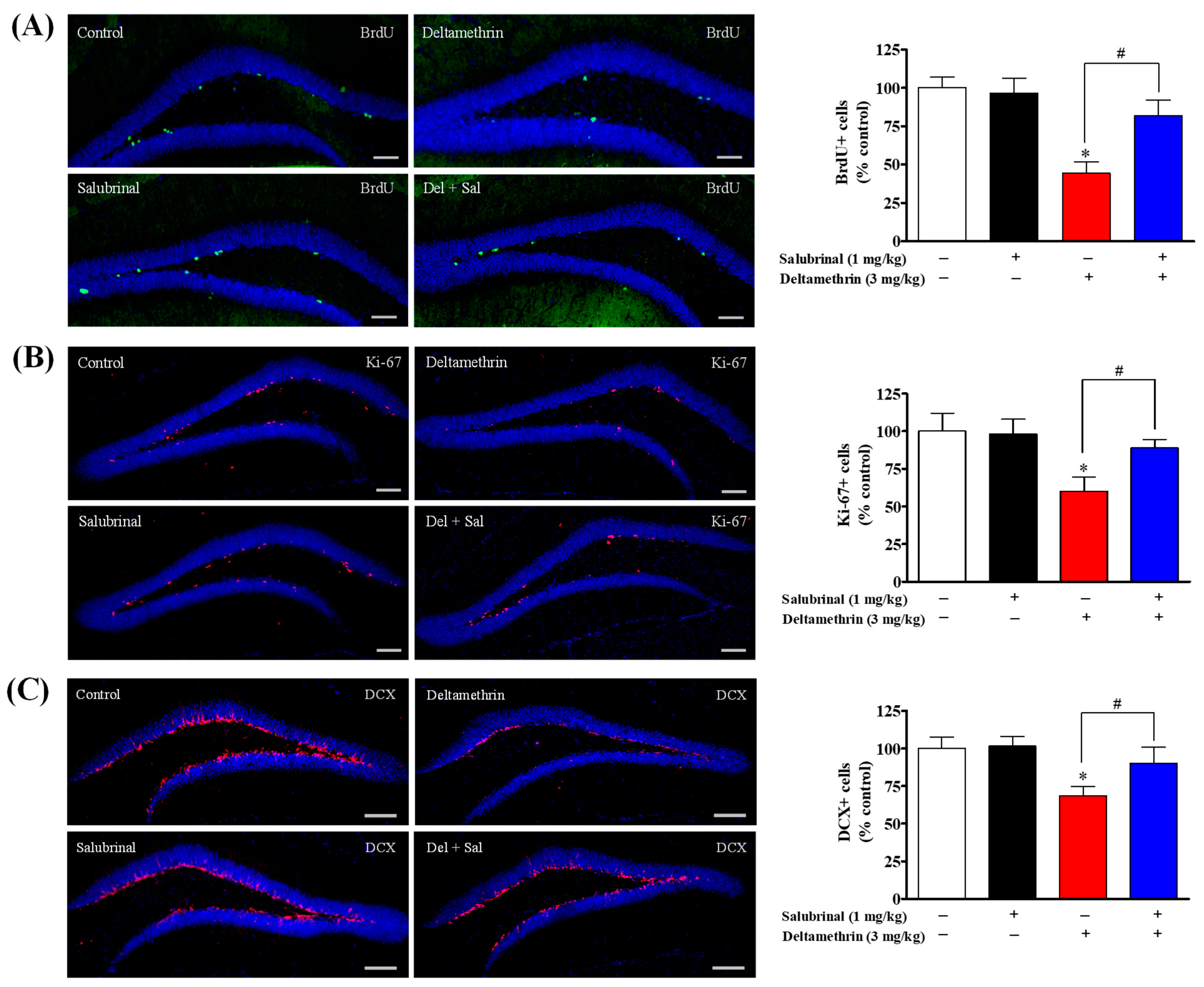
2.3. Inhibition of ER Stress with Salubrinal Attenuates Deltamethrin-Induced Reduction of Hippocampal Neurogenesis in Adult Mice
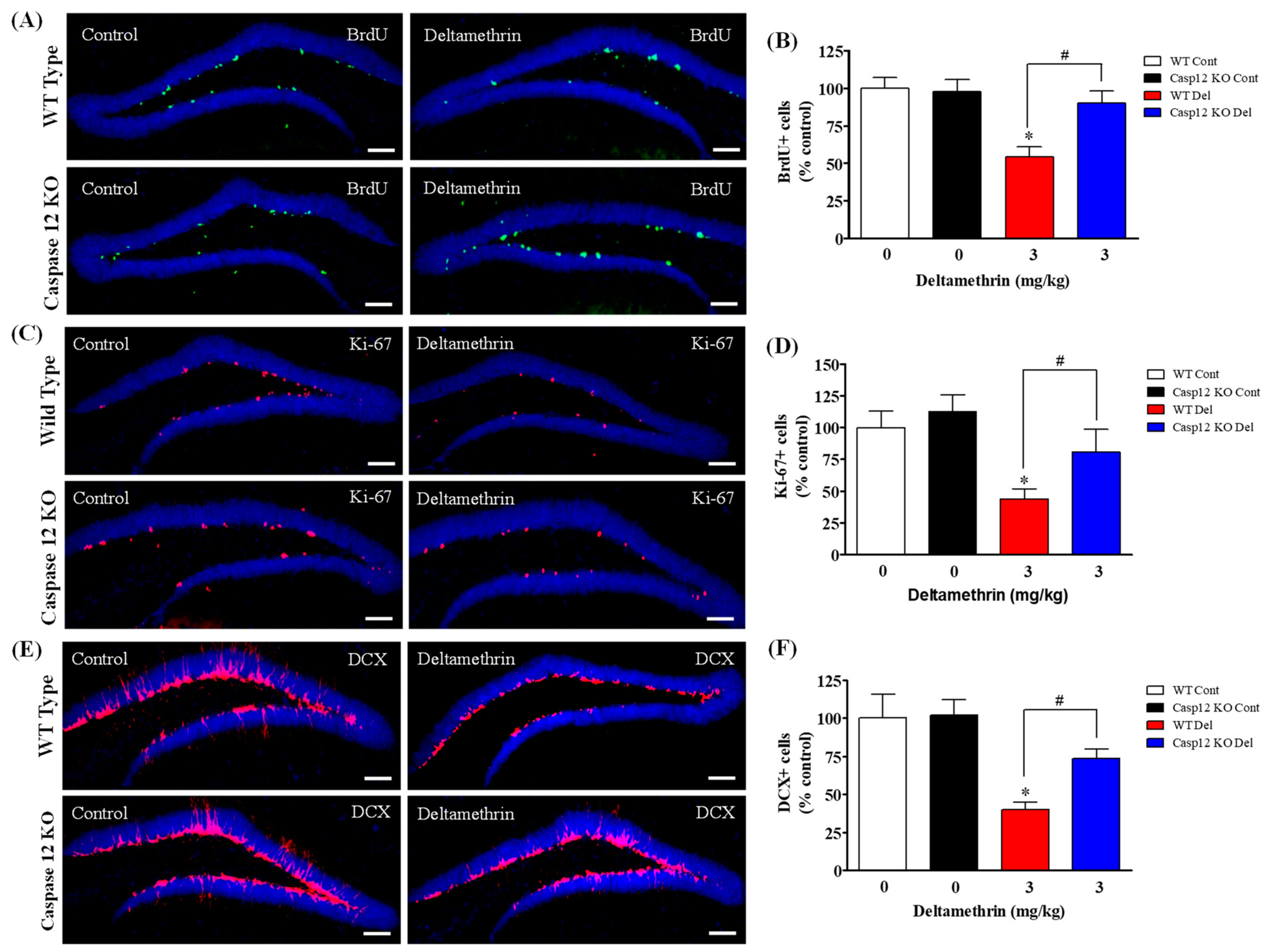
2.4. Caspase-12 KO Mice Are Protected from Deltamethrin-Induced Reduction of Hippocampal Neurogenesis in Adult Mice
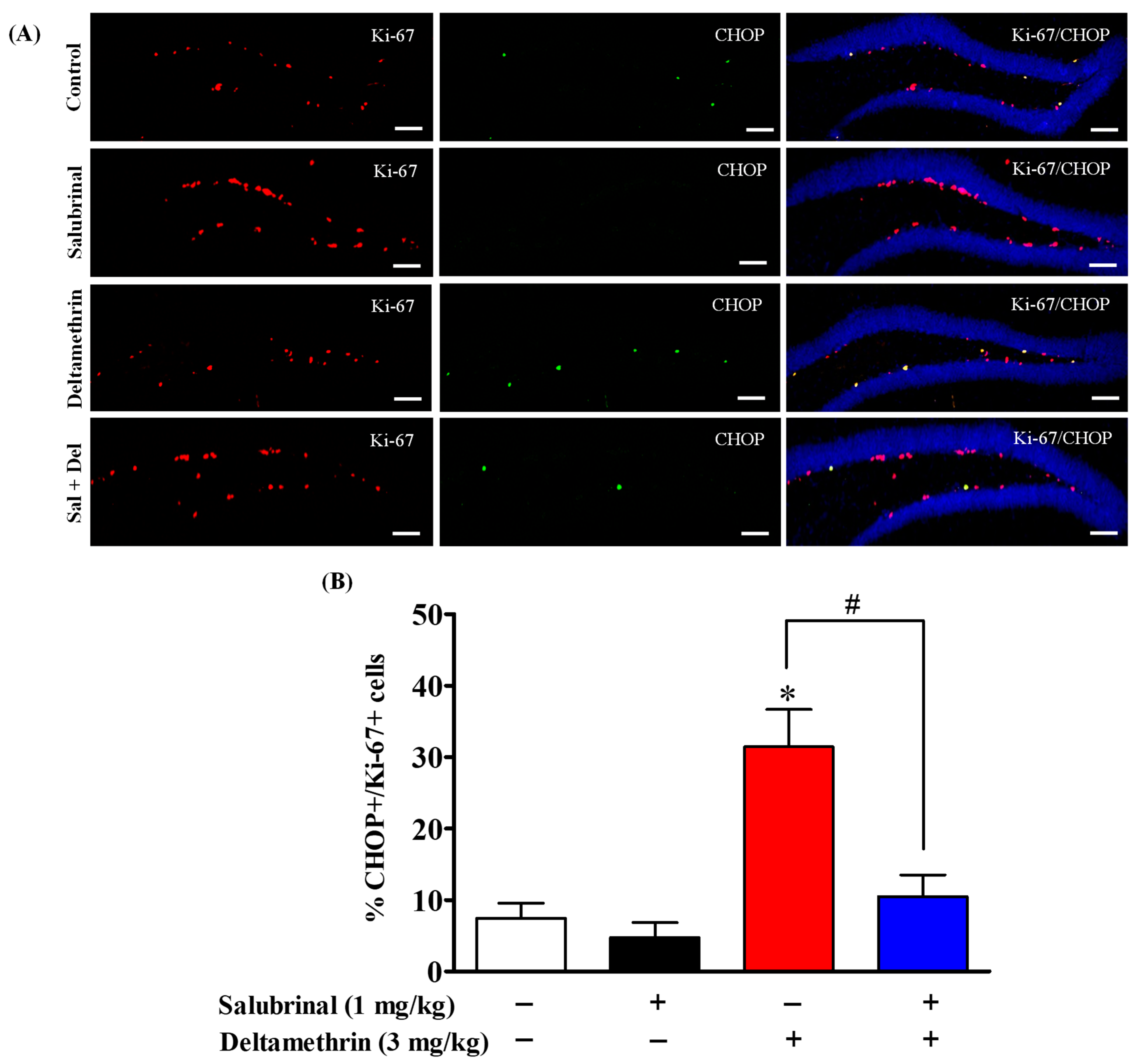
2.5. Salubrinal Treatment Attenuates the Expression of CHOP in Hippocampal NPCs in Deltamethrin-Treated Mice
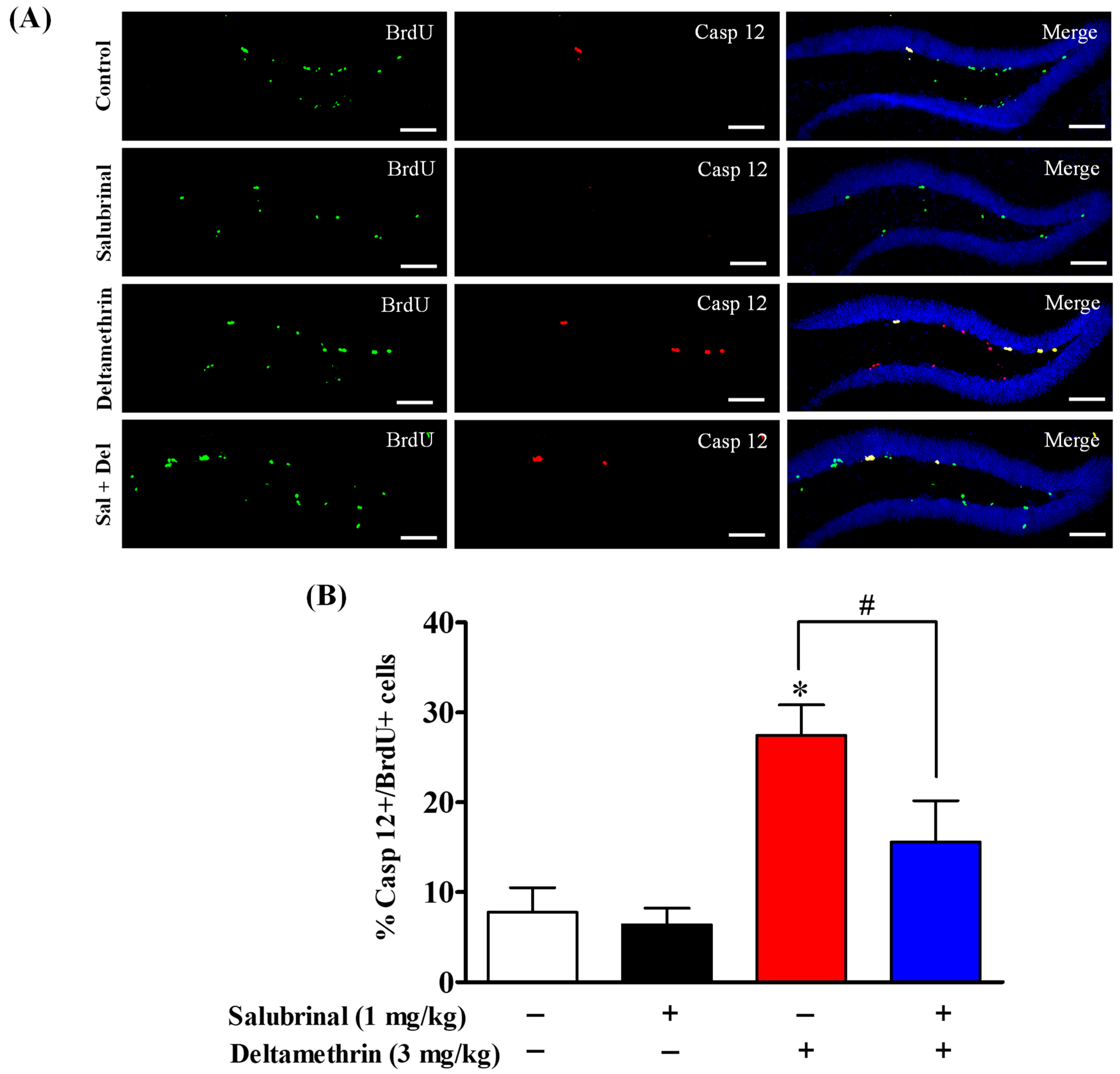
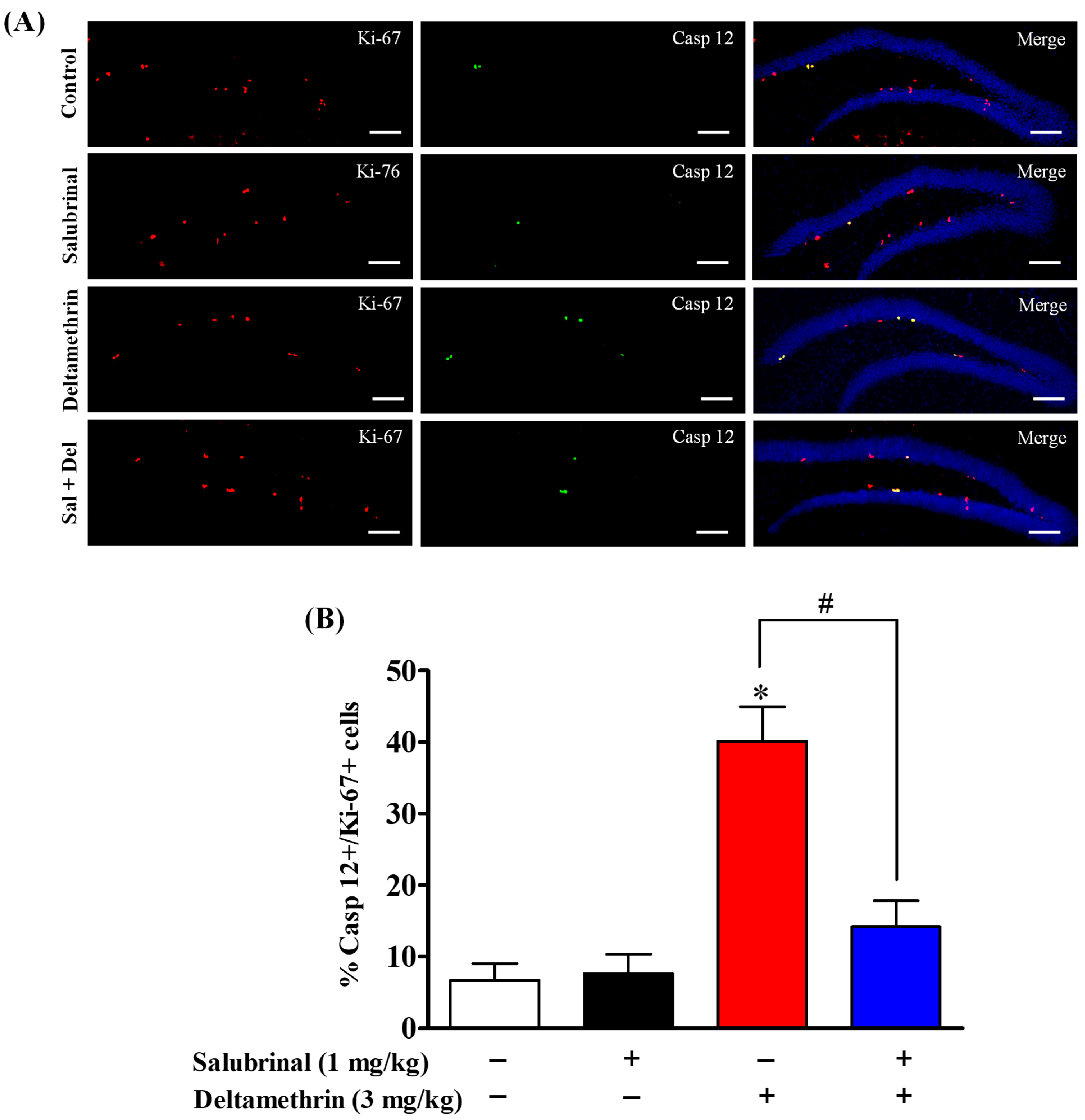
2.6. Salubrinal Attenuates the Expression of Caspase-12 in Hippocampal NPCs in Deltamethrin-Treated Mice
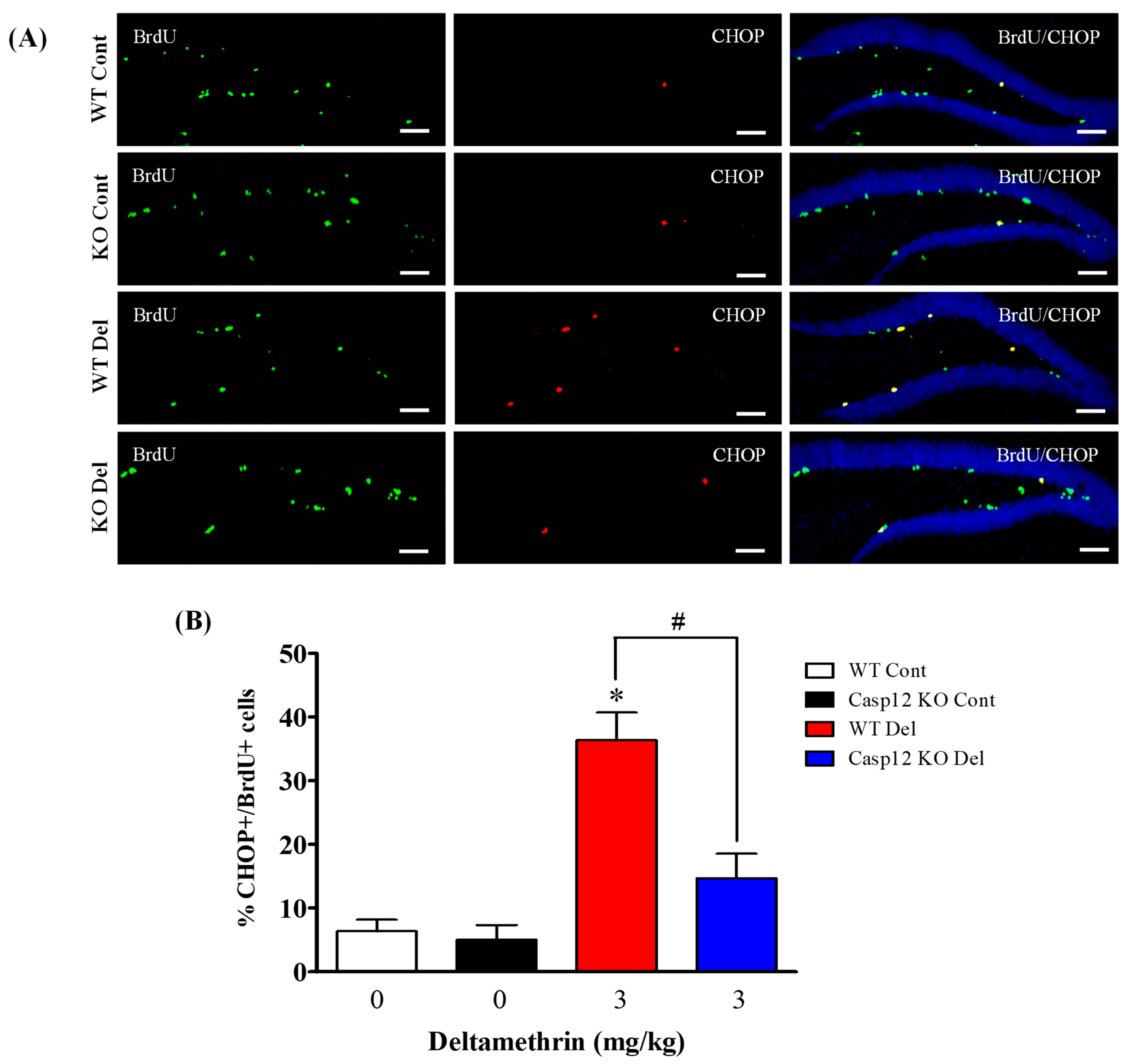
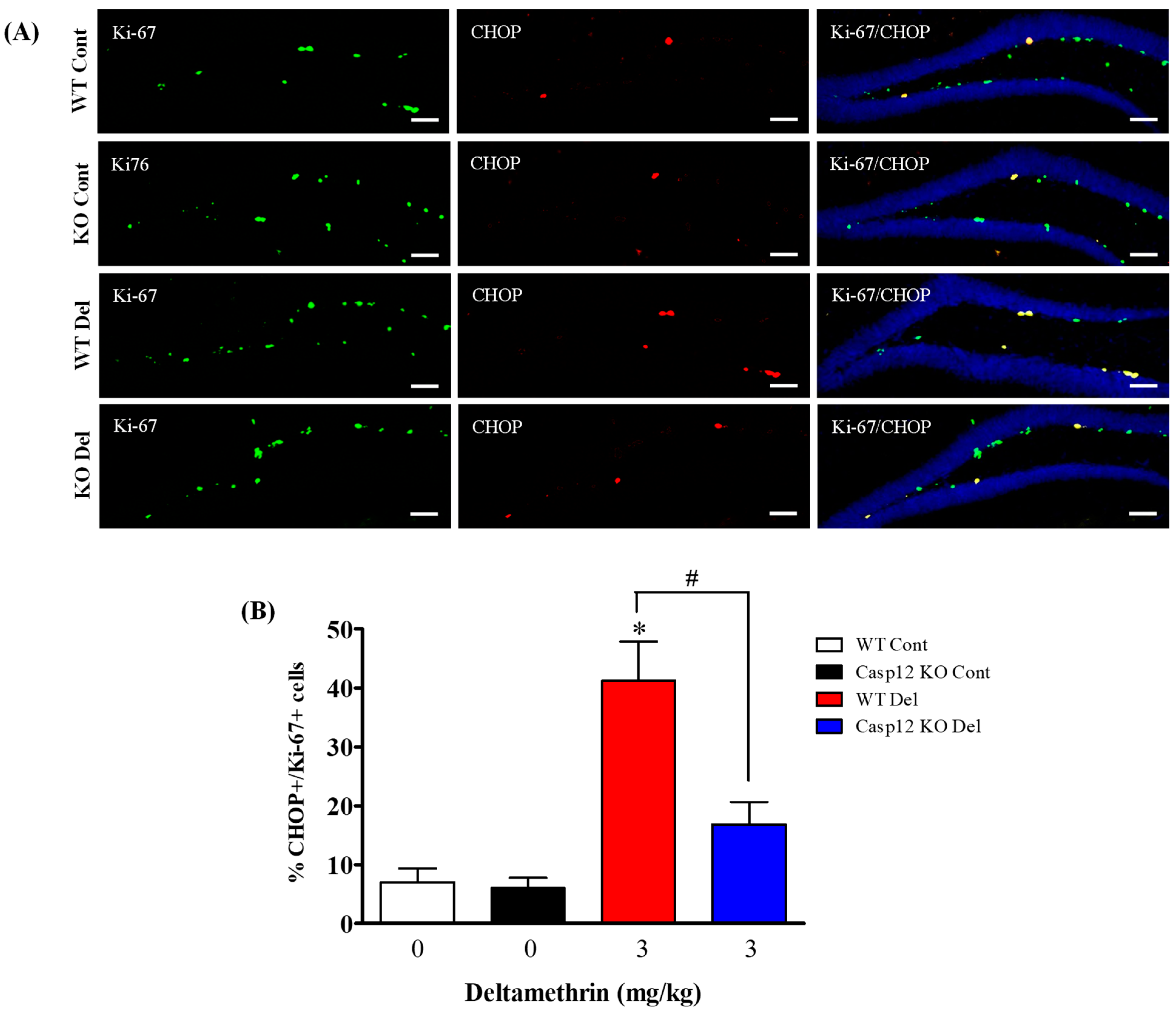
2.7. Caspase-12 Knockout Mice Are Protected against Deltamethrin-Induced Induction of ER Stress in Hippocampal NPCs
3. Discussion
4. Methods
4.1. Chemicals
4.2. Animals
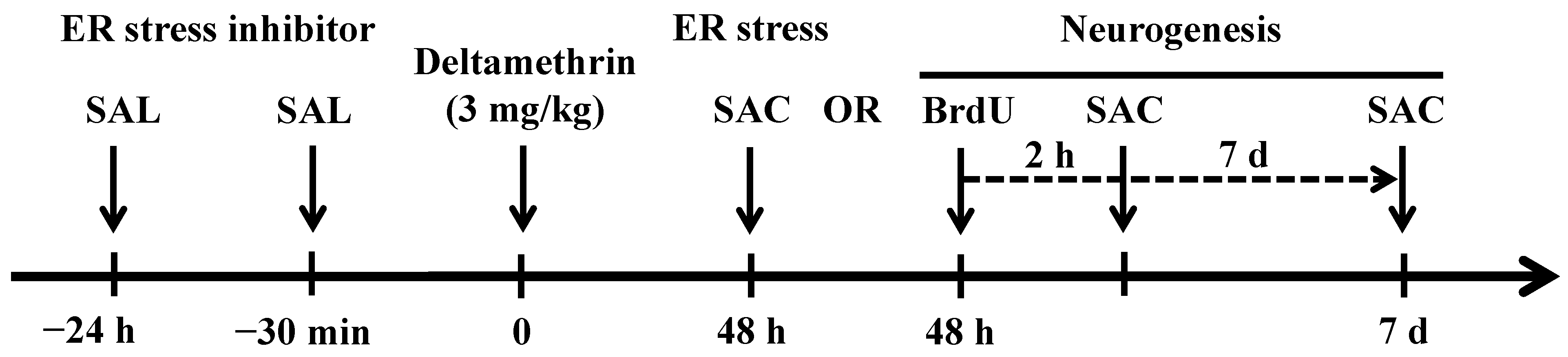
4.3. Treatment
4.4. BrdU Administration and Tissue Preparation
4.5. Western Immunoblotting
4.6. Immunofluorescence
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bao, W.; Liu, B.; Simonsen, D.W.; Lehmler, H.-J. Association between Exposure to Pyrethroid Insecticides and Risk of All-Cause and Cause-Specific Mortality in the General US Adult Population. JAMA Intern. Med. 2020, 180, 367–374. [Google Scholar] [CrossRef]
- Barr, D.B.; Olsson, A.O.; Wong, L.-Y.; Udunka, S.; Baker, S.E.; Whitehead, R.D.; Magsumbol, M.S.; Williams, B.L.; Needham, L.L. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: National health and nutrition examination survey 1999–2002. Environ. Health Perspect. 2010, 118, 742–748. [Google Scholar] [CrossRef]
- Trunnelle, K.J.; Bennett, D.H.; Ahn, K.C.; Schenker, M.B.; Tancredi, D.J.; Gee, S.J.; Stoecklin-Marois, M.T.; Hammock, B.D. Concentrations of the urinary pyrethroid metabolite 3-phenoxybenzoic acid in farm worker families in the MICASA study. Environ. Res. 2014, 131, 153–159. [Google Scholar] [CrossRef]
- Klimowska, A.; Amenda, K.; Rodzaj, W.; Wileńska, M.; Jurewicz, J.; Wielgomas, B. Evaluation of 1-year urinary excretion of eight metabolites of synthetic pyrethroids, chlorpyrifos, and neonicotinoids. Environ. Int. 2020, 145, 106119. [Google Scholar] [CrossRef]
- Rodzaj, W.; Wileńska, M.; Klimowska, A.; Dziewirska, E.; Jurewicz, J.; Walczak-Jędrzejowska, R.; Słowikowska-Hilczer, J.; Hanke, W.; Wielgomas, B. Concentrations of urinary biomarkers and predictors of exposure to pyrethroid insecticides in young, Polish, urban-dwelling men. Sci. Total Environ. 2021, 773, 145666. [Google Scholar] [CrossRef]
- Soderlund, D.M. Molecular mechanisms of pyrethroid insecticide neurotoxicity: Recent advances. Arch. Toxicol. 2012, 86, 165–181. [Google Scholar] [CrossRef]
- Clark, J.M.; Symington, S.B. Advances in the mode of action of pyrethroids. Top. Curr. Chem. 2012, 314, 49–72. [Google Scholar]
- Gupta, S. Racial and ethnic disparities in subjective cognitive decline: A closer look, United States, 2015–2018. BMC Public Health 2021, 21, 1173. [Google Scholar] [CrossRef]
- Lucchini, R.G.; Guazzetti, S.; Renzetti, S.; Conversano, M.; Cagna, G.; Fedrighi, C.; Giorgino, A.; Peli, M.; Placidi, D.; Zoni, S.; et al. Neurocognitive impact of metal exposure and social stressors among schoolchildren in Taranto, Italy. Environ. Health 2019, 18, 67. [Google Scholar] [CrossRef]
- Sasaki, N.; Carpenter, D.O. Associations between Metal Exposures and Cognitive Function in American Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 2327. [Google Scholar] [CrossRef]
- Calderon-Garciduenas, L.; Chávez-Franco, D.A.; Luévano-Castro, S.C.; Macías-Escobedo, E.; Hernández-Castillo, A.; Carlos-Hernández, E.; Franco-Ortíz, A.; Castro-Romero, S.P.; Cortés-Flores, M.; Crespo-Cortés, C.N.; et al. Metals, Nanoparticles, Particulate Matter, and Cognitive Decline. Front. Neurol. 2021, 12, 794071. [Google Scholar] [CrossRef] [PubMed]
- Aloizou, A.-M.; Siokas, V.; Vogiatzi, C.; Peristeri, E.; Docea, A.O.; Petrakis, D.; Provatas, A.; Folia, V.; Chalkia, C.; Vinceti, M.; et al. Pesticides, cognitive functions and dementia: A review. Toxicol. Lett. 2020, 326, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, S.-J.; Kim, S.-K.; Kim, C.-S.; Min, T.-H.K.S.-H.; Oh, S.-S.; Koh, S.-B. Pesticide exposure and cognitive decline in a rural South Korean population. PLoS ONE 2019, 14, e0213738. [Google Scholar]
- Dereumeaux, C.; Fillol, C.; Quenel, P.; Denys, S. Pesticide exposures for residents living close to agricultural lands: A review. Environ. Int. 2020, 134, 105210. [Google Scholar] [CrossRef] [PubMed]
- Friedman, E.; Hazlehurst, M.F.; Loftus, C.; Karr, C.; McDonald, K.N.; Suarez-Lopez, J.R. Residential proximity to greenhouse agriculture and neurobehavioral performance in Ecuadorian children. Int. J. Hyg. Environ. Health 2020, 223, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xiao, X.; Qi, Z.; Chen, L.; Chen, Y.; Xu, L.; Zhang, L.; Song, X.; Li, Y. Effects of prenatal and infant daily exposure to pyrethroid pesticides on the language development of 2-year-old toddlers: A prospective cohort study in rural Yunnan, China. NeuroToxicology 2022, 92, 180–190. [Google Scholar] [CrossRef]
- Kim, U.-J.; Hong, M.; Choi, Y.-H. Environmental Pyrethroid Exposure and Cognitive Dysfunction in U.S. Older Adults: The NHANES 2001–2002. Int. J. Environ. Res. Public Health 2021, 18, 12005. [Google Scholar] [CrossRef]
- Corazzari, M.; Gagliardi, M.; Fimia, G.M.; Piacentini, M. Endoplasmic Reticulum Stress, Unfolded Protein Response, and Cancer Cell Fate. Front. Oncol. 2017, 7, 78. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sivaram, G.; Richardson, J.R. Regional Susceptibility to ER Stress and Protection by Salubrinal Following a Single Exposure to Deltamethrin. Toxicol. Sci. 2019, 167, 249–257. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Suuronen, T.; Kaarniranta, K.; Ojala, J. ER stress in Alzheimer’s disease: A novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflamm. 2009, 6, 41. [Google Scholar] [CrossRef]
- Scheper, W.; Hoozemans, J.J.M. The unfolded protein response in neurodegenerative diseases: A neuropathological perspective. Acta Neuropathol. 2015, 130, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Shacham, T.; Sharma, N.; Lederkremer, G.Z. Protein Misfolding and ER Stress in Huntington’s Disease. Front Mol. Biosci. 2019, 6, 20. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; DiCicco-Bloom, E.; Richardson, J.R. Hippocampal ER stress and learning deficits following repeated pyrethroid exposure. Toxicol. Sci. 2015, 143, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Belkadi, A.; Zhou, X.; DiCicco-Bloom, E. Exposure to deltamethrin at the NOAEL causes ER stress and disruption of hippocampal neurogenesis in adult mice. NeuroToxicology 2022, 93, 233–243. [Google Scholar] [CrossRef]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef]
- Alam, M.J.; Kitamura, T.; Saitoh, Y.; Ohkawa, N.; Kondo, T.; Inokuchi, K. Adult Neurogenesis Conserves Hippocampal Memory Capacity. J. Neurosci. 2018, 38, 6854–6863. [Google Scholar] [CrossRef]
- Dard, R.F.; Dahan, L.; Rampon, C. Targeting hippocampal adult neurogenesis using transcription factors to reduce Alz-heimer’s disease-associated memory impairments. Hippocampus 2019, 29, 579–586. [Google Scholar] [CrossRef]
- Hong, M.; Kim, M.; Kim, T.-W.; Park, S.-S.; Kim, M.-K.; Park, Y.H.; Sung, Y.-H.; Shin, M.-S. Treadmill Exercise Improves Motor Function and Short-term Memory by Enhancing Synaptic Plasticity and Neurogenesis in Photothrombotic Stroke Mice. Int. Neurourol. J. 2020, 24, S28–S38. [Google Scholar] [CrossRef]
- Yau, S.-Y.; Li, A.; So, K.-F. Involvement of Adult Hippocampal Neurogenesis in Learning and Forgetting. Neural Plast. 2015, 2015, 717958. [Google Scholar] [CrossRef]
- Hollands, C.; Tobin, M.K.; Hsu, M.; Musaraca, K.; Yu, T.-S.; Mishra, R.; Kernie, S.G.; Lazarov, O. Depletion of adult neurogenesis exacerbates cognitive deficits in Alzheimer’s disease by compromising hippocampal inhibition. Mol. Neurodegener. 2017, 12, 64. [Google Scholar] [CrossRef]
- Bonds, J.A.; Shetti, A.; Stephen, T.K.L.; Bonini, M.G.; Minshall, R.D.; Lazarov, O. Deficits in hippocampal neurogenesis in obesity-dependent and -independent type-2 diabetes mellitus mouse models. Sci. Rep. 2020, 10, 16368. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Rodrigues, A.J.; Silva, J.M.; Tronche, F.; Almeida, O.F.X.; Sousa, N.; Sotiropoulos, I. Chronic Stress and Glucocorticoids: From Neuronal Plasticity to Neurodegeneration. Neural Plast. 2016, 2016, 6391686. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.L.; Ganaraja, B.; Murlimanju, B.V.; Joy, T.; Krishnamurthy, A.; Agrawal, A. Hippocampus and its involvement in Alzheimer’s disease: A review. 3 Biotech 2022, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Scopa, C.; Marrocco, F.; Latina, V.; Ruggeri, F.; Corvaglia, V.; La Regina, F.; Ammassari-Teule, M.; Middei, S.; Amadoro, G.; Meli, G.; et al. Impaired adult neurogenesis is an early event in Alzheimer’s disease neurodegeneration, mediated by intra-cellular Abeta oligomers. Cell Death Differ. 2020, 27, 934–948. [Google Scholar] [CrossRef] [PubMed]
- Cheyuo, C.; Aziz, M.; Wang, P. Neurogenesis in Neurodegenerative Diseases: Role of MFG-E8. Front. Neurosci. 2019, 13, 569. [Google Scholar] [CrossRef]
- Wang, H.; Matsushita, M.T. Heavy metals and adult neurogenesis. Curr. Opin. Toxicol. 2021, 26, 14–21. [Google Scholar] [CrossRef]
- Abbott, L.C.; Nigussie, F. Mercury Toxicity and Neurogenesis in the Mammalian Brain. Int. J. Mol. Sci. 2021, 22, 7520. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Z.; Bai, L.; Liu, R.; Ba, Y.; Zhang, H.; Cheng, X.; Zhou, G.; Huang, H. Resveratrol improved hippocampal neurogenesis following lead exposure in rats through activation of SIRT1 signaling. Environ. Toxicol. 2021, 36, 1664–1673. [Google Scholar] [CrossRef]
- Mishra, D.; Tiwari, S.K.; Agarwal, S.; Sharma, V.P.; Chaturvedi, R.K. Prenatal carbofuran exposure inhibits hippocampal neurogenesis and causes learning and memory deficits in offspring. Toxicol. Sci. 2012, 127, 84–100. [Google Scholar] [CrossRef]
- Hossain, M.M.; Belkadi, A.; Al-Haddad, S.; Richardson, J.R. Deltamethrin Exposure Inhibits Adult Hippocampal Neurogenesis and Causes Deficits in Learning and Memory in Mice. Toxicol. Sci. 2020, 178, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xiao, H.; Shao, Y.; Chang, X.; Zhang, Y.; Zhou, Z. Paraquat increases Interleukin-1beta in hippocampal dentate gyrus to impair hippocampal neurogenesis in adult mice. Ecotoxicol. Environ. Saf. 2020, 200, 110733. [Google Scholar] [CrossRef]
- Boda, E.; E Rigamonti, A.; Bollati, V. Understanding the effects of air pollution on neurogenesis and gliogenesis in the growing and adult brain. Curr. Opin. Pharmacol. 2020, 50, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. Neuropathological Mechanisms Associated with Pesticides in Alzheimer’s Disease. Toxics 2020, 8, 21. [Google Scholar] [CrossRef]
- Fagundes, B.H.F.; Nascimento, P.C.; Aragão, W.A.B.; Chemelo, V.S.; Bittencourt, L.O.; Eiró-Quirino, L.; Silva, M.C.F.; Freire, M.A.M.; Fernandes, L.M.P.; Maia, C.D.S.F.; et al. Methylmercury exposure during prenatal and postnatal neurodevelopment promotes oxidative stress associated with motor and cognitive damages in rats: An environmental-experimental toxicology study. Toxicol. Rep. 2022, 9, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Corcellas, C.; Feo, M.L.; Torres, J.P.; Malm, O.; Ocampo-Duque, W.; Eljarrat, E.; Barceló, D. Pyrethroids in human breast milk: Occurrence and nursing daily intake estimation. Environ. Int. 2012, 47, 17–22. [Google Scholar] [CrossRef]
- Mehta, R.V.; Sreenivasa, M.A.; Mathew, M.; Girard, A.W.; Taneja, S.; Ranjan, S.; Ramakrishnan, U.; Martorell, R.; Ryan, P.B.; Young, M.F. A mixed-methods study of pesticide exposures in Breastmilk and Community & Lactating Women’s per-spectives from Haryana, India. BMC Public Health 2020, 20, 1877. [Google Scholar]
- Kim, J.H.; Kim, S.; Hong, Y.-C. Household insecticide use and urinary 3-phenoxybenzoic acid levels in an elder population: A repeated measures data. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 1017–1031. [Google Scholar] [CrossRef]
- Morgan, M.; Jones, P.; Sobus, J.; Barr, D.B. Predictors of Urinary 3-Phenoxybenzoic Acid Levels in 50 North Carolina Adults. Int. J. Environ. Res. Public Health 2016, 13, 1172. [Google Scholar] [CrossRef]
- Xue, Q.; Pan, A.; Wen, Y.; Huang, Y.; Chen, D.; Yang, C.-X.; Wu, J.H.; Yang, J.; Pan, J.; Pan, X.-F. Association between pyrethroid exposure and cardiovascular disease: A national population-based cross-sectional study in the US. Environ. Int. 2021, 153, 106545. [Google Scholar] [CrossRef]
- Simaremare, S.R.S.; Hung, C.-C.; Hsieh, C.-J.; Yiin, L.-M. Relationship between Organophosphate and Pyrethroid Insecticides in Blood and Their Metabolites in Urine: A Pilot Study. Int. J. Environ. Res. Public Health 2019, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cheng, X.; Jiang, J.; Wang, J.; Xie, J.; Hu, X.; Huang, Y.; Song, L.; Liu, M.; Cai, L.; et al. The toxic influence of paraquat on hippocampal neurogenesis in adult mice. Food Chem. Toxicol. 2017, 106 Pt A, 356–366. [Google Scholar] [CrossRef]
- Chen, X.-P.; Chen, W.-F.; Wang, D.-W. Prenatal organophosphates exposure alternates the cleavage plane orientation of apical neural progenitor in developing neocortex. PLoS ONE 2014, 9, e95343. [Google Scholar] [CrossRef] [PubMed]
- Ojo, J.O.; Abdullah, L.; Evans, J.; Reed, J.M.; Montague, H.; Mullan, M.J.; Crawford, F.C. Exposure to an organophosphate pesticide, individually or in combination with other Gulf War agents, impairs synaptic integrity and neuronal differentiation, and is accompanied by subtle microvascular injury in a mouse model of Gulf War agent exposure. Neuropathology 2014, 34, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Sokolowski, K.; Obiorah, M.; Robinson, K.; McCandlish, E.; Buckley, B.; DiCicco-Bloom, E. Neural stem cell apoptosis after low-methylmercury exposures in postnatal hippocampus produce persistent cell loss and adolescent memory deficits. Dev. Neurobiol. 2013, 73, 936–949. [Google Scholar] [CrossRef]
- Xue, X.; Li, F.; Cai, M.; Hu, J.; Wang, Q.; Lou, S. Interactions between Endoplasmic Reticulum Stress and Autophagy: Implications for Apoptosis and Neuro-plasticity-Related Proteins in Palmitic Acid-Treated Prefrontal Cells. Neural. Plast. 2021, 2021, 8851327. [Google Scholar] [CrossRef]
- Xu, L.-H.; Xie, H.; Shi, Z.-H.; Du, L.-D.; Wing, Y.-K.; Li, A.M.; Ke, Y.; Yung, W.-H. Critical Role of Endoplasmic Reticulum Stress in Chronic Intermittent Hypoxia-Induced Deficits in Synaptic Plasticity and Long-Term Memory. Antioxid. Redox Signal. 2015, 23, 695–710. [Google Scholar] [CrossRef]
- Hoozemans, J.J.; van Haastert, E.S.; Nijholt, D.A.T.; Rozemuller, A.J.M.; Eikelenboom, P.; Scheper, W. The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am. J. Pathol. 2009, 174, 1241–1251. [Google Scholar] [CrossRef]
- Li, J.-Q.; Yu, J.-T.; Jiang, T.; Tan, L. Endoplasmic Reticulum Dysfunction in Alzheimer’s Disease. Mol. Neurobiol. 2015, 51, 383–395. [Google Scholar] [CrossRef]
- Yu, C.-L.; Yang, S.-F.; Hung, T.-W.; Lin, C.-L.; Hsieh, Y.-H.; Chiou, H.-L. Inhibition of eIF2α dephosphorylation accelerates pterostilbene-induced cell death in human hepatocellular carcinoma cells in an ER stress and autophagy-dependent manner. Cell Death Dis. 2019, 10, 418. [Google Scholar] [CrossRef]
- Teng, Y.; Gao, M.; Wang, J.; Kong, Q.; Hua, H.; Luo, T.; Jiang, Y. Inhibition of eIF2α dephosphorylation enhances TRAIL-induced apoptosis in hepatoma cells. Cell Death Dis. 2014, 5, e1060. [Google Scholar] [CrossRef]
- Nakagawa, T.; Zhu, H.; Morishima, N.; Li, E.; Xu, J.; Yankner, B.A.; Yuan, J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 2000, 403, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, J.; Katayama, T.; Taniguchi, M.; Honda, A.; Imaizumi, K.; Tohyama, M. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci. Lett. 2004, 357, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Heo, R.W.; Kim, H.; Yi, C.-O.; Shin, H.J.; Han, J.W.; Roh, G.S. Salubrinal, ER stress inhibitor, attenuates kainic acid-induced hippocampal cell death. J. Neural Transm. 2014, 121, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2018, 9, 3083. [Google Scholar] [CrossRef]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef]
- Klymenko, O.; Huehn, M.; Wilhelm, J.; Wasnick, R.; Shalashova, I.; Ruppert, C.; Henneke, I.; Hezel, S.; Guenther, K.; Mahavadi, P.; et al. Regulation and role of the ER stress transcription factor CHOP in alveolar epithelial type-II cells. J. Mol. Med. 2019, 97, 973–990. [Google Scholar] [CrossRef]
- Yin, Y.; Lv, G.; Zhang, W.; Yuan, J.; Yang, Y.; Wang, Y.; Liu, S.; Wang, S.; Yan, B.; Bo, H.; et al. Resveratrol glycoside mediates microglial endoplasmic reticulum stress to mitigate LPS-induced sepsis-associated cognitive dysfunction. Behav. Brain Res. 2023, 443, 114326. [Google Scholar] [CrossRef]
- Yang, Z.; Shao, Y.; Zhao, Y.; Li, Q.; Li, R.; Xiao, H.; Zhang, F.; Zhang, Y.; Chang, X.; Zhang, Y.; et al. Endoplasmic reticulum stress-related neuroinflammation and neural stem cells decrease in mice exposure to paraquat. Sci. Rep. 2020, 10, 17757. [Google Scholar] [CrossRef]
- Nakagawa, K.; Islam, S.; Ueda, M.; Nakagawa, T. Endoplasmic reticulum stress contributes to the decline in doublecortin expression in the immature neurons of mice with long-term obesity. Sci. Rep. 2022, 12, 1022. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Z.; Kumar, A.; Lipinski, M.M.; Loane, D.J.; Stoica, B.A.; Faden, A.I. Endoplasmic Reticulum Stress and Disrupted Neurogenesis in the Brain Are Associated with Cognitive Impairment and Depressive-Like Behavior after Spinal Cord Injury. J. Neurotrauma. 2016, 33, 1919–1935. [Google Scholar] [CrossRef] [PubMed]
- Mostafalou, S.; Abdollahi, M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, Y.; Zhao, Z.; Li, S.; Yin, J. The dynamic changes of endoplasmic reticulum stress pathway markers GRP78 and CHOP in the hippocampus of diabetic mice. Brain Res. Bull. 2015, 111, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Bertrand, M.J.; Gorman, A.M.; Vandenabeele, P.; Samali, A. The unfolded protein response at the crossroads of cellular life and death during endoplasmic reticulum stress. Biol. Cell 2012, 104, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.V.; Ellerby, H.M.; E Bredesen, D. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004, 11, 372–380. [Google Scholar] [CrossRef]
- Hossain, M.M.; Toltin, A.C.; Gamba, L.M.; Molina, M.A. Deltamethrin-Evoked ER Stress Promotes Neuroinflammation in the Adult Mouse Hippocampus. Cells 2022, 11, 1961. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toltin, A.C.; Belkadi, A.; Gamba, L.M.; Hossain, M.M. The Preventive Effects of Salubrinal against Pyrethroid-Induced Disruption of Adult Hippocampal Neurogenesis in Mice. Int. J. Mol. Sci. 2023, 24, 15614. https://doi.org/10.3390/ijms242115614
Toltin AC, Belkadi A, Gamba LM, Hossain MM. The Preventive Effects of Salubrinal against Pyrethroid-Induced Disruption of Adult Hippocampal Neurogenesis in Mice. International Journal of Molecular Sciences. 2023; 24(21):15614. https://doi.org/10.3390/ijms242115614
Chicago/Turabian StyleToltin, Abigail C., Abdelmadjid Belkadi, Laura M. Gamba, and Muhammad M. Hossain. 2023. "The Preventive Effects of Salubrinal against Pyrethroid-Induced Disruption of Adult Hippocampal Neurogenesis in Mice" International Journal of Molecular Sciences 24, no. 21: 15614. https://doi.org/10.3390/ijms242115614
APA StyleToltin, A. C., Belkadi, A., Gamba, L. M., & Hossain, M. M. (2023). The Preventive Effects of Salubrinal against Pyrethroid-Induced Disruption of Adult Hippocampal Neurogenesis in Mice. International Journal of Molecular Sciences, 24(21), 15614. https://doi.org/10.3390/ijms242115614






