Apolipoprotein D as a Potential Biomarker in Neuropsychiatric Disorders
Abstract
:1. Introduction
2. Apolipoprotein D
2.1. Basic Information
2.2. Apo D in Nervous System
2.3. Apo D in Neurodegenerative Diseases
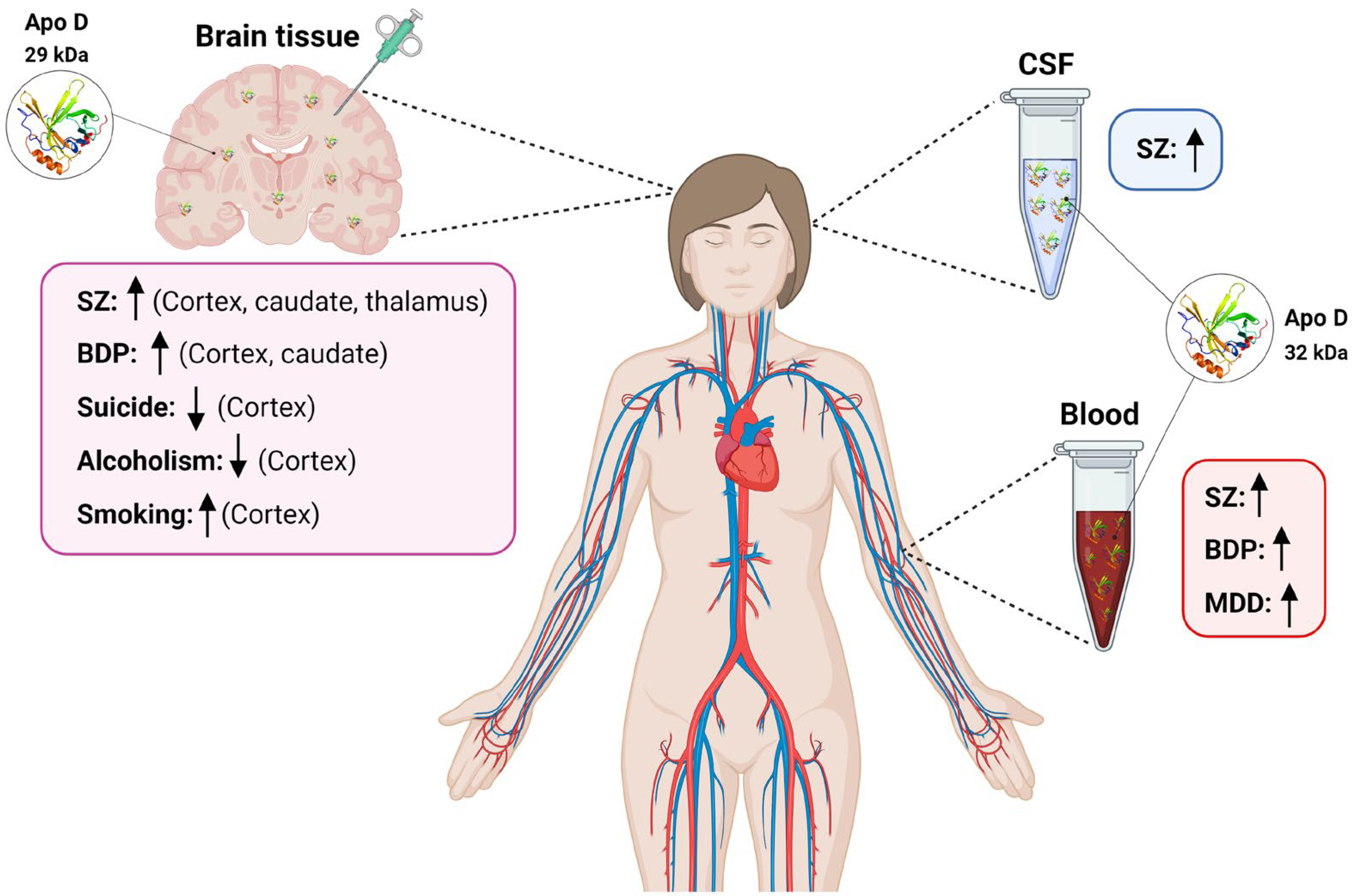
3. Role of Apo D as a Biomarker in Neuropsychiatric Disorders
3.1. Apo D in Schizophrenia
3.2. Apo D in Bipolar Disorders
3.3. Apo D in Major Depressive Disorder
3.4. Apo D and Other Neuropsychiatric Disorders
3.4.1. Apo D in Autism Spectrum Disorder
3.4.2. Apo D in Anxiety Disorders
3.4.3. Apo D in Addictions
4. Final Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Wittchen, H.U.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jönsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Susser, E.; Ritsner, M.S. Brain protection in neuropsychiatric disorders: Past, present and future challenges. In Brain Protection in Schizophrenia, Mood and Cognitive Disorders; Springer: Dordrecht, The Netherlands, 2010; pp. 3–25. ISBN 9789048185535. [Google Scholar]
- Woods, A.G.; Sokolowska, I.; Taurines, R.; Gerlach, M.; Dudley, E.; Thome, J.; Darie, C.C. Potential biomarkers in psychiatry: Focus on the cholesterol system. J. Cell. Mol. Med. 2012, 16, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Charlson, F.; van Ommeren, M.; Flaxman, A.; Cornett, J.; Whiteford, H.; Saxena, S. New WHO prevalence estimates of mental disorders in conflict settings: A systematic review and meta-analysis. Lancet 2019, 394, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J.; Taquet, M. Neuropsychiatric disorders following SARS-CoV-2 infection. Brain 2023, 146, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Collaborative Network. VizHub—GBD Results. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 26 September 2023).
- Taber, K.H.; Hurley, R.A.; Yudofsky, S.C. Diagnosis and treatment of neuropsychiatric disorders. Annu. Rev. Med. 2010, 61, 121–133. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, M.S.; Navarrete, F.; Sala, F.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front. Psychiatry 2020, 11, 432. [Google Scholar] [CrossRef]
- Elshourbagy, N.A.; Liao, W.S.; Mahley, R.W.; Taylor, J.M. Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc. Natl. Acad. Sci. USA 1985, 82, 203–207. [Google Scholar] [CrossRef]
- De Silva Harshini, V.; Harmony, J.A.K.; Stuart, W.D.; Gil, C.M.; Robbins, J. Apolipoprotein J: Structure and Tissue Distribution. Biochemistry 1990, 29, 5380–5389. [Google Scholar] [CrossRef]
- Drayna, D.; Fielding, C.; McLean, J.; Baer, B.; Castro, G.; Chen, E.; Comstock, L.; Henzel, W.; Kohr, W.; Rhee, L. Cloning and expression of human apolipoprotein D cDNA. J. Biol. Chem. 1986, 261, 16535–16539. [Google Scholar] [CrossRef]
- Elliott, D.A.; Weickert, C.S.; Garner, B. Apolipoproteins in the brain: Implications for neurological and psychiatric disorders. Clin. Lipidol. 2010, 5, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Forero, D.A.; López-León, S.; González-Giraldo, Y.; Dries, D.R.; Pereira-Morales, A.J.; Jiménez, K.M.; Franco-Restrepo, J.E. APOE gene and neuropsychiatric disorders and endophenotypes: A comprehensive review. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2018, 177, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Charnay, Y.; Imhof, A.; Vallet, P.G.; Kovari, E.; Bouras, C.; Giannakopoulos, P. Clusterin in neurological disorders: Molecular perspectives and clinical relevance. Brain Res. Bull. 2012, 88, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I.; Meaney, S.; Fogelman, A.M. Brain Cholesterol: Long Secret Life behind a Barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W. Outsourcing in the brain: Do neurons depend on cholesterol delivery by astrocytes? BioEssays 2003, 25, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Davison, J.; O’Gorman, A.; Brennan, L.; Cotter, D.R. A systematic review of metabolite biomarkers of schizophrenia. Schizophr. Res. 2018, 195, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Rapoport, S.I.; Rao, J.S. Altered arachidonic acid cascade enzymes in postmortem brain from bipolar disorder patients. Mol. Psychiatry 2011, 16, 419–428. [Google Scholar] [CrossRef]
- Parekh, A.; Smeeth, D.; Milner, Y.; Thuret, S. The role of lipid biomarkers in major depression. Healthcare 2017, 5, 5. [Google Scholar] [CrossRef]
- Rassart, E.; Desmarais, F.; Najyb, O.; Bergeron, K.F.; Mounier, C. Apolipoprotein D. Gene 2020, 756, 144874. [Google Scholar] [CrossRef]
- McConathy, W.J.; Alaupovic, P. Isolation and partial characterization of apolipoprotein D: A new protein moiety of the human plasma lipoprotein system. FEBS Lett. 1973, 37, 178–182. [Google Scholar] [CrossRef]
- Albers, J.J.; Cheung, M.C.; Ewens, S.L.; Tollefson, J.H. Characterization and immunoassay of apolipoprotein D. Atherosclerosis 1981, 39, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Holzfeind, P.; Merschak, P.; Dieplinger, H.; Redl, B. The human lacrimal gland synthesizes apolipoprotein D mRNA in addition to tear prealbumin mRNA, both species encoding members of the lipocalin superfamily. Exp. Eye Res. 1995, 61, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Borghini, I.; Barja, F.; Pometta, D.; James, R.W. Characterization of subpopulations of lipoprotein particles isolated from human cerebrospinal fluid. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1995, 1255, 192–200. [Google Scholar] [CrossRef]
- Sun, Q.; Disher, M.J.; Rustad, T.; Telian, S.A.; Andrews, P.C. AP30, a differential protein marker for perilymph and cerebrospinal fluid in middle ear fluid, has been purified and identified as human apolipoprotein D. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1998, 1384, 405–413. [Google Scholar] [CrossRef]
- Zeng, C.; Spielman, A.I.; Vowels, B.R.; Leyden, J.J.; Biemann, K.; Preti, G. A human axillary odorant is carried by apolipoprotein D. Proc. Natl. Acad. Sci. USA 1996, 93, 6626–6630. [Google Scholar] [CrossRef]
- Holmquist, L. Identification and quantification of apolipoprotein D in normal human urine. Electrophoresis 1990, 11, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Balbin, M.; Freije, J.M.P.; Fueyo, A.; Sanchez, L.M.; Lopez-Otin, C. Apolipoprotein D is the major protein component in cyst fluid from women with human breast gross cystic disease. Biochem. J. 1990, 271, 803–807. [Google Scholar] [CrossRef]
- Feig, M.A.; Hammer, E.; Völker, U.; Jehmlich, N. In-depth proteomic analysis of the human cerumen-A potential novel diagnostically relevant biofluid. J. Proteom. 2013, 83, 119–129. [Google Scholar] [CrossRef]
- Li, H.; Ruberu, K.; Karl, T.; Garner, B. Cerebral apolipoprotein-D Is hypoglycosylated compared to peripheral tissues and is variably expressed in mouse and human brain regions. PLoS ONE 2016, 11, e0148238. [Google Scholar] [CrossRef]
- Dilley, W.G.; Haagensen, D.E.; Cox, C.E.; Wells, S.A. Immunologic and steroid binding properties of the GCDFP-24 protein isolated from human breast gross cystic disease fluid. Breast Cancer Res. Treat. 1990, 16, 253–260. [Google Scholar] [CrossRef]
- Kielkopf, C.S.; Ghosh, M.; Anand, G.S.; Brown, S.H.J. HDX-MS reveals orthosteric and allosteric changes in apolipoprotein-D structural dynamics upon binding of progesterone. Protein Sci. 2019, 28, 365–374. [Google Scholar] [CrossRef]
- Lea, O.A. Binding properties of Progesterone-Binding Cyst Protein, PBCP. Steroids 1988, 52, 337–338. [Google Scholar] [CrossRef]
- Morais Cabral, J.H.; Atkins, G.L.; Sánchez, L.M.; López-Boado, Y.S.; López-Otin, C.; Sawyer, L. Arachidonic acid binds to apolipoprotein D: Implications for the protein’s function. FEBS Lett. 1995, 366, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Kim, W.S.; Shepherd, C.E.; Halliday, G.M. Apolipoprotein D Upregulation in Alzheimer’s Disease but Not Frontotemporal Dementia. J. Mol. Neurosci. 2019, 67, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kielkopf, C.S.; Low, J.K.K.; Mok, Y.F.; Bhatia, S.; Palasovski, T.; Oakley, A.J.; Whitten, A.E.; Garner, B.; Brown, S.H.J. Identification of a novel tetrameric structure for human apolipoprotein-D. J. Struct. Biol. 2018, 203, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, S.; Séguin, D.; Milne, R.; Rassart, E. Modulation of apolipoprotein D and apolipoprotein E mRNA expression by growth arrest and identification of key elements in the promoter. J. Biol. Chem. 2002, 277, 5514–5523. [Google Scholar] [CrossRef]
- Levros, L.C.; Labrie, M.; Charfi, C.; Rassart, E. Binding and repressive activities of apolipoprotein E3 and E4 isoforms on the human ApoD promoter. Mol. Neurobiol. 2013, 48, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Diez-Hermano, S.; Mejias, A.; Sanchez, D.; Gutierrez, G.; Ganfornina, M.D. Control of the neuroprotective Lipocalin Apolipoprotein D expression by alternative promoter regions and differentially expressed mRNA 5′UTR variants. PLoS ONE 2020, 15, e0234857. [Google Scholar] [CrossRef]
- Ganfornina, M.D.; Do Carmo, S.; Martínez, E.; Tolivia, J.; Navarro, A.; Rassart, E.; Sanchez, D. ApoD, a glia-derived apolipoprotein, is required for peripheral nerve functional integrity and a timely response to injury. Glia 2010, 58, 1320–1334. [Google Scholar] [CrossRef]
- Boyles, J.K.; Notterpek, L.M.; Anderson, L.J. Accumulation of apolipoproteins in the regenerating and remyelinating mammalian peripheral nerve: Identification of apolipoprotein D, apolipoprotein A-IV, apolipoprotein E, and apolipoprotein A-I. J. Biol. Chem. 1990, 265, 17805–17815. [Google Scholar] [CrossRef]
- Spreyer, P.; Schaal, H.; Kuhn, G.; Rothe, T.; Unterbeck, A.; Olek, K.; Muller, H.W. Regeneration-associated high level expression of apolipoprotein D mRNA in endoneurial fibroblasts of peripheral nerve. EMBO J. 1990, 9, 2479–2484. [Google Scholar] [CrossRef] [PubMed]
- García-Mateo, N.; Ganfornina, M.D.; Montero, O.; Gijón, M.A.; Murphy, R.C.; Sanchez, D. Schwann cell-derived Apolipoprotein D controls the dynamics of post-injury myelin recognition and degradation. Front. Cell. Neurosci. 2014, 8, 374. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Tolivia, J.; Astudillo, A.; Del Valle, E. Pattern of apolipoprotein D immunoreactivity in human brain. Neurosci. Lett. 1998, 254, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Rioseras, B.; del Valle, E.; Martínez-Pinilla, E.; Astudillo, A.; Tolivia, J. Expression pattern of Myelin-Related Apolipoprotein D in human multiple sclerosis lesions. Front. Aging Neurosci. 2018, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Loerch, P.M.; Lu, T.; Dakin, K.A.; Vann, J.M.; Isaacs, A.; Geula, C.; Wang, J.; Pan, Y.; Gabuzda, D.H.; Li, C.; et al. Evolution of the aging brain transcriptome and synaptic regulation. PLoS ONE 2008, 3, e3329. [Google Scholar] [CrossRef] [PubMed]
- De Magalhães, J.P.; Curado, J.; Church, G.M. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics 2009, 25, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.Y.; He, Y.; Suresh, S.; Patel, S.C. Differential expression of apolipoprotein D and apolipoprotein E in the kainic acid-lesioned rat hippocampus. Neuroscience 1997, 79, 359–367. [Google Scholar] [CrossRef]
- Franz, G.; Reindl, M.; Patel, S.C.; Beer, R.; Unterrichter, I.; Berger, T.; Schmutzhard, E.; Poewe, W.; Kampfl, A. Increased expression of apolipoprotein D following experimental traumatic brain injury. J. Neurochem. 1999, 73, 1615–1625. [Google Scholar] [CrossRef]
- Martínez, E.; Navarro, A.; Ordóñez, C.; Del Valle, E.; Tolivia, J. Oxidative stress induces apolipoprotein d overexpression in hippocampus during aging and alzheimer’s disease. J. Alzheimer’s Dis. 2013, 36, 129–144. [Google Scholar] [CrossRef]
- Martínez, E.; Navarro, A.; Ordóez, C.; Del Valle, E.; Tolivia, J. Amyloid-β25-35 induces apolipoprotein D synthesis and growth arrest in HT22 hippocampal cells. J. Alzheimer’s Dis. 2012, 30, 233–244. [Google Scholar] [CrossRef]
- Bajo-Grañeras, R.; Ganfornina, M.D.; Martín-Tejedor, E.; Sanchez, D. Apolipoprotein D mediates autocrine protection of astrocytes and controls their reactivity level, contributing to the functional maintenance of paraquat-challenged dopaminergic systems. Glia 2011, 59, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, S.; Levros, L.C.; Rassart, E. Modulation of apolipoprotein D expression and translocation under specific stress conditions. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2007, 1773, 954–969. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pinilla, E.; Rubio-Sardón, N.; Peláez, R.; García-álvarez, E.; Del Valle, E.; Tolivia, J.; Larráyoz, I.M.; Navarro, A. Neuroprotective effect of apolipoprotein d in cuprizone-induced cell line models: A potential therapeutic approach for multiple sclerosis and demyelinating diseases. Int. J. Mol. Sci. 2021, 22, 1260. [Google Scholar] [CrossRef] [PubMed]
- Bajo-Grañeras, R.; Sanchez, D.; Gutierrez, G.; González, C.; Do Carmo, S.; Rassart, E.; Ganfornina, M.D. Apolipoprotein D alters the early transcriptional response to oxidative stress in the adult cerebellum. J. Neurochem. 2011, 117, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Dassati, S.; Waldner, A.; Schweigreiter, R. Apolipoprotein D takes center stage in the stress response of the aging and degenerative brain. Neurobiol. Aging 2014, 35, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, C.; Navarro, A.; Perez, C.; Astudillo, A.; Martínez, E.; Tolivia, J. Apolipoprotein D expression in substantia nigra of Parkinson disease. Histol. Histopathol. 2006, 21, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Waldner, A.; Dassati, S.; Redl, B.; Smania, N.; Gandolfi, M. Apolipoprotein D Concentration in Human Plasma during Aging and in Parkinson’s Disease: A Cross-Sectional Study. Parkinsons. Dis. 2018, 2018, 3751516. [Google Scholar] [CrossRef]
- Muffat, J.; Walker, D.W. Apolipoprotein D: An overview of its role in aging and age-related diseases. Cell Cycle 2010, 9, 269–273. [Google Scholar] [CrossRef]
- Pascua-Maestro, R.; Diez-Hermano, S.; Lillo, C.; Ganfornina, M.D.; Sanchez, D. Protecting cells by protecting their vulnerable lysosomes: Identification of a new mechanism for preserving lysosomal functional integrity upon oxidative stress. PLoS Genet. 2017, 13, e1006603. [Google Scholar] [CrossRef]
- Fyfe-Desmarais, G.; Desmarais, F.; Rassart, É.; Mounier, C. Apolipoprotein D in Oxidative Stress and Inflammation. Antioxidants 2023, 12, 1027. [Google Scholar] [CrossRef]
- Sanchez, D.; Ganfornina, M.D. The Lipocalin Apolipoprotein D Functional Portrait: A Systematic Review. Front. Physiol. 2021, 12, 1587. [Google Scholar] [CrossRef] [PubMed]
- Glöckner, F.; Ohm, T.G. Hippocampal apolipoprotein D level depends on Braak stage and APOE genotype. Neuroscience 2003, 122, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Muffat, J.; Walker, D.W.; Benzer, S. Human ApoD, an apolipoprotein up-regulated in neurodegenerative diseases, extends lifespan and increases stress resistance in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 7088–7093. [Google Scholar] [CrossRef] [PubMed]
- Reindl, M.; Knipping, G.; Wicher, I.; Dilitz, E.; Egg, R.; Deisenhammer, F.; Berger, T. Increased intrathecal production of apolipoprotein D in multiple sclerosis. J. Neuroimmunol. 2001, 119, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Stoop, M.P.; Singh, V.; Dekker, L.J.; Titulaer, M.K.; Stingl, C.; Burgers, P.C.; Sillevis Smitt, P.A.E.; Hintzen, R.Q.; Luider, T.M. Proteomics comparison of cerebrospinal fluid of relapsing remitting and primary progressive multiple sclerosis. PLoS ONE 2010, 5, e12442. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, S.; Jacomy, H.; Talbot, P.J.; Rassart, E. Neuroprotective effect of apolipoprotein D against human coronavirus OC43-induced encephalitis in mice. J. Neurosci. 2008, 28, 10330–10338. [Google Scholar] [CrossRef]
- Lin, P.; Sun, J.; Lou, X.; Li, D.; Shi, Y.; Li, Z.; Ma, P.; Li, P.; Chen, S.; Jin, W.; et al. Consensus on potential biomarkers developed for use in clinical tests for schizophrenia. Gen. Psychiatry 2022, 35, e100685. [Google Scholar] [CrossRef] [PubMed]
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef]
- Mandal, P.K.; Gaur, S.; Roy, R.G.; Samkaria, A.; Ingole, R.; Goel, A. Schizophrenia, Bipolar and Major Depressive Disorders: Overview of Clinical Features, Neurotransmitter Alterations, Pharmacological Interventions, and Impact of Oxidative Stress in the Disease Process. ACS Chem. Neurosci. 2022, 13, 2784–2802. [Google Scholar] [CrossRef]
- Pagsberg, A.K. Schizophrenia spectrum and other psychotic disorders. Eur. Child Adolesc. Psychiatry 2013, 22, 3–9. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Wang, D.; Hu, X.; Jiang, J.; Qing, Y.; Yang, X.; Cui, G.; Wang, P.; Zhang, J.; et al. Impaired Membrane Lipid Homeostasis in Schizophrenia. Schizophr. Bull. 2022, 48, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.A.; Dean, B.; Pavey, G.; Sutcliffe, J.G. Increased CNS levels of apolipoprotein D in schizophrenic and bipolar subjects: Implications for the pathophysiology of psychiatric disorders. Proc. Natl. Acad. Sci. USA 2001, 98, 4066–4071. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.A.; Dean, B.; Scarr, E.; Copolov, D.; Sutcliffe, J.G. Differences in neuroanatomical sites of apoD elevation discriminate between schizophrenia and bipolar disorder. Mol. Psychiatry 2003, 8, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.A.; Danielson, P.E.; Austin Nelson, P.; Pribyl, T.M.; Hilbush, B.S.; Hasel, K.W.; Gregor Sutcliffe, J. Clozapine increases apolipoprotein D expression in rodent brain: Towards a mechanism for neuroleptic pharmacotherapy. J. Neurochem. 2001, 76, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Mahadik, S.P.; Khan, M.M.; Evans, D.R.; Parikh, V.V. Elevated plasma level of apolipoprotein D in schizophrenia and its treatment and outcome. Schizophr. Res. 2002, 58, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.O.; Jeffries, C.D.; Addington, J.; Bearden, C.E.; Cadenhead, K.S.; Cannon, T.D.; Cornblatt, B.A.; Mathalon, D.H.; McGlashan, T.H.; Seidman, L.J.; et al. Towards a Psychosis Risk Blood Diagnostic for Persons Experiencing High-Risk Symptoms: Preliminary Results from the NAPLS Project. Schizophr. Bull. 2015, 41, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.E.; Martinho, A.; Santa, C.; Madeira, N.; Coroa, M.; Santos, V.; Martins, M.J.; Pato, C.N.; Macedo, A.; Manadas, B. Systematic Review and Meta-Analysis of Mass Spectrometry Proteomics Applied to Human Peripheral Fluids to Assess Potential Biomarkers of Schizophrenia. Int. J. Mol. Sci. 2022, 23, 4917. [Google Scholar] [CrossRef]
- Raiszadeh, M.M.; Ross, M.M.; Russo, P.S.; Schaepper, M.A.; Zhou, W.; Deng, J.; Ng, D.; Dickson, A.; Dickson, C.; Strom, M.; et al. Proteomic analysis of eccrine sweat: Implications for the discovery of schizophrenia biomarker proteins. J. Proteome Res. 2012, 11, 2127–2139. [Google Scholar] [CrossRef]
- Csosz; Emri, G.; Kallõ, G.; Tsaprailis, G.; Tozsér, J. Highly abundant defense proteins in human sweat as revealed by targeted proteomics and label-free quantification mass spectrometry. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2024–2031. [Google Scholar] [CrossRef]
- Khan, M.M.; Parikh, V.V.; Mahadik, S.P. Antipsychotic drugs differentially modulate apolipoprotein D in rat brain. J. Neurochem. 2003, 86, 1089–1100. [Google Scholar] [CrossRef]
- Thomas, E.A.; George, R.C.; Gregor Sutcliffe, J. Apolipoprotein D modulates arachidonic acid signaling in cultured cells: Implications for psychiatric disorders. Prostaglandins Leukot. Essent. Fat. Acids 2003, 69, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kuiperij, H.B.; Hondius, D.C.; Kersten, I.; Versleijen, A.A.M.; Rozemuller, A.J.M.; Greenberg, S.M.; Schreuder, F.H.B.M.; Klijn, C.J.M.; Verbeek, M.M. Apolipoprotein D: A potential biomarker for cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol. 2020, 46, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Khoonsari, P.E.; Ossipova, E.; Lengqvist, J.; Svensson, C.I.; Kosek, E.; Kadetoff, D.; Jakobsson, P.J.; Kultima, K.; Lampa, J. The human CSF pain proteome. J. Proteom. 2019, 190, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kroksveen, A.C.; Guldbrandsen, A.; Vedeler, C.; Myhr, K.M.; Opsahl, J.A.; Berven, F.S. Cerebrospinal fluid proteome comparison between multiple sclerosis patients and controls. Acta Neurol. Scand. 2012, 126, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Miyajima, M.; Mineki, R.; Taka, H.; Murayama, K.; Arai, H. Analysis of potential diagnostic biomarkers in cerebrospinal fluid of idiopathic normal pressure hydrocephalus by proteomics. Acta Neurochir. 2006, 148, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Pokhriyal, R.; Khan, M.I.; Kumar, D.R.; Gupta, R.; Chadda, R.K.; Ramachandran, R.; Goyal, V.; Tripathi, M.; Hariprasad, G. Cerebrospinal fluid proteomics for identification of α2-macroglobulin as a potential biomarker to monitor pharmacological therapeutic efficacy in dopamine dictated disease states of Parkinson’s disease and schizophrenia. Neuropsychiatr. Dis. Treat. 2019, 15, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Lu, L.; Zhang, L.; Zhang, Q.; Ungvari, G.S.; Ng, C.H.; Yuan, Z.; Xiang, Y.; Wang, G.; Xiang, Y.T. Prevalence of suicide attempts in bipolar disorder: A systematic review and meta-analysis of observational studies. Epidemiol. Psychiatr. Sci. 2019, 29, e63. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Alda, M.; Baldessarini, R.J.; Bauer, M.; Berk, M.; Correll, C.U.; Fagiolini, A.; Fountoulakis, K.; Frye, M.A.; Grunze, H.; et al. The clinical characterization of the adult patient with bipolar disorder aimed at personalization of management. World Psychiatry 2022, 21, 364–387. [Google Scholar] [CrossRef]
- Magioncalda, P.; Martino, M. A unified model of the pathophysiology of bipolar disorder. Mol. Psychiatry 2022, 27, 202–211. [Google Scholar] [CrossRef]
- Dean, B.; Digney, A.; Sundram, S.; Thomas, E.; Scarr, E. Plasma apolipoprotein E is decreased in schizophrenia spectrum and bipolar disorder. Psychiatry Res. 2008, 158, 75–78. [Google Scholar] [CrossRef]
- Knöchel, C.; Kniep, J.; Cooper, J.D.; Stäblein, M.; Wenzler, S.; Sarlon, J.; Prvulovic, D.; Linden, D.E.J.; Bahn, S.; Stocki, P.; et al. Altered apolipoprotein C expression in association with cognition impairments and hippocampus volume in schizophrenia and bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, L.; Seregin, A.; Boksha, I.; Dmitrieva, E.; Simutkin, G.; Kornetova, E.; Savushkina, O.; Letova, A.; Bokhan, N.; Ivanova, S.; et al. The difference in serum proteomes in schizophrenia and bipolar disorder. BMC Genom. 2019, 20, 535. [Google Scholar] [CrossRef] [PubMed]
- Monroe, S.M.; Harkness, K.L. Major Depression and Its Recurrences: Life Course Matters. Annu. Rev. Clin. Psychol. 2022, 18, 329–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 2020, 126, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Dadkhah, M.; Jafarzadehgharehziaaddin, M.; Molaei, S.; Akbari, M.; Gholizadeh, N.; Fathi, F. Major depressive disorder: Biomarkers and biosensors. Clin. Chim. Acta 2023, 547, 117437. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.B.; Zhang, R.F.; Luo, D.; Zhou, Y.; Wang, Y.; Fang, L.; Li, W.J.; Mu, J.; Zhang, L.; Zhang, Y.; et al. Comparative proteomic analysis of plasma from major depressive patients: Identification of proteins associated with lipid metabolism and immunoregulation. Int. J. Neuropsychopharmacol. 2012, 15, 1413–1425. [Google Scholar] [CrossRef]
- Stelzhammer, V.; Haenisch, F.; Chan, M.K.; Cooper, J.D.; Steiner, J.; Steeb, H.; Martins-de-Souza, D.; Rahmoune, H.; Guest, P.C.; Bahn, S. Proteomic changes in serum of first onset, antidepressant drug-naïve major depression patients. Int. J. Neuropsychopharmacol. 2014, 17, 1599–1608. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kim, E.Y.; Kim, S.H.; Cho, K.C.; Ha, K.; Kim, K.P.; Ahn, Y.M. Discovery of serum protein biomarkers in drug-free patients with major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 69, 60–68. [Google Scholar] [CrossRef]
- Najjar, S.; Pearlman, D.M.; Devinsky, O.; Najjar, A.; Zagzag, D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: A review of clinical and experimental evidence. J. Neuroinflammation 2013, 10, 142. [Google Scholar] [CrossRef]
- Pan, S.J.; Tan, Y.L.; Yao, S.W.; Xin, Y.; Yang, X.; Liu, J.; Xiong, J. Fluoxetine induces lipid metabolism abnormalities by acting on the liver in patients and mice with depression. Acta Pharmacol. Sin. 2018, 39, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Benton, C.S.; Miller, B.H.; Skwerer, S.; Suzuki, O.; Schultz, L.E.; Cameron, M.D.; Marron, J.S.; Pletcher, M.T.; Wiltshire, T. Evaluating genetic markers and neurobiochemical analytes for fluoxetine response using a panel of mouse inbred strains. Psychopharmacology 2012, 221, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Rhee, S.J.; Kim, J.; Lee, Y.; Kim, H.; Lee, J.; Lee, K.; Shin, H.; Kim, H.; Lee, T.Y.; et al. Predictive protein markers for depression severity in mood disorders: A preliminary trans-diagnostic approach study. J. Psychiatr. Res. 2021, 142, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, M.; Kuloglu, M.; Tezcan, E.; Ustundag, B. Serum leptin and cholesterol values in violent and non-violent suicide attempters. Psychiatry Res. 2008, 158, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Olié, E.; Picot, M.C.; Guillaume, S.; Abbar, M.; Courtet, P. Measurement of total serum cholesterol in the evaluation of suicidal risk. J. Affect. Disord. 2011, 133, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Lalovic, A.; Levy, E.; Luheshi, G.; Canetti, L.; Grenier, E.; Sequeira, A.; Turecki, G. Cholesterol content in brains of suicide completers. Int. J. Neuropsychopharmacol. 2007, 10, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Freemantle, E.; Mechawar, N.; Turecki, G. Cholesterol and phospholipids in frontal cortex and synaptosomes of suicide completers: Relationship with endosomal lipid trafficking genes. J. Psychiatr. Res. 2013, 47, 272–279. [Google Scholar] [CrossRef]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.P.; Krämer-Albers, E.M. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell. Neurosci. 2013, 7, 182. [Google Scholar] [CrossRef]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J. Neuroinflammation 2017, 14, 47. [Google Scholar] [CrossRef]
- Kano, S.I.; Dohi, E.; Rose, I.V.L. Extracellular vesicles for research on psychiatric disorders. Schizophr. Bull. 2019, 45, 7–16. [Google Scholar] [CrossRef]
- Basso, M.; Bonetto, V. Extracellular vesicles and a novel form of communication in the brain. Front. Neurosci. 2016, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gui, Y.; Zhao, M.; Chen, X.; Li, H.; Tian, C.; Zhao, H.; Jiang, C.; Xu, P.; Zhang, S.; et al. The roles of extracellular vesicles in major depressive disorder. Front. Psychiatry 2023, 14, 1138110. [Google Scholar] [CrossRef] [PubMed]
- Pascua-Maestro, R.; González, E.; Lillo, C.; Ganfornina, M.D.; Falcón-Pérez, J.M.; Sanchez, D. Extracellular vesicles secreted by astroglial cells transport apolipoprotein D to neurons and mediate neuronal survival upon oxidative stress. Front. Cell. Neurosci. 2019, 12, 526. [Google Scholar] [CrossRef]
- Hirota, T.; King, B.H. Autism Spectrum Disorder: A Review. JAMA 2023, 329, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, B.; Wu, C.; Wang, J.; Sun, M. Autism Spectrum Disorder: Neurodevelopmental Risk Factors, Biological Mechanism, and Precision Therapy. Int. J. Mol. Sci. 2023, 24, 1819. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Vassall, S.; Kaur, G.; Lewis, C.; Karim, M.; Rossignol, D. Emerging biomarkers in autism spectrum disorder: A systematic review. Ann. Transl. Med. 2019, 7, 792. [Google Scholar] [CrossRef] [PubMed]
- Edlow, A.G.; Vora, N.L.; Hui, L.; Wick, H.C.; Cowan, J.M.; Bianchi, D.W. Maternal obesity affects fetal neurodevelopmental and metabolic gene expression: A pilot study. PLoS ONE 2014, 9, 88661. [Google Scholar] [CrossRef]
- Esnafoglu, E.; Cırrık, S. Apo D and Apo E levels in Autism spectrum disorders. Asian J. Psychiatr. 2022, 73, 103177. [Google Scholar] [CrossRef]
- Navarro, A.; Del Valle, E.; Juárez, A.; Martinez, E.; Ordóñez, C.; Astudillo, A.; Tolivia, J. Apolipoprotein D synthesis progressively increases in frontal cortex during human lifespan. Age 2010, 32, 85–96. [Google Scholar] [CrossRef]
- Camato, R.; Marcel, Y.L.; Milne, R.W.; Lussier-Cacan, S.; Weech, P.K. Protein polymorphism of a human plasma apolipoprotein D antigenic epitope. J. Lipid Res. 1989, 30, 865–875. [Google Scholar] [CrossRef]
- Hari, A.; Cruz, S.A.; Qin, Z.; Couture, P.; Vilmundarson, R.O.; Huang, H.; Stewart, A.F.R.; Chen, H.H. IRF2BP2-deficient microglia block the anxiolytic effect of enhanced postnatal care. Sci. Rep. 2017, 7, 9836. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, K.W.; Baek, I.S.; Lim, C.M.; Krishnan, V.; Lee, J.K.; Nestler, E.J.; Han, P.L. Adenylyl cyclase-5 activity in the nucleus accumbens regulates anxiety-related behavior. J. Neurochem. 2008, 107, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Wu, S.C.; Chiang, Y.S.; Chen, J.F. Correlation between serum lipid, lipoprotein concentrations and anxious state, depressive state or major depressive disorder. Psychiatry Res. 2003, 118, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Lewohl, J.M.; Wang, L.; Miles, M.F.; Zhang, L.; Dodd, P.R.; Adron Harris, R. Gene expression in human alcoholism: Microarray analysis of frontal cortex. Alcohol. Clin. Exp. Res. 2000, 24, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, R.D.; Lewohl, J.M.; Dodd, P.R.; Herlihy, A.; Liu, J.; Harris, R.A. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. J. Neurochem. 2002, 81, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Flatscher-Bader, T.; Van Der Brug, M.; Hwang, J.W.; Gochee, P.A.; Matsumoto, I.; Niwa, S.I.; Wilce, P.A. Alcohol-responsive genes in the frontal cortex and nucleus accumbens of human alcoholics. J. Neurochem. 2005, 93, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Flatscher-Bader, T.; Wilce, P.A. Chronic smoking and alcoholism change expression of selective genes in the human prefrontal cortex. Alcohol. Clin. Exp. Res. 2006, 30, 908–915. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Horn, K.H.; Greene, R.M.; Michele Pisano, M. Prenatal exposure to environmental tobacco smoke alters gene expression in the developing murine hippocampus. Reprod. Toxicol. 2010, 29, 164–175. [Google Scholar] [CrossRef]
- Befort, K.; Filliol, D.; Darcq, E.; Ghate, A.; Matifas, A.; Lardenois, A.; Muller, J.; Thibault, C.; Dembele, D.; Poch, O.; et al. Gene expression is altered in the lateral hypothalamus upon activation of the mu opioid receptor. Ann. N. Y. Acad. Sci. 2008, 1129, 175–184. [Google Scholar] [CrossRef]
- Zhou, Z.; Yuan, Q.; Mash, D.C.; Goldman, D. Substance-specific and shared transcription and epigenetic changes in the human hippocampus chronically exposed to cocaine and alcohol. Proc. Natl. Acad. Sci. USA 2011, 108, 6626–6631. [Google Scholar] [CrossRef]
| Brain Tissue | Serum/Plasma | CSF | Other | |||||
|---|---|---|---|---|---|---|---|---|
| SZ | ↑ BA9 and Caudate; BA46, BA11 and Thalamus (human) | [74,75] | ↓ (mouse) | [76] | ns (human) | [66] | = Sweat (human) | [81] |
| = Substantia nigra, BA18, CA1, CA3, Subiculum, Parahyppocampal gyrus and Cerebellum; BA40 and BA24 (human) | [74,75] | ↑(human) | [76] | |||||
| BPD | ↑ BA9 and Caudate; BA46 and BA40 (human) | [74,75] | ↑ (human) | [93,94] | nd | nd | ||
| = BA18; Thalamus (human) | [74,75] | = (human) | [92] | |||||
| Ns BA11 and BA24 (human) | [75] | |||||||
| MDD | nd | ↑ (human) | [101] | nd | nd | |||
| ns (human) | [99,100] | |||||||
| Suicide | ↓ BA10 (*) (human) | [109] | nd | nd | nd | |||
| ASD | nd | nd | nd | ↑ Amniotic fluid (*) (human) | [119] | |||
| Alcoholism | ↓ Superior frontal cortex (*) (human) | [126] | nd | nd | nd | |||
| ↑↓ Frontal cortex (*) (human) | [127] | |||||||
| ↓ Frontal cortex (*) (human) | [128] | |||||||
| ↑↓ Motor cortex (*) (human) | [127] | |||||||
| Smoking | ↑ Prefrontal cortex (*) (human) | [129] | ||||||
| ↓ Hippocampus (*) (mouse) | [130] | |||||||
| Morphine addiction | ↑ Lateral hypothalamus (*) (mouse) | [131] | ||||||
| Cocaine addiction | ns Hippocampus (*) (human) | [132] | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Valle, E.; Rubio-Sardón, N.; Menéndez-Pérez, C.; Martínez-Pinilla, E.; Navarro, A. Apolipoprotein D as a Potential Biomarker in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2023, 24, 15631. https://doi.org/10.3390/ijms242115631
del Valle E, Rubio-Sardón N, Menéndez-Pérez C, Martínez-Pinilla E, Navarro A. Apolipoprotein D as a Potential Biomarker in Neuropsychiatric Disorders. International Journal of Molecular Sciences. 2023; 24(21):15631. https://doi.org/10.3390/ijms242115631
Chicago/Turabian Styledel Valle, Eva, Nuria Rubio-Sardón, Carlota Menéndez-Pérez, Eva Martínez-Pinilla, and Ana Navarro. 2023. "Apolipoprotein D as a Potential Biomarker in Neuropsychiatric Disorders" International Journal of Molecular Sciences 24, no. 21: 15631. https://doi.org/10.3390/ijms242115631
APA Styledel Valle, E., Rubio-Sardón, N., Menéndez-Pérez, C., Martínez-Pinilla, E., & Navarro, A. (2023). Apolipoprotein D as a Potential Biomarker in Neuropsychiatric Disorders. International Journal of Molecular Sciences, 24(21), 15631. https://doi.org/10.3390/ijms242115631






