Exosomes Interactions with the Blood–Brain Barrier: Implications for Cerebral Disorders and Therapeutics
Abstract
1. Introduction
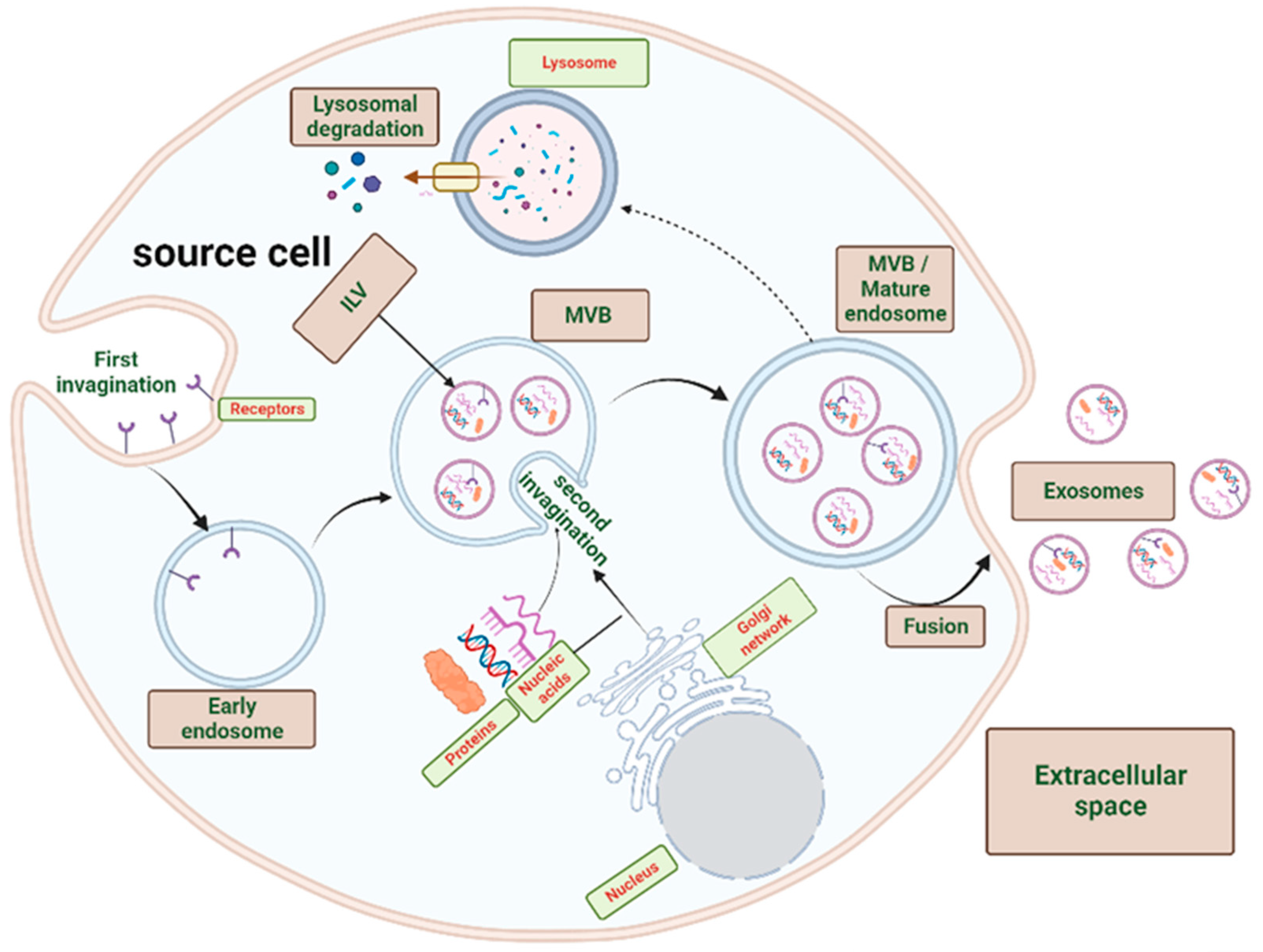
2. Exosomes Biogenesis
2.1. Exosomes Biogenesis
2.2. Exosomal Content
3. Transport of Exosomes through the Blood–Brain Barrier
4. Physiological Regulation of the Blood–Brain Barrier by Exosomes
5. Regulation of the Blood–Brain Barrier by Exosomes in Brain Diseases
5.1. Brain Glioma
5.2. Metastatic Brain Tumors
5.2.1. Metastatic Breast Cancer
5.2.2. Metastatic Lung Cancer
5.2.3. Other Types of Metastatic Cancer
5.3. Traumatic Brain Injury
5.4. Stroke
5.5. Neuroinflammation
5.6. Neurodegenerative Diseases
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palmer, A.M.; Alavijeh, M.S. Overview of Experimental Models of the Blood-Brain Barrier in CNS Drug Discovery. Curr. Protoc. Pharmacol. 2013, 62, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- Komarova, Y.A.; Kruse, K.; Mehta, D.; Malik, A.B. Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ. Res. 2017, 120, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular Pathways to Neurodegeneration in Alzheimer’s Disease and Other Disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Taddei, A.; Giampietro, C.; Conti, A.; Orsenigo, F.; Breviario, F.; Pirazzoli, V.; Potente, M.; Daly, C.; Dimmeler, S.; Dejana, E. Endothelial Adherens Junctions Control Tight Junctions by VE-Cadherin-Mediated Upregulation of Claudin-5. Nat. Cell Biol. 2008, 10, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Balusu, S.; Van Wonterghem, E.; De Rycke, R.; Raemdonck, K.; Stremersch, S.; Gevaert, K.; Brkic, M.; Demeestere, D.; Vanhooren, V.; Hendrix, A.; et al. Identification of a Novel Mechanism of Blood–Brain Communication during Peripheral Inflammation via Choroid Plexus-derived Extracellular Vesicles. EMBO Mol. Med. 2016, 8, 1162–1183. [Google Scholar] [CrossRef]
- Pan, B.-T.; Johnstone, R.M. Fate of the Transferrin Receptor during Maturation of Sheep Reticulocytes in Vitro: Selective Externalization of the Receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Z.; He, Y. Exosomes in the Pathogenesis, Progression, and Treatment of Osteoarthritis. Bioengineering 2022, 9, 99. [Google Scholar] [CrossRef]
- Isaac, R.; Reis, F.C.G.; Ying, W.; Olefsky, J.M. Exosomes as Mediators of Intercellular Crosstalk in Metabolism. Cell Metab. 2021, 33, 1744–1762. [Google Scholar] [CrossRef]
- Xu, M.; Feng, T.; Liu, B.; Qiu, F.; Xu, Y.; Zhao, Y.; Zheng, Y. Engineered Exosomes: Desirable Target-Tracking Characteristics for Cerebrovascular and Neurodegenerative Disease Therapies. Theranostics 2021, 11, 8926–8944. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain Metastatic Cancer Cells Release microRNA-181c-Containing Extracellular Vesicles Capable of Destructing Blood–Brain Barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, X.; Wang, J.; Zhao, Y.; Wang, Y.; Bihl, J.C.; Chen, Y.; Jiang, C. Glioma Stem Cells-Derived Exosomes Promote the Angiogenic Ability of Endothelial Cells through miR-21/VEGF Signal. Oncotarget 2017, 8, 36137–36148. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, P.; Sun, B.; Tang, N.; Pulliam, L. Immune Activated Monocyte Exosomes Alter microRNAs in Brain Endothelial Cells and Initiate an Inflammatory Response through the TLR4/MyD88 Pathway. Sci. Rep. 2017, 7, 9954. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, B.; Sun, R.; Chen, Y.; Feng, J. Exosome-Transported Long Non-Coding Ribonucleic Acid H19 Induces Blood–Brain Barrier Disruption in Cerebral Ischemic Stroke Via the H19/Micro Ribonucleic Acid-18a/Vascular Endothelial Growth Factor Axis. Neuroscience 2022, 500, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, G.; Yang, C.; Zhang, Y.; Chen, Y.; Zhong, C.; Li, Q. MicroRNA-550a-3-5p Controls the Brain Metastasis of Lung Cancer by Directly Targeting YAP1. Cancer Cell Int. 2021, 21, 491. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key Players in Cancer and Potential Therapeutic Strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Sahu, R.; Kaushik, S.; Clement, C.C.; Cannizzo, E.S.; Scharf, B.; Follenzi, A.; Potolicchio, I.; Nieves, E.; Cuervo, A.M.; Santambrogio, L. Microautophagy of Cytosolic Proteins by Late Endosomes. Dev. Cell 2011, 20, 131–139. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. IJN 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT Pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, J.; Lee, I.-H.; Iwasa, J.H.; Hurley, J.H. Reverse-Topology Membrane Scission by the ESCRT Proteins. Nat. Rev. Mol. Cell Biol. 2017, 18, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan–Syntenin–ALIX Regulates the Biogenesis of Exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-Modulated Exosome Secretion Promotes Clearance of Amyloid-β by Microglia. J. Biol. Chem. 2012, 287, 10977–10989. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, D.; Gutiérrez-Vázquez, C.; Jorge, I.; López-Martín, S.; Ursa, A.; Sánchez-Madrid, F.; Vázquez, J.; Yáñez-Mó, M. The Intracellular Interactome of Tetraspanin-Enriched Microdomains Reveals Their Function as Sorting Machineries toward Exosomes. J. Biol. Chem. 2013, 288, 11649–11661. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Z.; Ding, H.; Zhou, Y.; Doan, H.A.; Sin, K.W.T.; Zhu, Z.J.; Flores, R.; Wen, Y.; Gong, X.; et al. Tumor Induces Muscle Wasting in Mice through Releasing Extracellular Hsp70 and Hsp90. Nat. Commun. 2017, 8, 589. [Google Scholar] [CrossRef]
- Lauwers, E.; Wang, Y.-C.; Gallardo, R.; Van der Kant, R.; Michiels, E.; Swerts, J.; Baatsen, P.; Zaiter, S.S.; McAlpine, S.R.; Gounko, N.V.; et al. Hsp90 Mediates Membrane Deformation and Exosome Release. Mol. Cell 2018, 71, 689–702.e9. [Google Scholar] [CrossRef]
- van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A Common Pathway for a Specialized Function. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef]
- Blanc, L.; Vidal, M. New Insights into the Function of Rab GTPases in the Context of Exosomal Secretion. Small GTPases 2018, 9, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, N.; Lankar, D.; Faure, F.; Regnault, A.; Dumont, C.; Raposo, G.; Hivroz, C. TCR Activation of Human T Cells Induces the Production of Exosomes Bearing the TCR/CD3/ζ Complex. J. Immunol. 2002, 168, 3235–3241. [Google Scholar] [CrossRef] [PubMed]
- Leone, D.A.; Rees, A.J.; Kain, R. Dendritic Cells and Routing Cargo into Exosomes. Immunol. Cell Biol. 2018, 96, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, Biogenesis, and Mechanisms in Cancer Metastasis and Drug Resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Möbius, W.; Van Donselaar, E.; Ohno-Iwashita, Y.; Shimada, Y.; Heijnen, H.F.G.; Slot, J.W.; Geuze, H.J. Recycling Compartments and the Internal Vesicles of Multivesicular Bodies Harbor Most of the Cholesterol Found in the Endocytic Pathway: Cholesterol in the Endocytic Pathway. Traffic 2003, 4, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Phuyal, S.; Skotland, T.; Hessvik, N.P.; Simolin, H.; Øverbye, A.; Brech, A.; Parton, R.G.; Ekroos, K.; Sandvig, K.; Llorente, A. The Ether Lipid Precursor Hexadecylglycerol Stimulates the Release and Changes the Composition of Exosomes Derived from PC-3 Cells. J. Biol. Chem. 2015, 290, 4225–4237. [Google Scholar] [CrossRef] [PubMed]
- McAndrews, K.M.; Kalluri, R. Mechanisms Associated with Biogenesis of Exosomes in Cancer. Mol. Cancer 2019, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Wang, J.; Pan, S.; Zheng, L.; Wang, Z.-W.; Zhu, X. Nucleic Acids and Proteins Carried by Exosomes of Different Origins as Potential Biomarkers for Gynecologic Cancers. Mol. Ther. Oncolytics 2022, 24, 101–113. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Ahmed, M.; Osaid, Z.; Hamoudi, R.; Harati, R. Insights into Exosome Transport through the Blood–Brain Barrier and the Potential Therapeutical Applications in Brain Diseases. Pharmaceuticals 2023, 16, 571. [Google Scholar] [CrossRef]
- Gómez-Molina, C.; Sandoval, M.; Henzi, R.; Ramírez, J.P.; Varas-Godoy, M.; Luarte, A.; Lafourcade, C.A.; Lopez-Verrilli, A.; Smalla, K.-H.; Kaehne, T.; et al. Small Extracellular Vesicles in Rat Serum Contain Astrocyte-Derived Protein Biomarkers of Repetitive Stress. Int. J. Neuropsychopharmacol. 2019, 22, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Tachikawa, M.; Yagi, Y.; Umetsu, M.; Nurdin, A.; Miyauchi, E.; Watanabe, M.; Uchida, Y.; Terasaki, T. Cluster of Differentiation 46 Is the Major Receptor in Human Blood–Brain Barrier Endothelial Cells for Uptake of Exosomes Derived from Brain-Metastatic Melanoma Cells (SK-Mel-28). Mol. Pharm. 2019, 16, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh, M.; Gürsoy-Özdemir, Y.; Kaya, M.; Eslami Abriz, A.; Zarebkohan, A.; Rahbarghazi, R.; Sokullu, E. Exosomal Delivery of Therapeutic Modulators through the Blood–Brain Barrier; Promise and Pitfalls. Cell Biosci. 2021, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Sheng, L.; Li, N.; Bullock, K.; Song, N.; Shi, M.; Banks, W.A.; Zhang, J. Transmission of α-Synuclein-Containing Erythrocyte-Derived Extracellular Vesicles across the Blood-Brain Barrier via Adsorptive Mediated Transcytosis: Another Mechanism for Initiation and Progression of Parkinson’s Disease? Acta Neuropathol. Commun. 2017, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhu, Y.-L.; Hu, F.-H.; Wang, Y.-Y.; Huang, N.-P.; Xiao, Z.-D. Dynamics of Exosome Internalization and Trafficking. J. Cell. Physiol. 2013, 228, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.P.; Zhang, S.X.; Zimak, J.; Ségaliny, A.; et al. Elucidation of Exosome Migration Across the Blood–Brain Barrier Model In Vitro. Cell. Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mater. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.E.; Holst, M.R.; Nielsen, M.S. Vesicular Transport Machinery in Brain Endothelial Cells: What We Know and What We Do Not. Curr. Pharm. Des. 2020, 26, 1405–1416. [Google Scholar] [CrossRef]
- Hromada, C.; Mühleder, S.; Grillari, J.; Redl, H.; Holnthoner, W. Endothelial Extracellular Vesicles—Promises and Challenges. Front. Physiol. 2017, 8, 275. [Google Scholar] [CrossRef]
- Lachenal, G.; Pernet-Gallay, K.; Chivet, M.; Hemming, F.J.; Belly, A.; Bodon, G.; Blot, B.; Haase, G.; Goldberg, Y.; Sadoul, R. Release of Exosomes from Differentiated Neurons and Its Regulation by Synaptic Glutamatergic Activity. Mol. Cell. Neurosci. 2011, 46, 409–418. [Google Scholar] [CrossRef]
- Glebov, K.; Löchner, M.; Jabs, R.; Lau, T.; Merkel, O.; Schloss, P.; Steinhäuser, C.; Walter, J. Serotonin Stimulates Secretion of Exosomes from Microglia Cells: Serotonin Stimulates Microglial Exosome Release. Glia 2015, 63, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-Derived ATP Induces Vesicle Shedding and IL-1β Release from Microglia. J. Immunol. 2005, 174, 7268–7277. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Perrotta, C.; Novellino, L.; Francolini, M.; Riganti, L.; Menna, E.; Saglietti, L.; Schuchman, E.H.; Furlan, R.; Clementi, E.; et al. Acid Sphingomyelinase Activity Triggers Microparticle Release from Glial Cells. EMBO J. 2009, 28, 1043–1054. [Google Scholar] [CrossRef]
- Chatterjee, V.; Yang, X.; Ma, Y.; Cha, B.; Meegan, J.E.; Wu, M.; Yuan, S.Y. Endothelial Microvesicles Carrying Src-Rich Cargo Impair Adherens Junction Integrity and Cytoskeleton Homeostasis. Cardiovasc. Res. 2020, 116, 1525–1538. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhu, P.; Liu, S.; Jin, Z.; Li, D.; Zhao, H.; Zhu, X.; Shu, C.; Yan, D.; Dong, Z. IL-4-Polarized BV2 Microglia Cells Promote Angiogenesis by Secreting Exosomes. Adv. Clin. Exp. Med. 2019, 28, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Niu, F.; Yao, H.; Liao, K.; Chen, X.; Kook, Y.; Ma, R.; Hu, G.; Buch, S. Exosomal miR-9 Released from HIV Tat Stimulated Astrocytes Mediates Microglial Migration. J. Neuroimmune Pharmacol. 2018, 13, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Dozio, V.; Sanchez, J.-C. Characterisation of Extracellular Vesicle-Subsets Derived from Brain Endothelial Cells and Analysis of Their Protein Cargo Modulation after TNF Exposure. J. Extracell. Vesicles 2017, 6, 1302705. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Y.; Du, X.-F.; Li, J.; Zi, H.-X.; Bu, J.-W.; Yan, Y.; Han, H.; Du, J.-L. Neurons Secrete miR-132-Containing Exosomes to Regulate Brain Vascular Integrity. Cell Res. 2017, 27, 882–897. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, W.J.; Choi, Y.K.; Song, H.S.; Son, M.J.; Gelman, I.H.; Kim, Y.-J.; Kim, K.-W. SSeCKS Regulates Angiogenesis and Tight Junction Formation in Blood-Brain Barrier. Nat. Med. 2003, 9, 900–906. [Google Scholar] [CrossRef]
- Kriaučiūnaitė, K.; Kaušylė, A.; Pajarskienė, J.; Tunaitis, V.; Lim, D.; Verkhratsky, A.; Pivoriūnas, A. Immortalised Hippocampal Astrocytes from 3xTG-AD Mice Fail to Support BBB Integrity In Vitro: Role of Extracellular Vesicles in Glial-Endothelial Communication. Cell Mol. Neurobiol. 2021, 41, 551–562. [Google Scholar] [CrossRef]
- Mentor, S.; Fisher, D. High-Resolution Insights Into the in Vitro Developing Blood-Brain Barrier: Novel Morphological Features of Endothelial Nanotube Function. Front. Neuroanat. 2021, 15, 661065. [Google Scholar] [CrossRef] [PubMed]
- Virgintino, D.; Rizzi, M.; Errede, M.; Strippoli, M.; Girolamo, F.; Bertossi, M.; Roncali, L. Plasma Membrane-Derived Microvesicles Released from Tip Endothelial Cells during Vascular Sprouting. Angiogenesis 2012, 15, 761–769. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mentor, S.; Fisher, D. Exosomes Form Tunneling Nanotubes (TUNTs) in the Blood-Brain Barrier: A Nano-Anatomical Perspective of Barrier Genesis. Front. Mol. Neurosci. 2022, 15, 938315. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the Blood–Brain Barrier Really Disrupted in All Glioblastomas? A Critical Assessment of Existing Clinical Data. Neuro-Oncology 2018, 20, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Uzunalli, G.; Dieterly, A.M.; Kemet, C.M.; Weng, H.-Y.; Soepriatna, A.H.; Goergen, C.J.; Shinde, A.B.; Wendt, M.K.; Lyle, L.T. Dynamic Transition of the Blood-Brain Barrier in the Development of Non-Small Cell Lung Cancer Brain Metastases. Oncotarget 2019, 10, 6334–6348. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous Blood–Tumor Barrier Permeability Determines Drug Efficacy in Experimental Brain Metastases of Breast Cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Tumor-Derived Exosomes and Their Role in Cancer Progression. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 74, pp. 103–141. ISBN 978-0-12-804689-0. [Google Scholar]
- Wang, Z.-F.; Liao, F.; Wu, H.; Dai, J. Glioma Stem Cells-Derived Exosomal miR-26a Promotes Angiogenesis of Microvessel Endothelial Cells in Glioma. J. Exp. Clin. Cancer Res. 2019, 38, 201. [Google Scholar] [CrossRef]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-Secreted miR-105 Destroys Vascular Endothelial Barriers to Promote Metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef]
- Maher, E.A.; Furnari, F.B.; Bachoo, R.M.; Rowitch, D.H.; Louis, D.N.; Cavenee, W.K.; DePinho, R.A. Malignant Glioma: Genetics and Biology of a Grave Matter. Genes. Dev. 2001, 15, 1311–1333. [Google Scholar] [CrossRef]
- Schwartzbaum, J.A.; Fisher, J.L.; Aldape, K.D.; Wrensch, M. Epidemiology and Molecular Pathology of Glioma. Nat. Rev. Neurol. 2006, 2, 494–503. [Google Scholar] [CrossRef]
- Alves, T.R.; Lima, F.R.S.; Kahn, S.A.; Lobo, D.; Dubois, L.G.F.; Soletti, R.; Borges, H.; Neto, V.M. Glioblastoma Cells: A Heterogeneous and Fatal Tumor Interacting with the Parenchyma. Life Sci. 2011, 89, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Iacob, G.; Dinca, E.B. Current Data and Strategy in Glioblastoma Multiforme. J. Med. Life 2009, 2, 386–393. [Google Scholar] [PubMed]
- Miller, C.R.; Perry, A. Glioblastoma. Arch. Pathol. Lab. Med. 2007, 131, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Ohka, F.; Natsume, A.; Wakabayashi, T. Current Trends in Targeted Therapies for Glioblastoma Multiforme. Neurol. Res. Int. 2012, 2012, 878425. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and Molecular Prognostic Review of Glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Stefanik, D.F.; Fellows, W.K.; Rizkalla, L.R.; Rizkalla, W.M.; Stefanik, P.P.; Deleo, A.B.; Welch, W.C. Monoclonal Antibodies to Vascular Endothelial Growth Factor (VEGF) and the VEGF Receptor, FLT-1, Inhibit the Growth of C6 Glioma in a Mouse Xenograft. J. Neuro-Oncol. 2001, 55, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Hill, R.; Pilkington, G.; Madureira, P. The Role of Hypoxia in Glioblastoma Invasion. Cells 2017, 6, 45. [Google Scholar] [CrossRef]
- Engelhardt, S.; Al-Ahmad, A.J.; Gassmann, M.; Ogunshola, O.O. Hypoxia Selectively Disrupts Brain Microvascular Endothelial Tight Junction Complexes Through a Hypoxia-Inducible Factor-1 (HIF-1) Dependent Mechanism: HIF-1 MEDIATES TIGHT JUNCTION DISRUPTION. J. Cell. Physiol. 2014, 229, 1096–1105. [Google Scholar] [CrossRef]
- Schoch, H.J. Hypoxia-Induced Vascular Endothelial Growth Factor Expression Causes Vascular Leakage in the Brain. Brain 2002, 125, 2549–2557. [Google Scholar] [CrossRef]
- Treps, L.; Perret, R.; Edmond, S.; Ricard, D.; Gavard, J. Glioblastoma Stem-like Cells Secrete the pro-Angiogenic VEGF-A Factor in Extracellular Vesicles. J. Extracell. Vesicles 2017, 6, 1359479. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, H.; Xiong, C.; Liu, Y. Hypoxic Glioblastoma Release Exosomal VEGF-A Induce the Permeability of Blood-Brain Barrier. Biochem. Biophys. Res. Commun. 2018, 502, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; Delle Monache, S.; Di Francesco, M.; Sanità, P.; D’Ascenzo, S.; Gravina, G.L.; Festuccia, C.; Dolo, V. From Glioblastoma to Endothelial Cells through Extracellular Vesicles: Messages for Angiogenesis. Tumor Biol. 2016, 37, 12743–12753. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; Sathornsumetee, S.; Hao, Y.; Li, Z.; Hjelmeland, A.B.; Shi, Q.; McLendon, R.E.; Bigner, D.D.; Rich, J.N. Stem Cell–like Glioma Cells Promote Tumor Angiogenesis through Vascular Endothelial Growth Factor. Cancer Res. 2006, 66, 7843–7848. [Google Scholar] [CrossRef] [PubMed]
- Eramo, A.; Ricci-Vitiani, L.; Zeuner, A.; Pallini, R.; Lotti, F.; Sette, G.; Pilozzi, E.; Larocca, L.M.; Peschle, C.; De Maria, R. Chemotherapy Resistance of Glioblastoma Stem Cells. Cell Death Differ. 2006, 13, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.-L.; Hu, G.-W.; Chen, Y.; Liu, Y.; Tu, W.; Lu, Y.-M.; Wu, L.; Xu, G.-H. Glioma Cells Promote Angiogenesis through the Release of Exosomes Containing Long Non-Coding RNA POU3F3. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 959–972. [Google Scholar] [PubMed]
- Huo, H.; Yang, S.; Wu, H.; Sun, Y.; Zhao, R.; Ye, R.; Yan, D.; Shi, X.; Yang, J. Brain endothelial cells-derived extracellular vesicles overexpressing ECRG4 inhibit glioma proliferation through suppressing inflammation and angiogenesis. J. Tissue Eng. Regen. Med. 2021, 15, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, Y.; Wang, L.; Li, S.; Zhou, J.; Tan, Y.; Song, J.; Xing, H.; Yi, K.; Zhan, Q.; et al. Glioma-Derived Exosomes Hijack the Blood–Brain Barrier to Facilitate Nanocapsule Delivery via LCN2. J. Control. Release 2022, 345, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Sidu, R.K.; Zou, H.; Alam, M.K.; Yang, M.; Lee, Y. Inhibition of Glioma Cells’ Proliferation by Doxorubicin-Loaded Exosomes via Microfluidics. Int. J. Nanomed. 2020, 15, 8331–8343. [Google Scholar] [CrossRef]
- Zhang, C.; Song, J.; Lou, L.; Qi, X.; Zhao, L.; Fan, B.; Sun, G.; Lv, Z.; Fan, Z.; Jiao, B.; et al. Doxorubicin-loaded Nanoparticle Coated with Endothelial Cells-derived Exosomes for Immunogenic Chemotherapy of Glioblastoma. Bioeng. Transla Med. 2021, 6, e10203. [Google Scholar] [CrossRef]
- Lee, H.; Bae, K.; Baek, A.-R.; Kwon, E.-B.; Kim, Y.-H.; Nam, S.-W.; Lee, G.H.; Chang, Y. Glioblastoma-Derived Exosomes as Nanopharmaceutics for Improved Glioma Treatment. Pharmaceutics 2022, 14, 1002. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, T.; He, W.; Jin, H.; Liu, C.; Yang, Z.; Ren, J. Methotrexate-Loaded Extracellular Vesicles Functionalized with Therapeutic and Targeted Peptides for the Treatment of Glioblastoma Multiforme. ACS Appl. Mater. Interfaces 2018, 10, 12341–12350. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Ling, X.; Yang, Y.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Chen, B.; Li, H.; Wang, Y.; et al. Embryonic Stem Cells-Derived Exosomes Endowed with Targeting Properties as Chemotherapeutics Delivery Vehicles for Glioblastoma Therapy. Adv. Sci. 2019, 6, 1801899. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liang, Q.; Zhang, X.; Di, Z.; Wang, X.; Di, L. Tumor-Derived Exosomes Reversing TMZ Resistance by Synergistic Drug Delivery for Glioma-Targeting Treatment. Colloids Surf. B Biointerfaces 2022, 215, 112505. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.U.; Liu, Y.; Yang, Q.; Yang, H.; Liu, R.; Zhang, D.; Muhammad, P.; Liu, Y.; Hanif, S.; Ismail, M.; et al. Heme Oxygenase-1 Targeting Exosomes for Temozolomide Resistant Glioblastoma Synergistic Therapy. J. Control. Release 2022, 345, 696–708. [Google Scholar] [CrossRef] [PubMed]
- Boire, A.; Brastianos, P.K.; Garzia, L.; Valiente, M. Brain Metastasis. Nat. Rev. Cancer 2020, 20, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Schur, S.; Füreder, L.M.; Gatterbauer, B.; Dieckmann, K.; Widhalm, G.; Hainfellner, J.; Zielinski, C.C.; Birner, P.; Bartsch, R.; et al. Descriptive Statistical Analysis of a Real Life Cohort of 2419 Patients with Brain Metastases of Solid Cancers. ESMO Open 2016, 1, e000024. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Chao, S.T.; Sneed, P.K.; Luo, X.; Suh, J.; Roberge, D.; Bhatt, A.; Jensen, A.W.; Brown, P.D.; Shih, H.; et al. Diagnosis-Specific Prognostic Factors, Indexes, and Treatment Outcomes for Patients with Newly Diagnosed Brain Metastases: A Multi-Institutional Analysis of 4,259 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery with Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016, 316, 401. [Google Scholar] [CrossRef] [PubMed]
- Soffietti, R.; Kocher, M.; Abacioglu, U.M.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.-D.; Carrie, C.; et al. A European Organisation for Research and Treatment of Cancer Phase III Trial of Adjuvant Whole-Brain Radiotherapy Versus Observation in Patients with One to Three Brain Metastases from Solid Tumors After Surgical Resection or Radiosurgery: Quality-of-Life Results. J. Clin. Oncol. 2013, 31, 65–72. [Google Scholar] [CrossRef]
- Hoshide, R.; Jandial, R. The Role of the Neural Niche in Brain Metastasis. Clin. Exp. Metastasis 2017, 34, 369–376. [Google Scholar] [CrossRef]
- Shumakovich, M.A.; Mencio, C.P.; Siglin, J.S.; Moriarty, R.A.; Geller, H.M.; Stroka, K.M. Astrocytes from the Brain Microenvironment Alter Migration and Morphology of Metastatic Breast Cancer Cells. FASEB J. 2017, 31, 5049–5067. [Google Scholar] [CrossRef] [PubMed]
- Orr, F.W.; Wang, H.H.; Lafrenie, R.M.; Scherbarth, S.; Nance, D.M. Interactions between Cancer Cells and the Endothelium in Metastasis. J. Pathol. 2000, 190, 310–329. [Google Scholar] [CrossRef]
- Lee, T.-H.; Avraham, H.K.; Jiang, S.; Avraham, S. Vascular Endothelial Growth Factor Modulates the Transendothelial Migration of MDA-MB-231 Breast Cancer Cells through Regulation of Brain Microvascular Endothelial Cell Permeability. J. Biol. Chem. 2003, 278, 5277–5284. [Google Scholar] [CrossRef]
- Kinjyo, I.; Bragin, D.; Grattan, R.; Winter, S.S.; Wilson, B.S. Leukemia-derived Exosomes and Cytokines Pave the Way for Entry into the Brain. J. Leukoc. Biol. 2019, 105, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Alsabbagh, R.; Ahmed, M.; Alqudah, M.A.Y.; Hamoudi, R.; Harati, R. Insights into the Molecular Mechanisms Mediating Extravasation in Brain Metastasis of Breast Cancer, Melanoma, and Lung Cancer. Cancers 2023, 15, 2258. [Google Scholar] [CrossRef] [PubMed]
- Harati, R.; Hammad, S.; Tlili, A.; Mahfood, M.; Mabondzo, A.; Hamoudi, R. miR-27a-3p Regulates Expression of Intercellular Junctions at the Brain Endothelium and Controls the Endothelial Barrier Permeability. PLoS ONE 2022, 17, e0262152. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.; Mabondzo, A.; Hamoudi, R.; Harati, R. Regulation of P-Glycoprotein by miR-27a-3p at the Brain Endothelial Barrier. J. Pharm. Sci. 2022, 111, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Harati, R.; Mabondzo, A.; Tlili, A.; Khoder, G.; Mahfood, M.; Hamoudi, R. Combinatorial Targeting of microRNA-26b and microRNA-101 Exerts a Synergistic Inhibition on Cyclooxygenase-2 in Brain Metastatic Triple-Negative Breast Cancer Cells. Breast Cancer Res. Treat. 2021, 187, 695–713. [Google Scholar] [CrossRef]
- Harati, R.; Hafezi, S.; Mabondzo, A.; Tlili, A. Silencing miR-202-3p Increases MMP-1 and Promotes a Brain Invasive Phenotype in Metastatic Breast Cancer Cells. PLoS ONE 2020, 15, e0239292. [Google Scholar] [CrossRef]
- Harati, R.; Mohammad, M.G.; Tlili, A.; El-Awady, R.A.; Hamoudi, R. Loss of miR-101-3p Promotes Transmigration of Metastatic Breast Cancer Cells through the Brain Endothelium by Inducing COX-2/MMP1 Signaling. Pharmaceuticals 2020, 13, 144. [Google Scholar] [CrossRef]
- Hammash, D.; Mahfood, M.; Khoder, G.; Ahmed, M.; Tlili, A.; Hamoudi, R.; Harati, R. miR-623 Targets Metalloproteinase-1 and Attenuates Extravasation of Brain Metastatic Triple-Negative Breast Cancer Cells. BCTT 2022, 14, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Gill, C.M.; Cahill, D.P.; Santagata, S.; Brastianos, P.K. Treatment of Brain Metastases in the Modern Genomic Era. Pharmacol. Ther. 2017, 170, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, L.; Li, L.; Cao, Y. Exosomes Derived from Brain Metastatic Breast Cancer Cells Destroy the Blood-Brain Barrier by Carrying lncRNA GS1-600G8.5. BioMed Res. Int. 2020, 2020, 7461727. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Schattschneider, J.; Blechner, C.; Krisp, C.; Schlüter, H.; Schweizer, M.; Nalaskowski, M.; Oliveira-Ferrer, L.; Windhorst, S. Tubulin Tyrosine Ligase Like 4 (TTLL4) Overexpression in Breast Cancer Cells Is Associated with Brain Metastasis and Alters Exosome Biogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 205. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.; Hoshino, A.; Kenific, C.M.; Matei, I.R.; Steiner, L.; Freitas, D.; Kim, H.S.; Oxley, P.R.; Scandariato, I.; Casanova-Salas, I.; et al. Tumour Exosomal CEMIP Protein Promotes Cancer Cell Colonization in Brain Metastasis. Nat. Cell Biol. 2019, 21, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Fazakas, C.; Kozma, M.; Molnár, K.; Kincses, A.; Dér, A.; Fejér, A.; Horváth, B.; Wilhelm, I.; Krizbai, I.A.; Végh, A.G. Breast Adenocarcinoma-Derived Exosomes Lower First-Contact de-Adhesion Strength of Adenocarcinoma Cells to Brain Endothelial Layer. Colloids Surf. B Biointerfaces 2021, 204, 111810. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Ulahannan, D.; Khalifa, J.; Faivre-Finn, C.; Lee, S.-M. Emerging Treatment Paradigms for Brain Metastasis in Non-Small-Cell Lung Cancer: An Overview of the Current Landscape and Challenges Ahead. Ann. Oncol. 2017, 28, 2923–2931. [Google Scholar] [CrossRef]
- Gan, D.-X.; Wang, Y.-B.; He, M.-Y.; Chen, Z.-Y.; Qin, X.-X.; Miao, Z.-W.; Chen, Y.-H.; Li, B. Lung Cancer Cells-Controlled Dkk-1 Production in Brain Metastatic Cascade Drive Microglia to Acquire a Pro-Tumorigenic Phenotype. Front. Cell Dev. Biol. 2020, 8, 591405. [Google Scholar] [CrossRef]
- Geng, S.; Tu, S.; Bai, Z.; Geng, Y. Exosomal lncRNA LINC01356 Derived from Brain Metastatic Nonsmall-Cell Lung Cancer Cells Remodels the Blood–Brain Barrier. Front. Oncol. 2022, 12, 825899. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Deng, S.; Li, L.; Liu, T.; Zhang, T.; Li, J.; Yu, Y.; Xu, Y. TGF-Β1-Mediated Exosomal Lnc-MMP2-2 Increases Blood–Brain Barrier Permeability via the miRNA-1207-5p/EPB41L5 Axis to Promote Non-Small Cell Lung Cancer Brain Metastasis. Cell Death Dis. 2021, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- Ebben, J.D.; You, M. Brain Metastasis in Lung Cancer: Building a Molecular and Systems-Level Understanding to Improve Outcomes. Int. J. Biochem. Cell Biol. 2016, 78, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-H.; Miao, Z.-W.; Jiang, Q.-Z.; Gan, D.-X.; Wei, X.-G.; Xue, X.-Z.; Li, J.-Q.; Zheng, F.; Qin, X.-X.; Fang, W.-G.; et al. Brain Microvascular Endothelial Cell Exosome–Mediated S100A16 Up-regulation Confers Small-cell Lung Cancer Cell Survival in Brain. FASEB J. 2019, 33, 1742–1757. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, Y.; Chen, W.; Zhang, M.; Qin, J. Malignant Melanoma-Derived Exosomes Induce Endothelial Damage and Glial Activation on a Human BBB Chip Model. Biosensors 2022, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Marklund, N.; Hillered, L. Animal Modelling of Traumatic Brain Injury in Preclinical Drug Development: Where Do We Go from Here?: Animal Modelling of Traumatic Brain Injury. Br. J. Pharmacol. 2011, 164, 1207–1229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Eom, J.; Hunt, R.F. Transplanted Interneurons Improve Memory Precision after Traumatic Brain Injury. Nat. Commun. 2019, 10, 5156. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, S. Recent Advances in Pathophysiology of Traumatic Brain Injury. Curr. Neuropharmacol. 2018, 16, 1224–1238. [Google Scholar] [CrossRef]
- Di Pietro, V.; Ragusa, M.; Davies, D.; Su, Z.; Hazeldine, J.; Lazzarino, G.; Hill, L.J.; Crombie, N.; Foster, M.; Purrello, M.; et al. MicroRNAs as Novel Biomarkers for the Diagnosis and Prognosis of Mild and Severe Traumatic Brain Injury. J. Neurotrauma 2017, 34, 1948–1956. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.; Su, X.; He, J.; Bai, W.; He, X. MSCs-Derived Exosomes and Neuroinflammation, Neurogenesis and Therapy of Traumatic Brain Injury. Front. Cell. Neurosci. 2017, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Bhatti, U.F.; Brown, J.F.; Biesterveld, B.E.; Kathawate, R.G.; Graham, N.J.; Chtraklin, K.; Siddiqui, A.Z.; Dekker, S.E.; Andjelkovic, A.; et al. Early Single-Dose Treatment with Exosomes Provides Neuroprotection and Improves Blood-Brain Barrier Integrity in Swine Model of Traumatic Brain Injury and Hemorrhagic Shock. J. Trauma Acute Care Surg. 2020, 88, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Higgins, G.A.; Bhatti, U.F.; Biesterveld, B.E.; Dekker, S.E.; Kathawate, R.G.; Tian, Y.; Wu, Z.; Kemp, M.T.; Wakam, G.K.; et al. Early Treatment with Exosomes Following Traumatic Brain Injury and Hemorrhagic Shock in a Swine Model Promotes Transcriptional Changes Associated with Neuroprotection. J. Trauma Acute Care Surg. 2020, 89, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Wu, Z.; Bhatti, U.F.; Biesterveld, B.E.; Kemp, M.T.; Wakam, G.K.; Vercruysse, C.A.; Chtraklin, K.; Siddiqui, A.Z.; Pickell, Z.; et al. Early Single-Dose Exosome Treatment Improves Neurologic Outcomes in a 7-Day Swine Model of Traumatic Brain Injury and Hemorrhagic Shock. J. Trauma Acute Care Surg. 2020, 89, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Andrews, A.M.; Lutton, E.M.; Merkel, S.F.; Razmpour, R.; Ramirez, S.H. Mechanical Injury Induces Brain Endothelial-Derived Microvesicle Release: Implications for Cerebral Vascular Injury during Traumatic Brain Injury. Front. Cell. Neurosci. 2016, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Lei, Q.; Ma, J.; Gao, S.; Ju, R. Endothelial Progenitor Cell-Derived Microvesicles Promote Angiogenesis in Rat Brain Microvascular Endothelial Cells In Vitro. Front. Cell. Neurosci. 2021, 15, 638351. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, F.; Liu, L.; Xu, X.; Zhang, B.; Wu, Y.; Yin, D.; Zhou, S.; Sun, D.; Huang, Y.; et al. Endothelial Colony-Forming Cell-Derived Exosomes Restore Blood-Brain Barrier Continuity in Mice Subjected to Traumatic Brain Injury. Exp. Neurol. 2018, 307, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Duan, H.; Wang, W.; Zhao, S.; Khan, G.J.; Wang, M.; Zhang, Y.; Thakur, K.; Fang, X.; Wu, C.; et al. Ginsenoside Rg1 Ameliorates Blood–Brain Barrier Disruption and Traumatic Brain Injury via Attenuating Macrophages Derived Exosomes miR-21 Release. Acta Pharm. Sin. B 2021, 11, 3493–3507. [Google Scholar] [CrossRef]
- Alluri, H.; Wiggins-Dohlvik, K.; Davis, M.L.; Huang, J.H.; Tharakan, B. Blood–Brain Barrier Dysfunction Following Traumatic Brain Injury. Metab. Brain Dis. 2015, 30, 1093–1104. [Google Scholar] [CrossRef]
- Aoki, M.; Nata, T.; Morishita, R.; Matsushita, H.; Nakagami, H.; Yamamoto, K.; Yamazaki, K.; Nakabayashi, M.; Ogihara, T.; Kaneda, Y. Endothelial Apoptosis Induced by Oxidative Stress Through Activation of NF-κB: Antiapoptotic Effect of Antioxidant Agents on Endothelial Cells. Hypertension 2001, 38, 48–55. [Google Scholar] [CrossRef]
- Balkaya, M.; Kim, I.; Shakil, F.; Cho, S. CD36 Deficiency Reduces Chronic BBB Dysfunction and Scar Formation and Improves Activity, Hedonic and Memory Deficits in Ischemic Stroke. J. Cereb. Blood Flow. Metab. 2021, 41, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Reeson, P.; Tennant, K.A.; Gerrow, K.; Wang, J.; Weiser Novak, S.; Thompson, K.; Lockhart, K.-L.; Holmes, A.; Nahirney, P.C.; Brown, C.E. Delayed Inhibition of VEGF Signaling after Stroke Attenuates Blood-Brain Barrier Breakdown and Improves Functional Recovery in a Comorbidity-Dependent Manner. J. Neurosci. 2015, 35, 5128–5143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Graf, I.; Kuang, Y.; Zheng, X.; Haupt, M.; Majid, A.; Kilic, E.; Hermann, D.M.; Psychogios, M.-N.; Weber, M.S.; et al. Neural Progenitor Cell-Derived Extracellular Vesicles Enhance Blood-Brain Barrier Integrity by NF-κB (Nuclear Factor-κB)-Dependent Regulation of ABCB1 (ATP-Binding Cassette Transporter B1) in Stroke Mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1127–1145. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Y.; Song, J.-X.; Cai, H.; Wang, P.-P.; Yin, Q.-L.; Zhang, Y.-D.; Chen, J.; Li, M.; Song, J.-J.; Wang, Y.-L.; et al. Healthy Serum-Derived Exosomes Improve Neurological Outcomes and Protect Blood–Brain Barrier by Inhibiting Endothelial Cell Apoptosis and Reversing Autophagy-Mediated Tight Junction Protein Reduction in Rat Stroke Model. Front. Cell. Neurosci. 2022, 16, 841544. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Kuang, X.; Cai, S.; Wang, X.; Du, D.; Wang, J.; Wang, Y.; Chen, Y.; Bihl, J.; Chen, Y.; et al. miR-132-3p Priming Enhances the Effects of Mesenchymal Stromal Cell-Derived Exosomes on Ameliorating Brain Ischemic Injury. Stem Cell Res. Ther. 2020, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Luo, C.; Deng, M.; Zhao, M. Scutellarin-treated Exosomes Increase Claudin 5, Occludin and ZO1 Expression in Rat Brain Microvascular Endothelial Cells. Exp. Ther. Med. 2019, 18, 33–40. [Google Scholar] [CrossRef]
- Aragon, M.J.; Topper, L.; Tyler, C.R.; Sanchez, B.; Zychowski, K.; Young, T.; Herbert, G.; Hall, P.; Erdely, A.; Eye, T.; et al. Serum-Borne Bioactivity Caused by Pulmonary Multiwalled Carbon Nanotubes Induces Neuroinflammation via Blood–Brain Barrier Impairment. Proc. Natl. Acad. Sci. USA 2017, 114, E1968–E1976. [Google Scholar] [CrossRef]
- Pan, J.; He, R.; Huo, Q.; Shi, Y.; Zhao, L. Brain Microvascular Endothelial Cell Derived Exosomes Potently Ameliorate Cognitive Dysfunction by Enhancing the Clearance of Aβ Through Up-Regulation of P-Gp in Mouse Model of AD. Neurochem. Res. 2020, 45, 2161–2172. [Google Scholar] [CrossRef]
- Li, Q.; Nong, A.; Huang, Z.; Xu, Y.; He, K.; Jia, Y.; Huang, Y. Exosomes Containing miR-122-5p Secreted by LPS-Induced Neutrophils Regulate the Apoptosis and Permeability of Brain Microvascular Endothelial Cells by Targeting OCLN. Am. J. Transl. Res. 2021, 13, 4167–4181. [Google Scholar]
- Cerutti, C.; Edwards, L.J.; de Vries, H.E.; Sharrack, B.; Male, D.K.; Romero, I.A. MiR-126 and miR-126* Regulate Shear-Resistant Firm Leukocyte Adhesion to Human Brain Endothelium. Sci. Rep. 2017, 7, 45284. [Google Scholar] [CrossRef]
- Paul, D.; Baena, V.; Ge, S.; Jiang, X.; Jellison, E.R.; Kiprono, T.; Agalliu, D.; Pachter, J.S. Appearance of Claudin-5+ Leukocytes in the Central Nervous System during Neuroinflammation: A Novel Role for Endothelial-Derived Extracellular Vesicles. J. Neuroinflammation 2016, 13, 292. [Google Scholar] [CrossRef]
- Huang, K.; Huang, L.; Zhang, X.; Zhang, M.; Wang, Q.; Lin, H.; Yu, Z.; Li, X.; Liu, X.B.; Wu, Q.; et al. Mast Cells-Derived Exosomes Worsen the Development of Experimental Cerebral Malaria. Acta Trop. 2021, 224, 106145. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.D.; Ordain, N.S.; Kutscher, H.; Karki, S.; Reynolds, J.L. HIV Neuroinflammation: The Role of Exosomes in Cell Signaling, Prognostic and Diagnostic Biomarkers and Drug Delivery. Front. Cell Dev. Biol. 2021, 9, 637192. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.K.; Rutkai, I.; Kim, H.; Braun, S.E.; Abdel-Mageed, A.B.; Mondal, D.; Busija, D.W. Latent HIV-Exosomes Induce Mitochondrial Hyperfusion Due to Loss of Phosphorylated Dynamin-Related Protein 1 in Brain Endothelium. Mol. Neurobiol. 2021, 58, 2974–2989. [Google Scholar] [CrossRef] [PubMed]
- Raymond, A.D.; Diaz, P.; Chevelon, S.; Agudelo, M.; Yndart-Arias, A.; Ding, H.; Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Roy, U.; et al. Microglia-Derived HIV Nef+ Exosome Impairment of the Blood–Brain Barrier Is Treatable by Nanomedicine-Based Delivery of Nef Peptides. J. Neurovirol. 2016, 22, 129–139. [Google Scholar] [CrossRef] [PubMed]
- András, I.E.; Leda, A.; Contreras, M.G.; Bertrand, L.; Park, M.; Skowronska, M.; Toborek, M. Extracellular Vesicles of the Blood-Brain Barrier: Role in the HIV-1 Associated Amyloid Beta Pathology. Mol. Cell. Neurosci. 2017, 79, 12–22. [Google Scholar] [CrossRef] [PubMed]
- András, I.E.; Sewell, B.B.; Toborek, M. HIV-1 and Amyloid Beta Remodel Proteome of Brain Endothelial Extracellular Vesicles. Int. J. Mater. Sci. 2020, 21, 2741. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Pritzkow, S. Protein Misfolding, Aggregation, and Conformational Strains in Neurodegenerative Diseases. Nat. Neurosci. 2018, 21, 1332–1340. [Google Scholar] [CrossRef]
- Zenaro, E.; Piacentino, G.; Constantin, G. The Blood-Brain Barrier in Alzheimer’s Disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Barber, R.M.; Bhutta, Z.A.; Dandona, L.; Gething, P.W.; Hay, S.I.; Kinfu, Y.; Larson, H.J.; Liang, X.; Lim, S.S.; et al. Global, Regional, and National Levels of Maternal Mortality, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1775–1812. [Google Scholar] [CrossRef]
- Braak, H.; Tredici, K.D.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Honsho, M.; Zahn, T.R.; Keller, P.; Geiger, K.D.; Verkade, P.; Simons, K. Alzheimer’s Disease β-Amyloid Peptides Are Released in Association with Exosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 11172–11177. [Google Scholar] [CrossRef] [PubMed]
- Kuhnke, D.; Jedlitschky, G.; Grube, M.; Krohn, M.; Jucker, M.; Mosyagin, I.; Cascorbi, I.; Walker, L.C.; Kroemer, H.K.; Warzok, R.W.; et al. MDR1-P-Glycoprotein (ABCB1) Mediates Transport of Alzheimer’s Amyloid-β Peptides—Implications for the Mechanisms of Aβ Clearance at the Blood–Brain Barrier. Brain Pathol. 2007, 17, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s Disease: A Matter of Blood–Brain Barrier Dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef] [PubMed]
- Ridler, C. BACE1 Inhibitors Block New Aβ Plaque Formation. Nat. Rev. Neurol. 2018, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bodles-Brakhop, M.A.; Barger, W.S. A Role for P-Glycoprotein in Clearance of Alzheimer Amyloid β-Peptide from the Brain. Curr. Alzheimer Res. 2016, 13, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huber, C.C.; Wang, H. Disrupted Blood-Brain Barrier in 5×FAD Mouse Model of Alzheimer’s Disease Can Be Mimicked and Repaired in Vitro with Neural Stem Cell-Derived Exosomes. Biochem. Biophys. Res. Commun. 2020, 525, 192–196. [Google Scholar] [CrossRef]
- Darland, D.C.; D’Amore, P.A. TGF Beta Is Required for the Formation of Capillary-like Structures in Three-Dimensional Cocultures of 10T1/2 and Endothelial Cells. Angiogenesis 2001, 4, 11–20. [Google Scholar] [CrossRef]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-Primed Exosomes Potently Ameliorate Cognitive Function in AD Mice by Inhibiting Hyperphosphorylation of the Tau Protein through the AKT/GSK-3β Pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef]
- Chen, H.-X.; Liang, F.-C.; Gu, P.; Xu, B.-L.; Xu, H.-J.; Wang, W.-T.; Hou, J.-Y.; Xie, D.-X.; Chai, X.-Q.; An, S.-J. Exosomes Derived from Mesenchymal Stem Cells Repair a Parkinson’s Disease Model by Inducing Autophagy. Cell Death Dis. 2020, 11, 288. [Google Scholar] [CrossRef]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-Loaded Blood Exosomes Targeted to Brain for Better Treatment of Parkinson’s Disease. J. Control. Release 2018, 287, 156–166. [Google Scholar] [CrossRef]


| Transport Pathway | Mode of Transport | Energy Requirement | Reference |
|---|---|---|---|
| Fusion with the plasma membrane | Movement is achieved through physical interaction between the exosome and the cell plasma membrane | Low | [48] |
| Paracytosis | Movement of exosomes between the BMEC | Low | [43] |
| Transcytosis | Movement of exosomes across the BMEC | High | [45,46] |
| Micropinocytosis | The inward curvature of the cellular membrane and engulfment of exosomes | High | [43] |
| Type of Metastatic Cancer | Exosomal Content | Effect on the BBB | In-Vivo/In-Vitro | Reference |
|---|---|---|---|---|
| Breast cancer | miR-105 | Reduce the expression of TJs at BMEC | in-vitro and in-vivo | [69] |
| Breast cancer | miR-181c | Change the localization of TJs and actin filaments by inducing downregulation of PDPK1 gene in BMEC | in-vivo and in-vitro | [12] |
| Breast cancer | lncRNA GS1-600G8.5 | Reduce the expression of TJs at BMEC | in-vitro | [115] |
| Breast cancer | CEMIP | Increase the vascular co-option | in-vitro | [117] |
| Induce proinflammatory phenotype in BMEC | in-vivo | |||
| Lung cancer | miR-550a-3-5p | Reduce expression of Yes-associated protein 1 in BMEC | in-vitro | [16] |
| Lung cancer | LINC01356 | Reduce expression of TJs at BMEC | in-vitro | [122] |
| Lung cancer | Lnc-MMP2-2 | Upregulate EPB41L5 that induces endothelial-to-Mesenchymal Transition and causes a reciprocal repression of BMECs TJs | in-vitro and in-vivo | [123] |
| Condition | Trial Description | Interventions | Phase | Study Status | Enrollment | NCT Number |
|---|---|---|---|---|---|---|
| Cerebrovascular Disorders | Using MSC exosomes to improve functional recovery in poststroke patients. | MSC exosomes | Phase 1, Phase 2 | Unknown | N = 5 | NCT03384433 |
| Refractory Focal Epilepsy | Assess the safety, efficacy, and tolerability of nasal drops containing induced pluripotent stem cell (IPSC) exosomes in focal refractory epilepsy therapy. | IPSC exosomes | Early Phase 1 | Recruiting | N = 34 | NCT05886205 |
| Post-stroke Dementia | Investigating the significance of acupuncture-induced exosomes in post-stroke dementia therapy. | Device: Acupuncture | Not applicable | Recruiting | N = 30 | NCT05326724 |
| Alzheimer Disease | Assessing the efficacy and safety of allogenic adipose mesenchymal stem cell exosomes in AD patients with dementia. | low, mild, and high dosage MSC Exosomes administrated for nasal drip | Phase 1, Phase 2 | Unknown Status | N = 9 | NCT04388982 |
| Malignant Glioma of Brain | Activating the patient’s immune system using apoptosis released tumor exosomes loaded with tumor antigens. | Drug: IGF-1R/AS ODN | Phase 1 | Completed | N = 13 | NCT01550523 |
| Device: biodiffusion chamber |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osaid, Z.; Haider, M.; Hamoudi, R.; Harati, R. Exosomes Interactions with the Blood–Brain Barrier: Implications for Cerebral Disorders and Therapeutics. Int. J. Mol. Sci. 2023, 24, 15635. https://doi.org/10.3390/ijms242115635
Osaid Z, Haider M, Hamoudi R, Harati R. Exosomes Interactions with the Blood–Brain Barrier: Implications for Cerebral Disorders and Therapeutics. International Journal of Molecular Sciences. 2023; 24(21):15635. https://doi.org/10.3390/ijms242115635
Chicago/Turabian StyleOsaid, Zaynab, Mohamed Haider, Rifat Hamoudi, and Rania Harati. 2023. "Exosomes Interactions with the Blood–Brain Barrier: Implications for Cerebral Disorders and Therapeutics" International Journal of Molecular Sciences 24, no. 21: 15635. https://doi.org/10.3390/ijms242115635
APA StyleOsaid, Z., Haider, M., Hamoudi, R., & Harati, R. (2023). Exosomes Interactions with the Blood–Brain Barrier: Implications for Cerebral Disorders and Therapeutics. International Journal of Molecular Sciences, 24(21), 15635. https://doi.org/10.3390/ijms242115635









