Perilla-Leaf-Derived Extracellular Vesicles Selectively Inhibit Breast Cancer Cell Proliferation and Invasion
Abstract
:1. Introduction
2. Results and Discussion
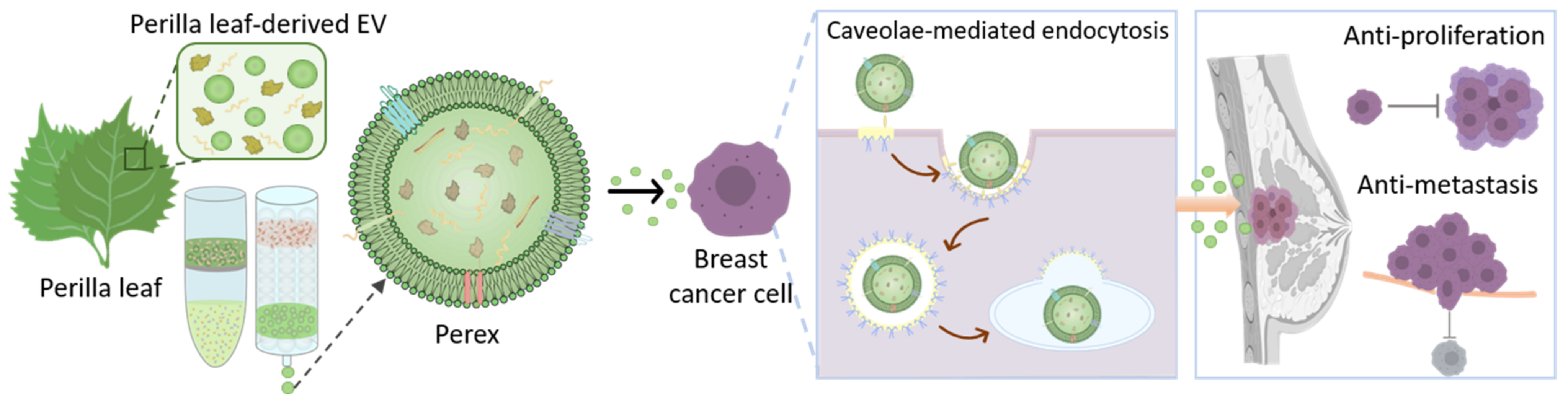
2.1. Isolation of Perex from Perilla Leaves Using Ultrafiltration and Size-Exclusion Chromatography
2.2. Characterization of Perilla-Leaf-Derived EVs
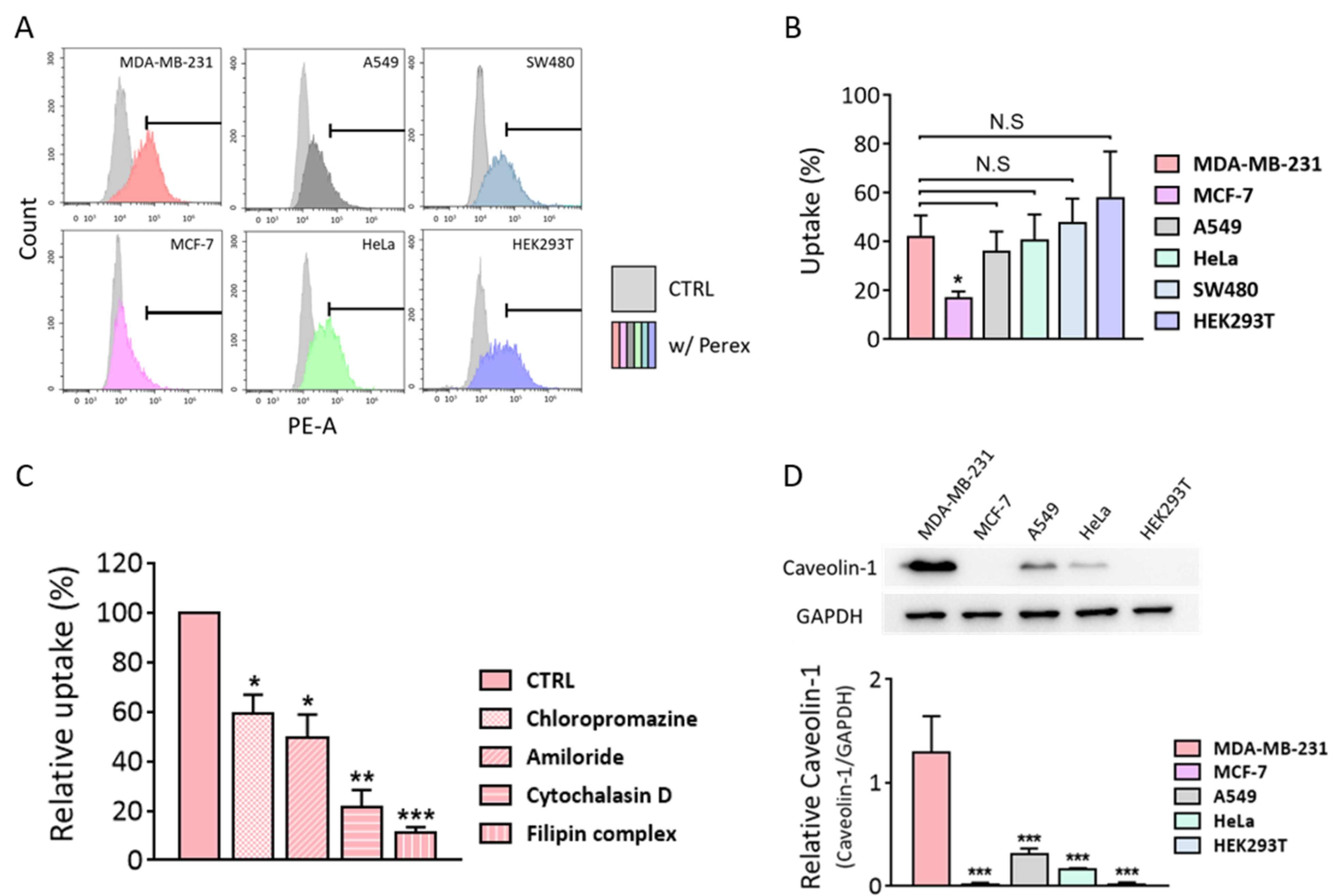
2.3. Perex Stability and Cellular Internalization of Perex in Breast Cancer Cells
2.4. Cytotoxic Effects of Perex against Various Cell Types
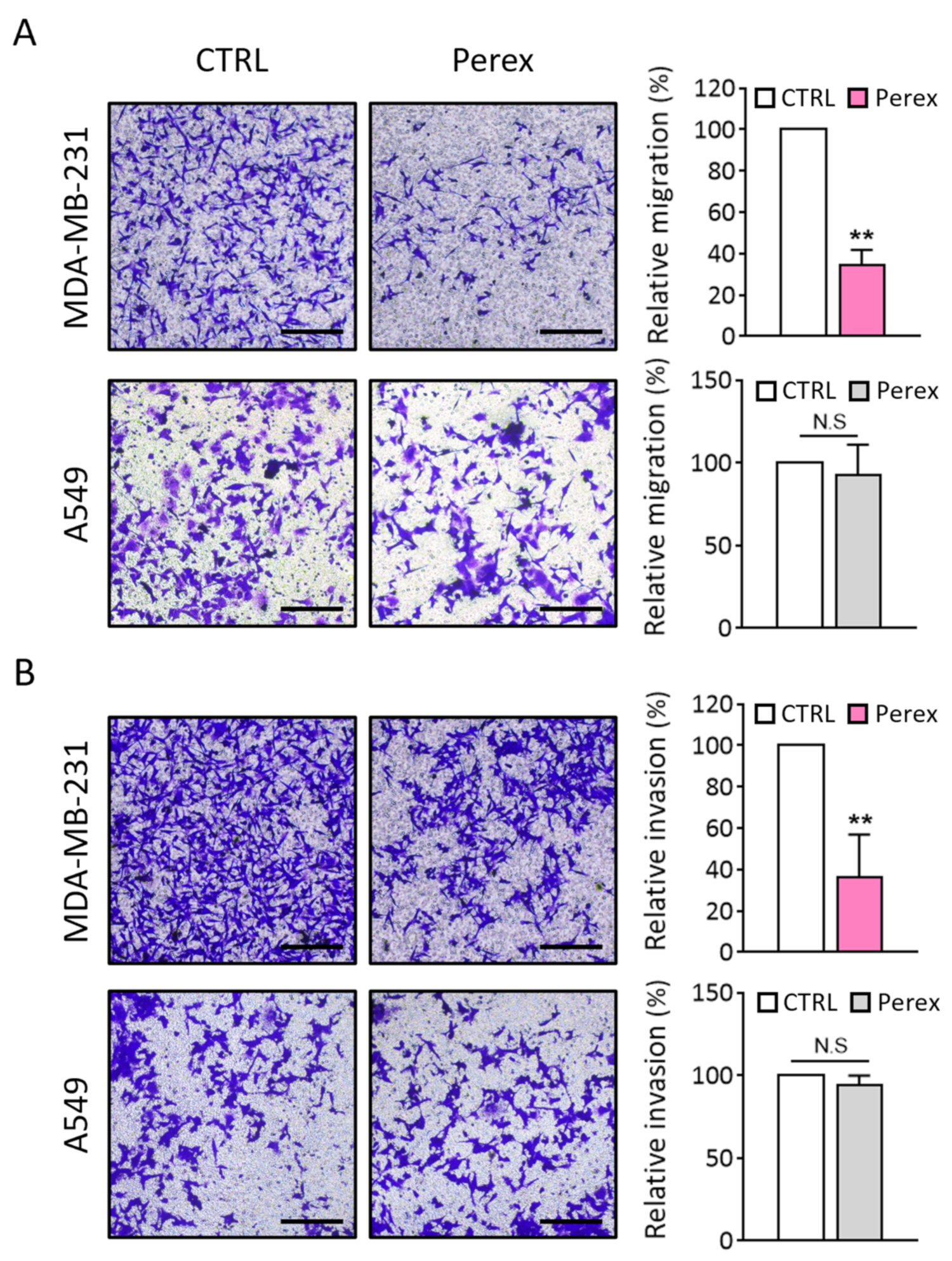
2.5. Effect of Perex on MDA-MB-231 Cell Migration and Invasion
2.6. Investigation of Selective Anti-Cancer Effects of Perex on MDA-MB-231 Cells
3. Materials and Methods
3.1. Cell Culture
3.2. Preparation and Isolation of Extracellular Vesicles
3.3. Characterization of Extracellular Vesicles
3.4. Internalization of Perex into Mammalian Cells
3.5. Measurement of Cell Proliferation
3.6. Cell Migration and Invasion Assays
3.7. Endocytosis Inhibitor Assay
3.8. Western Blot Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, F.; Zhang, S.; Liu, C.; Han, Z.; Liu, Y.; Deng, J.; Li, Y.; Wu, X.; Cai, L.; Qin, L. Protein analysis of extracellular vesicles to monitor and predict therapeutic response in metastatic breast cancer. Nat. Commun. 2021, 12, 2536. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Tang, Y.; Yu, H.; Li, H.-D. The role of ferroptosis in breast cancer patients: A comprehensive analysis. Cell Death Discov. 2021, 7, 93. [Google Scholar] [CrossRef]
- Jiang, K.; Song, X.; Yang, L.; Li, L.; Wan, Z.; Sun, X.; Gong, T.; Lin, Q.; Zhang, Z. Enhanced antitumor and anti-metastasis efficacy against aggressive breast cancer with a fibronectin-targeting liposomal doxorubicin. J. Control. Release 2018, 271, 21–30. [Google Scholar] [CrossRef]
- Medeiros, B.; Allan, A.L. Molecular Mechanisms of Breast Cancer Metastasis to the Lung: Clinical and Experimental Perspectives. Int. J. Mol. Sci. 2019, 20, 2272. [Google Scholar] [CrossRef]
- Theodossiou, T.A.; Ali, M.; Grigalavicius, M.; Grallert, B.; Dillard, P.; Schink, K.O.; Olsen, C.E.; Walchli, S.; Inderberg, E.M.; Kubin, A.; et al. Simultaneous defeat of MCF7 and MDA-MB-231 resistances by a hypericin PDT-tamoxifen hybrid therapy. NPJ Breast Cancer 2019, 5, 13. [Google Scholar] [CrossRef]
- Grubczak, K.; Kretowska-Grunwald, A.; Groth, D.; Poplawska, I.; Eljaszewicz, A.; Bolkun, L.; Starosz, A.; Holl, J.M.; Mysliwiec, M.; Kruszewska, J.; et al. Differential Response of MDA-MB-231 and MCF-7 Breast Cancer Cells to In Vitro Inhibition with CTLA-4 and PD-1 through Cancer-Immune Cells Modified Interactions. Cells 2021, 10, 2044. [Google Scholar] [CrossRef]
- Huang, K.S.; Wang, Y.T.; Byadgi, O.; Huang, T.Y.; Tai, M.H.; Shaw, J.F.; Yang, C.H. Screening of Specific and Common Pathways in Breast Cancer Cell Lines MCF-7 and MDA-MB-231 Treated with Chlorophyllides Composites. Molecules 2022, 27, 3950. [Google Scholar] [CrossRef]
- Lee, Y.K.; Bae, K.; Yoo, H.S.; Cho, S.H. Benefit of Adjuvant Traditional Herbal Medicine with Chemotherapy for Resectable Gastric Cancer. Integr. Cancer Ther. 2018, 17, 619–627. [Google Scholar] [CrossRef]
- Pedrosa, P.; Mendes, R.; Cabral, R.; Martins, L.; Baptista, P.V.; Fernandes, A.R. Combination of chemotherapy and Au-nanoparticle photothermy in the visible light to tackle doxorubicin resistance in cancer cells. Sci. Rep. 2018, 8, 11429. [Google Scholar] [CrossRef]
- Liu, C.T.; Chen, Y.H.; Huang, Y.C.; Chen, S.Y.; Tsai, M.Y. Chemotherapy in conjunction with traditional Chinese medicine for survival of patients with early female breast cancer: Protocol for a non-randomized, single center prospective cohort study. Trials 2019, 20, 741. [Google Scholar] [CrossRef]
- Hartjes, T.A.; Mytnyk, S.; Jenster, G.W.; van Steijn, V.; van Royen, M.E. Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches. Bioengineering 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X.; et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019, 9, 8001–8017. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Raghav, A.; Jeong, G.B. A systematic review on the modifications of extracellular vesicles: A revolutionized tool of nano-biotechnology. J. Nanobiotechnology 2021, 19, 459. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, L.; Kumar, P.; Wang, A. Engineering extracellular vesicles as nanotherapeutics for regenerative medicine. Biomolecules 2019, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The methods of choice for extracellular vesicles (EVs) characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, Y.; Wang, Q.; Jayasinghe, U.; Luo, X.; Wei, Q.; Wang, J.; Xiong, H.; Chen, C.; Xu, B. Exosome: Emerging biomarker in breast cancer. Oncotarget 2017, 8, 41717. [Google Scholar] [CrossRef]
- Yang, H.C.; Rhee, W.J. Single step in situ detection of surface protein and microRNA in clustered extracellular vesicles using flow cytometry. J. Clin. Med. 2021, 10, 319. [Google Scholar] [CrossRef]
- Cho, S.; Yang, H.C.; Rhee, W.J. Simultaneous multiplexed detection of exosomal microRNAs and surface proteins for prostate cancer diagnosis. Biosens. Bioelectron. 2019, 146, 111749. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Yu, Y.J.; You, J.Y.; Rhee, W.J.; Kim, J.A. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab Chip 2020, 20, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Rhee, W.J. Exosome-mediated let7c-5p delivery for breast cancer therapeutic development. Biotechnol. Bioprocess Eng. 2020, 25, 513–520. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.J.; Rhee, W.J. Phenylboronic Acid-conjugated Exosomes for Enhanced Anticancer Therapeutic Effect by Increasing Doxorubicin Loading Efficiency. Biotechnol. Bioprocess Eng. 2021, 26, 78–85. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, J.; Zheng, C.; Su, Y.; Bao, L.; Zhu, B.; Liu, S.; Wang, L.; Wang, X.; Wang, Y.; et al. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif. 2020, 53, e12830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Even, M.S.; Sandusky, C.B.; Barnard, N.D. Serum-free hybridoma culture: Ethical, scientific and safety considerations. Trends Biotechnol. 2006, 24, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Gottipamula, S.; Muttigi, M.; Kolkundkar, U.; Seetharam, R. Serum-free media for the production of human mesenchymal stromal cells: A review. Cell Prolif. 2013, 46, 608–627. [Google Scholar] [CrossRef] [PubMed]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant exosome-like nanovesicles: Emerging therapeutics and drug delivery nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Suharta, S.; Barlian, A.; Hidajah, A.C.; Notobroto, H.B.; Ana, I.D.; Indariani, S.; Wungu, T.D.K.; Wijaya, C.H. Plant-derived exosome-like nanoparticles: A concise review on its extraction methods, content, bioactivities, and potential as functional food ingredient. J. Food Sci. 2021, 86, 2838–2850. [Google Scholar] [CrossRef]
- Özkan, İ.; Koçak, P.; Yıldırım, M.; Ünsal, N.; Yılmaz, H.; Telci, D.; Şahin, F. Garlic (Allium sativum)-derived SEVs inhibit cancer cell proliferation and induce caspase mediated apoptosis. Sci. Rep. 2021, 11, 14773. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioac. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef]
- Yin, L.; Yan, L.; Yu, Q.; Wang, J.; Liu, C.; Wang, L.; Zheng, L. Characterization of the microRNA profile of ginger exosome-like nanoparticles and their anti-inflammatory effects in intestinal Caco-2 cells. J. Agric. Food Chem. 2022, 70, 4725–4734. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like nanoparticles from ginger rhizomes inhibited NLRP3 inflammasome activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef]
- Yamasaki, M.; Yamasaki, Y.; Furusho, R.; Kimura, H.; Kamei, I.; Sonoda, H.; Ikeda, M.; Oshima, T.; Ogawa, K.; Nishiyama, K. Onion (Allium cepa L.)-derived nanoparticles inhibited lps-induced nitrate production, however, their intracellular incorporation by endocytosis was not involved in this effect on RAW264 cells. Molecules 2021, 26, 2763. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Kim, D.K.; Rhee, W.J. Antioxidative Effects of Carrot-Derived Nanovesicles in Cardiomyoblast and Neuroblastoma Cells. Pharmaceutics 2021, 13, 1203. [Google Scholar] [CrossRef]
- Zhang, L.; He, F.; Gao, L.; Cong, M.; Sun, J.; Xu, J.; Wang, Y.; Hu, Y.; Asghar, S.; Hu, L. Engineering exosome-like nanovesicles derived from asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int. J. Nanomed. 2021, 16, 1575. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnology 2020, 18, 100. [Google Scholar] [CrossRef]
- Kim, K.; Yoo, H.J.; Jung, J.-H.; Lee, R.; Hyun, J.-K.; Park, J.-H.; Na, D.; Yeon, J.H. Cytotoxic effects of plant sap-derived extracellular vesicles on various tumor cell types. J. Funct. Biomater. 2020, 11, 22. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A review of exosomal isolation methods: Is size exclusion chromatography the best option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Guan, S.; Yu, H.; Yan, G.; Gao, M.; Sun, W.; Zhang, X. Characterization of urinary exosomes purified with size exclusion chromatography and ultracentrifugation. J. Proteome Res. 2020, 19, 2217–2225. [Google Scholar] [CrossRef]
- Ng, K.S.; Smith, J.A.; McAteer, M.P.; Mead, B.E.; Ware, J.; Jackson, F.O.; Carter, A.; Ferreira, L.; Bure, K.; Rowley, J.A. Bioprocess decision support tool for scalable manufacture of extracellular vesicles. Biotechnol. Bioeng. 2019, 116, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Ham, Y.M.; Kim, J.A.; Rhee, W.J. Single-step equipment-free extracellular vesicle concentration using super absorbent polymer beads. J. Extracell. Vesicles 2021, 10, e12074. [Google Scholar] [CrossRef] [PubMed]
- Miaczynska, M.; Stenmark, H. Mechanisms and functions of endocytosis. J. Cell Biol. 2008, 180, 7. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Harashima, H. Endocytosis of gene delivery vectors: From clathrin-dependent to lipid raft-mediated endocytosis. Mol. Ther. 2013, 21, 1118–1130. [Google Scholar] [CrossRef]
- Song, Y.; Wu, Y.; Xu, L.; Jiang, T.; Tang, C.; Yin, C. Caveolae-mediated endocytosis drives robust siRNA delivery of polymeric nanoparticles to macrophages. ACS Nano 2021, 15, 8267–8282. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Han, H.-H.; Sun, J.; Zhang, H.-T.; Wei, W.; Cui, S.-H.; Chen, X.; Wang, J.-C.; Zhang, Q. Regulating intracellular fate of siRNA by endoplasmic reticulum membrane-decorated hybrid nanoplexes. Nat. Commun. 2019, 10, 2702. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.J.; Larsen, J.D.; Sullivan, M.O. Polyplexes traffic through caveolae to the Golgi and endoplasmic reticulum en route to the nucleus. Mol. Pharm. 2012, 9, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.; Lv, Y.; Xin, X.; Qin, C.; Han, X.; Yang, L.; He, W.; Yin, L. Cytosolic co-delivery of miRNA-34a and docetaxel with core-shell nanocarriers via caveolae-mediated pathway for the treatment of metastatic breast cancer. Sci. Rep. 2017, 7, 46186. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Z.; He, W.; Qin, C.; Yao, J.; Zhou, J.; Yin, L. Globular protein-coated Paclitaxel nanosuspensions: Interaction mechanism, direct cytosolic delivery, and significant improvement in pharmacokinetics. Mol. Pharm. 2015, 12, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, S.; Li, J.; Zhu, D.; Wang, Z.; Cores, J.; Cheng, K.; Liu, G.; Huang, K. Extruded Mesenchymal Stem Cell Nanovesicles Are Equally Potent to Natural Extracellular Vesicles in Cardiac Repair. ACS Appl. Mater. Interfaces 2021, 13, 55767–55779. [Google Scholar] [CrossRef]
- Sawada, S.I.; Sato, Y.T.; Kawasaki, R.; Yasuoka, J.I.; Mizuta, R.; Sasaki, Y.; Akiyoshi, K. Nanogel hybrid assembly for exosome intracellular delivery: Effects on endocytosis and fusion by exosome surface polymer engineering. Biomater. Sci. 2020, 8, 619–630. [Google Scholar] [CrossRef]
- Nam, H.Y.; Kwon, S.M.; Chung, H.; Lee, S.Y.; Kwon, S.H.; Jeon, H.; Kim, Y.; Park, J.H.; Kim, J.; Her, S.; et al. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. J. Control. Release 2009, 135, 259–267. [Google Scholar] [CrossRef]
- Tse, E.Y.; Ko, F.C.; Tung, E.K.; Chan, L.K.; Lee, T.K.; Ngan, E.S.; Man, K.; Wong, A.S.; Ng, I.O.; Yam, J.W. Caveolin-1 overexpression is associated with hepatocellular carcinoma tumourigenesis and metastasis. J. Pathol. 2012, 226, 645–653. [Google Scholar] [PubMed]
- Ketteler, J.; Klein, D. Caveolin-1, cancer and therapy resistance. Int. J. Cancer 2018, 143, 2092–2104. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.K.; Kang, S.J.; Rhee, W.J. Perilla-Leaf-Derived Extracellular Vesicles Selectively Inhibit Breast Cancer Cell Proliferation and Invasion. Int. J. Mol. Sci. 2023, 24, 15633. https://doi.org/10.3390/ijms242115633
Kim DK, Kang SJ, Rhee WJ. Perilla-Leaf-Derived Extracellular Vesicles Selectively Inhibit Breast Cancer Cell Proliferation and Invasion. International Journal of Molecular Sciences. 2023; 24(21):15633. https://doi.org/10.3390/ijms242115633
Chicago/Turabian StyleKim, Do Kyung, Su Jin Kang, and Won Jong Rhee. 2023. "Perilla-Leaf-Derived Extracellular Vesicles Selectively Inhibit Breast Cancer Cell Proliferation and Invasion" International Journal of Molecular Sciences 24, no. 21: 15633. https://doi.org/10.3390/ijms242115633
APA StyleKim, D. K., Kang, S. J., & Rhee, W. J. (2023). Perilla-Leaf-Derived Extracellular Vesicles Selectively Inhibit Breast Cancer Cell Proliferation and Invasion. International Journal of Molecular Sciences, 24(21), 15633. https://doi.org/10.3390/ijms242115633






