Role of Exosomal miR-205-5p Cargo in Angiogenesis and Cell Migration
Abstract
1. Introduction
2. Results
2.1. sEVs Isolation and Characterization
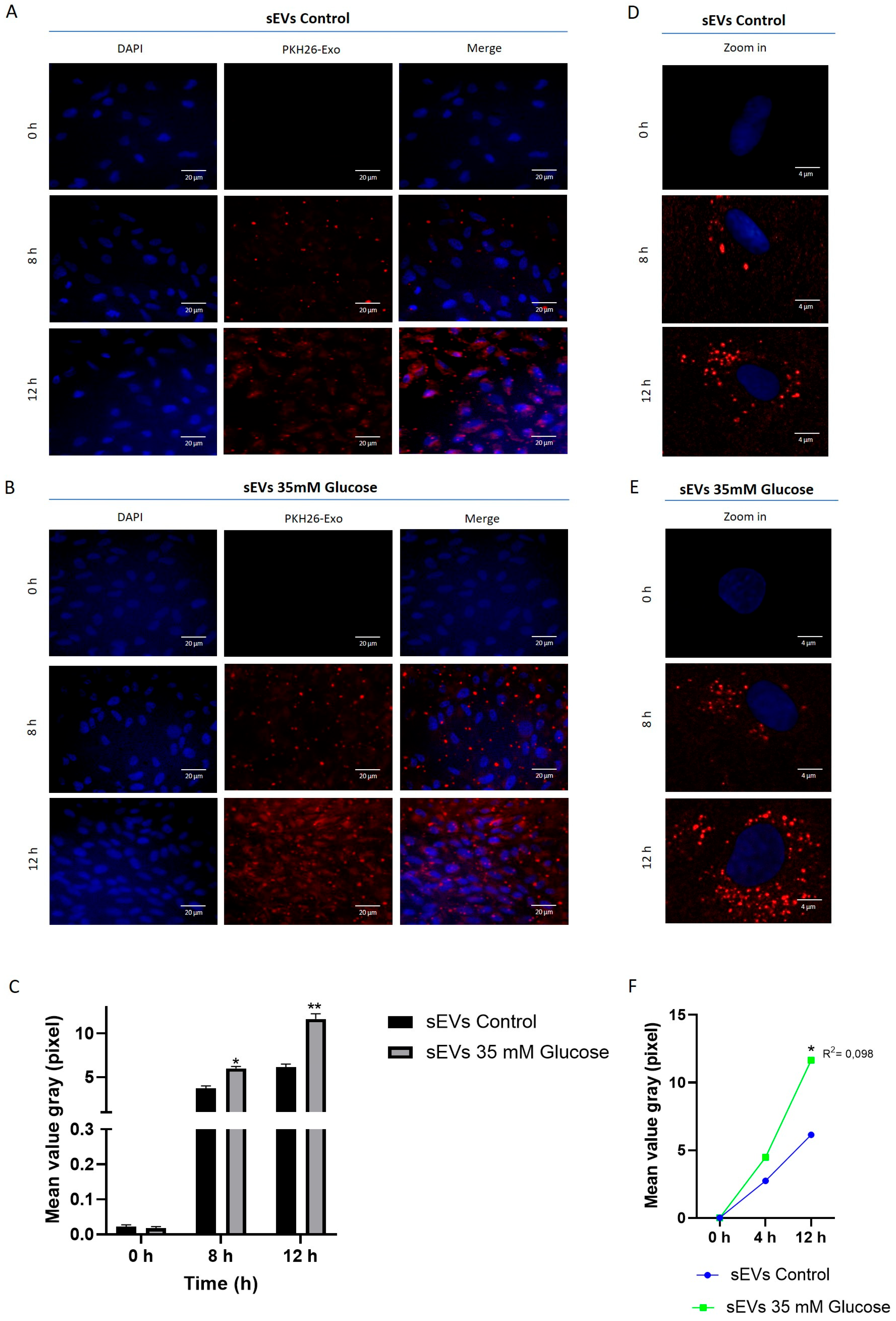
2.2. sEVs from ARPE-19 Are Incorporated into HUVEC
2.3. miR-205-5p Cargo in sEVs
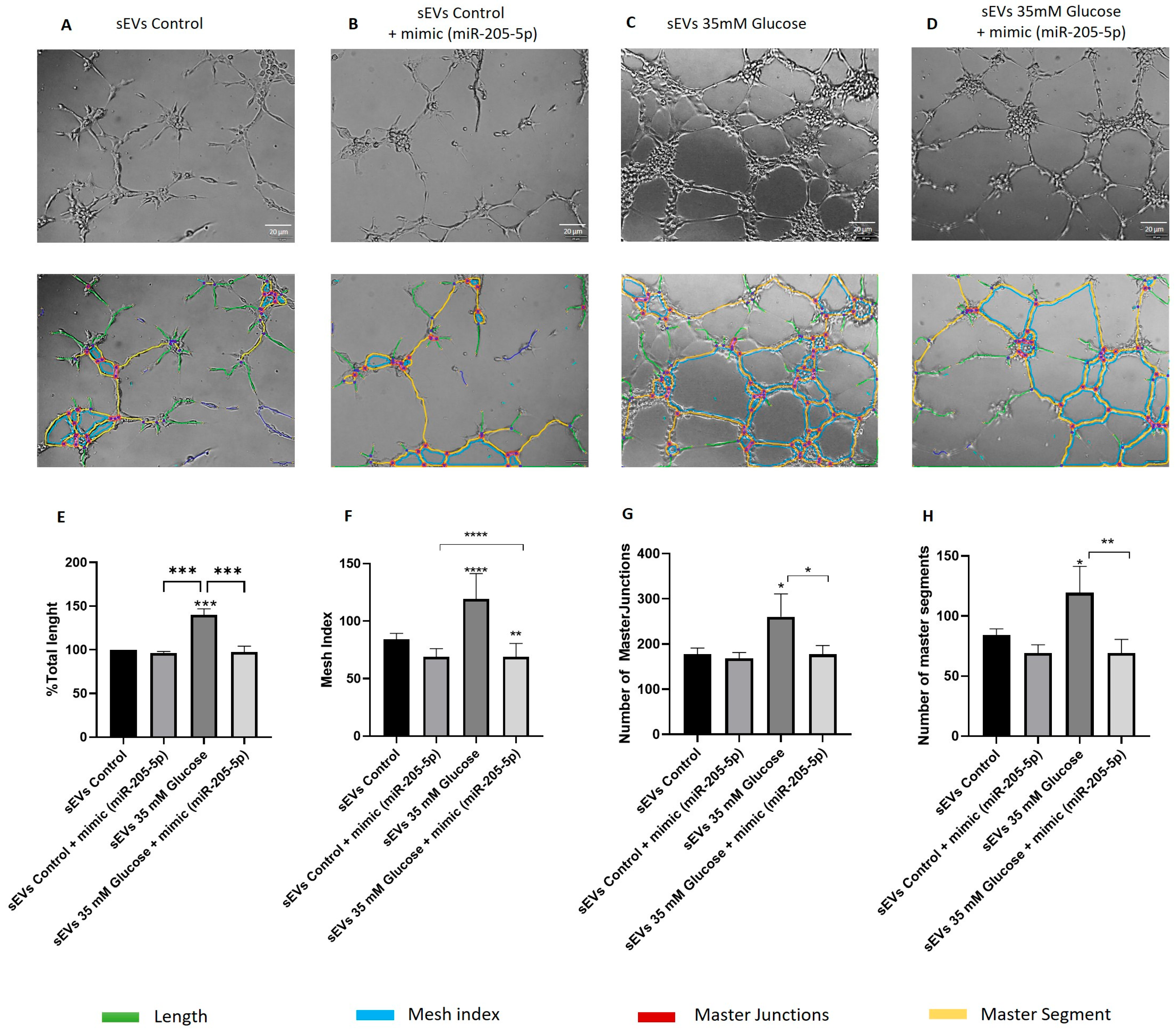
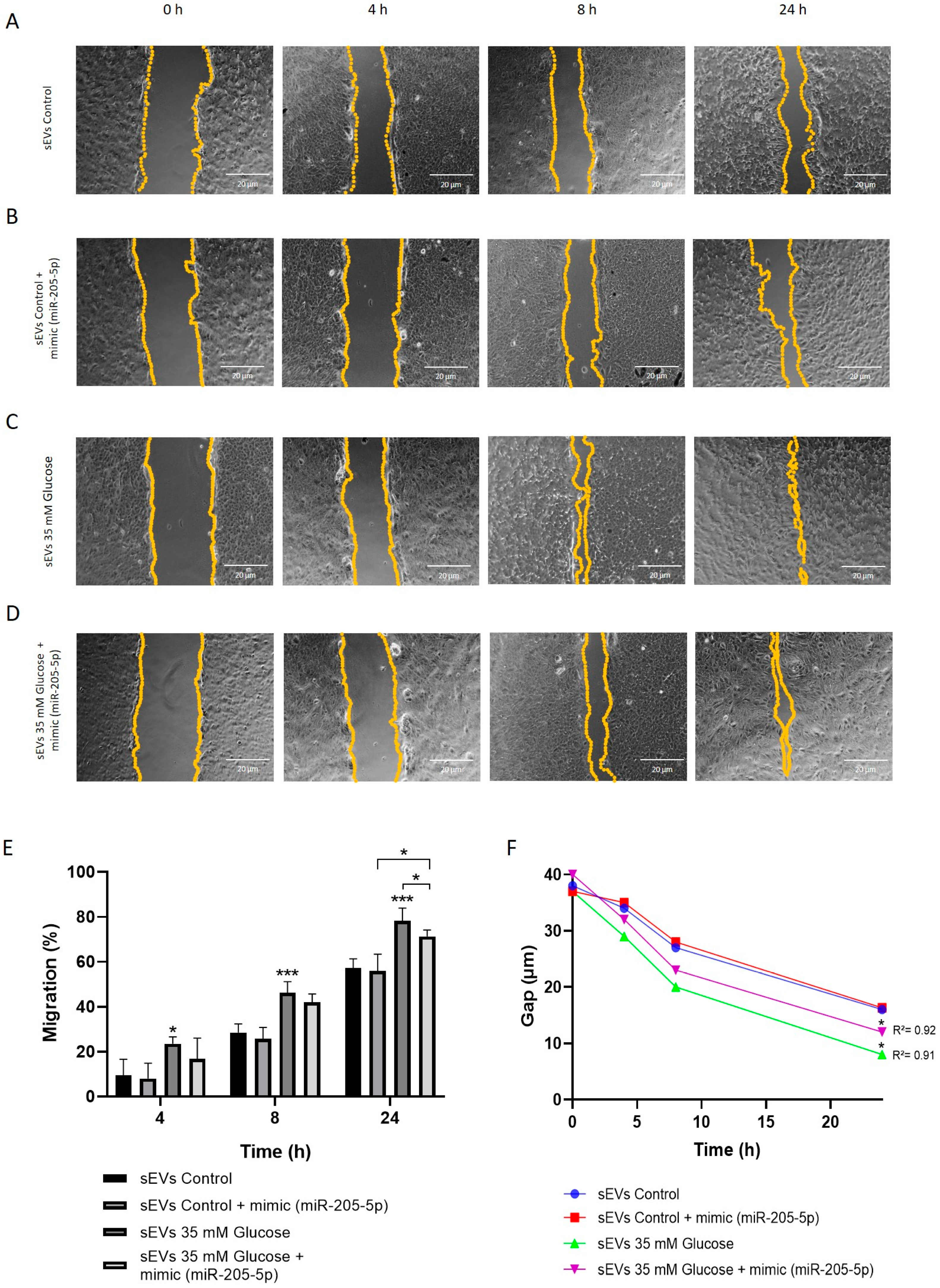
2.4. miR-205-5p sEVs Cargo Level Is Inversely Related to Angiogenesis and Migration
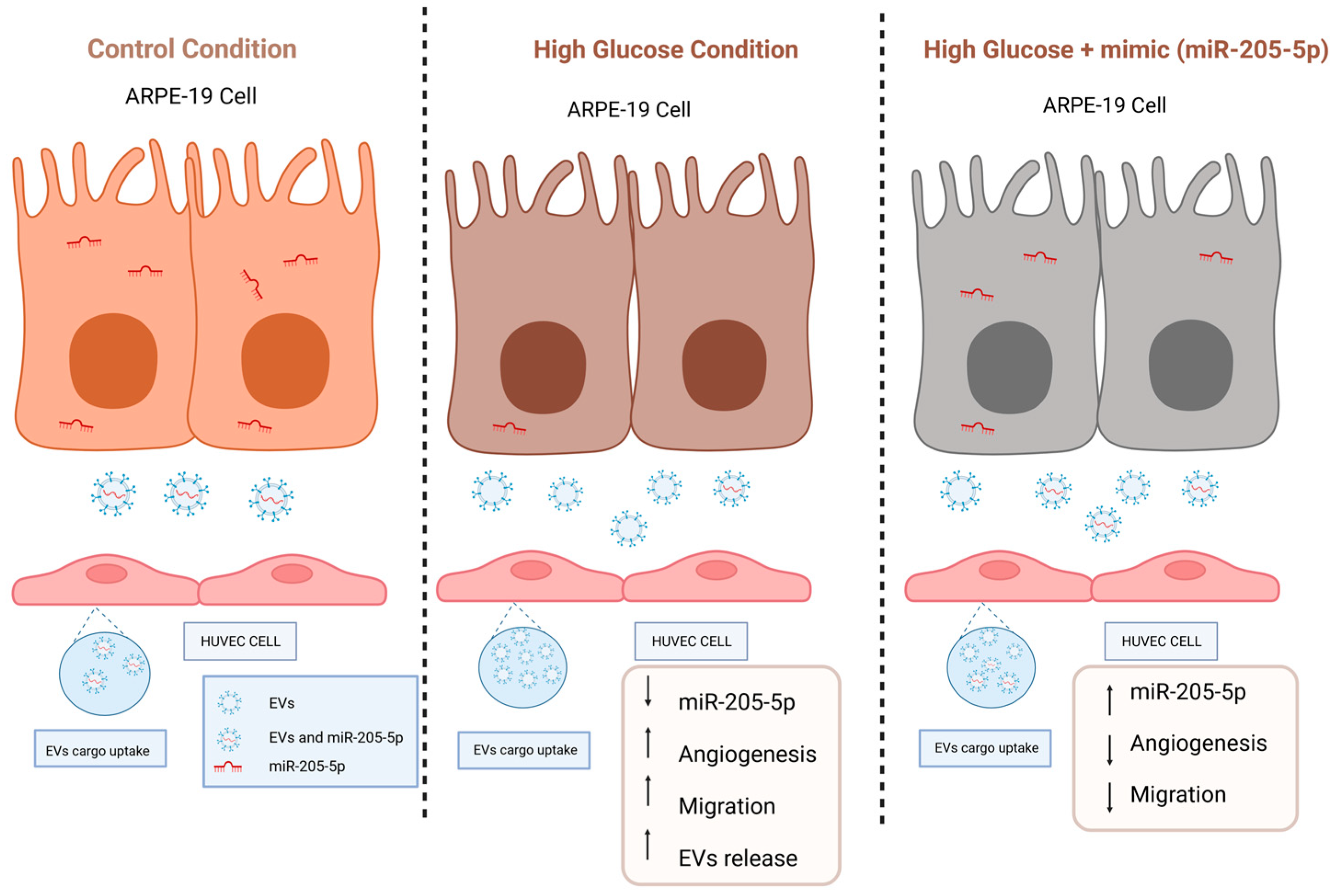
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. sEVs Isolation
4.3. sEVs Characterization
4.4. Western Blot
4.5. Fluorescence Labeling of sEVs and Internalization
4.6. Mimic Transfection
4.7. miRNA Expression Analysis
4.8. Vasculogenesis Assay
4.9. Scratch Wound Healing Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Zhao, Y.; Yin, Y.; Jia, X.; Mao, L. Mechanism of cargo sorting into small extracellular vesicles. Bioengineered 2021, 12, 8186–8201. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Gundu, C.; Arruri, V.K.; Yadav, P.; Navik, U.; Kumar, A.; Amalkar, V.S.; Vikram, A.; Gaddam, R.R. Dynamin-Independent Mechanisms of Endocytosis and Receptor Trafficking. Cells 2022, 1, 2557. [Google Scholar] [CrossRef]
- Fu, X.-L.; He, F.-T.; Li, M.-H.; Fu, C.-Y.; Chen, J.-Z. Up-regulation of miR-192-5p inhibits the ELAVL1/PI3Kδ axis and attenuates microvascular endothelial cell proliferation, migration and angiogenesis in diabetic retinopathy. Diabet. Med. 2023, 40, e15077. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Tian, Y.; Huang, W.; Tong, N.; Fu, X. Integrative biology of extracellular vesicles in diabetes mellitus and diabetic complications. Theranostics 2022, 12, 1342–1372. [Google Scholar] [CrossRef]
- Chiaradia, E.; Tancini, B.; Emiliani, C.; Delo, F.; Pellegrino, R.M.; Tognoloni, A.; Urbanelli, L.; Buratta, S. Extracellular Vesicles under Oxidative Stress Conditions: Biological Properties and Physiological Roles. Cells 2021, 12, 1763. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Qi, H.; Wang, Y.; Fa, S.; Yuan, C.; Yang, L. Extracellular Vesicles as Natural Delivery Carriers Regulate Oxidative Stress Under Pathological Conditions. Front. Bioeng. Biotechnol. 2021, 9, 752019. [Google Scholar] [CrossRef]
- Atienzar-Aroca, S.; Serrano-Heras, G.; Freire Valls, A.; Ruiz de Almodovar, C.; Muriach, M.; Barcia, J.M.; Garcia-Verdugo, J.M.; Romero, F.J.; Sancho-Pelluz, J. Role of retinal pigment epithelium-derived exosomes and autophagy in new blood vessel formation. J. Cell Mol. Med. 2018, 22, 5244–5256. [Google Scholar] [CrossRef]
- Wu, S.F.; Noren Hooten, N.; Freeman, D.W.; Mode, N.A.; Zonderman, A.B.; Evans, M.K. Extracellular vesicles in diabetes mellitus induce alterations in endothelial cell morphology and migration. J. Transl. Med. 2020, 18, 230. [Google Scholar] [CrossRef]
- Shah, N.; Ishii, M.; Brandon, C.; Ablonczy, Z.; Cai, J.; Liu, Y.; Chou, C.J.; Rohrer, B. Extracellular vesicle-mediated long-range communication in stressed retinal pigment epithelial cell monolayers. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2610–2622. [Google Scholar] [CrossRef] [PubMed]
- Toro, A.U.; Shukla, S.S.; Bansal, P. Micronome Revealed miR-205-5p as Key Regulator of VEGFA During Cancer Related Angiogenesis in Hepatocellular Carcinoma. Mol. Biotechnol. 2023, 65, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Li, T.; Ruan, L.; Yang, J.; Luo, Y.; Li, L.; Wu, X. Knockdown of Malat1 alleviates high-glucose-induced angiogenesis through regulating miR-205-5p/VEGF-A axis. Exp. Eye Res. 2021, 207, 108585. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics-challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gao, Y.; Xu, L.; Dang, W.; Yan, H.; Zou, D.; Zhu, Z.; Luo, L.; Tian, N.; Wang, X.; et al. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci. Rep. 2017, 7, 9371. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Li, Z.; Wang, H.; Liu, X. Differential lipidomics of HK-2 cells and exosomes under high glucose stimulation. Int. J. Med. Sci. 2022, 19, 393–401. [Google Scholar] [CrossRef]
- Kumar, A.; Sundaram, K.; Mu, J.; Dryden, G.W.; Sriwastva, M.K.; Lei, C.; Zhang, L.; Qiu, X.; Xu, F.; Yan, J.; et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat. Commun. 2021, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.J.; Zhu, M.; Xu, X.X.; Meng, X.M.; Wu, Y.G. Exosomes from high glucose-treated macrophages activate glomerular mesangial cells via TGF-beta1/Smad3 pathway in vivo and in vitro. FASEB J. 2019, 33, 9279–9290. [Google Scholar] [CrossRef]
- Huang, Y.; Li, R.; Zhang, L.; Chen, Y.; Dong, W.; Zhao, X.; Yang, H.; Zhang, S.; Xie, Z.; Ye, Z.; et al. Extracellular Vesicles From High Glucose-Treated Podocytes Induce Apoptosis of Proximal Tubular Epithelial Cells. Front. Physiol. 2020, 11, 579296. [Google Scholar] [CrossRef]
- Zhang, R.; Niu, S.; Rong, Z.; Li, F.; Ni, L.; Di, X.; Liu, C. A Potential Target for Diabetic Vascular Damage: High Glucose-Induced Monocyte Extracellular Vesicles Impair Endothelial Cells by Delivering miR-142-5p. Front. Bioeng. Biotechnol. 2022, 10, 913791. [Google Scholar] [CrossRef]
- Oltra, M.; Vidal-Gil, L.; Maisto, R.; Sancho-Pelluz, J.; Barcia, J.M. Oxidative stress-induced angiogenesis is mediated by miR-205-5p. J. Cell Mol. Med. 2020, 24, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Chiechi, A.; Couch, R.; Liotta, L.A.; Espina, V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp. Cell Res. 2013, 319, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Fu, W.; Wang, X.; Chen, H.; Wu, X.; Lao, G.; Wu, Y.; Hu, M.; Yang, C.; et al. MiR-195-5p and miR-205-5p in extracellular vesicles isolated from diabetic foot ulcer wound fluid decrease angiogenesis by inhibiting VEGFA expression. Aging 2021, 13, 19805–19821. [Google Scholar] [CrossRef] [PubMed]
- French, K.C.; Antonyak, M.A.; Cerione, R.A. Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Semin. Cell Dev. Biol. 2017, 67, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Stolz, D.B.; Sullivan, M.L.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 2012, 119, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.J.; Christianson, H.C.; Wittrup, A.; Bourseau-Guilmain, E.; Lindqvist, E.; Svensson, L.M.; Morgelin, M.; Belting, M. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 2013, 288, 17713–17724. [Google Scholar] [CrossRef]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef]
- Jurgielewicz, B.J.; Yao, Y.; Stice, S.L. Kinetics and Specificity of HEK293T Extracellular Vesicle Uptake using Imaging Flow Cytometry. Nanoscale Res. Lett. 2020, 15, 170. [Google Scholar] [CrossRef]
- Emam, S.E.; Abu Lila, A.S.; Elsadek, N.E.; Ando, H.; Shimizu, T.; Okuhira, K.; Ishima, Y.; Mahdy, M.A.; Ghazy, F.S.; Ishida, T. Cancer cell-type tropism is one of crucial determinants for the efficient systemic delivery of cancer cell-derived exosomes to tumor tissues. Eur. J. Pharm. Biopharm. 2019, 145, 27–34. [Google Scholar] [CrossRef]
- Ma, W.; Tang, F.; Xiao, L.; Han, S.; Yao, X.; Zhang, Q.; Zhou, J.; Wang, Y.; Zhou, J. miR-205-5p in exosomes divided from chondrogenic mesenchymal stem cells alleviated rheumatoid arthritis via regulating MDM2 in fibroblast-like synoviocytes. J. Musculoskelet. Neuronal Interact. 2022, 22, 132–141. [Google Scholar] [PubMed]
- Yang, W.; Tan, S.; Yang, L.; Chen, X.; Yang, R.; Oyang, L.; Lin, J.; Xia, L.; Wu, N.; Han, Y.; et al. Exosomal miR-205-5p enhances angiogenesis and nasopharyngeal carcinoma metastasis by targeting desmocollin-2. Mol. Ther. Oncolytics 2022, 24, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Mo, C.; Guo, S.; Zhuang, J.; Huang, B.; Mao, X. Human bone marrow mesenchymal stem cells-derived microRNA-205-containing exosomes impede the progression of prostate cancer through suppression of RHPN2. J. Exp. Clin. Cancer Res. CR 2019, 38, 495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, Z.; Wang, M.; Zhang, M.; Chen, Y.; Yang, X.; Zhou, C.; Liu, Y.; Hong, L.; Zhang, L. Detection of plasma exosomal miRNA-205 as a biomarker for early diagnosis and an adjuvant indicator of ovarian cancer staging. J. Ovarian Res. 2022, 15, 27. [Google Scholar] [CrossRef]
- Oltra, M.; Martínez-Santos, M.; Ybarra, M.; Rowland, H.; Muriach, M.; Romero, J.; Sancho-Pelluz, J.; Barcia, J.M. Oxidative-Induced Angiogenesis Is Modulated by Small Extracellular Vesicle miR-302a-3p Cargo in Retinal Pigment Epithelium Cells. Antioxidants 2022, 11, 818. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Santos, M.; Ybarra, M.; Oltra, M.; Muriach, M.; Romero, F.J.; Pires, M.E.; Sancho-Pelluz, J.; Barcia, J.M. Role of Exosomal miR-205-5p Cargo in Angiogenesis and Cell Migration. Int. J. Mol. Sci. 2024, 25, 934. https://doi.org/10.3390/ijms25020934
Martínez-Santos M, Ybarra M, Oltra M, Muriach M, Romero FJ, Pires ME, Sancho-Pelluz J, Barcia JM. Role of Exosomal miR-205-5p Cargo in Angiogenesis and Cell Migration. International Journal of Molecular Sciences. 2024; 25(2):934. https://doi.org/10.3390/ijms25020934
Chicago/Turabian StyleMartínez-Santos, Miriam, María Ybarra, María Oltra, María Muriach, Francisco J. Romero, Maria E. Pires, Javier Sancho-Pelluz, and Jorge M. Barcia. 2024. "Role of Exosomal miR-205-5p Cargo in Angiogenesis and Cell Migration" International Journal of Molecular Sciences 25, no. 2: 934. https://doi.org/10.3390/ijms25020934
APA StyleMartínez-Santos, M., Ybarra, M., Oltra, M., Muriach, M., Romero, F. J., Pires, M. E., Sancho-Pelluz, J., & Barcia, J. M. (2024). Role of Exosomal miR-205-5p Cargo in Angiogenesis and Cell Migration. International Journal of Molecular Sciences, 25(2), 934. https://doi.org/10.3390/ijms25020934






