Early Depletion of Neutrophils Reduces Retinal Inflammation and Neovascularization in Mice with Oxygen-Induced Retinopathy
Abstract
:1. Introduction
2. Results
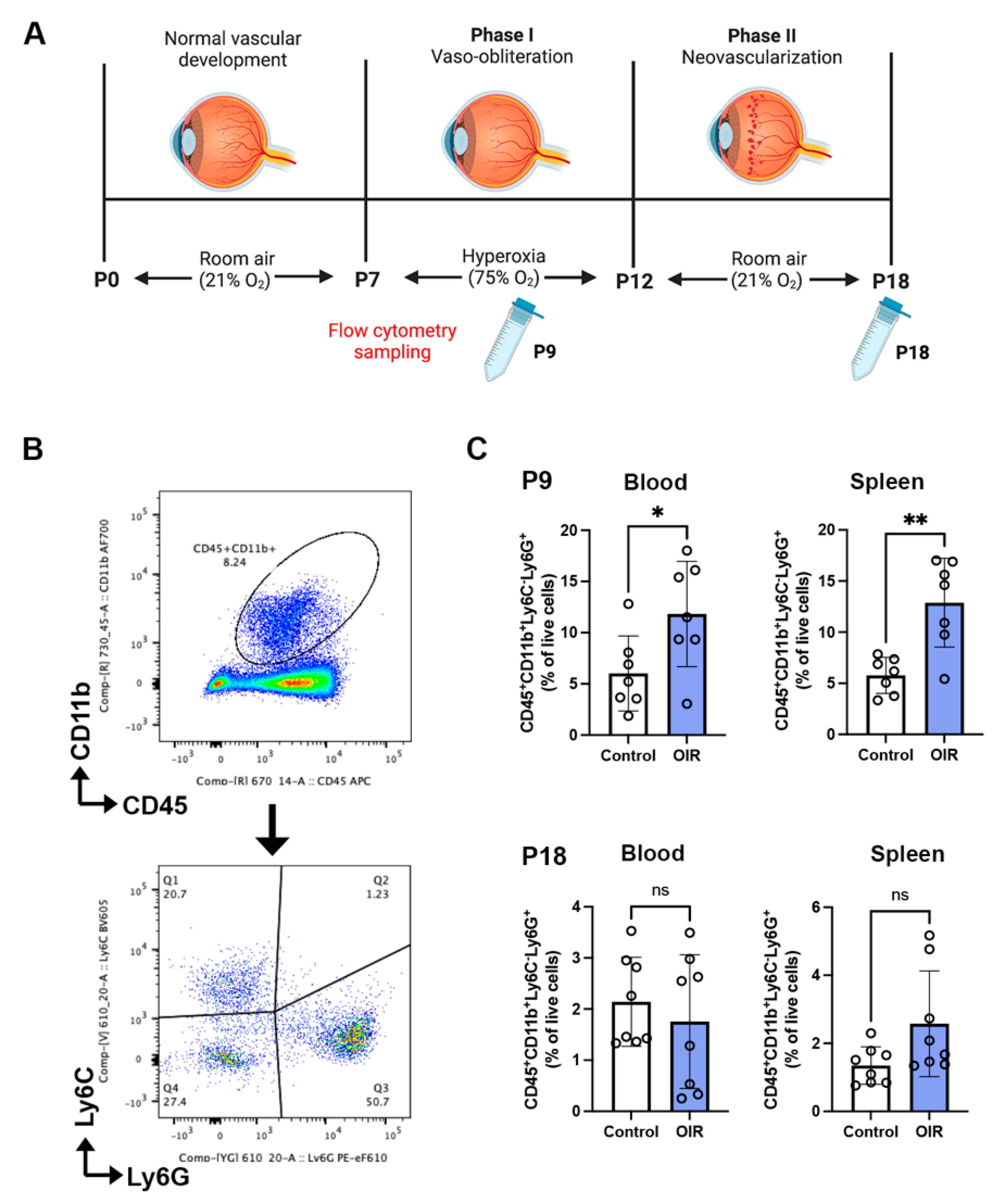
2.1. Neutrophils Are Increased in Phase I but Not Phase II OIR
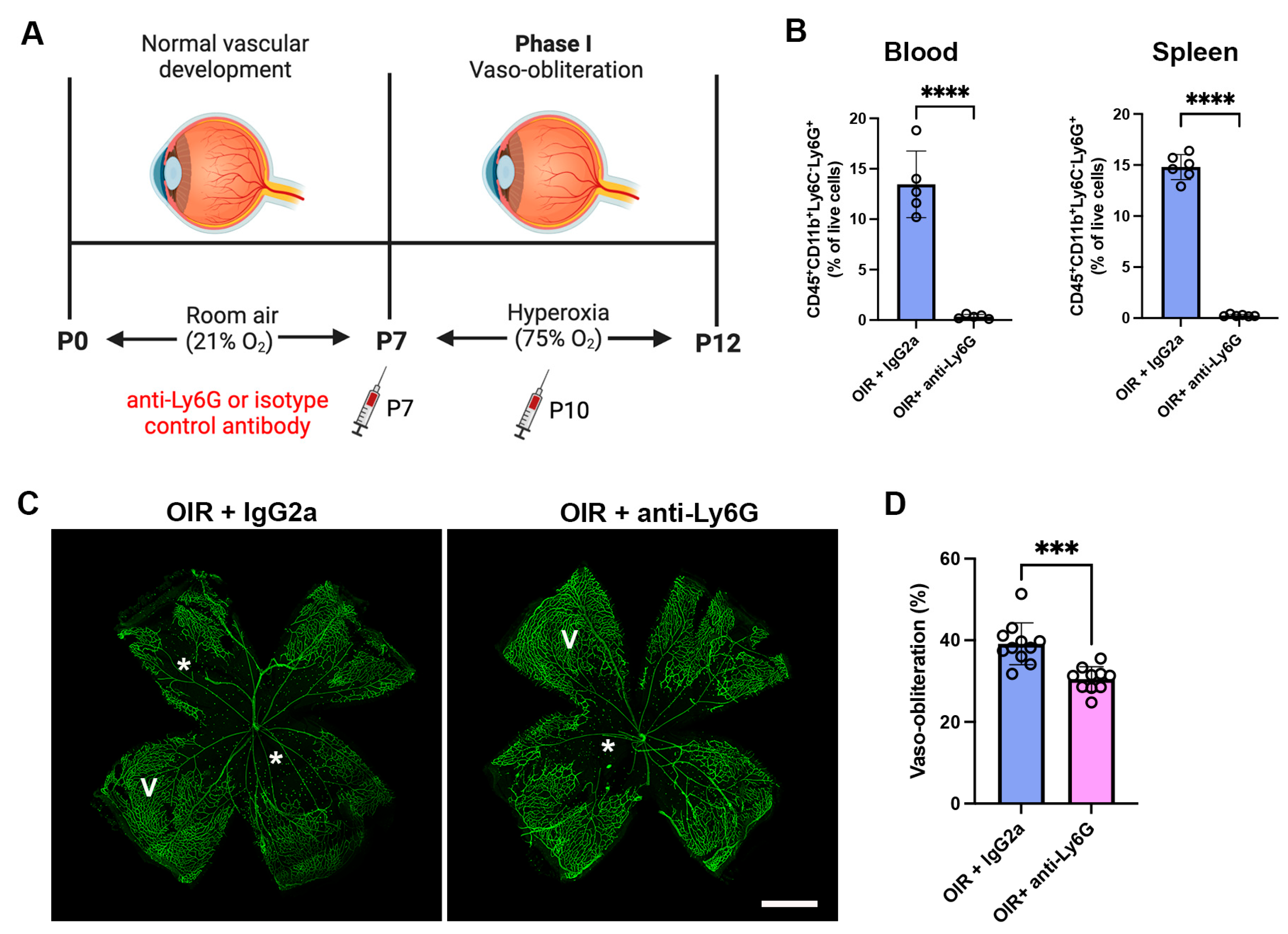
2.2. Neutrophil Depletion Reduced Neutrophils in the Blood and Spleen and Retinal Vaso-Obliteration in OIR Mice
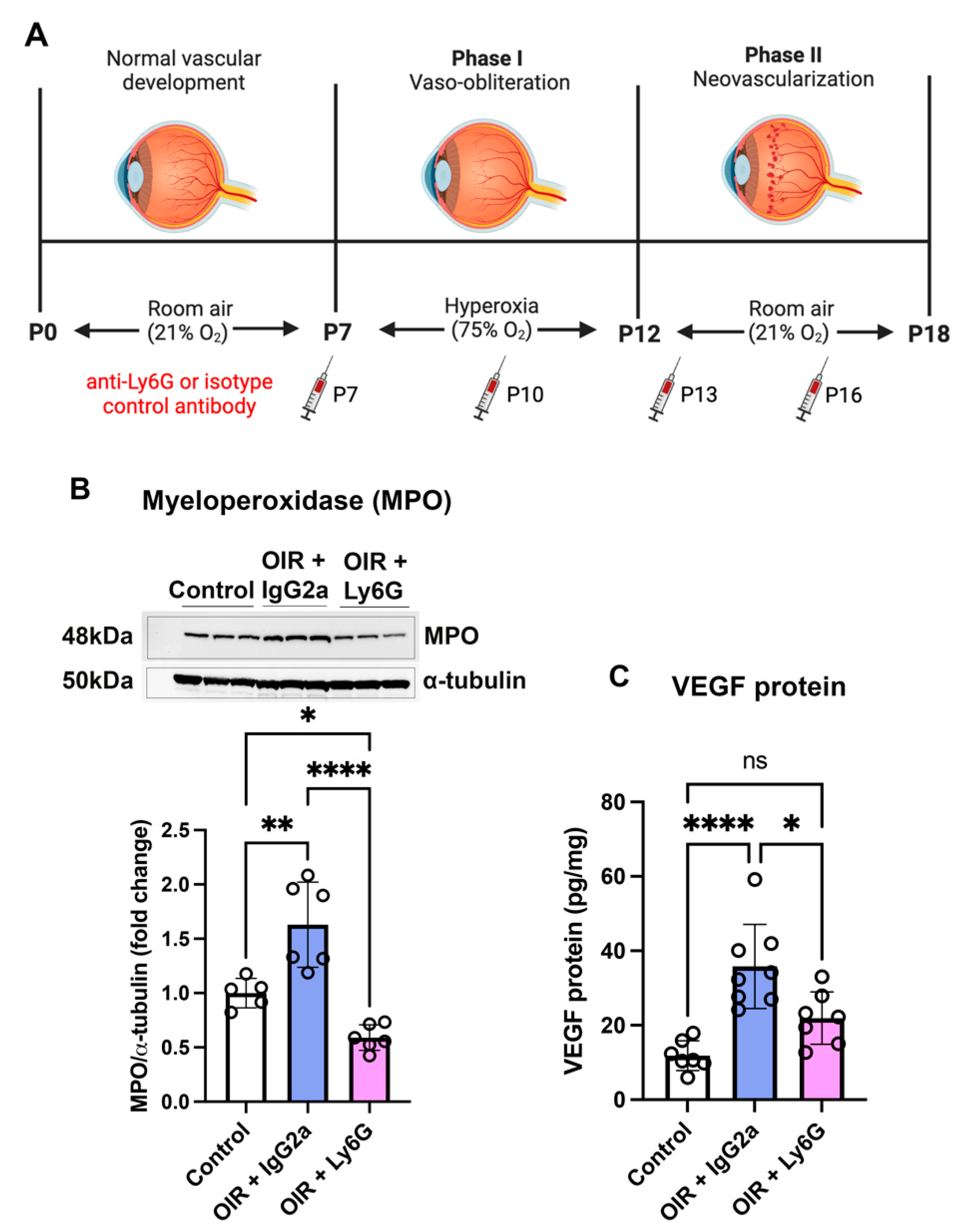
2.3. Neutrophil Depletion Reduced Myeloperoxidase and VEGF in the Retina of Early Phase II OIR
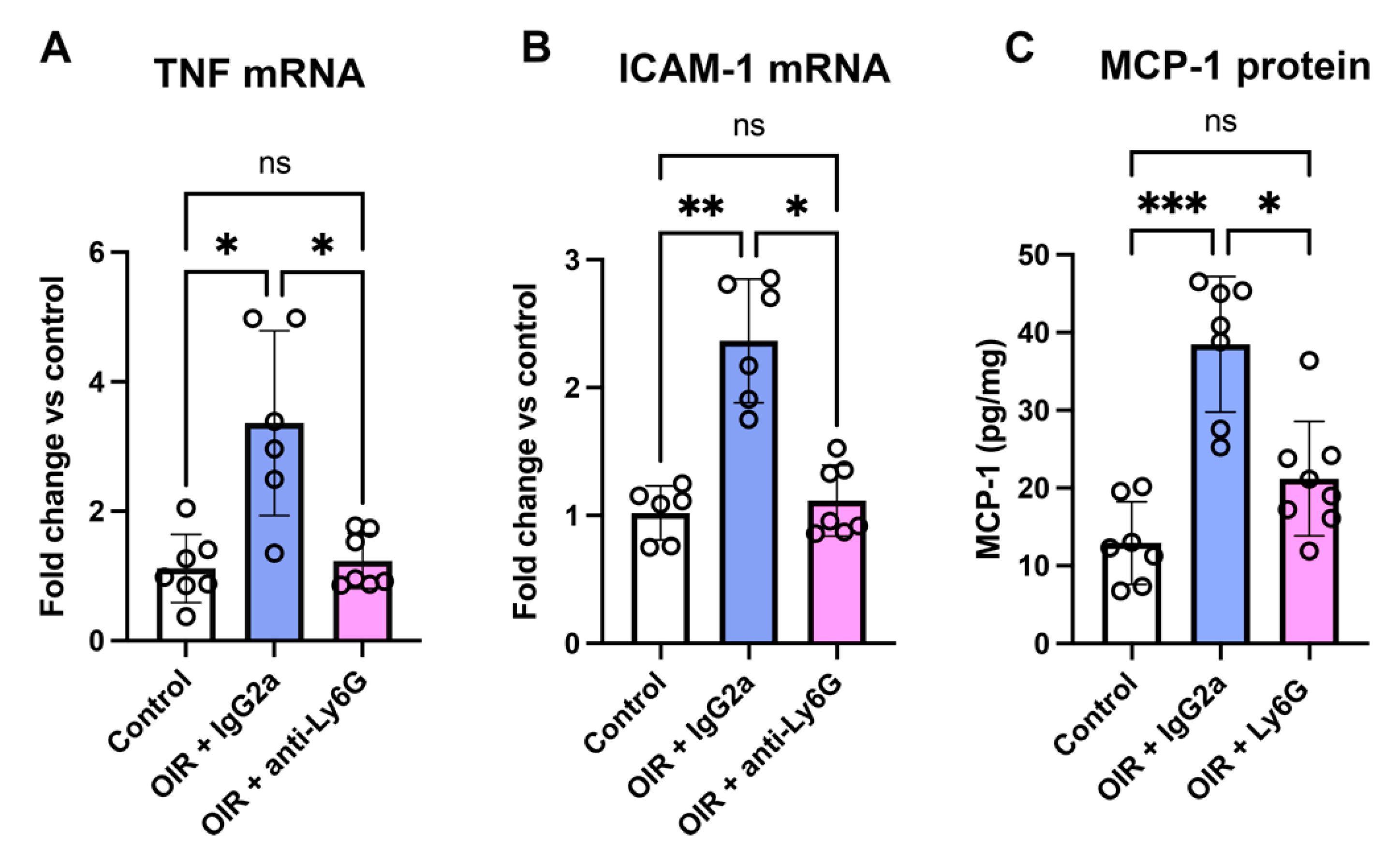
2.4. Neutrophil Depletion Reduced Inflammatory Factors in the Retina of Phase II OIR
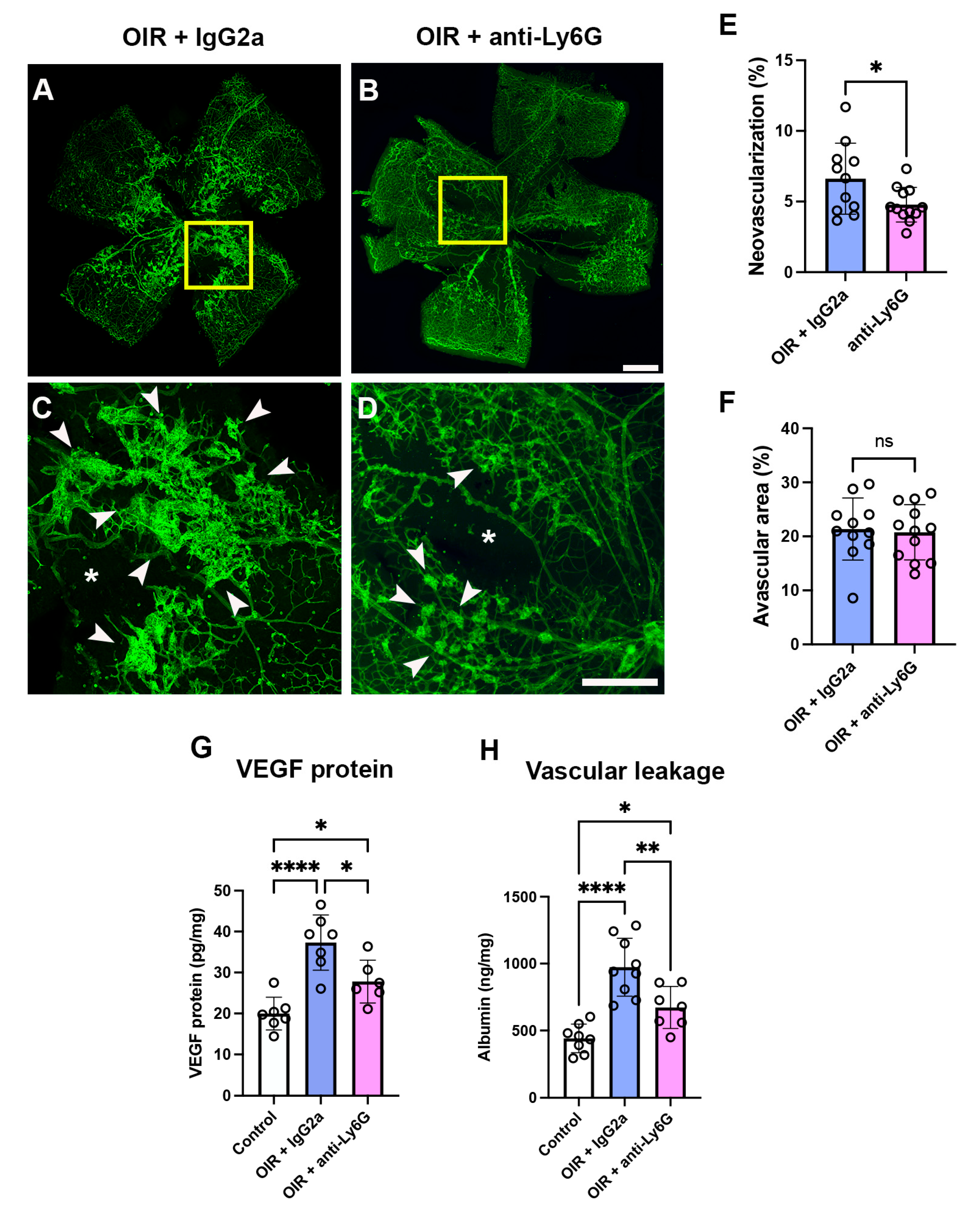
2.5. Neutrophil Depletion Reduced Retinal Vasculopathy in Phase II OIR
2.6. Body Weight
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Flow Cytometry
4.3. Retinal Vaso-Obliteration and Neovascularization
4.4. ELISA Assays for Vascular Leakage, VEGF, and MCP-1
4.5. Western Blotting
4.6. Quantitative Real-Time PCR
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, T.-E.; Wong, T.Y. Diabetic Retinopathy: Looking Forward to 2030. Front. Endocrinol. 2023, 13, 1077669. [Google Scholar] [CrossRef] [PubMed]
- Dammann, O.; Hartnett, M.E.; Stahl, A. Retinopathy of Prematurity. Dev. Med. Child Neurol. 2023, 65, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.H.; Shin, Y.U.; Cho, H. Retinopathy of Prematurity: A Review of Epidemiology and Current Treatment Strategies. Clin. Exp. Pediatr. 2022, 65, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Smith, L.E.H.; Dammann, O. Retinopathy of Prematurity. Lancet 2013, 382, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Fu, Z.; Lo, A.C.Y. Hypoxia-Induced Oxidative Stress in Ischemic Retinopathy. Oxid. Med. Cell. Longev. 2012, 2012, 426769. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; Deliyanti, D.; Figgett, W.A.; Talia, D.M.; De Haan, J.B.; Wilkinson-Berka, J.L. Ebselen by Modulating Oxidative Stress Improves Hypoxia-Induced Macroglial Müller Cell and Vascular Injury in the Retina. Exp. Eye Res. 2015, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Deliyanti, D.; Figgett, W.A.; Gebhardt, T.; Trapani, J.A.; Mackay, F.; Wilkinson-Berka, J.L. Cd8+ T Cells Promote Pathological Angiogenesis in Ocular Neovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 522–536. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.-S.; Li, X.-Q.; Hou, H.-Y.; Su, J.-B.; Yao, L.-B.; Zhang, J. Macrophages Promote Vasculogenesis of Retinal Neovascularization in an Oxygen-Induced Retinopathy Model in Mice. Cell Tissue Res. 2016, 364, 599–610. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in Chronic Inflammatory Diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Edwards, S.W. The Multifactorial Role of Neutrophils in Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2014, 10, 593–601. [Google Scholar] [CrossRef]
- Soehnlein, O. Multiple Roles for Neutrophils in Atherosclerosis. Circ. Res. 2012, 110, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Tsai, Y.F.; Pan, Y.L.; Hwang, T.L. Understanding the Role of Neutrophils in Acute Respiratory Distress Syndrome. Biomed. J. 2021, 44, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef] [PubMed]
- Lessieur, E.M.; Liu, H.; Saadane, A.; Du, Y.; Tang, J.; Kiser, J.; Kern, T.S. Neutrophil-Derived Proteases Contribute to the Pathogenesis of Early Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2021, 62, 7. [Google Scholar] [CrossRef] [PubMed]
- Haitao, L.; Timothy, S.K. Neutrophil Elastase Contributes to the Early Molecular Abnormalities of the Retina in Diabetes. Diabetes 2018, 67, 590. [Google Scholar] [CrossRef]
- Barliya, T.; Dardik, R.; Nisgav, Y.; Dachbash, M.; Gaton, D.; Kenet, G.; Ehrlich, R.; Weinberger, D.; Livnat, T. Possible Involvement of Netosis in Inflammatory Processes in the Eye: Evidence from a Small Cohort of Patients. Mol. Vis. 2017, 23, 922. [Google Scholar] [PubMed]
- Liu, H.; Lessieur, E.M.; Saadane, A.; Lindstrom, S.I.; Taylor, P.R.; Kern, T.S. Neutrophil Elastase Contributes to the Pathological Vascular Permeability Characteristic of Diabetic Retinopathy. Diabetologia 2019, 62, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Hata, Y.; Nakao, S.; Kita, T.; Miura, M.; Kawahara, S.; Zandi, S.; Almulki, L.; Shimokawa, H.; Hafezi-Moghadam, A.; et al. Rho Kinase Inhibition by Fasudil Ameliorates Diabetes-Induced Microvascular Damage. Diabetes 2009, 58, 215–226. [Google Scholar] [CrossRef]
- Woo, S.J.; Ahn, S.J.; Ahn, J.; Park, K.H.; Lee, K. Elevated Systemic Neutrophil Count in Diabetic Retinopathy and Diabetes: A Hospital-Based Cross-Sectional Study of 30,793 Korean Subjects. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7697–7703. [Google Scholar] [CrossRef]
- Guangyuan, L.; Alexander, A.V.; Ramaprasad Ravichandra, T.; Xiaoqi, W.; Rose, G.K.; Nader, S.; Timothy, S.K. Marrow-Derived Cells Regulate the Development of Early Diabetic Retinopathy and Tactile Allodynia in Mice. Diabetes 2012, 61, 3294–3303. [Google Scholar] [CrossRef]
- Jong-Kyu, P.; Jung-Hyun, K.; Gu, J.; Hwi Dong, Y.; Sung Keun, P.; Il Seong, N.-G.; Eunja, K.; Hyun Jung, K. Evaluation of Circulating Markers of Neutrophil Extracellular Trap (Net) Formation as Risk Factors for Diabetic Retinopathy in a Case-Control Association Study. Exp. Clin. Endocrinol. Diabetes 2016, 124, 557–561. [Google Scholar] [CrossRef]
- Scott, A.; Fruttiger, M. Oxygen-Induced Retinopathy: A Model for Vascular Pathology in the Retina. Eye 2010, 24, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Hypochlorous Acid Chemistry in Mammalian Cells: Influence on Infection and Role in Various Pathologies. Int. J. Mol. Sci. 2022, 23, 10735. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Seo, M.-S.; Ozaki, K.; Yamada, H.; Yamada, E.; Okamoto, N.; Hofmann, F.; Wood, J.M.; Campochiaro, P.A. Blockade of Vascular Endothelial Cell Growth Factor Receptor Signaling Is Sufficient to Completely Prevent Retinal Neovascularization. Am. J. Pathol. 2000, 156, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Deliyanti, D.; Talia, D.M.; Zhu, T.; Maxwell, M.J.; Agrotis, A.; Jerome, J.R.; Hargreaves, E.M.; Gerondakis, S.; Hibbs, M.L.; Mackay, F.; et al. Foxp3+ Tregs Are Recruited to the Retina to Repair Pathological Angiogenesis. Nat. Commun. 2017, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, T.A.; Gibson, D.S.; de Gooyer, T.E.; de la Cruz, V.F.; McDonald, D.M.; Stitt, A.W. Inhibition of Tumor Necrosis Factor-A Improves Physiological Angiogenesis and Reduces Pathological Neovascularization in Ischemic Retinopathy. Am. J. Pathol. 2005, 166, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Yoshida, A.; Ishibashi, T. Induction of Il-8, Mcp-1, and Bfgf by Tnf-A in Retinal Glial Cells: Implications for Retinal Neovascularization During Post-Ischemic Inflammation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.-P. Pericytes and the Pathogenesis of Diabetic Retinopathy. Horm. Metab. Res. 2005, 37, 39–43. [Google Scholar] [CrossRef]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B. A Central Role for Inflammation in the Pathogenesis of Diabetic Retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef]
- Stahl, A.; Connor, K.M.; Sapieha, P.; Chen, J.; Dennison, R.J.; Krah, N.M.; Seaward, M.R.; Willett, K.L.; Aderman, C.M.; Guerin, K.I. The Mouse Retina as an Angiogenesis Model. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, B.S.; de Winther, M.P.; Heeringa, P. Myeloperoxidase: Molecular Mechanisms of Action and Their Relevance to Human Health and Disease. Antioxid. Redox Signal. 2009, 11, 2899–2937. [Google Scholar] [CrossRef] [PubMed]
- Deliyanti, D.; Alrashdi, S.F.; Touyz, R.M.; Kennedy, C.R.; Jha, J.C.; Cooper, M.E.; Jandeleit-Dahm, K.A.; Wilkinson-Berka, J.L. Nox (Nadph Oxidase) 1, Nox4, and Nox5 Promote Vascular Permeability and Neovascularization in Retinopathy. Hypertension 2020, 75, 1091–1101. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L.; Deliyanti, D.; Rana, I.; Miller, A.G.; Agrotis, A.; Armani, R.; Szyndralewiez, C.; Wingler, K.; Touyz, R.M.; Cooper, M.E. Nadph Oxidase, Nox1, Mediates Vascular Injury in Ischemic Retinopathy. Antioxid. Redox Signal. 2014, 20, 2726–2740. [Google Scholar] [CrossRef]
- Augustin, A.; Breipohl, W.; Böker, T.; Lutz, J.; Spitznas, M. Increased Lipid Peroxide Levels and Myeloperoxidase Activity in the Vitreous of Patients Suffering from Proliferative Diabetic Retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 1993, 231, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.A.; Avery, R.L.; Foley, E.D.; Aiello, L.P.; Smith, L. Vascular Endothelial Growth Factor/Vascular Permeability Factor Expression in a Mouse Model of Retinal Neovascularization. Proc. Natl. Acad. Sci. USA 1995, 92, 905–909. [Google Scholar] [CrossRef]
- Le, Y.Z. Vegf Production and Signaling in Müller Glia Are Critical to Modulating Vascular Function and Neuronal Integrity in Diabetic Retinopathy and Hypoxic Retinal Vascular Diseases. Vis. Res. 2017, 139, 108–114. [Google Scholar] [CrossRef]
- Taichman, N.S.; Young, S.; Cruchley, A.T.; Taylor, P.; Paleolog, E. Human Neutrophils Secrete Vascular Endothelial Growth Factor. J. Leukoc. Biol. 1997, 62, 397–400. [Google Scholar] [CrossRef]
- Gong, Y.; Koh, D.R. Neutrophils Promote Inflammatory Angiogenesis Via Release of Preformed Vegf in an in Vivo Corneal Model. Cell Tissue Res. 2010, 339, 437–448. [Google Scholar] [CrossRef]
- Rivera, J.C.; Sitaras, N.; Noueihed, B.; Hamel, D.; Madaan, A.; Zhou, T.; Honoré, J.-C.; Quiniou, C.; Joyal, J.-S.; Hardy, P.; et al. Microglia and Interleukin-1β in Ischemic Retinopathy Elicit Microvascular Degeneration through Neuronal Semaphorin-3a. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Yoshida, A.; Ishibashi, T.; Elner, S.G.; Elner, V.M. Role of Mcp-1 and Mip-1α in Retinal Neovascularization During Postischemic Inflammation in a Mouse Model of Retinal Neovascularization. J. Leucoc. Biol. 2003, 73, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Davies, M.H.; Stempel, A.J.; Powers, M.R. Mcp-1 Deficiency Delays Regression of Pathologic Retinal Neovascularization in a Model of Ischemic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4195–4202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Pham, L.; Zhang, N.; He, S.; Gamulescu, M.-A.; Spee, C.; Ryan, S.J.; Hinton, D.R. Neutrophils Promote Experimental Choroidal Neovascularization. Mol. Vis. 2005, 11, 414–424. [Google Scholar] [PubMed]
- Tazzyman, S.; Lewis, C.E.; Murdoch, C. Neutrophils: Key Mediators of Tumour Angiogenesis. Int. J. Exp. Pathol. 2009, 90, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, A.; Seandel, M.; Kupriyanova, T.A.; Partridge, J.J.; Madsen, M.A.; Hahn-Dantona, E.A.; Quigley, J.P.; Deryugina, E.I. Proangiogenic Role of Neutrophil-Like Inflammatory Heterophils During Neovascularization Induced by Growth Factors and Human Tumor Cells. Blood 2006, 107, 317–327. [Google Scholar] [CrossRef]
- Binet, F.; Cagnone, G.; Crespo-Garcia, S.; Hata, M.; Neault, M.; Dejda, A.; Wilson, A.M.; Buscarlet, M.; Mawambo, G.T.; Howard, J.P. Neutrophil Extracellular Traps Target Senescent Vasculature for Tissue Remodeling in Retinopathy. Science 2020, 369, eaay5356. [Google Scholar] [CrossRef]
- Fischer, M.A.; Davies, M.L.; Reider, I.E.; Heipertz, E.L.; Epler, M.R.; Sei, J.J.; Ingersoll, M.A.; Van Rooijen, N.; Randolph, G.J.; Norbury, C.C. Cd11b+, Ly6g+ Cells Produce Type I Interferon and Exhibit Tissue Protective Properties Following Peripheral Virus Infection. PLoS Pathog. 2011, 7, e1002374. [Google Scholar] [CrossRef]
- Daley, J.M.; Thomay, A.A.; Connolly, M.D.; Reichner, J.S.; Albina, J.E. Use of Ly6g-Specific Monoclonal Antibody to Deplete Neutrophils in Mice. J. Leukoc. Biol. 2007, 83, 64–70. [Google Scholar] [CrossRef]
- Deliyanti, D.; Lee, J.Y.; Petratos, S.; Meyer, C.J.; Ward, K.W.; Wilkinson-Berka, J.L.; de Haan, J.B. A Potent Nrf2 Activator, Dh404, Bolsters Antioxidant Capacity in Glial Cells and Attenuates Ischaemic Retinopathy. Clin. Sci. 2016, 130, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deliyanti, D.; Suphapimol, V.; Ang, P.; Tang, X.; Jayasimhan, A.; Wilkinson-Berka, J.L. Early Depletion of Neutrophils Reduces Retinal Inflammation and Neovascularization in Mice with Oxygen-Induced Retinopathy. Int. J. Mol. Sci. 2023, 24, 15680. https://doi.org/10.3390/ijms242115680
Deliyanti D, Suphapimol V, Ang P, Tang X, Jayasimhan A, Wilkinson-Berka JL. Early Depletion of Neutrophils Reduces Retinal Inflammation and Neovascularization in Mice with Oxygen-Induced Retinopathy. International Journal of Molecular Sciences. 2023; 24(21):15680. https://doi.org/10.3390/ijms242115680
Chicago/Turabian StyleDeliyanti, Devy, Varaporn Suphapimol, Phoebe Ang, Xiuying Tang, Abhirup Jayasimhan, and Jennifer L. Wilkinson-Berka. 2023. "Early Depletion of Neutrophils Reduces Retinal Inflammation and Neovascularization in Mice with Oxygen-Induced Retinopathy" International Journal of Molecular Sciences 24, no. 21: 15680. https://doi.org/10.3390/ijms242115680
APA StyleDeliyanti, D., Suphapimol, V., Ang, P., Tang, X., Jayasimhan, A., & Wilkinson-Berka, J. L. (2023). Early Depletion of Neutrophils Reduces Retinal Inflammation and Neovascularization in Mice with Oxygen-Induced Retinopathy. International Journal of Molecular Sciences, 24(21), 15680. https://doi.org/10.3390/ijms242115680





