Changes in Polar Lipid Composition in Balsam Fir during Seasonal Cold Acclimation and Relationship to Needle Abscission
Abstract
:1. Introduction
2. Results
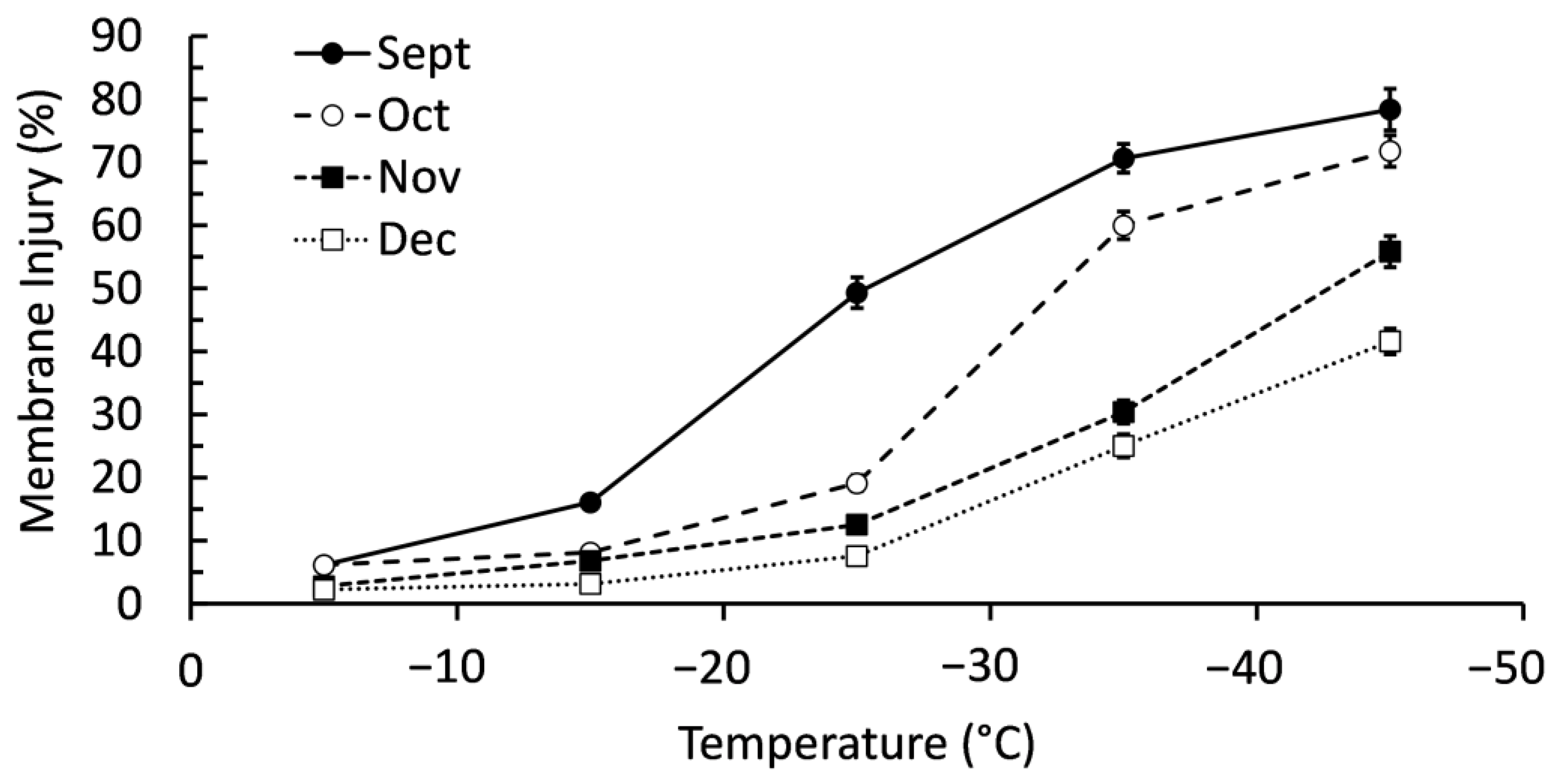
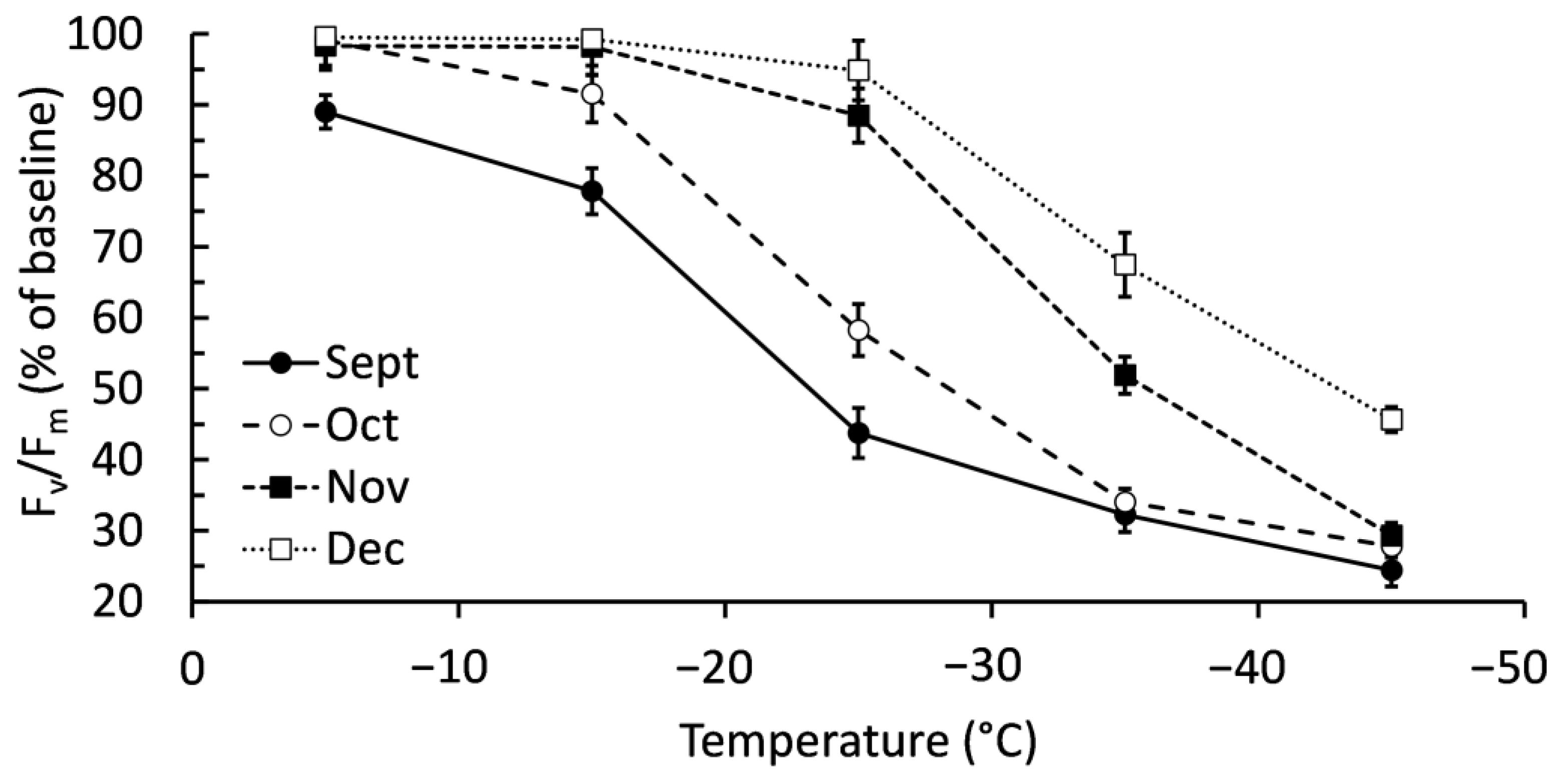
2.1. Confirming Cold Acclimation
2.2. Changes in Polar Lipids during Cold Acclimation
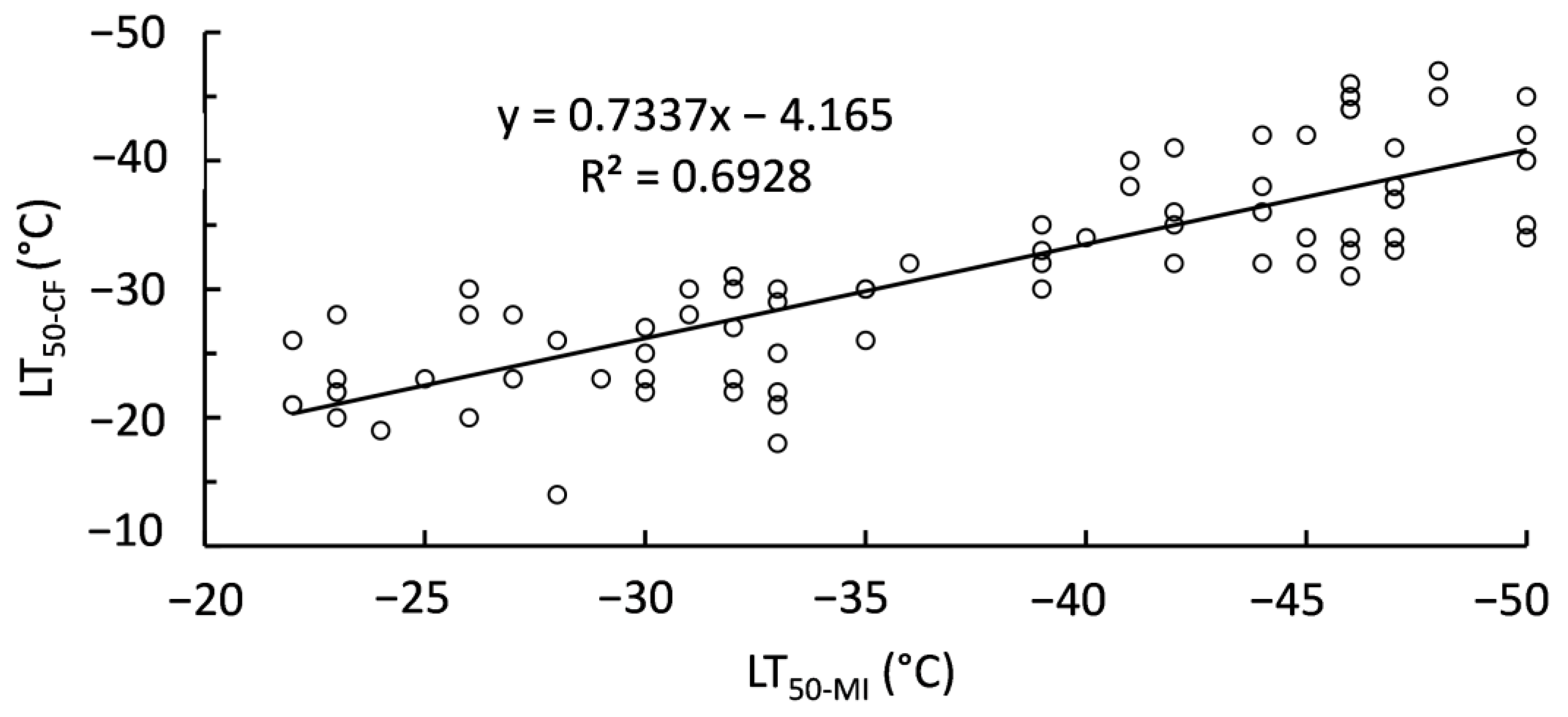
2.3. Relationship of Polar Lipids and Lethal Temperatures
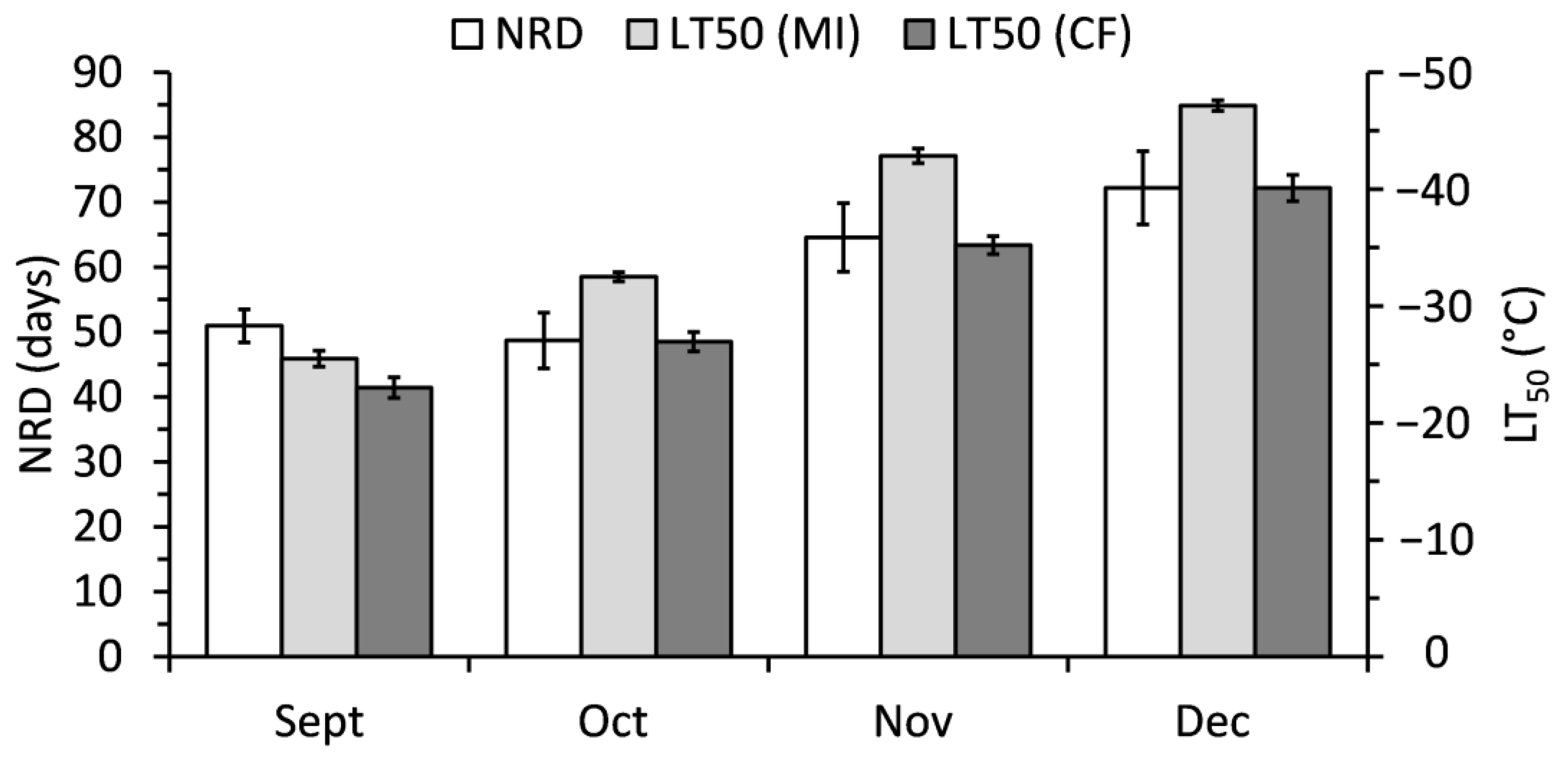
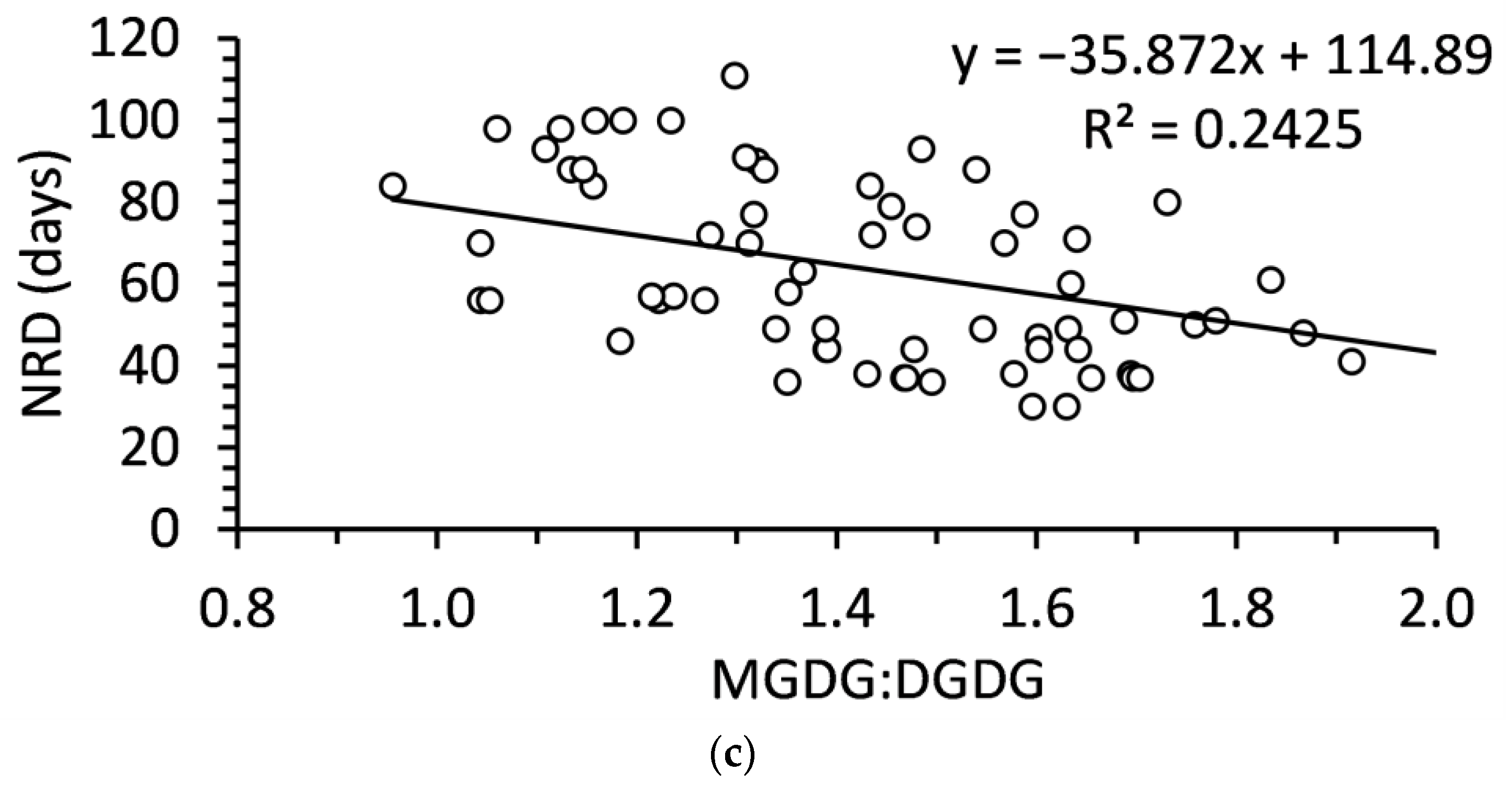
2.4. Relationship of Polar Lipids and Needle Abscission
3. Discussion
3.1. Cold Acclimation in Balsam Fir
3.2. Polar Lipids in Balsam Fir
3.3. Needle Retention in Balsam Fir
4. Materials and Methods
4.1. Sampling and Experimental Design
4.2. Environmental Conditions during Sampling Months
4.3. Display Conditions and Needle Retention
4.4. Freeze Testing to Evaluate Cold Tolerance
4.5. Lipid Extraction and Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collier, J.; MacLean, D.A.; D’Orangeville, L.; Taylor, A.R. A Review of Climate Change Effects on the Regeneration Dynamics of Balsam Fir. For. Chron. 2022, 98, 54–65. [Google Scholar] [CrossRef]
- Burns, R.M.; Honkala, B.H. Silvics of North America: 1. Conifers; US Department of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 1. [Google Scholar]
- Lada, R.R.; MacDonald, M.T. Understanding the Physiology of Postharvest Needle Abscission in Balsam Fir. Front. Plant Sci. 2015, 6, 1069. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, A.; MacDonald, M.T.; Lada, R. Environmental and Hormonal Physiology of Postharvest Needle Abscission in Christmas Trees. Crit. Rev. Plant Sci. 2016, 35, 1–17. [Google Scholar] [CrossRef]
- Environment Canada Historical Data - Climate - Environment and Climate Change Canada. Available online: https://climate.weather.gc.ca/historical_data/search_historic_data_e.html (accessed on 23 October 2023).
- Beck, E.H.; Fettig, S.; Knake, C.; Hartig, K.; Bhattarai, T. Specific and Unspecific Responses of Plants to Cold and Drought Stress. J. Biosci. 2007, 32, 501–510. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold Stress Effects on Reproductive Development in Grain Crops: An Overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Chang, C.Y.; Bräutigam, K.; Hüner, N.P.A.; Ensminger, I. Champions of Winter Survival: Cold Acclimation and Molecular Regulation of Cold Hardiness in Evergreen Conifers. N. Phytol. 2021, 229, 675–691. [Google Scholar] [CrossRef]
- Vogg, G.; Heim, R.; Gotschy, B.; Beck, E.; Hansen, J. Frost Hardening and Photosynthetic Performance of Scots Pine (Pinus sylvestris, L.). II. Seasonal Changes in the Fluidity of Thylakoid Membranes. Planta 1998, 204, 201–206. [Google Scholar] [CrossRef]
- Crosatti, C.; Rizza, F.; Badeck, F.W.; Mazzucotelli, E.; Cattivelli, L. Harden the Chloroplast to Protect the Plant. Physiol. Plant. 2013, 147, 55–63. [Google Scholar] [CrossRef]
- Ivanova, A.P.; Stefanov, K.L.; Yordanov, I.T. Effect of Cytokinin 4-PU-30 on the Lipid Composition of Water Stressed Bean Plants. Biol. Plant. 1998, 41, 155–159. [Google Scholar] [CrossRef]
- Degenkolbe, T.; Giavalisco, P.; Zuther, E.; Seiwert, B.; Hincha, D.K.; Willmitzer, L. Differential Remodeling of the Lipidome during Cold Acclimation in Natural Accessions of Arabidopsis thaliana: Lipidomics of Arabidopsis Cold Acclimation. Plant J. 2012, 72, 972–982. [Google Scholar] [CrossRef]
- Seiwert, D.; Witt, H.; Ritz, S.; Janshoff, A.; Paulsen, H. The Nonbilayer Lipid MGDG and the Major Light-Harvesting Complex (LHCII) Promote Membrane Stacking in Supported Lipid Bilayers. Biochemistry 2018, 57, 2278–2288. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Uemura, M. Protein and Lipid Compositions of Isolated Plasma Membranes from Orchard Grass (Dactylis glomerata L.) and Changes during Cold Acclimation. Plant Physiol. 1984, 75, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Nokhsorov, V.V.; Dudareva, L.V.; Senik, S.V.; Chirikova, N.K.; Petrov, K.A. Influence of Extremely Low Temperatures of the Pole of Cold on the Lipid and Fatty-Acid Composition of Aerial Parts of the Horsetail Family (Equisetaceae). Plants 2021, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.E.; Lada, R.R.; Caldwell, C.D.; Udenigwe, C.C.; MacDonald, M.T. Linking Changes in Fatty Acid Composition to Postharvest Needle Abscission Resistance in Balsam Fir Trees. Forests 2022, 13, 800. [Google Scholar] [CrossRef]
- Atucha Zamkova, A.-A.; Steele, K.A.; Smith, A.R. Methods for Measuring Frost Tolerance of Conifers: A Systematic Map. Forests 2021, 12, 1094. [Google Scholar] [CrossRef]
- Murray, M.B.; Cape, J.N.; Fowler, D. Quantification of Frost Damage in Plant Tissues by Rates of Electrolyte Leakage. N. Phytol. 1989, 113, 307–311. [Google Scholar] [CrossRef]
- Bachofen, C.; Wohlgemuth, T.; Ghazoul, J.; Moser, B. Cold Temperature Extremes during Spring Do Not Limit the Range Shift of Mediterranean Pines into Regions with Intermittent Frost. Funct. Ecol. 2016, 30, 856–865. [Google Scholar] [CrossRef]
- Adams, G.T.; Perkins, T.D. Assessing Cold Tolerance in Picea Using Chlorophyll Fluorescence. Environ. Exp. Bot. 1993, 33, 377–382. [Google Scholar] [CrossRef]
- Perks, M.P.; Osborne, B.A.; Mitchell, D.T. Rapid Predictions of Cold Tolerance in Douglas-Fir Seedlings Using Chlorophyll Fluorescence after Freezing. N. For. 2004, 28, 49–62. [Google Scholar] [CrossRef]
- Sakai, A. Comparative Study on Freezing Resistance of Conifers with Special Reference to Cold Adaptation and Its Evolutive Aspects. Can. J. Bot. 1983, 61, 2323–2332. [Google Scholar] [CrossRef]
- Norgaard Nielsen, C.C.; Rasmussen, H.N. Frost Hardening and Dehardening in Abies Procera and Other Conifers under Differing Temperature Regimes and Warm-Spell Treatments. Forestry 2009, 82, 43–59. [Google Scholar] [CrossRef]
- MacDonald, G.E.; Lada, R.R.; Caldwell, C.D.; Udenigwe, C.; MacDonald, M. Lipid and Fatty Acid Changes Linked to Postharvest Needle Abscission in Balsam Fir, Abies Balsamea. Trees 2020, 34, 297–305. [Google Scholar] [CrossRef]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.-E.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling Membrane Lipids in Plant Stress Responses. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef]
- Dörmann, P.; Benning, C. Galactolipids Rule in Seed Plants. Trends Plant Sci. 2002, 7, 112–118. [Google Scholar] [CrossRef]
- Oquist, G. Seasonally Induced Changes in Acyl Lipids and Fatty Acids of Chloroplast Thylakoids of Pinus silvestris: A Correlation between the Level of Unsaturation of Monogalactosyldiglyceride and the Rate of Electron Transport. Plant Physiol. 1982, 69, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Steponkus, P.L. Effect of Cold Acclimation on the Lipid Composition of the Lnner and Outer Membrane of the Chloroplast Envelope Lsolated from Rye Leaves. Plant Physiol. 1997, 114, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, I.; Mizusawa, N.; Wada, H.; Sato, N. Digalactosyldiacylglycerol Is Required for Stabilization of the Oxygen-Evolving Complex in Photosystem II. Plant Physiol. 2007, 145, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, J.W.M.; Storz, T.; Schmidt, R.R.; Heinz, E. Biosynthesis of Digalactosyldiacylglycerol in Plastids from 16:3 and 18:3 Plants. Plant Physiol. 1990, 93, 1286–1294. [Google Scholar] [CrossRef]
- Rocha, J.; Nitenberg, M.; Girard-Egrot, A.; Jouhet, J.; Maréchal, E.; Block, M.A.; Breton, C. Do Galactolipid Synthases Play a Key Role in the Biogenesis of Chloroplast Membranes of Higher Plants? Front. Plant Sci. 2018, 9, 126. [Google Scholar] [CrossRef]
- Kojima, M.; Shiraki, H.; Ohnishi, N.; Ito, S. Seasonal Change Sin Glycolipids and Phospholipids in Pinus nigra Needles. Res. Bull. Obihiro 1990, 17, 13–19. [Google Scholar]
- Orlova, I.V.; Serebriiskaya, T.S.; Popov, V.; Merkulova, N.; Nosov, A.M.; Trunova, T.I.; Tsydendambaev, V.D.; Los, D.A. Transformation of Tobacco with a Gene for the Thermophilic Acyl-Lipid Desaturase Enhances the Chilling Tolerance of Plants. Plant Cell Physiol. 2003, 44, 447–450. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell. Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Selvy, P.E.; Lavieri, R.R.; Lindsley, C.W.; Brown, H.A. Phospholipase D: Enzymology, Functionality, and Chemical Modulation. Chem. Rev. 2011, 111, 6064–6119. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid Signalling in Plant Responses to Abiotic Stress: Lipid Signalling in Plant Responses to Abiotic Stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef]
- Kiełbowicz-Matuk, A.; Banachowicz, E.; Turska-Tarska, A.; Rey, P.; Rorat, T. Expression and Characterization of a Barley Phosphatidylinositol Transfer Protein Structurally Homologous to the Yeast Sec14p Protein. Plant Sci. 2016, 246, 98–111. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.T.; Lada, R.R. Changes in Endogenous Hormone Levels Explains Seasonal Variation in Balsam Fir Needle Abscission Patterns. J. Plant Growth Regul. 2017, 36, 723–733. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Lada, R.R.; Veitch, R.S.; Thiagarajan, A.; Adams, A.D. Postharvest Needle Abscission Resistance of Balsam Fir (Abies Balsamea) Is Modified by Harvest Date. Can. J. For. Res. 2014, 44, 1394–1401. [Google Scholar] [CrossRef]
- Juurakko, C.L.; diCenzo, G.C.; Walker, V.K. Cold Acclimation and Prospects for Cold-Resilient Crops. Plant Stress 2021, 2, 100028. [Google Scholar] [CrossRef]
- Catalá, R.; López-Cobollo, R.; Mar Castellano, M.; Angosto, T.; Alonso, J.M.; Ecker, J.R.; Salinas, J. The Arabidopsis 14-3-3 Protein RARE COLD INDUCIBLE 1A Links Low-Temperature Response and Ethylene Biosynthesis to Regulate Freezing Tolerance and Cold Acclimation. Plant Cell 2014, 26, 3326–3342. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.; Bittner, A.; Prescher, A.; Van Buer, J. Preparing Plants for Improved Cold Tolerance by Priming. Plant Cell Environ. 2019, 42, 782–800. [Google Scholar] [CrossRef]
- Hassan, Q.K. Spatial Mapping of Growing Degree Days: An Application of MODIS-Based Surface Temperatures and Enhanced Vegetation Index. J. Appl. Remote Sens. 2007, 1, 013511. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Lada, R.R.; Dorais, M.; Pepin, S. Endogenous and Exogenous Ethylene Induces Needle Abscission and Cellulase Activity in Post-Harvest Balsam Fir (Abies balsamea L.). Trees 2011, 25, 947–952. [Google Scholar] [CrossRef]
- Man, R.; Lu, P.; Dang, Q.-L. Cold Tolerance of Black Spruce, White Spruce, Jack Pine, and Lodgepole Pine Seedlings at Different Stages of Spring Dehardening. N. For. 2021, 52, 317–328. [Google Scholar] [CrossRef]
- Odlum, K.D.; Blake, T.J. A Comparison of Analytical Approaches for Assessing Freezing Damage in Black Spruce Using Electrolyte Leakage Methods. Can. J. Bot. 1996, 74, 952–958. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Munnik, T.; Heilmann (Eds.) Plant Lipid Signaling Protocols; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1009, ISBN 978-1-62703-400-5. [Google Scholar]
- Brügger, B.; Erben, G.; Sandhoff, R.; Wieland, F.T.; Lehmann, W.D. Quantitative Analysis of Biological Membrane Lipids at the Low Picomole Level by Nano-Electrospray Ionization Tandem Mass Spectrometry. Proc. Natl. Acad. Sci. USA 1997, 94, 2339–2344. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, R.; Houjou, T.; Nakanishi, H.; Yamazaki, T.; Ishida, M.; Imagawa, M.; Shimizu, T. Focused Lipidomics by Tandem Mass Spectrometry. J. Chromatogr. B 2005, 823, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Isaac, G.; Jeannotte, R.; Esch, S.; Welti, R. New Mass Spectrometry-Based Strategies for Lipids. In Genetic Engineering; Springer: Boston, MA, USA, 2007; Volume 28. [Google Scholar]



| Lipid Class | September. | October | November | December | p-Value |
|---|---|---|---|---|---|
| DGDG | 26.87 ± 0.36 | 27.10 ± 0.43 | 28.33 ± 0.38 | 30.04 ± 0.37 | <0.001 |
| MGDG | 45.90 ± 0.87 | 40.98 ± 1.17 | 38.37 ± 0.81 | 33.86 ± 0.58 | <0.001 |
| PC | 15.01 ± 0.39 | 18.12 ± 0.61 | 18.48 ± 0.44 | 19.56 ± 0.40 | <0.001 |
| PG | 4.04 ± 0.20 | 3.89 ± 0.16 | 3.81 ± 0.15 | 5.11 ± 0.15 | <0.001 |
| PE | 2.62 ± 0.17 | 3.74 ± 0.18 | 3.75 ± 0.21 | 4.76 ± 0.24 | <0.001 |
| PI | 3.86 ± 0.15 | 3.99 ± 0.16 | 4.64 ± 0.17 | 4.75 ± 0.11 | <0.001 |
| PA | 0.47 ± 0.06 | 0.93 ± 0.09 | 1.19 ± 0.34 | 0.66 ± 0.05 | =0.049 |
| LPC | 0.05 ± 0.01 | 0.12 ± 0.02 | 0.14 ± 0.01 | 0.09 ± 0.01 | =0.041 |
| LPG | 1.05 ± 0.19 | 0.96 ± 0.15 | 1.14 ± 0.15 | 1.00 ± 0.20 | =0.344 |
| LPE | 0.11 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 | =0.071 |
| Lipid Class | LT50-MI | LT50-CF | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 (%) | p-Value | Slope | Constant | R2 (%) | p-Value | Slope | Constant | |
| DGDG | 31.5 | <0.001 | −2.23 | 25.8 | 29.5 | <0.001 | −1.87 | 21.3 |
| MGDG | 55.0 | <0.001 | 1.10 | −80.8 | 42.7 | <0.001 | 0.86 | −65.6 |
| PC | 36.7 | <0.001 | −1.86 | −3.80 | 26.0 | <0.001 | −1.36 | −7.01 |
| PG | 15.2 | =0.001 | −3.86 | −20.6 | 16.6 | =0.008 | −3.43 | −16.8 |
| PE | 35.4 | <0.001 | −4.40 | −20.6 | 27.7 | <0.001 | −3.41 | −18.6 |
| PI | 30.0 | <0.001 | −6.13 | −10.5 | 22.9 | <0.001 | −4.56 | −11.6 |
| PA | 2.5 | =0.180 | −1.65 | −35.5 | 3.4 | =0.411 | −0.73 | −30.7 |
| LPC | 5.1 | =0.060 | −29.0 | −34.0 | 7.8 | =0.193 | 24.2 | −28.85 |
| LPG | 0.7 | =0.498 | −0.69 | −36.2 | 3.4 | =0.399 | −1.10 | −30.1 |
| LPE | 3.1 | =0.130 | −30.3 | −32.8 | 4.6 | =0.208 | −28.4 | −27.5 |
| Sampling Date | CDD (Days) | Tmin (°C) | Tmax (°C) | Days Below 0 °C | Photoperiod (h) |
|---|---|---|---|---|---|
| 18 September | 0 | 5 | 21 | 0 | 12.4 |
| 28 October | 9 | −4 | 18 | 7 | 10.3 |
| 25 November | 102 | −8 | 17 | 28 | 9.2 |
| 30 December | 354 | −23 | 8.3 | 60 | 8.8 |
| Parameter | PA | PC/LPC | PE/LPE | PG | PI | PS | DGDG | MGDG |
|---|---|---|---|---|---|---|---|---|
| Typical Scan Time (min) | 3.51 | 1.28 | 3.34 | 3.21 | 4.00 | 4.01 | 1.67 | 1.67 |
| Depolarization Potential (V) | 100 | 100 | 100 | 100 | 100 | 100 | 90 | 90 |
| Exit Potential (V) | 14 | 14 | 14 | 14 | 14 | 14 | 10 | 10 |
| Collision Energy (V) | 25 | 40 | 28 | 20 | 25 | 26 | 24 | 21 |
| Collision Exit Potential (V) | 14 | 14 | 14 | 14 | 14 | 14 | 23 | 23 |
| Class | Ion Analyzed | Positive Ion Scan Mode | m/z Range | Reference |
|---|---|---|---|---|
| PA | (M + NH4)− | NL of 115.00 | 500–850 | [48] |
| PC/LPC | (M + H)− | Pre of m/z 184.07 | 450–960 | [49] |
| PE/LPE | (M + H)− | NL of 141.02 | 420–920 | [49] |
| PG | (M + NH4)− | NL of 189.04 | 650–1000 | [50] |
| LPG | (M-H)− | Prec 153 | - | [25] |
| PI | (M + NH4)− | NL of 277.06 | 790–950 | [50] |
| PS | (M + H)− | NL of 185.01 | 600–920 | [49] |
| DGDG | (M + NH4)− | NL of 341.13 | 890–1050 | [51] |
| MGDG | (M + NH4) | NL of 179.08 | 700–900 | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacDonald, M.T.; Lada, R.R.; MacDonald, G.E.; Caldwell, C.D.; Udenigwe, C.C. Changes in Polar Lipid Composition in Balsam Fir during Seasonal Cold Acclimation and Relationship to Needle Abscission. Int. J. Mol. Sci. 2023, 24, 15702. https://doi.org/10.3390/ijms242115702
MacDonald MT, Lada RR, MacDonald GE, Caldwell CD, Udenigwe CC. Changes in Polar Lipid Composition in Balsam Fir during Seasonal Cold Acclimation and Relationship to Needle Abscission. International Journal of Molecular Sciences. 2023; 24(21):15702. https://doi.org/10.3390/ijms242115702
Chicago/Turabian StyleMacDonald, Mason T., Rajasekaran R. Lada, Gaye E. MacDonald, Claude D. Caldwell, and Chibuike C. Udenigwe. 2023. "Changes in Polar Lipid Composition in Balsam Fir during Seasonal Cold Acclimation and Relationship to Needle Abscission" International Journal of Molecular Sciences 24, no. 21: 15702. https://doi.org/10.3390/ijms242115702
APA StyleMacDonald, M. T., Lada, R. R., MacDonald, G. E., Caldwell, C. D., & Udenigwe, C. C. (2023). Changes in Polar Lipid Composition in Balsam Fir during Seasonal Cold Acclimation and Relationship to Needle Abscission. International Journal of Molecular Sciences, 24(21), 15702. https://doi.org/10.3390/ijms242115702








