Fluoroquinolone-Based Organic Salts (GUMBOS) with Antibacterial Potential
Abstract
1. Introduction
2. Results and Discussion
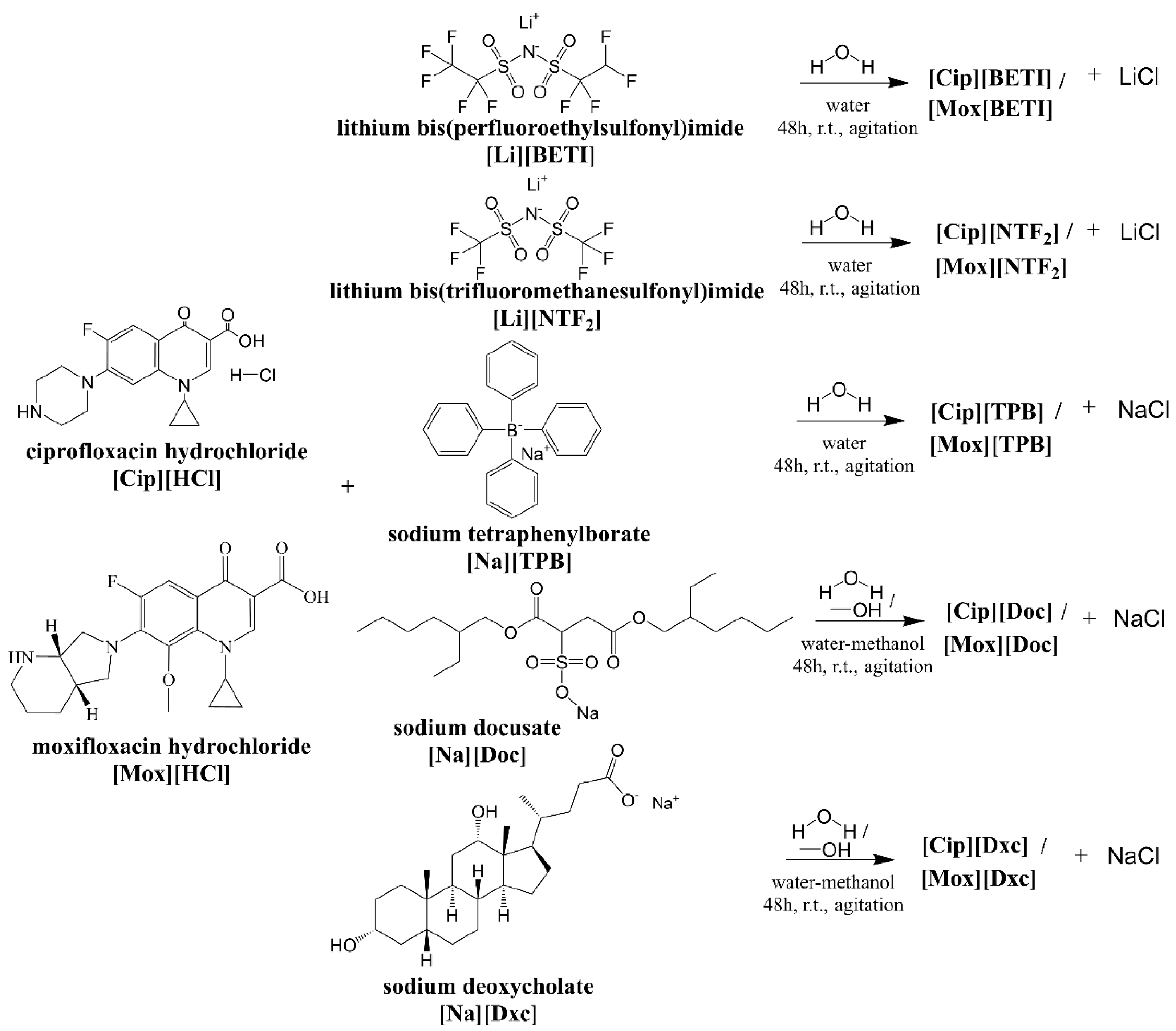
2.1. Synthesis of GUMBOS
2.2. Structural Characterization
2.2.1. 1H- and 13C-NMR
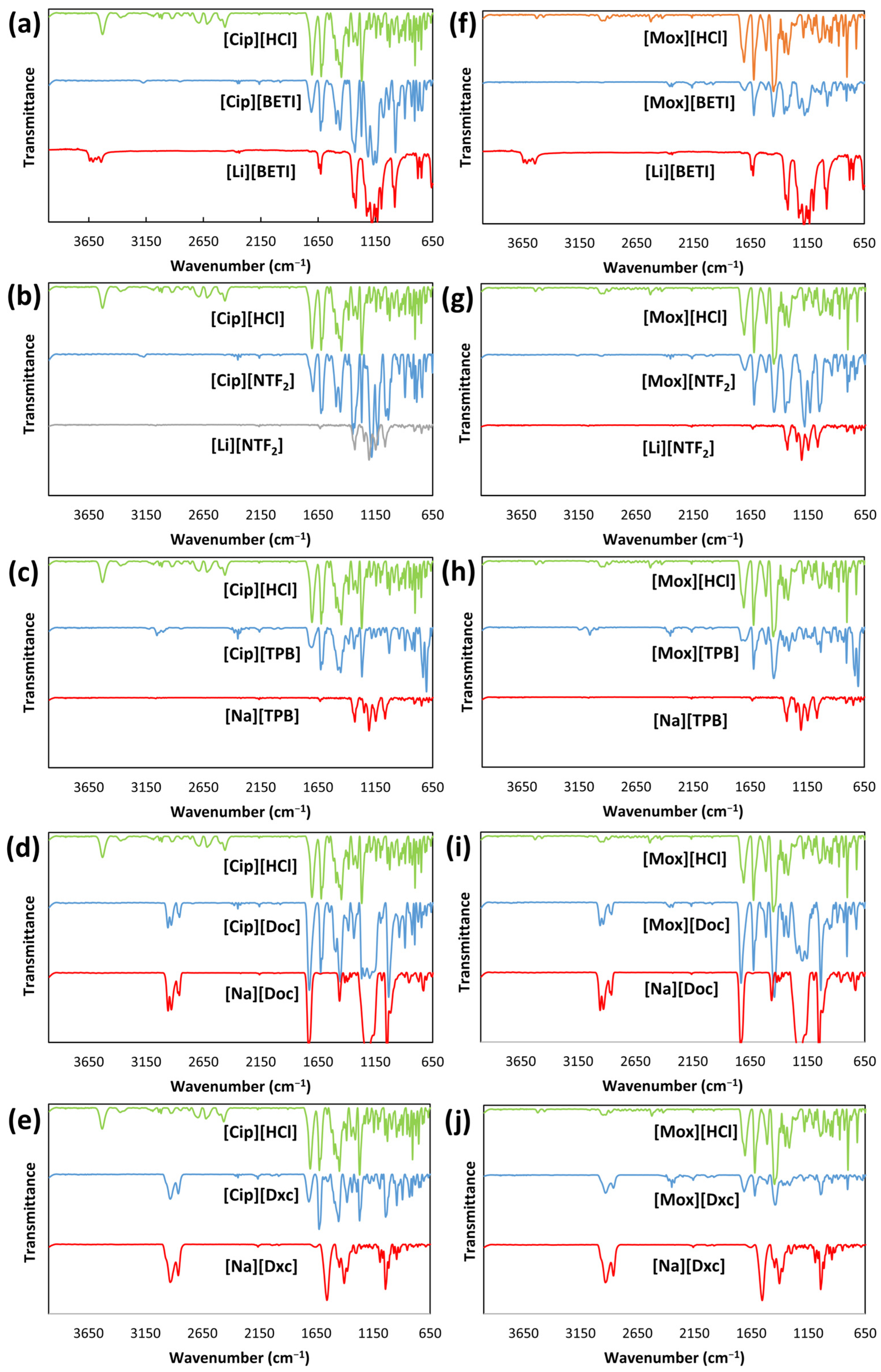
2.2.2. FT-IR Spectroscopy
2.2.3. ESI-MS
2.3. Thermal Stability Evaluation
2.4. Octanol–Water Partition Coefficients
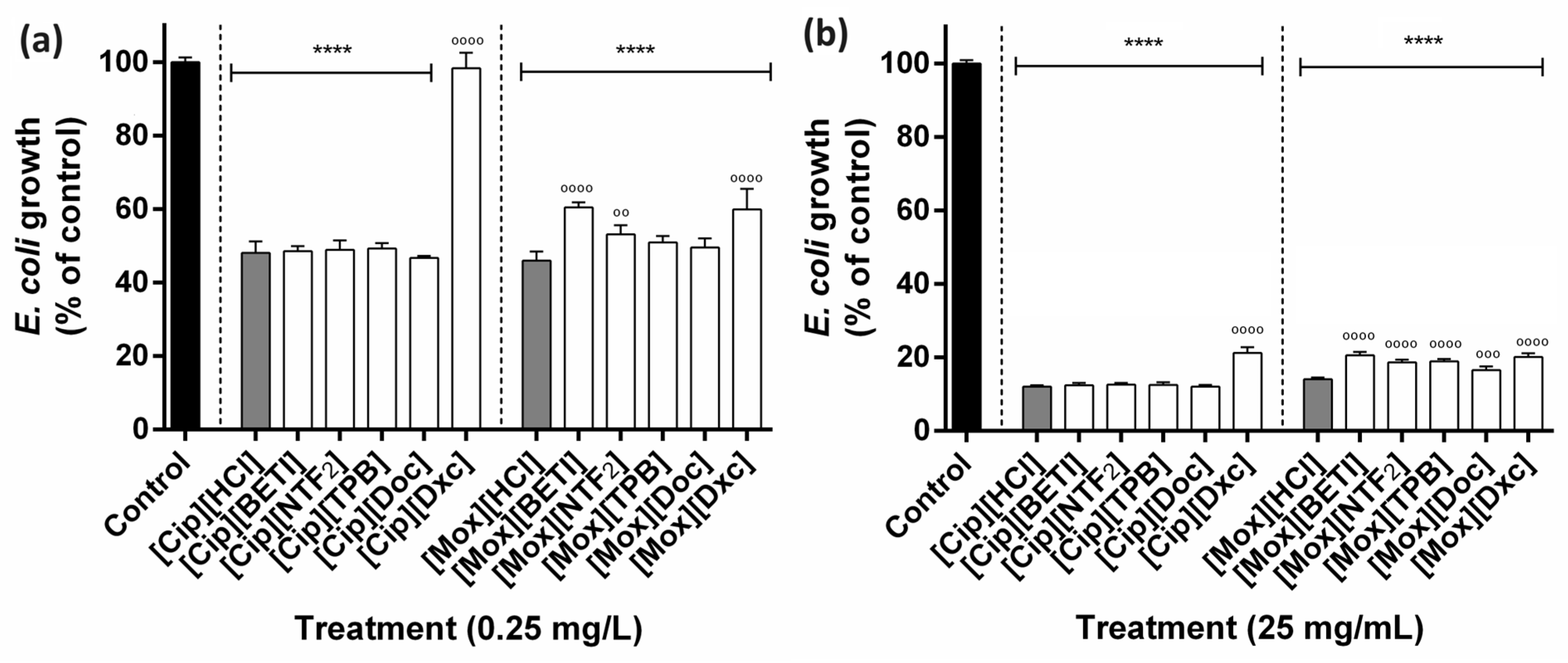
2.5. Antibacterial Susceptibility Testing
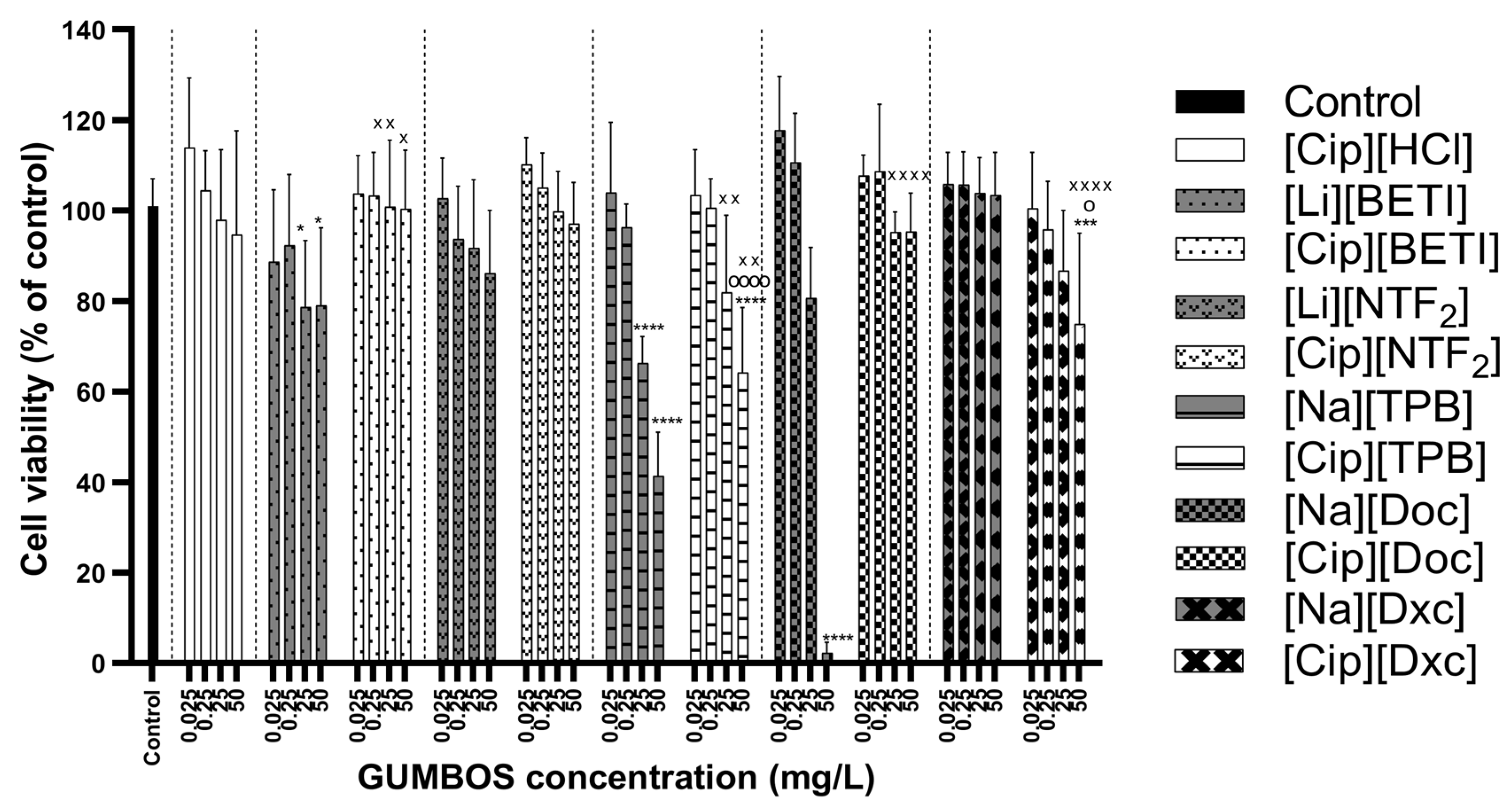
2.6. Cytotoxicity Evaluation
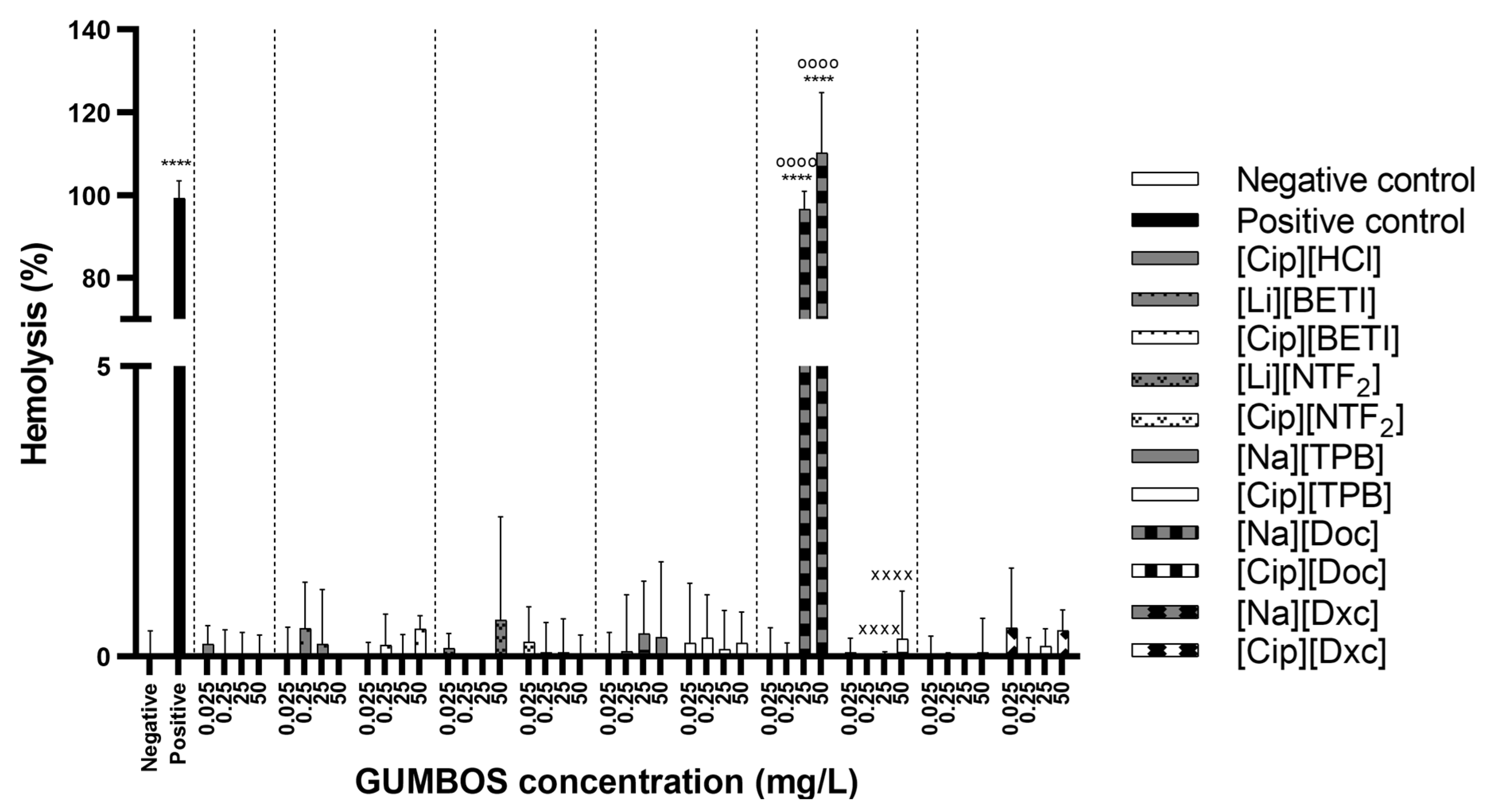
2.7. Hemolysis Assay
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of GUMBOS
3.3. Structural Characterization
3.3.1. 1H- and 13C-NMR
3.3.2. FT-IR Spectroscopy
3.3.3. ESI-MS
3.4. Thermal Stability Evaluation
3.5. Octanol–Water Partition Coefficients
3.6. Antibacterial Susceptibility Testing
3.7. Cytotoxicity Evaluation
3.8. Hemolysis Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Talbot, G.H.; Jezek, A.; Murray, B.E.; Jones, R.N.; Ebright, R.H.; Nau, G.J.; Rodvold, K.A.; Newland, J.G.; Boucher, H.W.; Infectious Diseases Society of America. The Infectious Diseases Society of America’s 10 × ‘20 Initiative (10 New Systemic Antibacterial Agents US Food and Drug Administration Approved by 2020): Is 20 × ‘20 a Possibility? Clin. Infect. Dis. 2019, 69, 1–11. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Kalan, L.; Wright, G.D. Antibiotic adjuvants: Multicomponent anti-infective strategies. Expert. Rev. Mol. Med. 2011, 13, e5. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Hagihara, M.; Crandon, J.L.; Nicolau, D.P. The efficacy and safety of antibiotic combination therapy for infections caused by Gram-positive and Gram-negative organisms. Expert. Opin. Drug Saf. 2012, 11, 221–233. [Google Scholar] [CrossRef]
- Little, W.; Black, C.; Smith, A.C. Clinical Implications of Polymicrobial Synergism Effects on Antimicrobial Susceptibility. Pathogens 2021, 10, 144. [Google Scholar] [CrossRef]
- Warner, I.M.; El-Zahab, B.; Siraj, N. Perspectives on moving ionic liquid chemistry into the solid phase. Anal. Chem. 2014, 86, 7184–7191. [Google Scholar] [CrossRef]
- Costa, F.M.S.; Saraiva, M.L.M.F.S.; Passos, M.L.C. Ionic liquids and organic salts with antimicrobial activity as a strategy against resistant microorganisms. J. Mol. Liq. 2022, 368, 120750. [Google Scholar] [CrossRef]
- Pérez, R.L.; Ayala, C.E.; Warner, I.M. Group of Uniform Materials Based on Organic Salts (GUMBOS): A Review of Their Solid State Properties and Applications; Murshed, S.M.S., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Cole, M.R.; Li, M.; Jadeja, R.; El-Zahab, B.; Hayes, D.; Hobden, J.A.; Janes, M.E.; Warner, I.M. Minimizing human infection from Escherichia coli O157:H7 using GUMBOS. J. Antimicrob. Chemother. 2013, 68, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.R.; Hobden, J.A.; Warner, I.M. Recycling antibiotics into GUMBOS: A new combination strategy to combat multi-drug-resistant bacteria. Molecules 2015, 20, 6466–6487. [Google Scholar] [CrossRef] [PubMed]
- Lopez, K.M.; Ravula, S.; Perez, R.L.; Ayala, C.E.; Losso, J.N.; Janes, M.E.; Warner, I.M. Hyaluronic Acid-Cellulose Composites as Patches for Minimizing Bacterial Infections. ACS Omega 2020, 5, 4125–4132. [Google Scholar] [CrossRef]
- Lopez, K.M.; Hobden, J.A.; Warner, I.M. Octenidine/carbenicillin GUMBOS as potential treatment for oropharyngeal gonorrhoea. J. Antimicrob. Chemother. 2020, 75, 3576–3581. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.; Santarem, N.; Santos, A.F.M.; Jacinto, M.L.; Cordeiro-da-Silva, A.; Prudencio, C.; Noronha, J.P.; Branco, L.C.; Petrovski, Z. Synthesis and Biological Evaluation of Amphotericin B Formulations Based on Organic Salts and Ionic Liquids against Leishmania infantum. Antibiotics 2022, 11, 1841. [Google Scholar] [CrossRef]
- Bento, C.M.; Silva, A.T.; Mansano, B.; Aguiar, L.; Teixeira, C.; Gomes, M.S.; Gomes, P.; Silva, T.; Ferraz, R. Improving the Antimycobacterial Drug Clofazimine through Formation of Organic Salts by Combination with Fluoroquinolones. Int. J. Mol. Sci. 2023, 24, 1402. [Google Scholar] [CrossRef]
- Ferraz, R.; Costa-Rodrigues, J.; Fernandes, M.H.; Santos, M.M.; Marrucho, I.M.; Rebelo, L.P.; Prudencio, C.; Noronha, J.P.; Petrovski, Z.; Branco, L.C. Antitumor Activity of Ionic Liquids Based on Ampicillin. ChemMedChem 2015, 10, 1480–1483. [Google Scholar] [CrossRef]
- Santos, M.M.; Raposo, L.R.; Carrera, G.; Costa, A.; Dionisio, M.; Baptista, P.V.; Fernandes, A.R.; Branco, L.C. Ionic Liquids and Salts from Ibuprofen as Promising Innovative Formulations of an Old Drug. ChemMedChem 2019, 14, 907–911. [Google Scholar] [CrossRef]
- Ferraz, R.; Silva, D.; Dias, A.R.; Dias, V.; Santos, M.M.; Pinheiro, L.; Prudencio, C.; Noronha, J.P.; Petrovski, Z.; Branco, L.C. Synthesis and Antibacterial Activity of Ionic Liquids and Organic Salts Based on Penicillin G and Amoxicillin hydrolysate Derivatives against Resistant Bacteria. Pharmaceutics 2020, 12, 221. [Google Scholar] [CrossRef]
- Santos, M.M.; Alves, C.; Silva, J.; Florindo, C.; Costa, A.; Petrovski, Z.; Marrucho, I.M.; Pedrosa, R.; Branco, L.C. Antimicrobial Activities of Highly Bioavailable Organic Salts and Ionic Liquids from Fluoroquinolones. Pharmaceutics 2020, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Lesher, G.Y.; Froelich, E.J.; Gruett, M.D.; Bailey, J.H.; Brundage, R.P. 1,8-Naphthyridine Derivatives. A New Class of Chemotherapeutic Agents. J. Med. Pharm. Chem. 1962, 91, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- Fabrega, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of action of and resistance to quinolones. Microb. Biotechnol. 2009, 2, 40–61. [Google Scholar] [CrossRef]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef]
- Naeem, A.; Badshah, S.L.; Muska, M.; Ahmad, N.; Khan, K. The Current Case of Quinolones: Synthetic Approaches and Antibacterial Activity. Molecules 2016, 21, 268. [Google Scholar] [CrossRef]
- Conley, Z.C.; Bodine, T.J.; Chou, A.; Zechiedrich, L. Wicked: The untold story of ciprofloxacin. PLoS Pathog. 2018, 14, e1006805. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Poeta, P.; Hebraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of quinolone action and resistance: Where do we stand? J. Med. Microbiol. 2017, 66, 551–559. [Google Scholar] [CrossRef]
- LeBel, M. Ciprofloxacin: Chemistry, mechanism of action, resistance, antimicrobial spectrum, pharmacokinetics, clinical trials, and adverse reactions. Pharmacotherapy 1988, 8, 3–33. [Google Scholar] [CrossRef]
- Dalhoff, A. Comparative in vitro and in vivo activity of the C-8 methoxy quinolone moxifloxacin and the C-8 chlorine quinolone BAY y 3118. Clin. Infect. Dis. 2001, 32 (Suppl. S1), S16–S22. [Google Scholar] [CrossRef]
- Cramariuc, O.; Rog, T.; Javanainen, M.; Monticelli, L.; Polishchuk, A.V.; Vattulainen, I. Mechanism for translocation of fluoroquinolones across lipid membranes. Biochim. Biophys. Acta 2012, 1818, 2563–2571. [Google Scholar] [CrossRef]
- Van Bambeke, F. Delafloxacin, a non-zwitterionic fluoroquinolone in Phase III of clinical development: Evaluation of its pharmacology, pharmacokinetics, pharmacodynamics and clinical efficacy. Future Microbiol. 2015, 10, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Varughese, L.R.; Rajpoot, M.; Goyal, S.; Mehra, R.; Chhokar, V.; Beniwal, V. Analytical profiling of mutations in quinolone resistance determining region of gyrA gene among UPEC. PLoS ONE 2018, 13, e0190729. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2021; ECDC: Stockholm, Sweden, 2022. [Google Scholar]
- Bag, P.P.; Ghosh, S.; Khan, H.; Devarapalli, R.; Malla Reddy, C. Drug–drug salt forms of ciprofloxacin with diflunisal and indoprofen. CrystEngComm 2014, 16, 7393–7396. [Google Scholar] [CrossRef]
- Osonwa, U.E.; Ugochukwu, J.I.; Ajaegbu, E.E.; Chukwu, K.I.; Azevedo, R.B.; Esimone, C.O. Enhancement of antibacterial activity of ciprofloxacin hydrochloride by complexation with sodium cholate. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 233–237. [Google Scholar] [CrossRef]
- Hamdan, I.I.; El-Sabawi, D.; Darwish, R.; Dahabiyeh, L.A. Preparation, characterization and antimicrobial assessment of selected ciprofloxacin salts. Acta Pharm. 2021, 71, 365–382. [Google Scholar] [CrossRef]
- Hibbard, T.; Nyambura, B.; Scholes, P.; Totolici, M.; Shankland, K.; Al-Obaidi, H. Preparation and Physiochemical Analysis of Novel Ciprofloxacin / Dicarboxylic Acid Salts. J. Pharm. Sci. 2023, 112, 195–203. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Roszkowski, P.; Bielenica, A.; Olejarz, W.; Stepien, K.; Struga, M. Anticancer and antimicrobial effects of novel ciprofloxacin fatty acids conjugates. Eur. J. Med. Chem. 2020, 185, 111810. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Struga, M.; Roszkowski, P.; Kolinski, M.; Kmiecik, S.; Jalbrzykowska, K.; Zabost, A.; Stefanska, J.; Augustynowicz-Kopec, E.; Wrzosek, M.; et al. The Effect of Conjugation of Ciprofloxacin and Moxifloxacin with Fatty Acids on Their Antibacterial and Anticancer Activity. Int. J. Mol. Sci. 2022, 23, 6261. [Google Scholar] [CrossRef]
- Ptaszynska, N.; Gucwa, K.; Olkiewicz, K.; Heldt, M.; Serocki, M.; Stupak, A.; Martynow, D.; Debowski, D.; Gitlin-Domagalska, A.; Lica, J.; et al. Conjugates of Ciprofloxacin and Levofloxacin with Cell-Penetrating Peptide Exhibit Antifungal Activity and Mammalian Cytotoxicity. Int. J. Mol. Sci. 2020, 21, 4696. [Google Scholar] [CrossRef]
- Upadhyay, R.; Kumar, R.; Jangra, M.; Rana, R.; Nayal, O.S.; Nandanwar, H.; Maurya, S.K. Synthesis of Bioactive Complex Small Molecule-Ciprofloxacin Conjugates and Evaluation of Their Antibacterial Activity. ACS Comb. Sci. 2020, 22, 440–445. [Google Scholar] [CrossRef]
- Madeira, D.; Alves, C.; Silva, J.; Florindo, C.; Costa, A.; Petrovski, Ž.; Marrucho, I.M.; Pedrosa, R.; Santos, M.M.; Branco, L.C. Fluoroquinolone-Based Organic Salts and Ionic Liquids as Highly Bioavailable Broad-Spectrum Antimicrobials. In Proceedings of the 1st International Electronic Conference on Pharmaceutics, Virtual, 1–15 December 2020. [Google Scholar]
- Kowalczuk, D.; Gladysz, A.; Pitucha, M.; Kaminski, D.M.; Baranska, A.; Drop, B. Spectroscopic Study of the Molecular Structure of the New Hybrid with a Potential Two-Way Antibacterial Effect. Molecules 2021, 26, 1442. [Google Scholar] [CrossRef]
- Al Omari, M.M.; Jaafari, D.S.; Al-Sou’od, K.A.; Badwan, A.A. Moxifloxacin hydrochloride. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 299–431. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Simone, M.I. Developing New Inexpensive Room-Temperature Ionic Liquids with High Thermal Stability and a Greener Synthetic Profile. ACS Omega 2020, 5, 12637–12648. [Google Scholar] [CrossRef] [PubMed]
- Sutar, Y.; Fulton, S.R.; Paul, S.; Altamirano, S.; Mhatre, S.; Saeed, H.; Patel, P.; Mallick, S.; Bhat, R.; Patravale, V.B.; et al. Docusate-Based Ionic Liquids of Anthelmintic Benzimidazoles Show Improved Pharmaceutical Processability, Lipid Solubility, and in Vitro Activity against Cryptococcus neoformans. ACS Infect. Dis. 2021, 7, 2637–2649. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Chauhan, S. Interactional Behavior of Sodium Cholate and Sodium Deoxycholate in the Presence of Ceftriaxone Sodium: Volumetric, Compressibility, Viscometric, and Proton Nuclear Magnetic Resonance Studies. J. Chem. Eng. Data 2020, 65, 4536–4546. [Google Scholar] [CrossRef]
- Sahoo, S.; Chakraborti, C.K.; Mishra, S.C.; Nanda, U.N.; Naik, S. FTIR and XRD investigations of some fluoroquinolones. Int. J. Pharm. Pharm. Sci. 2011, 3. [Google Scholar]
- Seku, K.; Yamala, A.K.; Kancherla, M.; Kumar, K.K.; Badathala, V. Synthesis of moxifloxacin–Au (III) and Ag (I) metal complexes and their biological activities. J. Anal. Sci. Technol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Kerner, M.; Plylahan, N.; Scheers, J.; Johansson, P. Thermal stability and decomposition of lithium bis(fluorosulfonyl)imide (LiFSI) salts. RSC Adv. 2016, 6, 23327–23334. [Google Scholar] [CrossRef]
- Ibrar, I.; Yadav, S.; Altaee, A.; Safaei, J.; Samal, A.K.; Subbiah, S.; Millar, G.; Deka, P.; Zhou, J. Sodium docusate as a cleaning agent for forward osmosis membranes fouled by landfill leachate wastewater. Chemosphere 2022, 308, 136237. [Google Scholar] [CrossRef]
- Sun, S.; Liang, N.; Kawashima, Y.; Xia, D.; Cui, F. Hydrophobic ion pairing of an insulin-sodium deoxycholate complex for oral delivery of insulin. Int. J. Nanomed. 2011, 6, 3049–3056. [Google Scholar] [CrossRef]
- Bhongade, B.; Talath, S.; Dhaneshwar, S. A Validated Method for the Quantitation of Ciprofloxacin Hydrochloride Using Diffuse Reflectance Infrared Fourier Transform Spectroscopy. Int. J. Spectrosc. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Dholakia, M.; Dave, R.; Thakkar, V.; Rana, H.; Gohel, M.; Patel, N. Newer Ophthalmic in Situ Gel of Moxifloxacin Hydrochloride: Optimization Using Box Behnken Statistical Design. Int. J. Pharm. Pharm. Sci. 2018, 10, 5–13. [Google Scholar] [CrossRef]
- Gnanaraj, J.S.; Zinigrad, E.; Asraf, L.; Gottlieb, H.E.; Sprecher, M.; Aurbach, D.; Schmidt, M. The use of accelerating rate calorimetry (ARC) for the study of the thermal reactions of Li-ion battery electrolyte solutions. J. Power Sources 2003, 119–121, 794–798. [Google Scholar] [CrossRef]
- Sa’adun, N.N.; Subramaniam, R.; Kasi, R. Development and characterization of poly(1-vinylpyrrolidone-co-vinyl acetate) copolymer based polymer electrolytes. Sci. World J. 2014, 2014, 254215. [Google Scholar] [CrossRef]
- Grabowska, O.; Kogut, M.M.; Zamojc, K.; Samsonov, S.A.; Makowska, J.; Tesmar, A.; Chmur, K.; Wyrzykowski, D.; Chmurzynski, L. Effect of Tetraphenylborate on Physicochemical Properties of Bovine Serum Albumin. Molecules 2021, 26, 6565. [Google Scholar] [CrossRef]
- Kamel, A.H.; Ezzat, S.; Ahmed, M.A.; Abd El-Galil, E.A.; Abdulrahman, A.A.; Mohamed, A.-O. Modified Potentiometric Screen-Printed Electrodes Based on Imprinting Character for Sodium Deoxycholate Determination. Biomolecules 2020, 10, 251. [Google Scholar] [CrossRef]
- Chen, M.; Perez, R.L.; Du, P.; Bhattarai, N.; McDonough, K.C.; Ravula, S.; Kumar, R.; Mathis, J.M.; Warner, I.M. Tumor-Targeting NIRF NanoGUMBOS with Cyclodextrin-Enhanced Chemo/Photothermal Antitumor Activities. ACS Appl. Mater. Interfaces 2019, 11, 27548–27557. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley Online Library: Hoboken, NJ, USA, 2008; Volume 1. [Google Scholar]
- Abbas, N.; Qamar, N.; Hussain, A.; Latif, S.; Arshad, M.S.; Ijaz, Q.A.; Mahmood, F.; Bukhari, N.I. Fabrication of modified-release custom-designed ciprofloxacin tablets via fused deposition modeling 3D printing. J. 3D Print. Med. 2020, 4, 17–27. [Google Scholar] [CrossRef]
- Barnes, M.J. Excess Sodium Tetraphenylborate and Intermediates Decomposition Studies; Savannah River Site: Aiken, SC, USA, 1998. [Google Scholar]
- Crawford, C.L.; Barnes, M.J.; Peterson, R.A.; Wilmarth, W.R.; Hyder, M.L. Copper-catalyzed sodium tetraphenylborate, triphenylborane, diphenylborinic acid and phenylboronic acid decomposition kinetic studies in aqueous alkaline solutions. J. Organomet. Chem. 1999, 581, 194–206. [Google Scholar] [CrossRef]
- Volgyi, G.; Vizseralek, G.; Takacs-Novak, K.; Avdeef, A.; Tam, K.Y. Predicting the exposure and antibacterial activity of fluoroquinolones based on physicochemical properties. Eur. J. Pharm. Sci. 2012, 47, 21–27. [Google Scholar] [CrossRef]
- Sadakane, K.; Fujii, K.; Tsuzuki, S.; Watanabe, H.; Umebayashi, Y. Solvation state of sodium tetraphenylborate in 3-methylpyridine and its aqueous solutions. J. Mol. Liq. 2017, 248, 53–59. [Google Scholar] [CrossRef]
- Sadakane, K.; Seto, H. Membrane Formation in Liquids by Adding an Antagonistic Salt. Front. Phys. 2018, 6, 26. [Google Scholar] [CrossRef]
- Kumar Sar, S. Complex Behavior of Bile Salts (SC and SDC) at Various Temperatures under the Influence of Tricyclic Antidepressant Drug (Imipramine) in Aqueous Solution. Int. J. Appl. Sci. Res. Rev. 2016, 3, 1237. [Google Scholar] [CrossRef][Green Version]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Ali Khan, M.; Rossi, F.; Rossetti, A.; Nazarzadeh Zare, E.; et al. Antimicrobial Ionic Liquid-Based Materials for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2104148. [Google Scholar] [CrossRef]
- Bhattarai, N.; Chen, M.L.; Pérez, R.; Ravula, S.M.; Strongin, R.; McDonough, K.M.; Warner, I. Comparison of Chemotherapeutic Activities of Rhodamine-Based GUMBOS and NanoGUMBOS. Molecules 2020, 25, 3272. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of Mics and Zone Diameters, version 12.0; EUCAST: Växjö, Sweden, 2022.
- Sistrunk, J.R.; Nickerson, K.P.; Chanin, R.B.; Rasko, D.A.; Faherty, C.S. Survival of the Fittest: How Bacterial Pathogens Utilize Bile to Enhance Infection. Clin. Microbiol. Rev. 2016, 29, 819–836. [Google Scholar] [CrossRef]
- Zgurskaya, H.I.; Lopez, C.A.; Gnanakaran, S. Permeability Barrier of Gram-Negative Cell Envelopes and Approaches to Bypass It. ACS Infect. Dis. 2015, 1, 512–522. [Google Scholar] [CrossRef]
- Arslan, S.; Logoglu, E.; Oktemer, A. Antimicrobial activity studies on some piperidine and pyrrolidine substituted halogenobenzene derivatives. J. Enzyme Inhib. Med. Chem. 2006, 21, 211–214. [Google Scholar] [CrossRef]
- Brunel, F.; Lautard, C.; Garzino, F.; Giorgio, S.; Raimundo, J.M.; Bolla, J.M.; Camplo, M. Antibacterial activities of fluorescent nano assembled triphenylamine phosphonium ionic liquids. Bioorg. Med. Chem. Lett. 2016, 26, 3770–3773. [Google Scholar] [CrossRef]
- Anvari, S.; Hajfarajollah, H.; Mokhtarani, B.; Enayati, M.; Sharifi, A.; Mirzaei, M. Antibacterial and anti-adhesive properties of ionic liquids with various cationic and anionic heads toward pathogenic bacteria. J. Mol. Liq. 2016, 221, 685–690. [Google Scholar] [CrossRef]
- Araque, P.; Herrera, A.; Montaño, D.; Yepes, A.; García, E.; Sepúlveda, J.; Torijano, S.; Cardona-G, W. Antimicrobial Activity and in Silico Study of Methylimidazolium-Furanchalcone Hybrids and 1-Alkyl-3-Methylimidazolium Salts. J. Chil. Chem. Soc. 2019, 64, 4547–4552. [Google Scholar] [CrossRef]
- Anandkumar, B.; George, R.P.; Philip, J. Efficacy of imidazolium and piperidinium based ionic liquids on inhibiting biofilm formation on titanium and carbon steel surfaces. Anal. Chim. Acta 2020, 1126, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Bromberger, B.; Sommer, J.; Robben, C.; Trautner, C.; Kalb, R.; Rossmanith, P.; Mester, P.-J. Evaluation of the antimicrobial activity of pyrithione-based ionic liquids. Sep. Purif. Technol. 2020, 251, 117309. [Google Scholar] [CrossRef]
- Gong, Y.; Wen, A.; Cheung, D.; Wong, M.; Sacks, S.L. Preclinical evaluation of docusate as protective agent from herpes simplex viruses. Antivir. Res. 2001, 52, 25–32. [Google Scholar] [CrossRef]
- Saeed, H.K.; Sutar, Y.; Patel, P.; Bhat, R.; Mallick, S.; Hatada, A.E.; Koomoa, D.T.; Lange, I.; Date, A.A. Synthesis and Characterization of Lipophilic Salts of Metformin to Improve Its Repurposing for Cancer Therapy. ACS Omega 2021, 6, 2626–2637. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Costa, A.; Matos, C.; Nunes, S.L.; Matias, A.N.; Duarte, C.M.; Rebelo, L.P.; Branco, L.C.; Marrucho, I.M. Novel organic salts based on fluoroquinolone drugs: Synthesis, bioavailability and toxicological profiles. Int. J. Pharm. 2014, 469, 179–189. [Google Scholar] [CrossRef]
- EMA. Strategies to Identify and Mitigate Risks for First-In-Human and Early Clinical Trials with Investigational Medicinal Products; EMA: Amsterdam, The Nederland, 2017. [Google Scholar]
- Sansone, S.; Rottensteiner, J.; Stocker, J.; Rosanelli, C.; Wiedermann, C.J. Ciprofloxacin-induced acute haemolytic anaemia in a patient with glucose-6-phosphate dehydrogenase Mediterranean deficiency: A case report. Ann. Hematol. 2010, 89, 935–937. [Google Scholar] [CrossRef]
- Saebo, I.P.; Bjoras, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- Arese, P.; Gallo, V.; Pantaleo, A.; Turrini, F. Life and Death of Glucose-6-Phosphate Dehydrogenase (G6PD) Deficient Erythrocytes—Role of Redox Stress and Band 3 Modifications. Transfus. Med. Hemother 2012, 39, 328–334. [Google Scholar] [CrossRef]
- Bastos, J.C.; Vieira, N.S.M.; Gaspar, M.M.; Pereiro, A.B.; Araújo, J.M.M. Human Cytotoxicity, Hemolytic Activity, Anti-Inflammatory Activity and Aqueous Solubility of Ibuprofen-Based Ionic Liquids. Sustain. Chem. 2022, 3, 358–375. [Google Scholar] [CrossRef]
- Vieira, N.S.M.; Oliveira, A.L.S.; Araújo, J.M.M.; Gaspar, M.M.; Pereiro, A.B. Ecotoxicity and Hemolytic Activity of Fluorinated Ionic Liquids. Sustain. Chem. 2021, 2, 115–126. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Hakansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 107: Partition Coefficient (n-Octanol/Water): Shake Flask Method; OECD: Paris, France, 1995. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts—Fourth Edition; CLSI standard M27; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Smith, S.M.; Eng, R.H. Activity of ciprofloxacin against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 1985, 27, 688–691. [Google Scholar] [CrossRef]
- Firsov, A.A.; Lubenko, I.Y.; Smirnova, M.V.; Strukova, E.N.; Zinner, S.H. Enrichment of fluoroquinolone-resistant Staphylococcus aureus: Oscillating ciprofloxacin concentrations simulated at the upper and lower portions of the mutant selection window. Antimicrob. Agents Chemother. 2008, 52, 1924–1928. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Argudin, M.A.; Deplano, A.; Nhung, P.H.; Nguyen, H.A.; Tulkens, P.M.; Dodemont, M.; Van Bambeke, F. Antibiotic Resistance, Biofilm Formation, and Intracellular Survival as Possible Determinants of Persistent or Recurrent Infections by Staphylococcus aureus in a Vietnamese Tertiary Hospital: Focus on Bacterial Response to Moxifloxacin. Microb. Drug Resist. 2020, 26, 537–544. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition; CLSI standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- ATCC. Staphylococcus aureus subsp. aureus Rosenbach (ATCC 25923). In Product Sheet; ATCC, Ed.; American Type Culture Collection: Manassas, VA, USA, 2023; BSL 2. [Google Scholar]
- Yemmireddy, V.K.; Cason, C.; Moreira, J.; Adhikari, A. Effect of pecan variety and the method of extraction on the antimicrobial activity of pecan shell extracts against different foodborne pathogens and their efficacy on food matrices. Food Control 2020, 112, 107098. [Google Scholar] [CrossRef]
- Page, B.; Page, M.; Noel, C. A New Fluorometric Assay for Cytotoxicity Measurements in-Vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef]




| [Cip]-Based GUMBOS | Yield (%) | [Mox]-Based GUMBOS | Yield (%) |
|---|---|---|---|
| [Cip][BETI] | 74% | [Mox][BETI] | 69% |
| [Cip][NTF2] | 74% | [Mox][NTF2] | 64% |
| [Cip][TPB] | 52% | [Mox][TPB] | 53% |
| [Cip][Doc] | 81% | [Mox][Doc] | 67% |
| [Cip][Dxc] | 64% | [Mox][Dxc] | 48% |
| GUMBOS | Ionic Formula | ESI-MS (Positive Mode) | ESI-MS (Negative Mode) | |||||
|---|---|---|---|---|---|---|---|---|
| Cation | Anion | Expected Mass (m/z) | Experimental Mass (m/z) | Mass Error (ppm) | Expected Mass (m/z) | Experimental Mass (m/z) | Mass Error (ppm) | |
| [Cip][BETI] | C17H18FN3O3+ | C4F10NO4S2- | 332.1410 | 332.1476 | ±19.8711 | 379.9114 | 379.9124 | ±2.6322 |
| [Cip][NTF2] | C2F6NO4S2- | 332.1414 | ±1.2043 | 279.9178 | 279.9190 | ±4.2870 | ||
| [Cip][TPB] | (C6H5)4B- | 332.1405 | ±1.5054 | 319.1663 | 319.1674 | ±3.4465 | ||
| [Cip][Doc] | C20H37O7S- | 332.1421 | ±3.3118 | 421.2260 | 421.2267 | ±1.6618 | ||
| [Cip][Dxc] | C24H39O4- | 332.1411 | ±0.3011 | 391.2853 | 391.2845 | ±2.0445 | ||
| [Mox][BETI] | C21H24FN3O4+ | C4F10NO4S2- | 402.1829 | 402.1838 | ±2.2378 | 379.9109 | 379.9164 | ±14.4771 |
| [Mox][NTF2] | C2F6NO4S2- | 402.1832 | ±0.7459 | 279.9178 | 279.9188 | ±3.5725 | ||
| [Mox][TPB] | (C6H5)4B- | 402.1841 | ±2.9837 | 319.1664 | 319.1674 | ±3.1332 | ||
| [Mox][Doc] | C20H37O7S- | 402.1843 | ±3.4810 | 421.2260 | 421.2269 | ±2.1366 | ||
| [Mox][Dxc] | C24H39O4- | 402.1841 | ±2.9837 | 391.2853 | 391.2848 | ±1.2778 | ||
| Precursors | Tonset | Tpeak |
|---|---|---|
| [Cip][HCl] | 322.7 °C | 325.7 °C |
| [Mox][HCl] | 253.5 °C | 257.5 °C |
| [Li][BETI] | 327.8 °C | 330.0 °C |
| [Li][NTF2] | 151.1 °C | 165 °C |
| [Na][TPB] | 64.5 °C | 73.7 °C |
| [Na][Doc] | 239.5 °C | 282.4 °C |
| [Na][Dxc] | 353.7 °C | 355.8 °C |
| GUMBOS | Tonset | Tpeak |
|---|---|---|
| [Cip][BETI] | 210.8 °C | 213.2 °C |
| [Cip][NTF2] | 202.9 °C | 208.0 °C |
| [Cip][TPB] | 112.4 °C | 127.6 °C |
| [Cip][Doc] | 269.0 °C | 270.6 °C |
| 275.1 °C | 282.9 °C | |
| [Cip][Dxc] | 140.2 °C | 147.1 °C |
| 334.9 °C | 339.4 °C | |
| [Mox][BETI] | 260.2 °C | 278.7 °C |
| [Mox][NTF2] | 263.3 °C | 279.6 °C |
| [Mox][TPB] | * | * |
| [Mox][Doc] | 269.4 °C | 299.3 °C |
| [Mox][Dxc] | 125.3 °C | 131.7 °C |
| GUMBOS | Tstart | Tonset | Tpeak | Residue at 500 °C |
|---|---|---|---|---|
| [Cip][HCl] * | 150 °C | 300 °C | 318 °C | N/A |
| [Cip][BETI] | 266.25 °C | 284.61 °C | 294.04 °C | 35.43% |
| [Cip][NTF2] | 263.57 °C | 287.79 °C | 295.18 °C | 39.88% |
| [Cip][TPB] | 98.94 °C | 117.85 °C | 122.95 °C | 36.93% |
| [Cip][Doc] | 252.18 °C | 286.49 °C | 286.64 °C | 24.26% |
| [Cip][Dxc] | 211.93 °C | 326.23 °C | 332.30 °C | 19.75% |
| [Mox][HCl] * | 220 °C | 240 °C | 260 °C | N/A |
| [Mox][BETI] | 266.98 °C | 307.46 °C | 322.97 °C | 32.31% |
| [Mox][NTF2] | 297.72 °C | 319.64 °C | 327.51 °C | 24.57% |
| [Mox][TPB] | 144.02 °C | 176.37 °C | 177.21 °C | 42.13% |
| [Mox][Doc] | 249.91 °C | 271.04 °C | 275.90 °C | 33.55% |
| [Mox][Dxc] | 208.92 °C | 275.22 °C | 284.97 °C | 15.60% |
| ± SD * | ||
|---|---|---|
| Anion Counterpart | [Cip]-Based GUMBOS | [Mox]-Based GUMBOS |
| [BETI] | −0.314 ± 0.094 | −0.170 ± 0.116 |
| [NTF2] | 0.145 ± 0.075 | −0.191 ± 0.031 |
| [TPB] | −0.464 ± 0.006 | 0.487 ± 0.075 |
| [Doc] | 0.162 ± 0.015 | 0.187 ± 0.027 |
| [Dxc] | −1.110 ± 0.018 | 1.086 ± 0.136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, F.M.S.; Granja, A.; Pérez, R.L.; Warner, I.M.; Reis, S.; Passos, M.L.C.; Saraiva, M.L.M.F.S. Fluoroquinolone-Based Organic Salts (GUMBOS) with Antibacterial Potential. Int. J. Mol. Sci. 2023, 24, 15714. https://doi.org/10.3390/ijms242115714
Costa FMS, Granja A, Pérez RL, Warner IM, Reis S, Passos MLC, Saraiva MLMFS. Fluoroquinolone-Based Organic Salts (GUMBOS) with Antibacterial Potential. International Journal of Molecular Sciences. 2023; 24(21):15714. https://doi.org/10.3390/ijms242115714
Chicago/Turabian StyleCosta, Fábio M. S., Andreia Granja, Rocío L. Pérez, Isiah M. Warner, Salette Reis, Marieta L. C. Passos, and M. Lúcia M. F. S. Saraiva. 2023. "Fluoroquinolone-Based Organic Salts (GUMBOS) with Antibacterial Potential" International Journal of Molecular Sciences 24, no. 21: 15714. https://doi.org/10.3390/ijms242115714
APA StyleCosta, F. M. S., Granja, A., Pérez, R. L., Warner, I. M., Reis, S., Passos, M. L. C., & Saraiva, M. L. M. F. S. (2023). Fluoroquinolone-Based Organic Salts (GUMBOS) with Antibacterial Potential. International Journal of Molecular Sciences, 24(21), 15714. https://doi.org/10.3390/ijms242115714










