Aggressive PitNETs and Potential Target Therapies: A Systematic Review of Molecular and Genetic Pathways
Abstract
1. Introduction
2. Material and Method
Search Strategy and Data Extraction
3. Results
4. Discussion
4.1. Tumor Microenvironment
4.1.1. Macrophages
4.1.2. Lymphocytes
4.1.3. Stromal Cells
4.1.4. Folliculo-Stellate Cells
4.1.5. Cytokines, Chemokines, and Growth Factors
4.1.6. The Role of Immunotherapy in Pituitary Tumors: Response and Outcomes
4.2. PI3K/Akt/mTOR and RAS/MEK/ERK Pathways
Target Therapy against the PI3K/Akt/mTOR and Ras/Raf/MEK/ERK Pathways
4.3. Receptors
4.3.1. Somatostatin and Dopamine Receptors
4.3.2. Peptide Receptor Radionuclide Therapy
4.3.3. Transforming Growth Factor Receptor
4.3.4. Fibroblast Growth Factor Receptors
4.3.5. Folate Receptor
4.3.6. Estrogen Modulators
4.3.7. Wnt/β-Catenin and E-Cadherin
4.3.8. Galectin-3
4.4. Matrix MetalloProteinases
4.5. Angiogenesis
4.5.1. Vascular Endothelial Growth Factor
4.5.2. Endocan
4.6. Genetic Aspects in Aggressive PitNETs
4.6.1. Germline Mutations
4.6.2. Somatic Mutations
GNAS
USP8
4.7. Future Perspectives in PitNETs: The Role of Epigenetic
4.7.1. DNA Methylation
4.7.2. MicroRNA
4.8. Molecular Target Therapy and Clinical Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melmed, S. Pituitary-Tumor Endocrinopathies. N. Engl. J. Med. 2020, 382, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Saeger, W.; Lüdecke, D.K.; Buchfelder, M.; Fahlbusch, R.; Quabbe, H.J.; Petersenn, S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur. J. Endocrinol. 2007, 156, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, J.; Jaffrain-Rea, M.L.; Vasiljevic, A.; Raverot, G.; Roncaroli, F.; Villa, C. How to Classify the Pituitary Neuroendocrine Tumors (PitNET)s in 2020. Cancers 2020, 12, 514. [Google Scholar] [CrossRef]
- Serioli, S.; Doglietto, F.; Fiorindi, A.; Biroli, A.; Mattavelli, D.; Buffoli, B.; Ferrari, M.; Cornali, C.; Rodella, L.; Maroldi, R.; et al. Pituitary Adenomas and Invasiveness from Anatomo-Surgical, Radiological, and Histological Perspectives: A Systematic Literature Review. Cancers 2019, 11, 1936. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Casar-Borota, O.; Chanson, P.; Delgrange, E.; Earls, P.; Ezzat, S.; Grossman, A.; Ikeda, H.; Inoshita, N.; Karavitaki, N.; et al. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): An International Pituitary Pathology Club proposal. Endocr. Relat. Cancer 2017, 24, C5–C8. [Google Scholar] [CrossRef]
- Raverot, G.; Ilie, M.D.; Lasolle, H.; Amodru, V.; Trouillas, J.; Castinetti, F.; Brue, T. Aggressive pituitary tumors and pituitary carcinomas. Nat. Rev. Endocrinol. 2021, 17, 671–684. [Google Scholar] [CrossRef]
- Raverot, G.; Burman, P.; McCormack, A.; Heaney, A.; Petersenn, S.; Popovic, V.; Trouillas, J.; Dekkers, O.M. European Society of Endocrinology. Clinical Practice Guidelines for the management of aggressive pituitary tumors and carcinomas. Eur. J. Endocrinol. 2018, 178, G1–G24. [Google Scholar] [CrossRef]
- Ilie, M.D.; Jouanneau, E.; Raverot, G. Aggressive Pituitary Adenomas and Carcinomas. Endocrinol. Metab. Clin. N. Am. 2020, 49, 505–515. [Google Scholar] [CrossRef]
- Ji, Y.; Vogel, R.I.; Lou, E. Temozolomide treatment of pituitary carcinomas and atypical adenomas: Systematic review of case reports. Neurooncol. Pract. 2016, 3, 188–195. [Google Scholar] [CrossRef]
- Lasolle, H.; Cortet, C.; Castinetti, F.; Cloix, L.; Caron, P.; Delemer, B.; Desailloud, R.; Jublanc, C.; Lebrun-Frenay, C.; Sadoul, J.L.; et al. Temozolomide treatment can improve overall survival in aggressive pituitary tumors and pituitary carcinomas. Eur. J. Endocrinol. 2017, 176, 769–777. [Google Scholar] [CrossRef]
- Chiloiro, S.; De Marinis, L. The immune microenviroment in somatotropinomas: From biology to personalized and target therapy. Rev. Endocr. Metab. Disord. 2023, 24, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.D.; Vasiljevic, A.; Bertolino, P.; Raverot, G. Biological and Therapeutic Implications of the Tumor Microenvironment in Pituitary Adenomas. Endocr. Rev. 2023, 44, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nakao, T.; Ogawa, W.; Fukuoka, H. Aggressive Cushing’s Disease: Molecular Pathology and Its Therapeutic Approach. Front. Endocrinol. 2021, 12, 650791. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- DeLellis, R.A.; Lloyd, R.V.; Heitz, P.U.; Eng, C. (Eds.) World Health Organization classification of tumors: Pathology and genetics of tumors of endocrine organs. In Tumor of the Pituitary Gland; IARC Press: Lyon, France, 2004; Chapter 1; pp. 10–47. [Google Scholar]
- Critical Appraisal Skills Programme. CASP Checklist. 2022. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 29 June 2023).
- Lockwood, C.; Munn, Z.; Porritt, K. Qualitative research synthesis: Methodological guidance for systematic reviewers utilizing meta-aggregation. Int. J. Evid. Based Healthc. 2015, 13, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.D.; Vasiljevic, A.; Raverot, G.; Bertolino, P. The Microenvironment of Pituitary Tumors—Biological and Therapeutic Implications. Cancers 2019, 11, 1605. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, C.; Song, Q.; Zhu, M.; Xu, Y.; Xiao, M.; Zheng, W. Identifying cancer-associated fibroblasts as emerging targets for hepatocellular carcinoma. Cell Biosci. 2020, 10, 127. [Google Scholar] [CrossRef]
- Marques, P.; Korbonits, M. Tumor microenvironment and pituitary tumor behaviour. J. Endocrinol. Investig. 2023, 46, 1047–1063. [Google Scholar] [CrossRef]
- Principe, M.; Chanal, M.; Ilie, M.D.; Ziverec, A.; Vasiljevic, A.; Jouanneau, E.; Hennino, A.; Raverot, G.; Bertolino, P. Immune landscape of pituitary tumors reveals association between macrophages and gonadotroph tumor invasion. J. Clin. Endocrinol. Metab. 2020, 105, dgaa520. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, C.; Zhang, D.; Peng, J.; Ma, S.; Wang, X.; Guan, X.; Li, P.; Li, D.; Jia, G.; et al. Comprehensive analysis of the immunological landscape of pituitary adenomas: Implications of immunotherapy for pituitary adenomas. J. Neurooncol. 2020, 149, 473–487. [Google Scholar]
- Lyu, L.; Jiang, Y.; Ma, W.; Li, H.; Liu, X.; Li, L.; Shen, A.; Yu, Y.; Jiang, S.; Li, H.; et al. Single-cell sequencing of PIT1- positive pituitary adenoma highlights the pro-tumor microenvironment mediated by IFN-gamma-induced tumor-associated fbroblasts remodelling. Br. J. Cancer 2023, 128, 1117–1133. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, H.M.; Kujas, M.; Casanova, S.; Wollan, P.C.; Racadot, J.; VAN Effenterre, R.; Derome, P.J.; Turpin, G. Prevalence of lymphocytic infiltrate in 1400 pituitary adenomas. Endocr. J. 1998, 45, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.; Barry, S.; Carlsen, E.; Collier, D.; Ronaldson, A.; Awad, S.; Dorward, N.; Grieve, J.; Mendoza, N.; Muquit, S.; et al. Chemokines modulate the tumor microenvironment in pituitary neuroendocrine tumors. Acta Neuropathol. Commun. 2019, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, J.; Wu, Y.; Sheng, L.; Li, Y.; Zha, B.; Sun, T.; Yang, J.; Zang, S.; Liu, J. Alterations in CD8+ Tregs, CD56+ Natural Killer Cells and IL-10 Are Associated with Invasiveness of Nonfunctioning Pituitary Adenomas (NFPAs). Pathol. Oncol. Res. 2021, 27, 598887. [Google Scholar] [CrossRef]
- Wang, P.-F.; Wang, T.-J.; Yang, Y.-K.; Yao, K.; Li, Z.; Li, Y.M.; Yan, C.X. The expression profile of PD-L1 and CD8(+) lymphocyte in pituitary adenomas indicating for immunotherapy. J. Neuro Oncol. 2018, 139, 89–95. [Google Scholar] [CrossRef]
- Mei, Y.; Bi, W.L.; Agolia, J.; Hu, C.; Larsen, A.M.G.; Meredith, D.M.; Al Abdulmohsen, S.; Bale, T.; Dunn, G.P.; Abedalthagafi, M.; et al. Immune profiling of pituitary tumors reveals variations in immune infiltration and checkpoint molecule expression. Pituitary 2021, 24, 359–373. [Google Scholar]
- Moldovan, I.M.; Şuşman, S.; Pîrlog, R.; Jianu, E.M.; Leucuţa, D.C.; Melincovici, C.S.; Crişan, D.; Florian, I.Ş. Molecular markers in the diagnosis of invasive pituitary adenomas—An immunohistochemistry study. Rom. J. Morphol. Embryol. 2017, 58, 1357–1364. [Google Scholar]
- Qu, X.; Yang, W.; Jiang, M.; Han, T.; Han, L.; Qu, Y.; Wang, G.; Shi, D.; Xu, G. CD147 expression in pituitary adenomas and its significance for clinical outcome. Hum. Pathol. 2010, 41, 1165–1171. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, S.; Hu, Y.; Zhou, P.; Gao, L.; Wang, M.; Sun, Z.; Chen, C.; Yin, S.; Wang, X.; et al. Invasive pituitary adenomaderived tumor-associated fibroblasts promote tumor progression both in vitro and in vivo. Exp. Clin. Endocrinol. Diabetes 2018, 126, 213–221. [Google Scholar] [CrossRef]
- Azorín, E.; Solano-Agama, C.; Mendoza-Garrido, M.E. The invasion mode of GH(3) cells is conditioned by collagen subtype, and its efficiency depends on cell-cell adhesion. Arch. Biochem. Biophys. 2012, 528, 148–155. [Google Scholar] [CrossRef]
- Marques, P.; Barry, S.; Carlsen, E.; Collier, D.; Ronaldson, A.; Awad, S.; Dorward, N.; Grieve, J.; Mendoza, N.; Muquit, S.; et al. Pituitary tumor fibroblast derived cytokines influence tumor aggressiveness. Endocr. Relat. Cancer 2019, 26, 853–865. [Google Scholar] [CrossRef]
- Zhang, D.; Hugo, W.; Bergsneider, M.; Wang, M.B.; Kim, W.; Vinters, H.V.; Heaney, A.P. Single-cell RNA sequencing in silent corticotroph tumors confirms impaired POMC processing and provides new insights into their invasive behavior. Eur. J. Endocrinol. 2022, 187, 49–64. [Google Scholar] [CrossRef]
- Devnath, S.; Inoue, K. An insight to pituitary folliculo-stellate cells. J. Neuroendocr. 2008, 20, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.D.; Vasiljevic, A.; Chanal, M.; Gadot, N.; Chinezu, L.; Jouanneau, E.; Hennino, A.; Raverot, G.; Bertolino, P. Intratumoral spatial distribution of S100B + folliculostellate cells is associated with proliferation and expression of FSH and ERalpha in gonadotroph tumors. Acta Neuropathol. Commun. 2022, 10, 18. [Google Scholar] [CrossRef]
- Voit, D.; Saeger, W.; Lüdecke, D.K. Folliculo-stellate cells in pituitary adenomas of patients with acromegaly. Pathol. Res. Pract. 1999, 195, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Xu, Y.; Xu, H.; Ren, J.; Meng, T.; Ni, Y.; Zhu, Q.; Zhang, W.B.; Pan, Y.B.; Jin, J.; et al. Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting CCL17. Theranostics 2021, 11, 3839–3852. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, J.H. Transcriptome Analysis Identifies an Attenuated Local Immune Response in Invasive Nonfunctioning Pituitary Adenomas. Endocrinol. Metab. 2019, 34, 314–322. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, Z.; Wang, Z.; Jiang, Q.; Zhang, Z.; Liu, X.; Xing, B.; Li, S.; Guo, X.; Liu, Y.; et al. PKCθ Regulates Pituitary Adenoma Bone Invasion by Activating Osteoclast in NF-κB/IL-1β-Dependent Manner. Cancers 2023, 15, 1624. [Google Scholar] [CrossRef]
- Voellger, B.; Zhang, Z.; Benzel, J.; Wang, J.; Lei, T.; Nimsky, C.; Bartsch, J.W. Targeting Aggressive Pituitary Adenomas at the Molecular Level—A Review. J. Clin. Med. 2021, 11, 124. [Google Scholar]
- Duhamel, C.; Ilie, M.D.; Salle, H.; Nassouri, A.S.; Gaillard, S.; Deluche, E.; Assaker, R.; Mortier, L.; Cortet, C.; Raverot, G. Immunotherapy in Corticotroph and Lactotroph Aggressive Tumors and Carcinomas: Two Case Reports and a Review of the Literature. J. Pers. Med. 2020, 10, 88. [Google Scholar] [CrossRef]
- Majd, N.; Waguespack, S.G.; Janku, F.; Fu, S.; Penas-Prado, M.; Xu, M.; Alshawa, A.; Kamiya-Matsuoka, C.; Raza, S.M.; McCutcheon, I.E.; et al. Efficacy of pembrolizumab in patients with pituitary carcinoma: Report of four cases from a phase II study. J. Immunother. Cancer 2020, 8, e001532. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.L.; Tabar, V.; Young, R.J.; Cohen, M.; Cuaron, J.; Yang, T.J.; Rosenblum, M.; Rudneva, V.A.; Geer, E.B.; Bodei, L. Synergism of Checkpoint Inhibitors and Peptide Receptor Radionuclide Therapy in the Treatment of Pituitary Carcinoma. J. Endocr. Soc. 2021, 5, bvab133. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, S.; Giampietro, A.; Gessi, M.; Lauretti, L.; Mattogno, P.P.; Cerroni, L.; Carlino, A.; De Alessandris, Q.G.; Olivi, A.; Rindi, G.; et al. CD68+ and CD8+ immune cells are associated with the growth pattern of somatotroph tumors and response to first generation somatostatin analogs. J. Neuroendocr. 2023, 35, e13263. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, X.; Yao, K.; Yang, Y.; Zhang, L.; Liu, N.; Yan, C.; Qi, X.; Han, S. Targeting the Tumor Immune Microenvironment Could Become a Potential Therapeutic Modality for Aggressive Pituitary Adenoma. Brain Sci. 2023, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, H.; Komohara, Y.; Yano, H.; Fujiwara, Y.; Kai, K.; Yamada, R.; Yoshii, D.; Uekawa, K.; Shinojima, N.; Mikami, Y.; et al. Macrophage colony-stimulating factor potentially induces recruitment and maturation of macrophages in recurrent pituitary neuroendocrine tumors. Microbiol. Immunol. 2022, 67, 90–98. [Google Scholar] [CrossRef]
- Ilie, M.D.; Villa, C.; Cuny, T.; Cortet, C.; Assie, G.; Baussart, B.; Cancel, M.; Chanson, P.; Decoudier, B.; Deluche, E.; et al. Real-life efficacy and predictors of response to immunotherapy in pituitary tumors: A cohort study. Eur. J. Endocrinol. 2022, 187, 685–696. [Google Scholar] [CrossRef]
- Xi, Z.; Jones, P.S.; Mikamoto, M.; Jiang, X.; Faje, A.T.; Nie, C.; Labelle, K.E.; Zhou, Y.; Miller, K.K.; Soberman, R.J.; et al. The upregulation of molecules related to tumor immune escape in human pituitary adenomas. Front. Endocrinol. 2021, 12, 726448. [Google Scholar] [CrossRef]
- Thiele, J.O.; Lohrer, P.; Schaaf, L.; Feirer, M.; Stummer, W.; Losa, M.; Lange, M.; Tichomirowa, M.; Arzt, E.; Stalla, G.K.; et al. Functional in vitro studies on the role and regulation of interleukin-6 in human somatotroph pituitary adenomas. Eur. J. Endocrinol. 2003, 149, 455–461. [Google Scholar] [CrossRef][Green Version]
- Zatelli, M.C.; Piccin, D.; Vignali, C.; Tagliati, F.; Ambrosio, M.R.; Bondanelli, M.; Cimino, V.; Bianchi, A.; Schmid, H.A.; Scanarini, M.; et al. Pasireotide, a multiple somatostatin receptor subtypes ligand, reduces cell viability in non-functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Endocr. Relat. Cancer 2007, 14, 91–102. [Google Scholar] [CrossRef]
- Beck, G.C.H.; Brinkkoetter, P.; Hanusch, C.; Schulte, J.; van Ackern, K.; van der Woude, F.J.; Yard, B.A. Clinical review: Immunomodulatory effects of dopamine in general inflammation. Crit. Care 2004, 8, 485–491. [Google Scholar] [CrossRef]
- Casanueva, F.F.; Molitch, M.E.; Schlechte, J.A.; Abs, R.; Bonert, V.; Bronstein, M.D.; Brue, T.; Cappabianca, P.; Colao, A.; Fahlbusch, R.; et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin. Endocrinol. 2006, 65, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Recouvreux, M.V.; Camilletti, M.A.; Rifkin, D.B.; Díaz-Torga, G. The pituitary TGFbeta1 system as a novel target for the treatment of resistant prolactinomas. J. Endocrinol. 2016, 228, R73–R83. [Google Scholar] [CrossRef]
- Chauvet, N.; Romanò, N.; Lafont, C.; Guillou, A.; Galibert, E.; Bonnefont, X.; Le Tissier, P.; Fedele, M.; Fusco, A.; Mollard, P.; et al. Complementary actions of dopamine D2 receptor agonist and anti-vegf therapy on tumoral vessel normalization in a transgenic mouse model. Int. J. Cancer 2017, 140, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, B.; Chen, Y.; Li, C.; Zhang, Y. Analysis of the Prognostic and Immunological Role of HSPB1 in Pituitary Adenoma: A Potential Target for Therapy. Medicina 2023, 59, 885. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 2007, 1773, 1263–1284. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Vlotides, G.; Siegel, E.; Donangelo, I.; Gutman, S.; Ren, S.G.; Melmed, S. Rat Prolactinoma cell growth regulation by Epidermal Growth Factor receptor ligands. Cancer Res. 2008, 68, 6377–6386. [Google Scholar] [CrossRef]
- Wei, D.; Yu, Z.; Cheng, Y.; Jiawei, H.; Jian, G.; Hua, G.; Guilan, D. Dysregulated miR-137 and its target EGFR contribute to the progression of pituitary adenomas. Mol. Cell Endocrinol. 2021, 520, 111083. [Google Scholar] [CrossRef]
- Wei, D.; Yu, Z.; Cheng, Y.; Jiawei, H.; Jian, G.; Hua, G.; Guilan, D. ADAM12 induces EMT and promotes cell migration, invasion and proliferation in pituitary adenomas via EGFR/ERK signaling pathway. Biomed. Pharmacother. 2018, 97, 1066–1077. [Google Scholar] [CrossRef]
- Rose, A.; Froment, P.; Perrot, V.; Quon, M.J.; LeRoith, D.; Dupont, J. The Luteinizing Hormone-releasing Hormone Inhibits the Anti-apoptotic Activity of Insulin-like Growth Factor-1 in Pituitary T3 Cells by Protein Kinase C-mediated Negative Regulation of Akt. J. Biol. Chem. 2004, 279, 52500–52516. [Google Scholar] [CrossRef]
- Fernández, M.; Sánchez-Franco, F.; Palacios, N.; Sánchez, I.; Fernández, C.; Cacicedo, L. IGF-I inhibits apoptosis through the activation of the phosphatidylinositol 3-kinase/Akt pathway in pituitary cells. J. Mol. Endocrinol. 2004, 33, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Kowarik, M.; Onofri, C.; Colaco, T.; Stalla, G.K.; Renner, U. Platelet-derived Growth Factor (PDGF) and PDGF Receptor Expression and Function in Folliculostellate Pituitary Cells. Exp. Clin. Endocrinol. Diabetes 2010, 118, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Murat, C.B.; Braga, P.B.; Fortes, M.A.; Bronstein, M.D.; Corrêa-Giannella, M.L.; Giorgi, R.R. Mutation and genomic amplification of the PIK3CA proto-oncogene in pituitary adenomas. Braz. J. Med. Biol. Res. 2012, 45, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jiang, X.; Shen, Y.; Li, M.; Ma, H.; Xing, M.; Lu, Y. Frequent mutations and amplifications of the PIK3CA gene in pituitary tumors. Endocr. Relat. Cancer 2009, 16, 301–310. [Google Scholar] [CrossRef]
- Musat, M.; Korbonits, M.; Kola, B.; Borboli, N.; Hanson, M.R.; Nanzer, A.M.; Grigson, J.; Jordan, S.; Morris, D.G.; Gueorguiev, M.; et al. Enhanced protein kinase B/Akt signalling in pituitary tumors. Endocr. Relat. Cancer 2005, 12, 423–433. [Google Scholar] [CrossRef]
- Jia, W.; Sanders, A.J.; Jia, G.; Liu, X.; Lu, R.; Jiang, W.G. Expression of the mTOR pathway regulators in human pituitary adenomas indicates the clinical course. Anticancer Res. 2013, 33, 3123–3131. [Google Scholar]
- Xu, Q.; Yu, Z.X.; Xie, Y.L.; Bai, L.; Liang, S.R.; Ji, Q.H.; Zhou, J. MicroRNA-137 inhibits pituitary prolactinoma proliferation by targeting AKT2. J. Endocrinol. Investig. 2023, 46, 1145–1154. [Google Scholar] [CrossRef]
- Noh, T.W.; Jeong, H.J.; Lee, M.K.; Kim, T.S.; Kim, S.H.; Lee, E.J. Predicting Recurrence of Nonfunctioning Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2009, 94, 4406–4413. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, T.; Fan, Y.; Serick Du, G.; Wu, P.; Geng, D. MicroRNA-106b promotes pituitary tumor cell proliferation and invasion through PI3K/AKT signaling pathway by targeting PTEN. Tumor Biol. 2016, 37, 13469–13477. [Google Scholar] [CrossRef]
- Zhang, D. Effect of Everolimus in Treatment of Aggressive Prolactin-Secreting Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2019, 104, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Willingham, M.C.; Furuya, F.; Cheng, S.Y. Activation of Phosphatidylinositol 3-Kinase Signaling Promotes Aberrant Pituitary Growth in a Mouse Model of Thyroid-Stimulating Hormone-Secreting Pituitary Tumors. Endocrinology 2008, 149, 3339–3345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gorshtein, A.; Rubinfeld, H.; Kendler, E.; Theodoropoulou, M.; Cerovac, V.; Stalla, G.K.; Cohen, Z.R.; Hadani, M.; Shimon, I. Mammalian target of rapamycin inhibitors rapamycin and RAD001 (everolimus) induce anti-proliferative effects in GH-secreting pituitary tumor cells in vitro. Endocr. Relat. Cancer 2009, 16, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, E.A.; Zieliński, G.; Maksymowicz, M.; Hutnik, Ł.; Bednarczuk, T.; Włodarski, P. mTOR is Frequently Active in GH-Secreting Pituitary Adenomas without Influencing their Morpho-pathological Features. Endocr. Pathol. 2013, 24, 11–19. [Google Scholar] [CrossRef]
- Dworakowska, D.; Wlodek, E.; Leontiou, C.A.; Igreja, S.; Cakir, M.; Teng, M.; Prodromou, N.; Góth, M.I.; Grozinsky-Glasberg, S.; Gueorguiev, M.; et al. Activation of RAF/MEK/ERK and PI3K/AKT/mTOR pathways in pituitary adenomas and their effects on downstream effectors. Endocr. Relat. Cancer 2009, 16, 1329–1338. [Google Scholar] [CrossRef]
- Yao, H.; Tang, H.; Zhang, Y.; Zhang, Q.F.; Liu, X.Y.; Liu, Y.T.; Gu, W.T.; Zheng, Y.Z.; Shang, H.B.; Wang, Y.; et al. DEPTOR inhibits cell proliferation and confers sensitivity to dopamine agonist in pituitary adenoma. Cancer Lett. 2019, 459, 135–144. [Google Scholar] [CrossRef]
- Jian, F.; Chen, Y.; Ning, G.; Fu, W.; Tang, H.; Chen, X.; Zhao, Y.; Zheng, L.; Pan, S.; Wang, W.; et al. Cold inducible RNA binding protein upregulation in pituitary corticotroph adenoma induces corticotroph cell proliferation via Erk signaling pathway. Oncotarget 2016, 7, 9175–9187. [Google Scholar] [CrossRef]
- Cheng, S.Q.; Fan, H.Y.; Xu, X.; Gao, W.W.; Lv, S.G.; Ye, M.H.; Wu, M.J.; Shen, X.L.; Cheng, Z.J.; Zhu, X.G.; et al. Over-expression of LRIG1 Suppresses Biological Function of Pituitary Adenoma via Attenuation of PI3K/AKT and Ras/Raf/ERK Pathways In Vivo and In Vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 558–563. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Tian, W.; Xu, Y.; Li, R.; Zhao, K.; You, C.; Zhu, Y.; Bartsch, J.W.; Niu, H.; et al. PDCD10 promotes the aggressive behaviors of pituitary adenomas by up-regulating CXCR2 and activating downstream AKT/ERK signaling. Aging 2022, 14, 6066–6080. [Google Scholar] [CrossRef]
- Romano, D.; Pertuit, M.; Rasolonjanahary, R.; Barnier, J.V.; Magalon, K.; Enjalbert, A.; Gerard, C. Regulation of the RAP1/RAF-1/Extracellularly Regulated Kinase-1/2 Cascade and Prolactin Release by the Phosphoinositide 3-Kinase/AKT Pathway in Pituitary Cells. Endocrinology 2006, 147, 6036–6045. [Google Scholar] [CrossRef]
- Ewing, I.; Pedder-Smith, S.; Franchi, G.; Ruscica, M.; Emery, M.; Vax, V.; Garcia, E.; Czirják, S.; Hanzély, Z.; Kola, B.; et al. A mutation and expression analysis of the oncogene BRAF in pituitary adenomas. Clin. Endocrinol. 2007, 66, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Identification of recurrent USP48 and BRAF mutations in Cushing’s disease. Nat. Commun. 2018, 9, 3171. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Melmed, S.; Scheithauer, B.; Kovacs, K.; Prager, D. H-ras mutations in human pituitary carcinoma metastases. J. Clin. Endocrinol. Metab. 1994, 78, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Sukumari-Ramesh, S.; Singh, N.; Dhandapani, K.M.; Vender, J.R. mTOR inhibition reduces cellular proliferation and sensitizes pituitary adenoma cells to ionizing radiation. Surg. Neurol. Int. 2011, 2, 22. [Google Scholar] [CrossRef]
- Pivonello, C.; Patalano, R.; Solari, D.; Auriemma, R.S.; Frio, F.; Vitulli, F.; Grasso, L.F.S.; Di Cera, M.; De Martino, M.C.; Cavallo, L.M.; et al. Effect of combined treatment with a pan-PI3K inhibitor or an isoform-specific PI3K inhibitor and everolimus on cell proliferation in GH-secreting pituitary tumor in an experimental setting. Endocrine 2018, 62, 663–680. [Google Scholar] [CrossRef] [PubMed]
- Zatelli, M.C.; Minoia, M.; Filieri, C.; Tagliati, F.; Buratto, M.; Ambrosio, M.R.; Lapparelli, M.; Scanarini, M.; Degli Uberti, E.C. Effect of Everolimus on Cell Viability in Nonfunctioning Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2010, 95, 968–976. [Google Scholar] [CrossRef]
- Cerovac, V.; Monteserin-Garcia, J.; Rubinfeld, H.; Buchfelder, M.; Losa, M.; Florio, T.; Paez-Pereda, M.; Stalla, G.K.; Theodoropoulou, M. The Somatostatin Analogue Octreotide Confers Sensitivity to Rapamycin Treatment on Pituitary Tumor Cells. Cancer Res. 2010, 70, 666–674. [Google Scholar] [CrossRef]
- Theodoropoulou, M.; Zhang, J.; Laupheimer, S.; Paez-Pereda, M.; Erneux, C.; Florio, T.; Pagotto, U.; Stalla, G.K. Octreotide, a Somatostatin Analogue, Mediates Its Antiproliferative Action in Pituitary Tumor Cells by Altering Phosphatidylinositol 3-Kinase Signaling and Inducing Zac1 Expression. Cancer Res. 2006, 66, 1576–1582. [Google Scholar] [CrossRef]
- Chanal, M.; Chevallier, P.; Raverot, V.; Fonteneau, G.; Lucia, K.; Garcia, J.L.M.; Rachwan, A.; Jouanneau, E.; Trouillas, J.; Honnorat, J.; et al. Differential Effects of PI3K and Dual PI3K/mTOR Inhibition in Rat Prolactin-Secreting Pituitary Tumors. Mol. Cancer Ther. 2016, 15, 1261–1270. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Ma, D.; Li, X. The Potential Role of CERS1 in Autophagy Through PI3K/AKT Signaling Pathway in Hypophysoma. Technol. Cancer Res. Treat. 2020, 19, 1533033820977536. [Google Scholar] [CrossRef]
- Lee, M.; Theodoropoulou, M.; Graw, J.; Roncaroli, F.; Zatelli, M.C.; Pellegata, N.S. Levels of p27 Sensitize to Dual PI3K/mTOR Inhibition. Mol. Cancer Ther. 2011, 10, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Wiedemann, T.; Gross, C.; Leinhäuser, I.; Roncaroli, F.; Braren, R.; Pellegata, N.S. Targeting PI3K/mTOR Signaling Displays Potent Antitumor Efficacy against Nonfunctioning Pituitary Adenomas. Clin. Cancer Res. 2015, 21, 3204–3215. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zhang, B.; Liu, X.; Ma, S.; Yang, Y.; Yao, Y.; Feng, M.; Bao, X.; Li, G.; Wang, J.; et al. Inhibition of PI3K/AKT/mTOR Pathway Enhances Temozolomide-Induced Cytotoxicity in Pituitary Adenoma Cell Lines in Vitro and Xenografted Pituitary Adenoma in Female Nude Mice. Endocrinology 2013, 154, 1247–1259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeng, J.; See, A.P.; Aziz, K.; Thiyagarajan, S.; Salih, T.; Gajula, R.P.; Armour, M.; Phallen, J.; Terezakis, S.; Kleinberg, L.; et al. Nelfinavir induces radiation sensitization in pituitary adenoma cells. Cancer Biol. Ther. 2011, 12, 657–663. [Google Scholar] [CrossRef][Green Version]
- Reubi, J.C.; Waser, B.; Schaer, J.C.; Laissue, J.A. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur. J. Nucl. Med. 2001, 28, 836–846. [Google Scholar] [CrossRef]
- Behling, F.; Honegger, J.; Skardelly, M.; Gepfner-Tuma, I.; Tabatabai, G.; Tatagiba, M.; Schittenhelm, J. High Expression of Somatostatin Receptors 2A, 3, and 5 in Corticotroph Pituitary Adenoma. Int. J. Endocrinol. 2018, 2018, 1763735. [Google Scholar] [CrossRef]
- Magagna-Poveda, A.; Leske, H.; Schmid, C.; Bernays, R.; Rushing, E.J. Expression of somatostatin receptors, angiogenesis and proliferation markers in pituitary adenomas: An immunohistochemical study with diagnostic and therapeutic implications. Swiss Med. Wkly. 2013, 143, w13895. [Google Scholar] [CrossRef]
- Ramírez, C.; Cheng, S.; Vargas, G.; Asa, S.L.; Ezzat, S.; González, B.; Cabrera, L.; Guinto, G.; Mercado, M. Expression of Ki-67, PTTG1, FGFR4, and SSTR 2, 3, and 5 in nonfunctioning pituitary adenomas: A high throughput TMA, immunohistochemical study. J. Clin. Endocrinol. Metab. 2012, 97, 1745–1751. [Google Scholar] [CrossRef]
- Brzana, J.; Yedinak, C.G.; Gultekin, S.H.; Delashaw, J.B.; Fleseriu, M. Growth hormone granulation pattern and somatostatin receptor subtype 2A correlate with postoperative somatostatin receptor ligand response in acromegaly: A large single center experience. Pituitary 2013, 16, 490–498. [Google Scholar] [CrossRef]
- Venegas-Moreno, E.; Vazquez-Borrego, M.C.; Dios, E.; Gros-Herguido, N.; Flores-Martinez, A.; Rivero-Cortés, E.; Madrazo-Atutxa, A.; Japón, M.A.; Luque, R.M.; Castaño, J.P.; et al. Association between dopamine and somatostatin receptor expression and pharmacological response to somatostatin analogues in acromegaly. J. Cell Mol. Med. 2018, 22, 1640–1649. [Google Scholar] [CrossRef]
- Peverelli, E.; Giardino, E.; Treppiedi, D.; Catalano, R.; Mangili, F.; Locatelli, M.; Lania, A.G.; Arosio, M.; Spada, A.; Mantovani, G. A novel pathway activated by somatostatin receptor type 2 (SST2): Inhibition of pituitary tumor cell migration and invasion through cytoskeleton protein recruitment. Int. J. Cancer 2018, 142, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Peverelli, E.; Giardino, E.; Treppiedi, D.; Locatelli, M.; Vaira, V.; Ferrero, S.; Bosari, S.; Lania, A.G.; Spada, A.; Mantovani, G. Dopamine receptor type 2 (DRD2) inhibits migration and invasion of human tumorous pituitary cells through ROCK-mediated cofilin inactivation. Cancer Lett. 2016, 381, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Boscaro, M.; Ludlam, W.H.; Atkinson, B.; Glusman, J.E.; Petersenn, S.; Reincke, M.; Snyder, P.; Tabarin, A.; Biller, B.M.; Findling, J.; et al. Treatment of pituitary-dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): A multicenter, phase II trial. J. Clin. Endocrinol. Metab. 2009, 94, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Moreno, P.; Ibáñez-Costa, A.; Venegas-Moreno, E.; Fuentes-Fayos, A.C.; Alhambra-Expósito, M.R.; Fajardo-Montañana, C.; García-Martínez, A.; Dios, E.; Vázquez-Borrego, M.C.; Remón-Ruiz, P.; et al. Integrative Clinical, Radiological, and Molecular Analysis for Predicting Remission and Recurrence of Cushing Disease. J. Clin. Endocrinol. Metab. 2022, 107, e2938–e2951. [Google Scholar] [CrossRef]
- Luque, R.M.; Ibáñez-Costa, A.; Neto, L.V.; Taboada, G.F.; Hormaechea-Agulla, D.; Kasuki, L.; Venegas-Moreno, E.; Moreno-Carazo, A.; Gálvez, M.Á.; Soto-Moreno, A.; et al. Truncated somatostatin receptor variant sst5TMD4 confers aggressive features (proliferation, invasion and reduced octreotide response) to somatotropinomas. Cancer Lett. 2015, 359, 299–306. [Google Scholar] [CrossRef]
- Ibáñez-Costa, A.; López-Sánchez, L.M.; Gahete, M.D.; Rivero-Cortés, E.; Vázquez-Borrego, M.C.; Gálvez, M.A.; de la Riva, A.; Venegas-Moreno, E.; Jiménez-Reina, L.; Moreno-Carazo, A.; et al. BIM-23A760 influences key functional endpoints in pituitary adenomas and normal pituitaries: Molecular mechanisms underlying the differential response in adenomas. Sci. Rep. 2017, 7, 42002. [Google Scholar] [CrossRef]
- De Bruin, C.; Pereira, A.M.; Feelders, R.A.; Romijn, J.A.; Roelfsema, F.; Sprij-Mooij, D.M.; van Aken, M.O.; van der Lelij, A.J.; de Herder, W.W.; Lamberts, S.W.; et al. Coexpression of dopamine and somatostatin receptor subtypes in corticotroph adenomas. J. Clin. Endocrinol. Metab. 2009, 94, 1118–1124. [Google Scholar] [CrossRef]
- Lee, M.; Lupp, A.; Mendoza, N.; Martin, N.; Beschorner, R.; Honegger, J.; Schlegel, J.; Shively, T.; Pulz, E.; Schulz, S.; et al. SSTR3 is a putative target for the medical treatment of gonadotroph adenomas of the pituitary. Endocr. Relat. Cancer 2015, 22, 111–119. [Google Scholar] [CrossRef]
- Hofland, J.; Brabander, T.; Verburg, F.A.; Feelders, R.A.; de Herder, W.W. Peptide Receptor Radionuclide Therapy. J. Clin. Endocrinol. Metab. 2022, 107, 3199–3208. [Google Scholar] [CrossRef]
- Gonzalez, P.; Debnath, S.; Chen, Y.A.; Hernandez, E.; Jha, P.; Dakanali, M.; Hsieh, J.T.; Sun, X. A Theranostic Small-Molecule Prodrug Conjugate for Neuroendocrine Prostate Cancer. Pharmaceutics 2023, 15, 481. [Google Scholar] [CrossRef]
- Gupta, S.K.; Singla, S.; Damle, N.A.; Agarwal, K.; Bal, C. Diagnosis of Men-I Syndrome on (68)Ga-DOTANOC PET-CT and Role of Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE. Int. J. Endocrinol. Metab. 2012, 10, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Ferraù, F.; Alafaci, C.; Herberg, A.; Granata, F.; Militano, V.; Salpietro, F.M.; Trimarchi, F.; Cannavò, S. First demonstration of the effectiveness of peptide receptor radionuclide therapy (PRRT) with 111In-DTPA-octreotide in a giant PRL-secreting pituitary adenoma resistant to conventional treatment. Pituitary 2012, 15 (Suppl. S1), S57–S60. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, G.; Ferraù, F.; Laudicella, R.; Cotta, O.R.; Messina, E.; Granata, F.; Angileri, F.F.; Vento, A.; Alibrandi, A.; Baldari, S.; et al. Peptide receptor radionuclide therapy for aggressive pituitary tumors: A monocentric experience. Endocr. Connect. 2019, 8, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Priola, S.M.; Esposito, F.; Cannavò, S.; Conti, A.; Abbritti, R.V.; Barresi, V.; Baldari, S.; Ferraù, F.; Germanò, A.; Tomasello, F.; et al. Aggressive Pituitary Adenomas: The Dark Side of the Moon. World Neurosurg. 2017, 97, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Kovács, G.L.; Góth, M.; Rotondo, F.; Scheithauer, B.W.; Carlsen, E.; Saadia, A.; Hubina, E.; Kovács, L.; Szabolcs, I.; Nagy, P.; et al. ACTH-secreting Crooke cell carcinoma of the pituitary. Eur. J. Clin. Investig. 2013, 43, 20–26. [Google Scholar] [CrossRef]
- Komor, J.; Reubi, J.C.; Christ, E.R. Peptide receptor radionuclide therapy in a patient with disabling non-functioning pituitary adenoma. Pituitary 2014, 17, 227–231. [Google Scholar] [CrossRef]
- Maclean, J.; Aldridge, M.; Bomanji, J.; Short, S.; Fersht, N. Peptide receptor radionuclide therapy for aggressive atypical pituitary adenoma/carcinoma: Variable clinical response in preliminary evaluation. Pituitary 2014, 17, 530–538. [Google Scholar] [CrossRef]
- Bengtsson, D.; Schrøder, H.D.; Andersen, M.; Maiter, D.; Berinder, K.; Rasmussen, U.F.; Rasmussen, Å.K.; Johannsson, G.; Hoybye, C.; van der Lely, A.J.; et al. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J. Clin. Endocrinol. Metab. 2015, 100, 1689–1698. [Google Scholar] [CrossRef]
- Burman, P.; Trouillas, J.; Losa, M.; McCormack, A.; Petersenn, S.; Popovic, V.; Theodoropoulou, M.; Raverot, G.; Dekkers, O.M.; ESE Survey Collaborators. Aggressive pituitary tumors and carcinomas, characteristics and management of 171 patients. Eur. J. Endocrinol. 2022, 187, 593–605. [Google Scholar] [CrossRef]
- Novruzov, F.; Aliyev, A.; Wan, M.Y.S.; Syed, R.; Mehdi, E.; Aliyeva, I.; Giammarile, F.; Bomanji, J.B.; Kayani, I. The value of [68Ga]Ga-DOTA-TATE PET/CT in diagnosis and management of suspected pituitary tumors. Eur. J. Hybrid Imaging 2021, 5, 10. [Google Scholar] [CrossRef]
- Waligórska-Stachura, J.; Gut, P.; Sawicka-Gutaj, N.; Liebert, W.; Gryczyńska, M.; Baszko-Błaszyk, D.; Blanco-Gangoo, A.R.; Ruchała, M. Growth hormone-secreting macroadenoma of the pituitary gland successfully treated with the radiolabeled somatostatin analog (90)Y-DOTATATE: Case report. J. Neurosurg. 2016, 125, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Assadi, M.; Nemati, R.; Shooli, H.; Rekabpour, S.J.; Nabipour, I.; Jafari, E.; Gholamrezanezhad, A.; Amini, A.; Ahmadzadehfar, H. An aggressive functioning pituitary adenoma treated with peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1015–1016. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Zhou, N.; Wu, C.-Y.; Li, S.; Chen, Y.-A.; Debnath, S.; Hofstad, M.; Ma, S.; Raj, G.V.; He, D.; et al. Validation of SV2A-Targeted PET Imaging for Noninvasive Assessment of Neuroendocrine Differentiation in Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 13085. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Massagué, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Jiang, M.; Mou, C.Z.; Han, T.; Wang, M.; Yang, W. Thrombospondin-1 and transforming growth factor-β1 levels in prolactinoma and their clinical significance. J. Int. Med. Res. 2012, 40, 1284–1294. [Google Scholar] [CrossRef]
- Elenkova, A.; Atanassova, I.; Kirilov, G.; Vasilev, V.; Kalinov, K.; Zacharieva, S. Transforming growth factor β1 is not a reliable biomarker for valvular fibrosis but could be a potential serum marker for invasiveness of prolactinomas (pilot study). Eur. J. Endocrinol. 2013, 169, 299–306. [Google Scholar] [CrossRef]
- Dallago, C.M.; Barbosa-Coutinho, L.M.; Ferreira, N.P.; Meurer, R.; Pereira-Lima, J.F.; Oliveira, M.d.C. Determination of cell proliferation using Mcm2 antigen and evaluation of apoptosis and TGF-beta1 expression in GH-secreting or clinically nonfunctioning pituitary adenomas. Endocr. Pathol. 2010, 21, 32–39. [Google Scholar] [CrossRef]
- Zhu, H.; Yao, X.; Wu, L.; Li, C.; Bai, J.; Gao, H.; Ji, H.; Zhang, Y. Association of TGF-β1 and WIF1 Expression with 36 Paired Primary/Recurrent Nonfunctioning Pituitary Adenomas: A High-Throughput Tissue Microarrays Immunohistochemical Study. World Neurosurg. 2018, 119, e23–e31. [Google Scholar] [CrossRef]
- Duan, J.; Hu, C.; Zhang, Q.; Zhu, J. Exploration of the Effects of TGF-β Pathway-Based Pituitary Tumor of Rats on GH3 Cell Line after Intervention with Different Concentrations of TGZ. Contrast Media Mol. Imaging 2022, 2022, 7445042. [Google Scholar] [CrossRef]
- Gu, Y.H.; Feng, Y.G. Down-regulation of TGF-β RII expression is correlated with tumor growth and invasion in non-functioning pituitary adenomas. J. Clin. Neurosci. 2018, 47, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Petiti, J.P.; Sosa, L.D.V.; Picech, F.; Crespo, G.D.M.; Rojas, J.Z.A.; Pérez, P.A.; Guido, C.B.; Leimgruber, C.; Sabatino, M.E.; García, P.; et al. Trastuzumab inhibits pituitary tumor cell growth modulating the TGFB/SMAD2/3 pathway. Endocr. Relat. Cancer 2018, 25, 837–852. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Wu, D.; Li, C.; Zhang, Y. Smad3 and phospho-Smad3 are potential markers of invasive nonfunctioning pituitary adenomas. Onco Targets Ther. 2016, 9, 2265–2271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eswarakumar, V.P.; Lax, I.; Schlessinger, J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor. Rev. 2005, 16, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Giraldi, F.P.; Moro, M.; Cavagnini, F.; Study Group on the Hypothalamo-Pituitary-Adrenal Axis of the Italian Society of Endocrinology. Gender-related differences in the presentation and course of Cushing’s disease. J. Clin. Endocrinol. Metab. 2003, 88, 1554–1558. [Google Scholar] [CrossRef]
- Qian, Z.R.; Sano, T.; Asa, S.L.; Yamada, S.; Horiguchi, H.; Tashiro, T.; Li, C.C.; Hirokawa, M.; Kovacs, K.; Ezzat, S. Cytoplasmic expression of fibroblast growth factor receptor-4 in human pituitary adenomas: Relation to tumor type, size, proliferation, and invasiveness. J. Clin. Endocrinol. Metab. 2004, 89, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.P.; Lerário, A.M.; Bronstein, M.D.; Soares, I.C.; Mendonca, B.B.; Fragoso, M.C. Influence of the fibroblast growth factor receptor 4 expression and the G388R functional polymorphism on Cushing’s disease outcome. J. Clin. Endocrinol. Metab. 2010, 95, E271–E279. [Google Scholar] [CrossRef]
- Morita, K.; Takano, K.; Yasufuku-Takano, J.; Yamada, S.; Teramoto, A.; Takei, M.; Osamura, R.Y.; Sano, T.; Fujita, T. Expression of pituitary tumor-derived, N-terminally truncated isoform of fibroblast growth factor receptor 4 (ptd-FGFR4) correlates with tumor invasiveness but not with G-protein alpha subunit (gsp) mutation in human GH-secreting pituitary adenomas. Clin. Endocrinol. 2008, 68, 435–441. [Google Scholar] [CrossRef]
- Ezzat, S.; Zheng, L.; Winer, D.; Asa, S.L. Targeting N-cadherin through fibroblast growth factor receptor-4: Distinct pathogenetic and therapeutic implications. Mol. Endocrinol. 2006, 20, 2965–2975. [Google Scholar] [CrossRef]
- Nawaz, F.Z.; Kipreos, E.T. Emerging roles for folate receptor FOLR1 in signaling and cancer. Trends Endocrinol. Metab. 2022, 33, 159–174. [Google Scholar] [CrossRef]
- Evans, C.O.; Reddy, P.; Brat, D.J.; O’Neill, E.B.; Craige, B.; Stevens, V.L.; Oyesiku, N.M. Differential expression of folate receptor in pituitary adenomas. Cancer Res. 2003, 63, 4218–4224. [Google Scholar] [PubMed]
- Ding, Z.; Li, B.; Wang, Q.; Miao, Y.; Lu, X. Increase in folate receptor alpha expression in nonfunctional pituitary adenomas. Turk. Neurosurg. 2015, 25, 298–304. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Ma, S.; Yao, Y.; Li, G.; Feng, M.; Deng, K.; Dai, C.; Cai, F.; Li, Y.; Zhang, B.; et al. Differential expression of folate receptor alpha in pituitary adenomas and its relationship to tumor behavior. Neurosurgery 2012, 70, 1274–1280, discussion 1280. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Cai, F.; Hwang, K.C.; Zhou, Y.; Zhang, Z.; Liu, X.; Ma, S.; Yang, Y.; Yao, Y.; Feng, M.; et al. Folate receptor-mediated boron-10 containing carbon nanoparticles as potential delivery vehicles for boron neutron capture therapy of nonfunctional pituitary adenomas. Sci. China Life Sci. 2013, 56, 163–173. [Google Scholar] [CrossRef]
- Larysz, D.; Zebracka-Gala, J.; Rudnik, A.; Hasse-Lazar, K.; Kowalska, M.; Jarząb, M.; Król, A.; Szpak-Ulczok, S.; Bażowski, P.; Jarząb, B. Expression of genes FOLR1, BAG1 and LAPTM4B in functioning and non-functioning pituitary adenomas. Folia Neuropathol. 2012, 50, 277–286. [Google Scholar] [CrossRef]
- Moreno, C.S.; Evans, C.O.; Zhan, X.; Okor, M.; Desiderio, D.M.; Oyesiku, N.M. Novel molecular signaling and classification of human clinically nonfunctional pituitary adenomas identified by gene expression profiling and proteomic analyses. Cancer Res. 2005, 65, 10214–10222. [Google Scholar] [CrossRef]
- Yao, C.; Evans, C.O.; Stevens, V.L.; Owens, T.R.; Oyesiku, N.M. Folate receptor alpha regulates cell proliferation in mouse gonadotroph alphaT3-1 cells. Exp. Cell Res. 2009, 315, 3125–3132. [Google Scholar] [CrossRef]
- Galt, J.R.; Halkar, R.K.; Evans, C.O.; Osman, N.A.; LaBorde, D.; Fox, T.H.; Faraj, B.A.; Kumar, K.; Wang, H.; Oyesiku, N.M. In vivo assay of folate receptors in nonfunctional pituitary adenomas with 99mTc-folate SPECT/CT. J. Nucl. Med. 2010, 51, 1716–1723. [Google Scholar] [CrossRef]
- Liu, X.; Ma, S.; Dai, C.; Cai, F.; Yao, Y.; Yang, Y.; Feng, M.; Deng, K.; Li, G.; Ma, W.; et al. Antiproliferative, antiinvasive, and proapoptotic activity of folate receptor α-targeted liposomal doxorubicin in nonfunctional pituitary adenoma cells. Endocrinology 2013, 154, 1414–1423. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef]
- Yaşar, P.; Ayaz, G.; User, S.D.; Güpür, G.; Muyan, M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod. Med. Biol. 2016, 16, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Shen, P.; Loggie, B.W.; Chang, Y.; Deuel, T.F. A variant of estrogen receptor-{alpha}, hER-{alpha}36: Transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 9063–9068. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, Z.Q.; Chu, M.; Wang, Z.; Zhang, W.G.; Wang, L.Z.; Li, C.G.; Wang, J.S. Resveratrol inhibited GH3 cell growth and decreased prolactin level via estrogen receptors. Clin. Neurol. Neurosurg. 2012, 114, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Hannen, R.; Steffani, M.; Voellger, B.; Carl, B.; Wang, J.; Bartsch, J.W.; Nimsky, C. Effects of anti-estrogens on cell invasion and survival in pituitary adenoma cells: A systematic study. J. Steroid Biochem. Mol. Biol. 2019, 187, 88–96. [Google Scholar] [CrossRef]
- Kansra, S.; Yamagata, S.; Sneade, L.; Foster, L.; Ben-Jonathan, N. Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release. Mol. Cell Endocrinol. 2005, 239, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xue, Y.; Cao, L.; Liu, Q.; Liu, C.; Shan, X.; Wang, H.; Gu, Y.; Zhang, Y. ESR1 and its antagonist fulvestrant in pituitary adenomas. Mol. Cell. Endocrinol. 2017, 443, 32–41. [Google Scholar] [CrossRef]
- Wierinckx, A.; Delgrange, E.; Bertolino, P.; François, P.; Chanson, P.; Jouanneau, E.; Lachuer, J.; Trouillas, J.; Raverot, G. Sex-Related Differences in Lactotroph Tumor Aggressiveness Are Associated with a Specific Gene-Expression Signature and Genome Instability. Front. Endocrinol. 2018, 9, 706. [Google Scholar] [CrossRef]
- Trouillas, J.; Delgrange, E.; Wierinckx, A.; Vasiljevic, A.; Jouanneau, E.; Burman, P.; Raverot, G. Clinical, Pathological, and Molecular Factors of Aggressiveness in Lactotroph Tumors. Neuroendocrinology 2019, 109, 70–76. [Google Scholar] [CrossRef]
- Delgrange, E.; Vasiljevic, A.; Wierinckx, A.; François, P.; Jouanneau, E.; Raverot, G.; Trouillas, J. Expression of estrogen receptor alpha is associated with prolactin pituitary tumor prognosis and supports the sex-related difference in tumor growth. Eur. J. Endocrinol. 2015, 172, 791–801. [Google Scholar] [CrossRef]
- Bima, C.; Chiloiro, S.; Giampietro, A.; Gessi, M.; Mattogno, P.P.; Lauretti, L.; Anile, C.; Rindi, G.; Pontecorvi, A.; De Marinis, L.; et al. Galectin-3 and Estrogen Receptor Alpha as Prognostic Markers in Prolactinoma: Preliminary Results from a Pilot Study. Front. Endocrinol. 2021, 12, 684055. [Google Scholar] [CrossRef]
- Burdman, J.A.; Pauni, M.; Sereno, G.M.H.; Bordón, A.E. Estrogen receptors in human pituitary tumors. Horm. Metab. Res. 2008, 40, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Z.; Gui, S.B.; Zong, X.Y.; Zhang, Y.Z. The Expression of Estrogen Receptor Subtypes in Prolactinomas and Their Relationship to Tumor Biological Behavior. Biomed. Env. Sci. 2015, 28, 820–822. [Google Scholar] [CrossRef]
- Mahboobifard, F.; Bidari-Zerehpoosh, F.; Davoudi, Z.; Panahi, M.; Dargahi, L.; Pourgholami, M.H.; Sharifi, G.; Izadi, N.; Jorjani, M. Expression patterns of ERα66 and its novel variant isoform ERα36 in lactotroph pituitary adenomas and associations with clinicopathological characteristics. Pituitary 2020, 23, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, H.; Tamura, K.; Iida, H.; Kutsukake, M.; Endo, A.; Ikeda, Y.; Haraoka, J. Co-expression of somatostatin receptor subtypes and estrogen receptor-α mRNAs by non-functioning pituitary adenomas in young patients. Mol. Cell. Endocrinol. 2011, 331, 73–78. [Google Scholar] [CrossRef]
- Manoranjan, B.; Salehi, F.; Scheithauer, B.W.; Rotondo, F.; Kovacs, K.; Cusimano, M.D. Estrogen receptors alpha and beta immunohistochemical expression: Clinicopathological correlations in pituitary adenomas. Anticancer. Res. 2010, 30, 2897–2904. [Google Scholar]
- Su, Y.X.; Du, G.L.; Shen, H.L.; Wang, W.; Bao, J.L.; Aierken, A.; Wang, B.W.; Jiang, S.; Zhu, J.; Gao, X.M. Increased expression of aromatase cytochrome P450 enzyme is associated with prolactinoma invasiveness in post-menopausal women. J. Int. Med. Res. 2019, 47, 3115–3126. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, X.; Zhang, K.; Liu, Z.; Shao, Z.; Song, C.; Wang, X.; Li, Z. Estrogen receptor α/prolactin receptor bilateral crosstalk promotes bromocriptine resistance in prolactinomas. Int. J. Med. Sci. 2020, 17, 3174–3189, Erratum in Int. J. Med. Sci. 2022, 19, 831–832. [Google Scholar] [CrossRef]
- Lv, H.; Li, C.; Gui, S.; Zhang, Y. Expression of estrogen receptor α and growth factors in human prolactinoma and its correlation with clinical features and gender. J. Endocrinol. Investig. 2012, 35, 174–180. [Google Scholar]
- Zhou, W.; Song, Y.; Xu, H.; Zhou, K.; Zhang, W.; Chen, J.; Qin, M.; Yi, H.; Gustafsson, J.A.; Yang, H.; et al. In nonfunctional pituitary adenomas, estrogen receptors and slug contribute to the development of invasiveness. J. Clin. Endocrinol. Metab. 2011, 96, E1237–E1245. [Google Scholar] [CrossRef]
- Pereira-Lima, J.F.; Marroni, C.P.; Pizarro, C.B.; Barbosa-Coutinho, L.M.; Ferreira, N.P.; Oliveira, M.C. Immunohistochemical detection of estrogen receptor alpha in pituitary adenomas and its correlation with cellular replication. Neuroendocrinology 2004, 79, 119–124. [Google Scholar] [CrossRef]
- Øystese, K.A.; Casar-Borota, O.; Normann, K.R.; Zucknick, M.; Berg, J.P.; Bollerslev, J. Estrogen Receptor α, a Sex-Dependent Predictor of Aggressiveness in Nonfunctioning Pituitary Adenomas: SSTR and Sex Hormone Receptor Distribution in NFPA. J. Clin. Endocrinol. Metab. 2017, 102, 3581–3590. [Google Scholar] [CrossRef] [PubMed]
- Voellger, B.; Waldt, N.; Rupa, R.; Kirches, E.; Melhem, O.; Ochel, H.J.; Mawrin, C.; Firsching, R. Combined effects of resveratrol and radiation in GH3 and TtT/GF pituitary adenoma cells. J. Neuro Oncol. 2018, 139, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bartsch, J.W.; Benzel, J.; Lei, T.; Nimsky, C.; Voellger, B. Selective estrogen receptor modulators decrease invasiveness in pituitary adenoma cell lines AtT-20 and TtT/GF by affecting expression of MMP-14 and ADAM12. FEBS Open Bio. 2020, 10, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Voellger, B.; Kirches, E.; Wilisch-Neumann, A.; Weise, A.; Tapia-Perez, J.H.; Rupa, R.; Mawrin, C.; Firsching, R. Resveratrol decreases B-cell lymphoma-2 expression and viability in GH3 pituitary adenoma cells of the rat. Onco Targets Ther. 2013, 9, 1269–1276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wright, D.J.; Earnhardt, J.N.; Perry, R.; Bailey, S.; Komm, B.; Minck, D.R.; Cukierski, M.A. Carcinogenicity and hormone studies with the tissue-selective estrogen receptor modulator bazadoxifene. J. Cell Physiol. 2013, 228, 724–733. [Google Scholar] [CrossRef]
- Wang, C.; Bai, M.; Wang, X.; Tan, C.; Zhang, D.; Chang, L.; Li, G.; Xie, L.; Su, J.; Wen, Y. Estrogen receptor antagonist fulvestrant inhibits proliferation and promotes apoptosis of prolactinoma cells by regulating the IRE1/XBP1 signaling pathway. Mol. Med. Rep. 2018, 18, 4037–4041. [Google Scholar] [CrossRef]
- Dimaraki, E.V.; Symons, K.V.; Barkan, A.L. Raloxifene decreases serum IGF-I in male patients with active acromegaly. Eur. J. Endocrinol. 2004, 150, 481–487. [Google Scholar] [CrossRef][Green Version]
- Choudhary, C.; Hamrahian, A.H.; Bena, J.F.; Recinos, P.; Kennedy, L.; Dobri, G. The effect of raloxifene on serum prolactin level in patients with prolactinoma. Endocr. Pract. 2019, 25, 684–688. [Google Scholar] [CrossRef]
- Ceccato, F.; Lizzul, L.; Voltan, G.; Barbot, M.; Scaroni, C. Anastrozole as add-on therapy for cabergoline-resistant prolactin-secreting pituitary adenomas: Real-life experience in male patients. Pituitary 2021, 24, 914–921. [Google Scholar] [CrossRef]
- Balili, I.; Barkan, A. Tamoxifen as a therapeutic agent in acromegaly. Pituitary 2014, 17, 500–504. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, Z.; Niu, B.; Zhang, J.; Tan, T.K.; Lee, S.R.; Zhao, Y.; Harris, D.C.; Zheng, G. E-cadherin/β-catenin complex and the epithelial barrier. J. Biomed. Biotechnol. 2011, 2011, 567305. [Google Scholar] [CrossRef]
- Chauvet, N.; Romanò, N.; Meunier, A.C.; Galibert, E.; Fontanaud, P.; Mathieu, M.N.; Osterstock, G.; Osterstock, P.; Baccino, E.; Rigau, V.; et al. Combining Cadherin Expression with Molecular Markers Discriminates Invasiveness in Growth Hormone and Prolactin Pituitary Adenomas. J. Neuroendocr. 2016, 28, 12352. [Google Scholar] [CrossRef]
- Mendes, G.A.; Haag, T.; Trott, G.; Rech, C.G.S.L.; Ferreira, N.P.; Oliveira, M.C.; Kohek, M.B.; Pereira-Lima, J.F.S. Expression of E-cadherin, Slug and NCAM and its relationship to tumor invasiveness in patients with acromegaly. Braz. J. Med. Biol. Res. 2017, 51, e6808. [Google Scholar] [CrossRef]
- Qian, Z.R.; Sano, T.; Yoshimoto, K.; Asa, S.L.; Yamada, S.; Mizusawa, N.; Kudo, E. Tumor-specific downregulation and methylation of the CDH13 (H-cadherin) and CDH1 (E-cadherin) genes correlate with aggressiveness of human pituitary adenomas. Mod. Pathol. 2007, 20, 1269–1277. [Google Scholar] [CrossRef]
- Øystese, K.A.B.; Casar-Borota, O.; Berg-Johnsen, J.; Berg, J.P.; Bollerslev, J. Distribution of E- and N-cadherin in subgroups of non-functioning pituitary neuroendocrine tumors. Endocrine 2022, 77, 151–159. [Google Scholar] [CrossRef]
- Elston, M.S.; Gill, A.J.; Conaglen, J.V.; Clarkson, A.; Cook, R.J.; Little, N.S.; Robinson, B.G.; Clifton-Bligh, R.J.; McDonald, K.L. Nuclear accumulation of e-cadherin correlates with loss of cytoplasmic membrane staining and invasion in pituitary adenomas. J. Clin. Endocrinol. Metab. 2009, 94, 1436–1442. [Google Scholar] [CrossRef]
- Øystese, K.A.B.; Berg, J.P.; Normann, K.R.; Zucknick, M.; Casar-Borota, O.; Bollerslev, J. The role of E and N-cadherin in the postoperative course of gonadotroph pituitary tumors. Endocrine 2018, 62, 351–360. [Google Scholar] [CrossRef]
- Zhou, K.; Jin, H.; Luo, Y. Expression and significance of E-cadherin and β-catenins in pituitary adenoma. Int. J. Surg. Pathol. 2013, 21, 363–367. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Yu, S.; Bai, J.; Li, C.; Wu, D.; Zhang, Y. Increased β catenin and c-myc expression predict aggressive growth of non-functioning pituitary adenomas: An assessment using a tissue microarray-based approach. Mol. Med. Rep. 2017, 15, 1793–1799. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Hong, L.; Liu, C.; Yu, S.; Yu, G.; Zhang, Y. Low expression of secreted frizzled-related protein 2 and nuclear accumulation of β-catenin in aggressive nonfunctioning pituitary adenoma. Oncol. Lett. 2016, 12, 199–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demarchi, G.; Valla, S.; Perrone, S.; Chimento, A.; Bonadeo, N.; Vitale, D.L.; Spinelli, F.M.; Cervio, A.; Sevlever, G.; Alaniz, L.; et al. β-Catenin is reduced in membranes of human prolactinoma cells and it is inhibited by temozolomide in prolactin secreting tumor models. Tumor Biol. 2022, 44, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.; Jin, L.; Zhang, S.; Stilling, G.; Scheithauer, B.W.; Kovacs, K.; Lloyd, R.V. Identification and consequences of galectin-3 expression in pituitary tumors. Mol. Cell Endocrinol. 2010, 326, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Riss, D.; Jin, L.; Qian, X.; Bayliss, J.; Scheithauer, B.W.; Young, W.F., Jr.; Vidal, S.; Kovacs, K.; Raz, A.; Lloyd, R.V. Differential expression of galectin-3 in pituitary tumors. Cancer Res. 2003, 63, 2251–2255. [Google Scholar]
- Yoshii, T.; Fukumori, T.; Honjo, Y.; Inohara, H.; Kim, H.R.; Raz, A. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J. Biol. Chem. 2002, 277, 6852–6857. [Google Scholar] [CrossRef]
- Righi, A.; Morandi, L.; Leonardi, E.; Farnedi, A.; Marucci, G.; Sisto, A.; Frank, G.; Faustini-Fustini, M.; Zoli, M.; Mazzatenta, D.; et al. Galectin-3 expression in pituitary adenomas as a marker of aggressive behavior. Hum. Pathol. 2013, 44, 2400–2409. [Google Scholar] [CrossRef]
- Dai, D.; Li, Y.; Lu, Q.; Yu, L.; Min, W.; Wang, L.; Cao, Y.; Yue, Z. GAL3 protein expression is related to clinical features of prolactin-secreting pituitary microadenoma and predicts its recurrence after surgical treatment. Cell. Physiol. Biochem. 2014, 33, 1026–1035. [Google Scholar] [CrossRef]
- Kawamoto, H.; Kawamoto, K.; Mizoue, T.; Uozumi, T.; Arita, K.; Kurisu, K. Matrix metalloproteinase-9 secretion by human pituitary adenomas detected by cell immunoblot analysis. Acta Neurochir. 1996, 138, 1442–1448. [Google Scholar] [CrossRef]
- Liu, W.; Kunishio, K.; Matsumoto, Y.; Okada, M.; Nagao, S. Matrix metalloproteinase-2 expression correlates with cavernous sinus invasion in pituitary adenomas. J. Clin. Neurosci. 2005, 12, 791–794. [Google Scholar] [CrossRef]
- Turner, H.E.; Nagy, Z.; Esiri, M.M.; Harris, A.L.; Wass, J.A. Role of matrix metalloproteinase 9 in pituitary tumor behavior. J. Clin. Endocrinol. Metab. 2000, 85, 2931–2935. [Google Scholar] [CrossRef]
- Gültekin, G.D.; Çabuk, B.; Vural, Ç.; Ceylan, S. Matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-2: Prognostic biological markers in invasive prolactinomas. J. Clin. Neurosci. 2015, 22, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, S.; Wu, T.; Lv, Z.; Ba, J.; Gu, W.; Mu, Y. Expression and clinical significance of Cathepsin K and MMPs in invasive non-functioning pituitary adenomas. Front. Oncol. 2022, 12, 901647. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, M.; Zhang, Y.; Dai, C.; Sun, B.; Bao, X.; Deng, K.; Yao, Y.; Wang, R. Expression of Matrix Metalloproteinase-9, Pituitary Tumor Transforming Gene, High Mobility Group A 2, and Ki-67 in Adrenocorticotropic Hormone-Secreting Pituitary Tumors and Their Association with Tumor Recurrence. World Neurosurg. 2018, 113, e213–e221. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhao, Y.; Abdel-Fattah, R.; Amos, S.; Xiao, A.; Lopes, M.B.; Hussaini, I.M.; Laws, E.R. Matrix metalloproteinase-9, a potential biological marker in invasive pituitary adenomas. Pituitary 2008, 11, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, I.M.; Trotter, C.; Zhao, Y.; Abdel-Fattah, R.; Amos, S.; Xiao, A.; Agi, C.U.; Redpath, G.T.; Fang, Z.; Leung, G.K.; et al. Matrix metalloproteinase-9 is differentially expressed in nonfunctioning invasive and noninvasive pituitary adenomas and increases invasion in human pituitary adenoma cell line. Am. J. Pathol. 2007, 170, 356–365. [Google Scholar] [CrossRef]
- Beaulieu, E.; Kachra, Z.; Mousseau, N.; Delbecchi, L.; Hardy, J.; Béliveau, R. Matrix metalloproteinases and their inhibitors in human pituitary tumors. Neurosurgery 1999, 45, 1432–1441. [Google Scholar] [CrossRef]
- Alvaro, V.; Lévy, L.; Dubray, C.; Roche, A.; Peillon, F.; Quérat, B.; Joubert, D. Invasive human pituitary tumors express a point-mutated alpha-protein kinase-C. J. Clin. Endocrinol. Metab. 1993, 77, 1125–1129. [Google Scholar] [CrossRef]
- Alvaro, V.; Touraine, P.; Vozari, R.R.; Bai-Grenier, F.; Birman, P.; Joubert, D. Protein kinase C activity and expression in normal and adenomatous human pituitaries. Int. J. Cancer 1992, 50, 724–730. [Google Scholar] [CrossRef]
- Feng, J.; Yu, S.Y.; Li, C.Z.; Li, Z.Y.; Zhang, Y.Z. Integrative proteomics and transcriptomics revealed that activation of the IL-6R/JAK2/STAT3/MMP9 signaling pathway is correlated with invasion of pituitary null cell adenomas. Mol. Cell. Endocrinol. 2016, 436, 195–203. [Google Scholar] [CrossRef]
- Knappe, U.J.; Hagel, C.; Lisboa, B.W.; Wilczak, W.; Lüdecke, D.K.; Saeger, W. Expression of serine proteases and metalloproteinases in human pituitary adenomas and anterior pituitary lobe tissue. Acta Neuropathol. 2003, 106, 471–478. [Google Scholar] [CrossRef]
- Mao, J.H.; Guo, H.; Si, N.; Qiu, L.; Guo, L.F.; Sun, Z.S.; Xiang, Y.; Yang, X.H.; Zhao, W.G.; Zhang, W.C. Regulating effect of MMP-9 and TIMP-1 in pituitary adenoma invasion. Genet. Mol. Res. 2015, 14, 17091–17098. [Google Scholar] [CrossRef] [PubMed]
- Hui, P.; Xu, X.; Xu, L.; Hui, G.; Wu, S.; Lan, Q. Expression of MMP14 in invasive pituitary adenomas: Relationship to invasion and angiogenesis. Int. J. Clin. Exp. Pathol. 2015, 8, 3556–3567. [Google Scholar]
- Deryugina, E.I.; Soroceanu, L.; Strongin, A.Y. Up-regulation of vascular endothelial growth factor by membrane-type 1 matrix metalloproteinase stimulates human glioma xenograft growth and angiogenesis. Cancer Res. 2002, 62, 580–588. [Google Scholar] [PubMed]
- Yao, G.; He, P.; Chen, L.; Hu, X.; Gu, F.; Ye, C. MT1-MMP in breast cancer: Induction of VEGF-C correlates with metastasis and poor prognosis. Cancer Cell Int. 2013, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Ruskyte, K.; Liutkevicienė, R.; Vilkeviciute, A.; Vaitkiene, P.; Valiulytė, I.; Glebauskiene, B.; Kriauciuniene, L.; Zaliuniene, D. MMP-14 and TGFβ-1 methylation in pituitary adenomas. Oncol. Lett. 2016, 12, 3013–3017. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Matano, F.; Yoshida, D.; Ishii, Y.; Tahara, S.; Teramoto, A.; Morita, A. Endocan, a new invasion and angiogenesis marker of pituitary adenomas. J. Neuro Oncol. 2014, 117, 485–491. [Google Scholar] [CrossRef]
- Nomura, R.; Yoshida, D.; Teramoto, A. Stromal cell-derived factor-1 expression in pituitary adenoma tissues and upregulation in hypoxia. J. Neurooncol. 2009, 94, 173–181. [Google Scholar] [CrossRef]
- Niveiro, M.; Aranda, F.I.; Peiró, G.; Alenda, C.; Picó, A. Immunohistochemical analysis of tumor angiogenic factors in human pituitary adenomas. Hum. Pathol. 2005, 36, 1090–1095. [Google Scholar] [CrossRef]
- Sánchez-Ortiga, R.; Sánchez-Tejada, L.; Moreno-Perez, O.; Riesgo, P.; Niveiro, M.; Alfonso, A.M.P. Over-expression of vascular endothelial growth factor in pituitary adenomas is associated with extrasellar growth and recurrence. Pituitary 2013, 16, 370–377. [Google Scholar] [CrossRef]
- Di Ieva, A.; Weckman, A.; Di Michele, J.; Rotondo, F.; Grizzi, F.; Kovacs, K.; Cusimano, M.D. Microvascular morphometrics of the hypophysis and pituitary tumors: From bench to operating theatre. Microvasc. Res. 2013, 89, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Yamada, S.; Teramoto, A. Correlation between tumor vascularity and clinical findings in patients with pituitary adenomas. Endocr. Pathol. 2004, 15, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Kovacs, K.; Horvath, E.; Scheithauer, B.W.; Kuroki, T.; Lloyd, R.V. Microvessel density in pituitary adenomas and carcinomas. Virchows Arch. 2001, 438, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Viacava, P.; Gasperi, M.; Acerbi, G.; Manetti, L.; Cecconi, E.; Bonadio, A.G.; Naccarato, A.G.; Acerbi, F.; Parenti, G.; Lupi, I.; et al. Microvascular density and vascular endothelial growth factor expression in normal pituitary tissue and pituitary adenomas. J. Endocrinol. Investig. 2003, 26, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. VEGF receptor protein-tyrosine kinases: Structure and regulation. Biochem. Biophys. Res. Commun. 2008, 375, 287–291. [Google Scholar] [CrossRef]
- Nör, J.E.; Christensen, J.; Mooney, D.J.; Polverini, P.J. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am. J. Pathol. 1999, 154, 375–384. [Google Scholar] [CrossRef]
- Dai, C.; Liang, S.; Sun, B.; Li, Y.; Kang, J. Anti-VEGF Therapy in Refractory Pituitary Adenomas and Pituitary Carcinomas: A Review. Front. Oncol. 2021, 11, 773905. [Google Scholar] [CrossRef]
- Yilmaz, M.; Vural, E.; Koc, K.; Ceylan, S. Cavernous sinus invasion and effect of immunohistochemical features on remission in growth hormone secreting pituitary adenomas. Turk. Neurosurg. 2015, 25, 380–388. [Google Scholar] [CrossRef]
- Borg, S.A.; Kerry, K.E.; Royds, J.A.; Battersby, R.D.; Jones, T.H. Correlation of VEGF production with IL1 alpha and IL6 secretion by human pituitary adenoma cells. Eur. J. Endocrinol. 2005, 152, 293–300. [Google Scholar] [CrossRef]
- Ghadir, M.; Khamseh, M.E.; Panahi-Shamsabad, M.; Ghorbani, M.; Akbari, H.; Mehrjardi, A.Z.; Honardoost, M.; Jafar-Mohammadi, B. Cell proliferation, apoptosis, and angiogenesis in non-functional pituitary adenoma: Association with tumor invasiveness. Endocrine 2020, 69, 596–603. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Tohti, M.; Hu, Y.; Wang, S.; Li, W.; Lu, Z.; Ma, C. The expression profile of Dopamine D2 receptor, MGMT and VEGF in different histological subtypes of pituitary adenomas: A study of 197 cases and indications for the medical therapy. J. Exp. Clin. Cancer Res. 2014, 33, 56. [Google Scholar] [CrossRef] [PubMed]
- Cristina, C.; Díaz-Torga, G.; Baldi, A.; Góngora, A.; Rubinstein, M.; Low, M.J.; Becú-Villalobos, D. Increased pituitary vascular endothelial growth factor-a in dopaminergic D2 receptor knockout female mice. Endocrinology 2005, 146, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Korsisaari, N.; Ross, J.; Wu, X.; Kowanetz, M.; Pal, N.; Hall, L.; Eastham-Anderson, J.; Forrest, W.F.; Van Bruggen, N.; Peale, F.V.; et al. Blocking vascular endothelial growth factor-A inhibits the growth of pituitary adenomas and lowers serum prolactin level in a mouse model of multiple endocrine neoplasia type 1. Clin. Cancer Res. 2008, 14, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hu, Y.; Zhu, W.; Nie, C.; Zhao, W.; Faje, A.T.; Labelle, K.E.; Swearingen, B.; Lee, H.; Hedley-Whyte, E.T.; et al. Sprouting Angiogenesis in Human Pituitary Adenomas. Front. Oncol. 2022, 12, 875219. [Google Scholar] [CrossRef]
- Miyajima, K.; Takekoshi, S.; Itoh, J.; Kakimoto, K.; Miyakoshi, T.; Osamura, R.Y. Inhibitory effects of anti-VEGF antibody on the growth and angiogenesis of estrogen-induced pituitary prolactinoma in Fischer 344 Rats: Animal model of VEGF-targeted therapy for human endocrine tumors. Acta Histochem. Cytochem. 2010, 43, 33–44. [Google Scholar] [CrossRef][Green Version]
- Kurosaki, M.; Saegert, W.; Abe, T.; Lüdecke, D.K. Expression of vascular endothelial growth factor in growth hormone-secreting pituitary adenomas: Special reference to the octreotide treatment. Neurol. Res. 2008, 30, 518–522. [Google Scholar] [CrossRef]
- Amato, R.; Catalani, E.; Monte, M.D.; Cammalleri, M.; Di Renzo, I.; Perrotta, C.; Cervia, D.; Casini, G. Autophagy-mediated neuroprotection induced by octreotide in an ex vivo model of early diabetic retinopathy. Pharmacol. Res. 2018, 128, 167–178. [Google Scholar] [CrossRef]
- Pan, K.F.; Yang, Y.C.; Lee, W.J.; Hua, K.T.; Chien, M.H. Proteoglycan Endocan: A multifaceted therapeutic target in Cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188672. [Google Scholar] [CrossRef]
- Miao, Y.; Zong, M.; Jiang, T.; Yuan, X.; Guan, S.; Wang, Y.; Zhou, D. A comparative analysis of ESM-1 and vascular endothelial cell marker (CD34/CD105) expression on pituitary adenoma invasion. Pituitary 2016, 19, 194–201. [Google Scholar] [CrossRef]
- Cornelius, A.; Cortet-Rudelli, C.; Assaker, R.; Kerdraon, O.; Gevaert, M.H.; Prévot, V.; Lassalle, P.; Trouillas, J.; Delehedde, M.; Maurage, C.A. Endothelial expression of endocan is strongly associated with tumor progression in pituitary adenoma. Brain Pathol. 2012, 22, 757–764. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Z.; Wei, L.; Zhang, J. Endothelial cell-specific molecule-1 as an invasiveness marker for pituitary null cell adenoma. BMC Endocr. Disord. 2019, 19, 90. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Liu, X.; Zhang, Q.; Chen, G.; Ling, F. Associations of Endocan, FGF2, and PDGF Expression with Pituitary Neuroendocrine Tumor (PitNET) Invasiveness. Turk. Neurosurg. 2022; epub ahead of print. [Google Scholar] [CrossRef]
- Roudnicky, F.; Poyet, C.; Wild, P.; Krampitz, S.; Negrini, F.; Huggenberger, R.; Rogler, A.; Stöhr, R.; Hartmann, A.; Provenzano, M.; et al. Endocan is upregulated on tumor vessels in invasive bladder cancer where it mediates VEGF-A-induced angiogenesis. Cancer Res. 2013, 73, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.F.; Chang, Y.C.; Li, C.H.; Chan, M.H.; Chen, C.L.; Tsai, W.C.; Hsiao, M. Type V collagen alpha 1 chain promotes the malignancy of glioblastoma through PPRC1-ESM1 axis activation and extracellular matrix remodeling. Cell Death Discov. 2021, 7, 313. [Google Scholar] [CrossRef]
- Sun, L.; Sun, C.; Sun, J.; Yang, W. Downregulation of ENDOCAN in myeloid leukemia cells inhibits proliferation and promotes apoptosis by suppressing nuclear factor κB activity. Mol. Med. Rep. 2019, 19, 3247–3254. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Leng, Z.G.; Guo, Y.H.; Lin, S.J.; Wu, Z.R.; Su, Z.P.; Lu, J.L.; Wei, L.F.; Zhuge, Q.C.; Jin, K.; et al. Dopamine agonist resistance-related endocan promotes angiogenesis and cells viability of prolactinomas. Endocrine 2016, 52, 641–651. [Google Scholar] [CrossRef]
- Vierimaa, O.; Georgitsi, M.; Lehtonen, R.; Vahteristo, P.; Kokko, A.; Raitila, A.; Tuppurainen, K.; Ebeling, T.M.; Salmela, P.I.; Paschke, R.; et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science 2006, 312, 1228–1230. [Google Scholar] [CrossRef]
- Re, A.; Ferraù, F.; Cafiero, C.; Spagnolo, F.; Barresi, V.; Romeo, D.P.; Ragonese, M.; Grassi, C.; Pontecorvi, A.; Farsetti, A.; et al. Somatic Deletion in Exon 10 of Aryl Hydrocarbon Receptor Gene in Human GH-Secreting Pituitary Tumors. Front. Endocrinol. 2020, 11, 591039. [Google Scholar] [CrossRef]
- De Pinho, L.K.J.; Neto, L.V.; Wildemberg, L.E.A.; Gasparetto, E.L.; Marcondes, J.; de Almeida Nunes, B.; Takiya, C.M.; Gadelha, M.R. Low aryl hydrocarbon receptor-interacting protein expression is a better marker of invasiveness in somatotropinomas than Ki-67 and p53. Neuroendocrinology 2011, 94, 39–48. [Google Scholar] [CrossRef]
- Bogner, E.M.; Daly, A.F.; Gulde, S.; Karhu, A.; Irmler, M.; Beckers, J.; Mohr, H.; Beckers, A.; Pellegata, N.S. miR-34a is upregulated in AIP-mutated somatotropinomas and promotes octreotide resistance. Int. J. Cancer 2020, 147, 3523–3538. [Google Scholar] [CrossRef]
- Ritvonen, E.; Pitkänen, E.; Karppinen, A.; Vehkavaara, S.; Demir, H.; Paetau, A.; Schalin-Jäntti, C.; Karhu, A. Impact of AIP and inhibitory G protein alpha 2 proteins on clinical features of sporadic GH-secreting pituitary adenomas. Eur. J. Endocrinol. 2017, 176, 243–252. [Google Scholar] [CrossRef]
- Dutta, P.; Reddy, K.S.; Rai, A.; Madugundu, A.K.; Solanki, H.S.; Bhansali, A.; Radotra, B.D.; Kumar, N.; Collier, D.; Iacovazzo, D.; et al. Surgery, Octreotide, Temozolomide, Bevacizumab, Radiotherapy, and Pegvisomant Treatment of an AIP Mutation–Positive Child. J. Clin. Endocrinol. Metab. 2019, 104, 3539–3544. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Chen, S.; Yu, X.; Zhang, J.; Liang, W.; Zhang, Y.; Chen, Y.; Chen, S.; Hong, Y.; Yan, W.; et al. Transcription factor GTF2B regulates AIP protein expression in growth hormone-secreting pituitary adenomas and influences tumor phenotypes. Neuro Oncol. 2022, 24, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Neou, M.; Villa, C.; Armignacco, R.; Jouinot, A.; Raffin-Sanson, M.L.; Septier, A.; Letourneur, F.; Diry, S.; Diedisheim, M.; Izac, B.; et al. Pangenomic Classification of Pituitary Neuroendocrine Tumors. Cancer Cell 2020, 37, 123–134.e5. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, S.; Horiguchi, K.; Tosaka, M.; Yamada, S.; Yamada, M. Whole-Exome Sequencing Study of Thyrotropin-Secreting Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2017, 102, 566–575. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Q.; Zhu, J.; Yao, B.; Ma, C.; Qiao, N.; He, S.; Ye, Z.; Wang, Y.; Han, R.; et al. Integrated proteogenomic characterization across major histological types of pituitary neuroendocrine tumors. Cell Res. 2022, 32, 1047–1067. [Google Scholar] [CrossRef]
- Landis, C.A.; Harsh, G.; Lyons, J.; Davis, R.L.; McCormick, F.; Bourne, H.R. Clinical characteristics of acromegalic patients whose pituitary tumors contain mutant Gs protein. J. Clin. Endocrinol. Metab. 1990, 71, 1416–1420. [Google Scholar] [CrossRef]
- Buchfelder, M.; Fahlbusch, R.; Merz, T.; Symowski, H.; Adams, E.F. Analysis of GNAS mutations in 60 growth hormone secreting pituitary tumors: Correlation with clinical and pathological characteristics and surgical outcome based on highly sensitive GH and IGF-I criteria for remission. Pituitary 2007, 10, 275–282. [Google Scholar] [CrossRef]
- Buchfelder, M.; Fahlbusch, R.; Merz, T.; Symowski, H.; Adams, E.F. Clinical correlates in acromegalic patients with pituitary tumors expressing GSP oncogenes. Pituitary 1999, 1, 181–185. [Google Scholar] [CrossRef]
- Efstathiadou, Z.A.; Bargiota, A.; Chrisoulidou, A.; Kanakis, G.; Papanastasiou, L.; Theodoropoulou, A.; Tigas, S.K.; Vassiliadi, D.A.; Alevizaki, M.; Tsagarakis, S. Impact of gsp mutations in somatotroph pituitary adenomas on growth hormone response to somatostatin analogs: A meta-analysis. Pituitary 2015, 18, 861–867. [Google Scholar] [CrossRef]
- Foltran, R.K.; Amorim, P.V.G.H.; Duarte, F.H.; Grande, I.P.P.; Freire, A.C.T.B.; Frassetto, F.P.; Dettoni, J.B.; Alves, V.A.; Castro, I.; Trarbach, E.B.; et al. Study of major genetic factors involved in pituitary tumorigenesis and their impact on clinical and biological characteristics of sporadic somatotropinomas and non-functioning pituitary adenomas. Braz. J. Med. Biol. Res. 2018, 51, e7427. [Google Scholar] [CrossRef]
- Niendorf, S.; Oksche, A.; Kisser, A.; Löhler, J.; Prinz, M.; Schorle, H.; Feller, S.; Lewitzky, M.; Horak, I.; Knobeloch, K.P. Essential role of ubiquitin-specific protease 8 for receptor tyrosine kinase stability and endocytic trafficking in vivo. Mol. Cell. Biol. 2007, 27, 5029–5039. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Lee, S.Y.; Lee, J.; Jeong, C.H.; Farrand, L.; Lim, S.; Reddy, K.; Kim, J.Y.; Lee, M.H.; Lee, H.J.; et al. USP8 is a novel target for overcoming gefitinib resistance in lung cancer. Clin. Cancer Res. 2013, 19, 3894–3904. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Y.; Song, Z.J.; Chen, J.H.; Wang, Y.F.; Li, S.Q.; Zhou, L.F.; Mao, Y.; Li, Y.M.; Hu, R.G.; Zhang, Z.Y.; et al. Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 2015, 25, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rivas, L.G.; Theodoropoulou, M.; Ferraù, F.; Nusser, C.; Kawaguchi, K.; Stratakis, C.A.; Faucz, F.R.; Wildemberg, L.E.; Assié, G.; Beschorner, R.; et al. The Gene of the Ubiquitin-Specific Protease 8 Is Frequently Mutated in Adenomas Causing Cushing’s Disease. J. Clin. Endocrinol. Metab. 2015, 100, E997–E1004. [Google Scholar] [CrossRef]
- Albani, A.; Pérez-Rivas, L.G.; Dimopoulou, C.; Zopp, S.; Colón-Bolea, P.; Roeber, S.; Honegger, J.; Flitsch, J.; Rachinger, W.; Buchfelder, M.; et al. The USP8 mutational status may predict long-term remission in patients with Cushing’s disease. Clin. Endocrinol. 2018, 89, 454–458. [Google Scholar] [CrossRef]
- Faucz, F.R.; Tirosh, A.; Tatsi, C.; Berthon, A.; Hernández-Ramírez, L.C.; Settas, N.; Angelousi, A.; Correa, R.; Papadakis, G.Z.; Chittiboina, P.; et al. Somatic USP8 Gene Mutations Are a Common Cause of Pediatric Cushing Disease. J. Clin. Endocrinol. Metab. 2017, 102, 2836–2843. [Google Scholar] [CrossRef]
- Wanichi, I.Q.; de Paula Mariani, B.M.; Frassetto, F.P.; Siqueira, S.A.C.; de Castro Musolino, N.R.; Cunha-Neto, M.B.C.; Ochman, G.; Cescato, V.A.S.; Machado, M.C.; Trarbach, E.B.; et al. Cushing’s disease due to somatic USP8 mutations: A systematic review and meta-analysis. Pituitary 2019, 22, 435–442. [Google Scholar] [CrossRef]
- Hayashi, K.; Inoshita, N.; Kawaguchi, K.; Ardisasmita, A.I.; Suzuki, H.; Fukuhara, N.; Okada, M.; Nishioka, H.; Takeuchi, Y.; Komada, M.; et al. The USP8 mutational status may predict drug susceptibility in corticotroph adenomas of Cushing’s disease. Eur. J. Endocrinol. 2016, 174, 213–226. [Google Scholar] [CrossRef]
- Bruns, C.; Lewis, I.; Briner, U.; Meno-Tetang, G.; Weckbecker, G. SOM230: A novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 2002, 146, 707–716. [Google Scholar] [CrossRef]
- Duong, C.V.; Emes, R.D.; Wessely, F.; Yacqub-Usman, K.; Clayton, R.N.; Farrell, W.E. Quantitative, genome-wide analysis of the DNA methylome in sporadic pituitary adenomas. Endocr. Relat. Cancer 2012, 19, 805–816. [Google Scholar] [CrossRef]
- Kober, P.; Boresowicz, J.; Rusetska, N.; Maksymowicz, M.; Goryca, K.; Kunicki, J.; Bonicki, W.; Siedlecki, J.A.; Bujko, M. DNA methylation profiling in nonfunctioning pituitary adenomas. Mol. Cell Endocrinol. 2018, 473, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Dudley, K.J.; Revill, K.; Clayton, R.N.; Farrell, W.E. Pituitary tumors: All silent on the epigenetics front. J. Mol. Endocrinol. 2009, 42, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Pease, M.; Shi, L.; Punj, V.; Shiroishi, M.S.; Commins, D.; Weisenberger, D.J.; Wang, K.; Zada, G. A pilot genome-scale profiling of DNA methylation in sporadic pituitary macroadenomas: Association with tumor invasion and histopathological subtype. PLoS ONE 2014, 9, e96178. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Li, C.; Xie, W.; Miao, Y.; Guo, J.; Wang, J.; Zhang, Y. Integrated analysis of DNA methylation and mRNA expression profiles to identify key genes involved in the regrowth of clinically non-functioning pituitary adenoma. Aging 2020, 12, 2408–2427. [Google Scholar] [CrossRef]
- Wang, R.Q.; Lan, Y.L.; Lou, J.C.; Lyu, Y.Z.; Hao, Y.C.; Su, Q.F.; Ma, B.B.; Yuan, Z.B.; Yu, Z.K.; Zhang, H.Q.; et al. Expression and methylation status of LAMA2 are associated with the invasiveness of nonfunctioning PitNET. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018818821296. [Google Scholar] [CrossRef]
- Gadelha, M.R.; Kasuki, L.; Dénes, J.; Trivellin, G.; Korbonits, M. MicroRNAs: Suggested role in pituitary adenoma pathogenesis. J. Endocrinol. Investig. 2013, 36, 889–895. [Google Scholar] [CrossRef]
- Nadhamuni, V.S.; Korbonits, M. Novel Insights into Pituitary Tumorigenesis: Genetic and Epigenetic Mechanisms. Endocr. Rev. 2020, 41, 821–846. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Z.; Gao, H. Advancements in the study of miRNA regulation during the cell cycle in human pituitary adenomas. J. Neurooncol 2017, 134, 253–258. [Google Scholar] [CrossRef]
- Renjie, W.; Haiqian, L. MiR-132, miR-15a and miR-16 synergistically inhibit pituitary tumor cell proliferation, invasion and migration by targeting Sox5. Cancer Lett. 2015, 356, 568–578. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, W.; Feng, Q.; Hao, B.; Cheng, C.; Cheng, Y.; Li, Y.; Fan, X.; Chen, Z. Comprehensive circular RNA profiling reveals that hsa_circ_0001368 is involved in growth hormone-secreting pituitary adenoma development. Brain Res. Bull. 2020, 161, 65–77. [Google Scholar] [CrossRef]
- Shen, D.W.; Li, Y.L.; Hou, Y.J.; Xu, Z.D.; Li, Y.Z.; Chang, J.Y. MicroRNA-543 promotes cell invasion and impedes apoptosis in pituitary adenoma via activating the Wnt/β-catenin pathway by negative regulation of Smad7. Biosci. Biotechnol. Biochem. 2019, 83, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Wierinckx, A.; Croze, S.; Rey, C.; Legras-Lachuer, C.; Morel, A.P.; Fusco, A.; Raverot, G.; Trouillas, J.; Lachuer, J. Deregulation of miR-183 and KIAA0101 in Aggressive and Malignant Pituitary Tumors. Front. Med. 2015, 2, 54. [Google Scholar] [CrossRef] [PubMed]
- Garbicz, F.; Mehlich, D.; Rak, B.; Sajjad, E.; Maksymowicz, M.; Paskal, W.; Zieliński, G.; Włodarski, P.K. Increased expression of the microRNA 106b~25 cluster and its host gene MCM7 in corticotroph pituitary adenomas is associated with tumor invasion and Crooke’s cell morphology. Pituitary 2017, 20, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhang, Y.; Zhang, Z.; Yang, Y.; Song, T. Effect of miR-106b on Invasiveness of Pituitary Adenoma via PTEN-PI3K/AKT. Med. Sci. Monit. 2017, 23, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, T.; Faucz, F.R.; Azevedo, M.; Xekouki, P.; Iliopoulos, D.; Stratakis, C.A. Functional screen analysis reveals miR-26b and miR-128 as central regulators of pituitary somatomammotrophic tumor growth through activation of the PTEN-AKT pathway. Oncogene 2013, 32, 1651–1659. [Google Scholar] [CrossRef]
- Duan, J.; Lu, G.; Li, Y.; Zhou, S.; Zhou, D.; Tao, H. Increased miR-338-3p expression correlates with invasiveness of GH-producing pituitary adenomas. Endocrine 2017, 58, 184–189. [Google Scholar] [CrossRef]
- Duan, J.; Lu, G.; Li, Y.; Zhou, S.; Zhou, D.; Tao, H. miR-137 functions as a tumor suppressor gene in pituitary adenoma by targeting AKT2. Int. J. Clin. Exp. Pathol. 2019, 12, 1557–1564. [Google Scholar]
- Lei, C.; Jing, G.; Jichao, W.; Xiaohui, L.; Fang, Q.; Hua, G.; Yazhou, M.; Zhang, Y. MiR-137’s Tumor Suppression on Prolactinomas by Targeting MITF and Modulating Wnt Signaling Pathway. J. Clin. Endocrinol. Metab. 2019, 104, 6391–6402. [Google Scholar] [CrossRef]
- Song, Z.J.; Reitman, Z.J.; Ma, Z.Y.; Chen, J.H.; Zhang, Q.L.; Shou, X.F.; Huang, C.X.; Wang, Y.F.; Li, S.Q.; Mao, Y.; et al. The genome-wide mutational landscape of pituitary adenomas. Cell Res. 2016, 26, 1255–1259. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Klicka, K.; Rak, B.; Mehlich, D.; Garbicz, F.; Zieliński, G.; Maksymowicz, M.; Sajjad, E.; Włodarski, P.K. Lineage-dependent role of miR-410-3p as oncomiR in gonadotroph and corticotroph pituitary adenomas or tumor suppressor miR in somatotroph adenomas via MAPK, PTEN/AKT, and STAT3 signaling pathways. Endocrine 2019, 65, 646–655. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. Chapter 01: Tumours of the pituitary gland. In WHO Classification of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; Volume 10, pp. 12–44. [Google Scholar]
- Mete, O.; Osamura, R.Y.; Asa, L.S. Chapter 02: Pituitary Tumours. In WHO Classification of Tumours Editorial Board. Endocrine and Neuroendocrine Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2022; Volume 10. Available online: https://publications.iarc.fr (accessed on 30 June 2023).
- Villa, C.; Baussart, B.; Assié, G.; Raverot, G.; Roncaroli, F. The World Health Organization classifications of pituitary neuroendocrine tumours: A clinico-pathological appraisal. Endocr. Relat. Cancer 2023, 30, e230021. [Google Scholar] [CrossRef] [PubMed]
- Lenders, N.F.; Earls, P.E.; Inder, W.J.; McCormack, A.I. The evolution in pituitary tumour classification: A clinical perspective. Endocr. Oncol. 2023, 3, e220079. [Google Scholar] [CrossRef]
- Daly, A.F.; Tichomirowa, M.A.; Petrossians, P.; Heliövaara, E.; Jaffrain-Rea, M.L.; Barlier, A.; Naves, L.A.; Ebeling, T.; Karhu, A.; Raappana, A.; et al. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: An international collaborative study. J. Clin. Endocrinol. Metab. 2010, 95, E373–E383. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, S.; De Marinis, L. From Pituitary Adenoma to Pituitary Neuroendocrine Tumors: How Molecular Pathways may Impact the Therapeutic Management? Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1744–1759. [Google Scholar] [CrossRef] [PubMed]
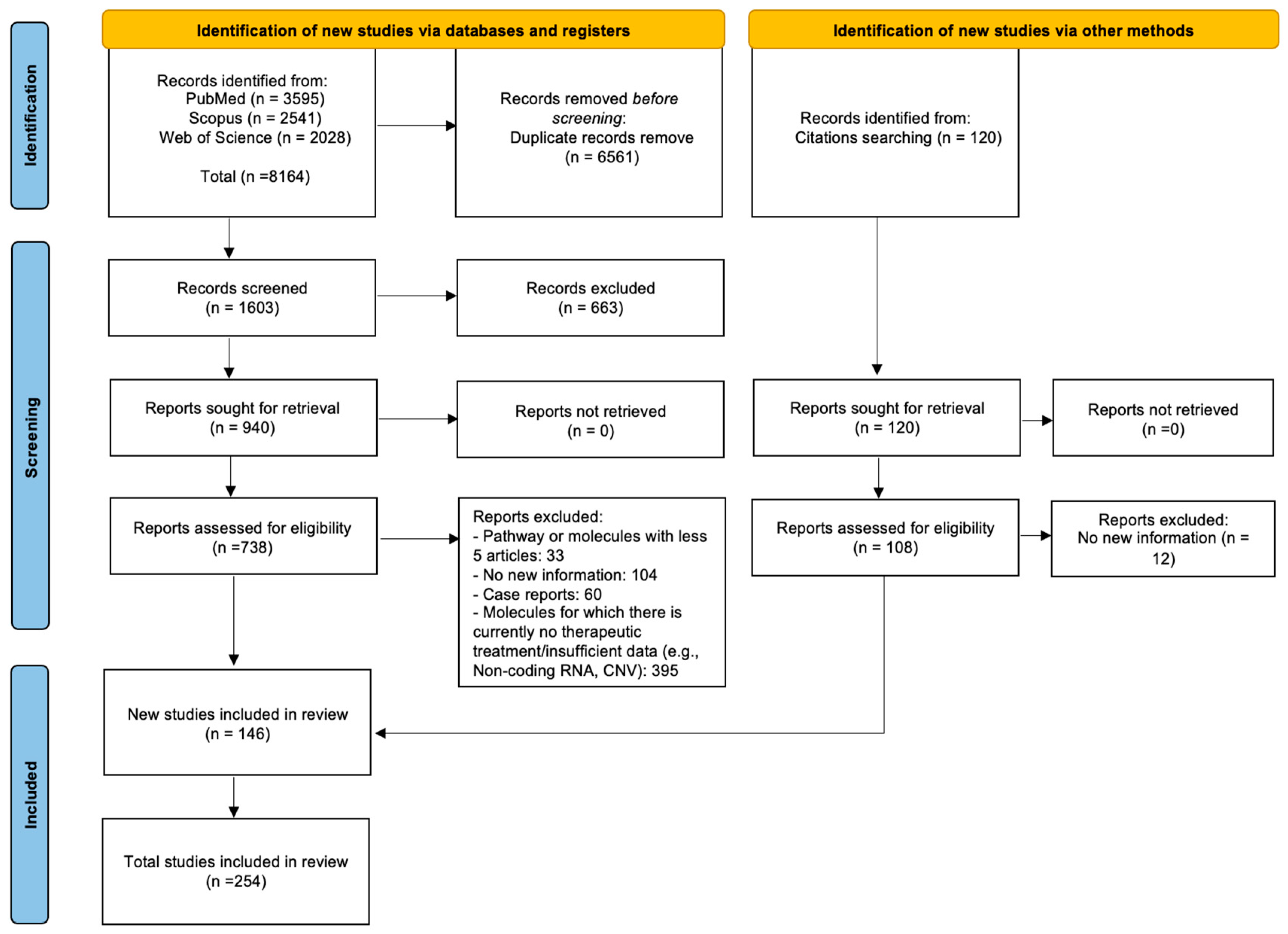
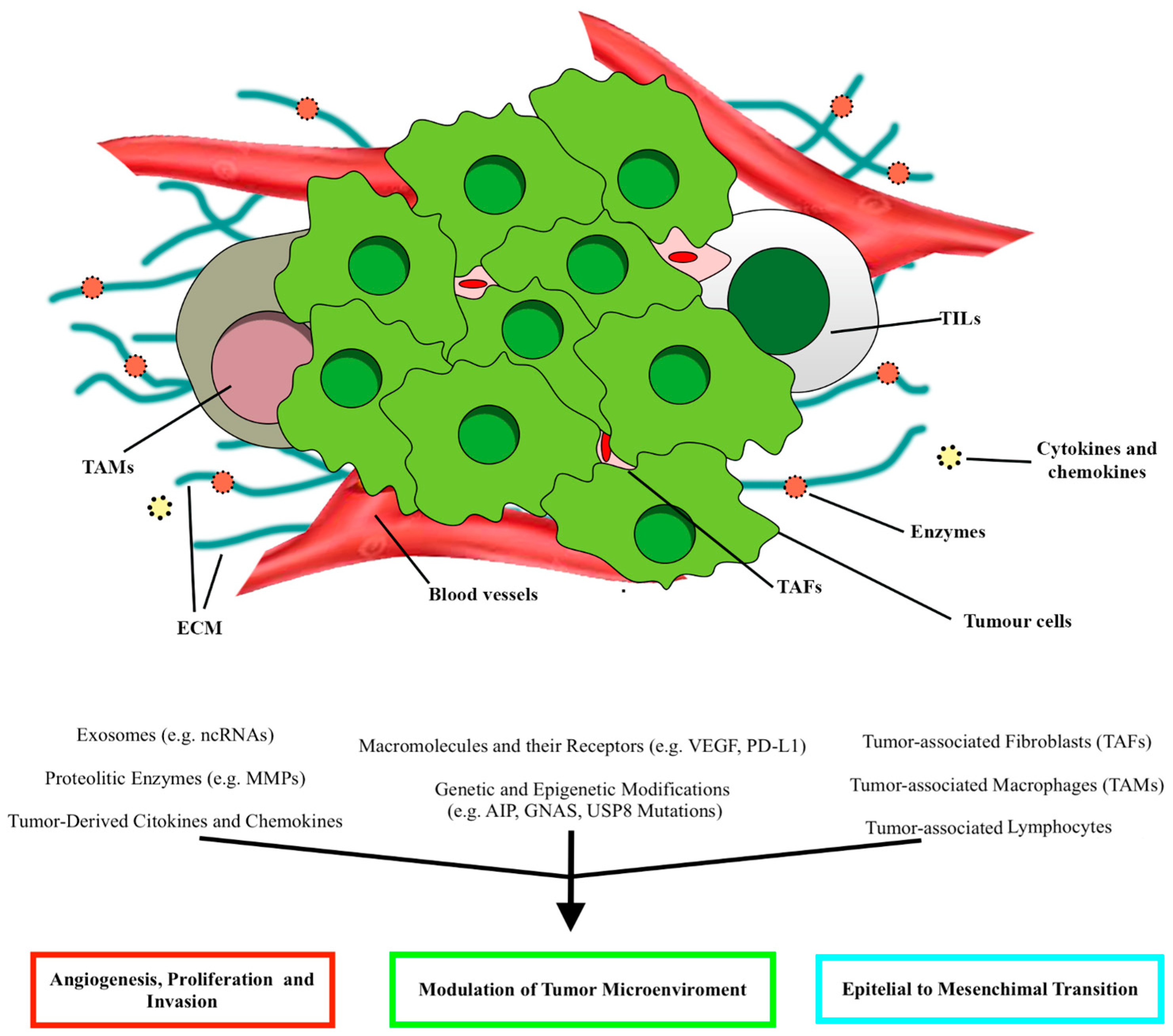
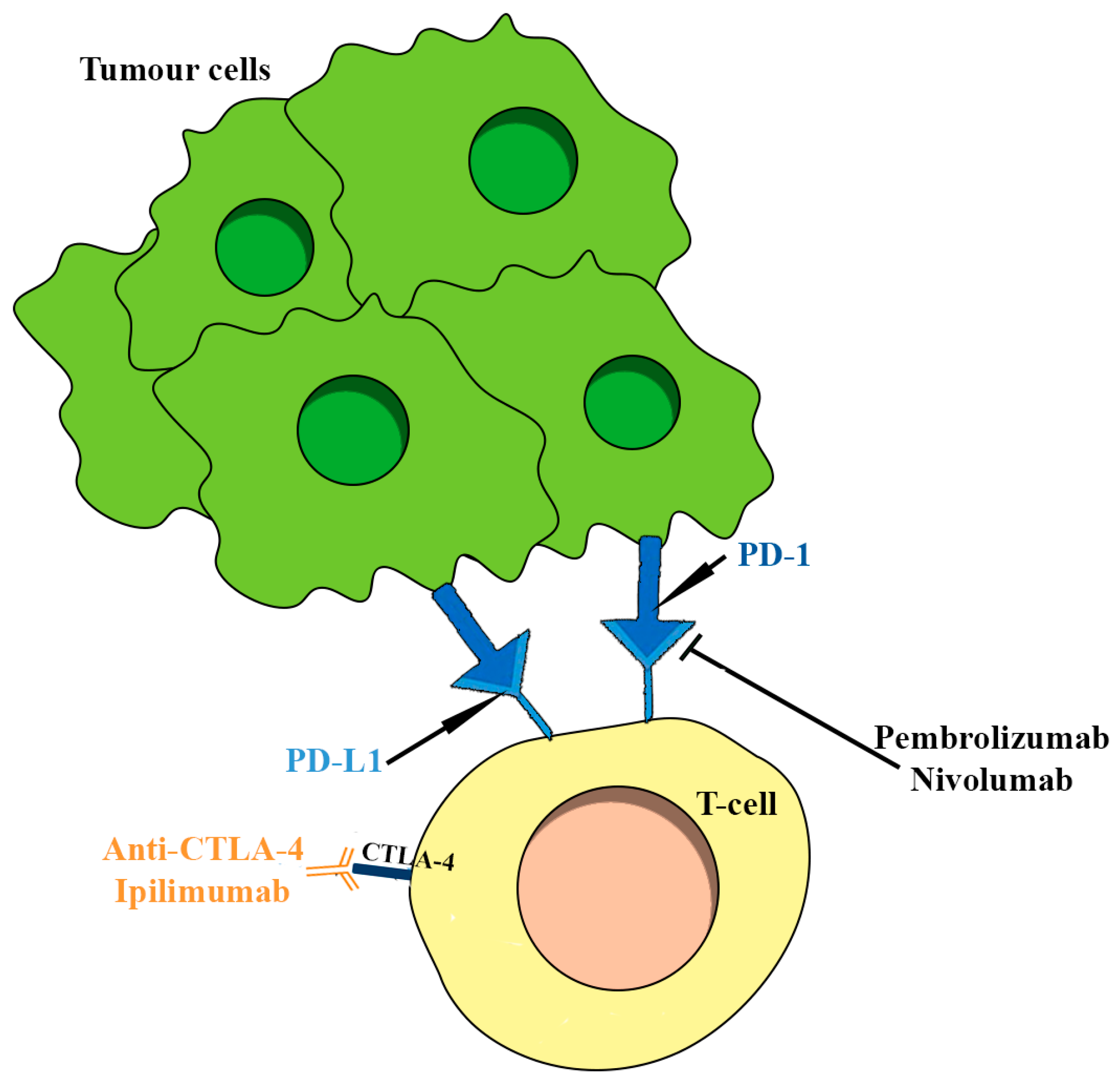
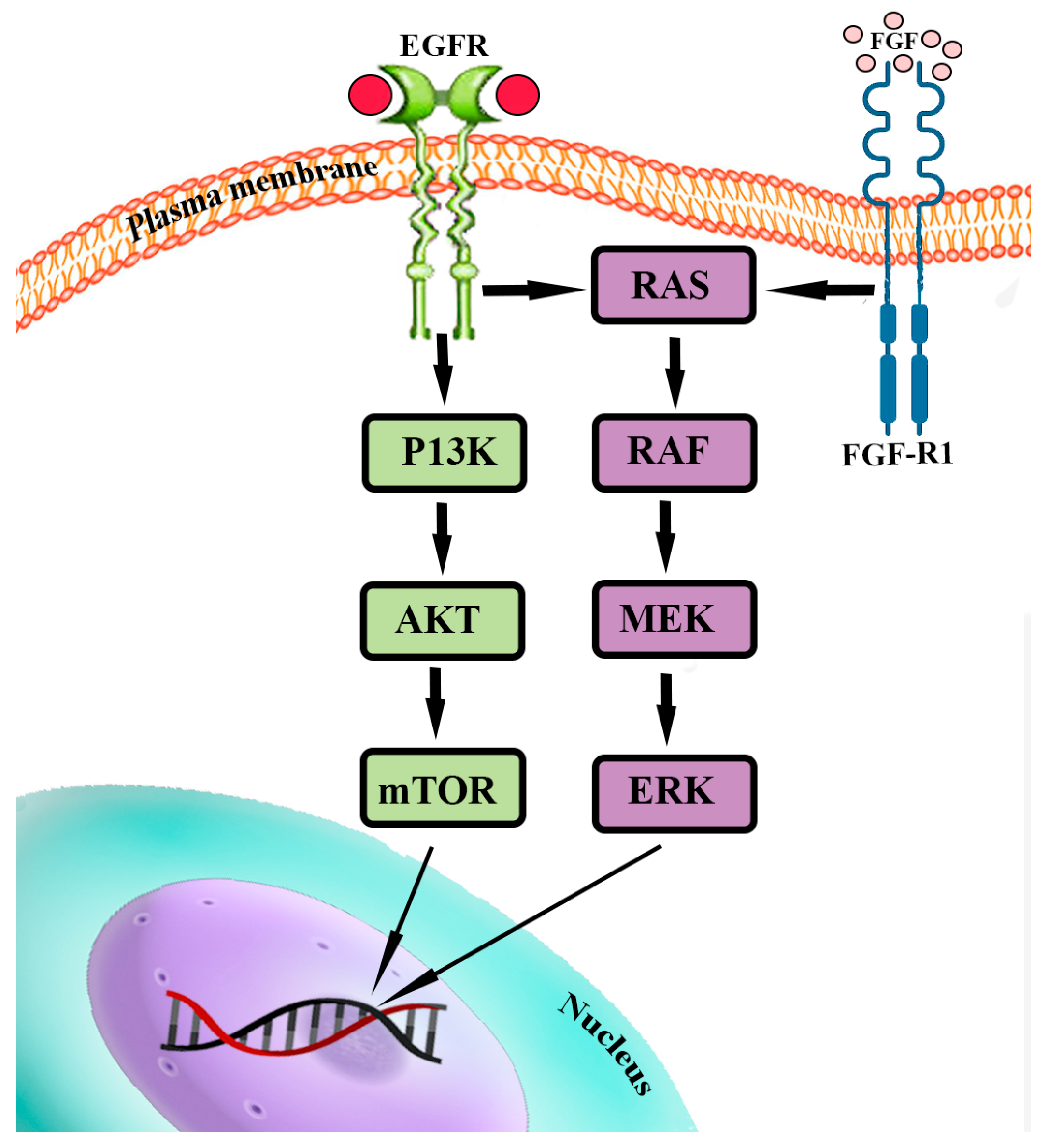


| Molecular Mechanism/Pathway | Molecular Therapies |
|---|---|
| Immune-checkpoint inhibitors (ICIs) | Anti-CTLA-4: Ipilimumab Anti-PD-1/PD-L1: Nivolumab, Pembrolizumab |
| DNA repair mechanisms | Temozolomide |
| PI3K/Akt/mTOR and RAS/RAF/MEK/ERK pathways | mTOR Inhibitors: Rapamycin/Sirolimus, Everolimus/RAD001 PI3K Inhibitors: Buparlisib, Alpelisib PI3K-mTOR inhibitors: Dactolisib, Voxtalisib BRAF inhibitor: Vemurafenib |
| EGFR | anti-EGFR tyrosine kinase inhibitor: Gefitinib, Lapatinib |
| VEGF/VEGFR | VEGFA Inhibitor: Bevacizumab VEGFR Inhibitor: Sunitinib, Apatinib, Axitinib (also combined with Bromocriptine) |
| Estrogen Modulators | SERMs and SERDs with/without DA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serioli, S.; Agostini, L.; Pietrantoni, A.; Valeri, F.; Costanza, F.; Chiloiro, S.; Buffoli, B.; Piazza, A.; Poliani, P.L.; Peris-Celda, M.; et al. Aggressive PitNETs and Potential Target Therapies: A Systematic Review of Molecular and Genetic Pathways. Int. J. Mol. Sci. 2023, 24, 15719. https://doi.org/10.3390/ijms242115719
Serioli S, Agostini L, Pietrantoni A, Valeri F, Costanza F, Chiloiro S, Buffoli B, Piazza A, Poliani PL, Peris-Celda M, et al. Aggressive PitNETs and Potential Target Therapies: A Systematic Review of Molecular and Genetic Pathways. International Journal of Molecular Sciences. 2023; 24(21):15719. https://doi.org/10.3390/ijms242115719
Chicago/Turabian StyleSerioli, Simona, Ludovico Agostini, Alberto Pietrantoni, Federico Valeri, Flavia Costanza, Sabrina Chiloiro, Barbara Buffoli, Amedeo Piazza, Pietro Luigi Poliani, Maria Peris-Celda, and et al. 2023. "Aggressive PitNETs and Potential Target Therapies: A Systematic Review of Molecular and Genetic Pathways" International Journal of Molecular Sciences 24, no. 21: 15719. https://doi.org/10.3390/ijms242115719
APA StyleSerioli, S., Agostini, L., Pietrantoni, A., Valeri, F., Costanza, F., Chiloiro, S., Buffoli, B., Piazza, A., Poliani, P. L., Peris-Celda, M., Iavarone, F., Gaudino, S., Gessi, M., Schinzari, G., Mattogno, P. P., Giampietro, A., De Marinis, L., Pontecorvi, A., Fontanella, M. M., ... Doglietto, F. (2023). Aggressive PitNETs and Potential Target Therapies: A Systematic Review of Molecular and Genetic Pathways. International Journal of Molecular Sciences, 24(21), 15719. https://doi.org/10.3390/ijms242115719







