A Serine Protease Inhibitor, Camostat Mesilate, Suppresses Urinary Plasmin Activity and Alleviates Hypertension and Podocyte Injury in Dahl Salt-Sensitive Rats
Abstract
1. Introduction
2. Results
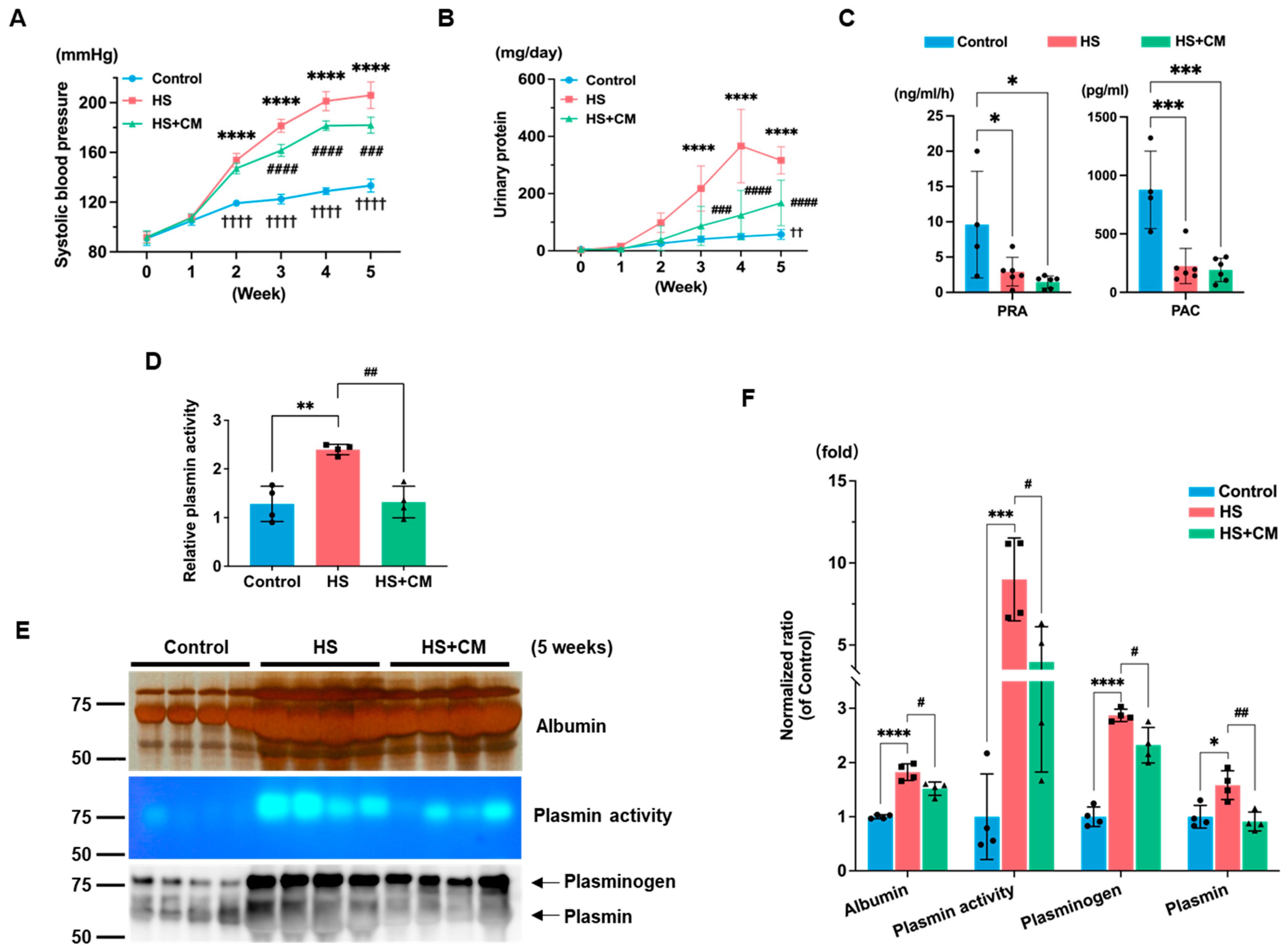
2.1. The Effects of CM on Body Weight, Organ Weight, Systolic Blood Pressure, Urinary Protein, and Blood Parameters
2.2. The Effects of CM on Urinary Plasmin
2.3. The Effects of CM on Renal ENaC Expression
2.4. The Effects of CM on Glomerular Sclerosis, Podocyte Injury, and Glomerular Apoptosis
2.5. The Effects of CM on Protease-Activated Receptors (PARs) and Kidney Injury Markers
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Pathological Examination
4.3. Urinary Serine Protease Activities
4.4. Chromogenic Assay for Urinary Plasmin Activity
4.5. Western Blotting
4.6. Immunofluorescence
4.7. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
4.8. Quantitative Real-Time Polymerase Chain Reaction
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masilamani, S.; Kim, G.H.; Mitchell, C.; Wade, J.B.; Knepper, M.A. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J. Clin. Investig. 1999, 104, R19–R23. [Google Scholar]
- Kitamura, K.; Tomita, K. Proteolytic activation of the epithelial sodium channel and therapeutic application of a serine protease inhibitor for the treatment of salt-sensitive hypertension. Clin. Exp. Nephrol. 2012, 16, 44–48. [Google Scholar]
- Svenningsen, P.; Bistrup, C.; Friis, U.G.; Bertog, M.; Haerteis, S.; Krueger, B.; Stubbe, J.; Jensen, O.N.; Thiesson, H.C.; Uhrenholt, T.R.; et al. Plasmin in nephrotic urine activates the epithelial sodium channel. J. Am. Soc. Nephrol. 2009, 20, 299–310. [Google Scholar]
- Anand, D.; Hummler, E.; Rickman, O.J. ENaC activation by proteases. Acta Physiol. 2022, 235, e13811. [Google Scholar]
- Passero, C.J.; Mueller, G.M.; Rondon-Berrios, H.; Tofovic, S.P.; Hughey, R.P.; Kleyman, T.R. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J. Biol. Chem. 2008, 283, 36586–36591. [Google Scholar]
- Raij, L.; Tian, R.; Wong, J.S.; He, J.C.; Campbell, K.N. Podocyte injury: The role of proteinuria, urinary plasminogen, and oxidative stress. Am. J. Physiol. Renal Physiol. 2016, 311, F1308–F1317. [Google Scholar] [CrossRef]
- Egerman, M.A.; Wong, J.S.; Runxia, T.; Mosoyan, G.; Chauhan, K.; Reyes-Bahamonde, J.; Anandakrishnan, N.; Wong, N.J.; Bagiella, E.; Salem, F.; et al. Plasminogenuria is associated with podocyte injury, edema, and kidney dysfunction in incident glomerular disease. FASEB J. 2020, 34, 16191–16204. [Google Scholar]
- Tamura, Y.; Hirado, M.; Okamura, K.; Minato, Y.; Fujii, S. Synthetic inhibitors of trypsin, plasmin, kallikrein, thrombin, C1r, and C1 esterase. Biochim. Biophys. Acta 1977, 484, 417–422. [Google Scholar]
- Maekawa, A.; Kakizoe, Y.; Miyoshi, T.; Wakida, N.; Ko, T.; Shiraishi, N.; Adachi, M.; Tomita, K.; Kitamura, K. Camostat mesilate inhibits prostasin activity and reduces blood pressure and renal injury in salt-sensitive hypertension. J. Hypertens. 2009, 27, 181–189. [Google Scholar]
- Uchimura, K.; Kakizoe, Y.; Onoue, T.; Hayata, M.; Morinaga, J.; Yamazoe, R.; Ueda, M.; Mizumoto, T.; Adachi, M.; Miyoshi, T.; et al. In vivo contribution of serine proteases to the proteolytic activation of gammaENaC in aldosterone-infused rats. Am. J. Physiol. Renal Physiol. 2012, 303, F939–F943. [Google Scholar]
- Ueda, M.; Uchimura, K.; Narita, Y.; Miyasato, Y.; Mizumoto, T.; Morinaga, J.; Hayata, M.; Kakizoe, Y.; Adachi, M.; Miyoshi, T.; et al. The serine protease inhibitor camostat mesilate attenuates the progression of chronic kidney disease through its antioxidant effects. Nephron 2015, 129, 223–232. [Google Scholar] [PubMed]
- Kakizoe, Y.; Miyasato, Y.; Onoue, T.; Nakagawa, T.; Hayata, M.; Uchimura, K.; Morinaga, J.; Mizumoto, T.; Adachi, M.; Miyoshi, T.; et al. A serine protease inhibitor attenuates aldosterone-induced kidney injuries via the suppression of plasmin activity. J. Pharmacol. Sci. 2016, 132, 145–153. [Google Scholar] [PubMed]
- Mizumoto, T.; Kakizoe, Y.; Nakagawa, T.; Iwata, Y.; Miyasato, Y.; Uchimura, K.; Adachi, M.; Deng, Q.; Hayata, M.; Morinaga, J.; et al. A serine protease inhibitor camostat mesilate prevents podocyte apoptosis and attenuates podocyte injury in metabolic syndrome model rats. J. Pharmacol. Sci. 2021, 146, 192–199. [Google Scholar] [PubMed]
- Kakizoe, Y.; Kitamura, K.; Ko, T.; Wakida, N.; Maekawa, A.; Miyoshi, T.; Shiraishi, N.; Adachi, M.; Zhang, Z.; Masilamani, S.; et al. Aberrant ENaC activation in Dahl salt-sensitive rats. J. Hypertens. 2009, 27, 1679–1689. [Google Scholar] [PubMed]
- Deng, Q.; Kakizoe, Y.; Iwata, Y.; Nakagawa, T.; Miyasato, Y.; Nakagawa, M.; Nishiguchi, K.; Nagayoshi, Y.; Adachi, M.; Narita, Y.; et al. The serine protease plasmin plays detrimental roles in epithelial sodium channel activation and podocyte injury in Dahl salt-sensitive rats. Hypertens. Res. 2023, 46, 50–62. [Google Scholar]
- Pavlov, T.S.; Levchenko, V.; O’Connor, P.M.; Ilatovskaya, D.V.; Palygin, O.; Mori, T.; Mattson, D.L.; Sorokin, A.; Lombard, J.H.; Cowley, A.W., Jr.; et al. Deficiency of renal cortical EGF increases ENaC activity and contributes to salt-sensitive hypertension. J. Am. Soc. Nephrol. 2013, 24, 1053–1062. [Google Scholar]
- Sharma, R.; Waller, A.P.; Agrawal, S.; Wolfgang, K.J.; Luu, H.; Shahzad, K.; Isermann, B.; Smoyer, W.E.; Nieman, M.T.; Kerlin, B.A. Thrombin-Induced Podocyte Injury Is Protease-Activated Receptor Dependent. J. Am. Soc. Nephrol. 2017, 28, 2618–2630. [Google Scholar]
- Bohnert, B.N.; Gonzalez-Menendez, I.; Dörffel, T.; Schneider, J.C.; Xiao, M.; Janessa, A.; Kalo, M.Z.; Fehrenbacher, B.; Schaller, M.; Casadei, N.; et al. Essential role of DNA-PKcs and plasminogen for the development of doxorubicin-induced glomerular injury in mice. Dis. Model. Mech. 2021, 14, dmm049038. [Google Scholar]
- Andersen, H.; Friis, U.G.; Hansen, P.B.; Svenningsen, P.; Henriksen, J.E.; Jensen, B.L. Diabetic nephropathy is associated with increased urine excretion of proteases plasmin, prostasin and urokinase and activation of amiloride-sensitive current in collecting duct cells. Nephrol. Dial. Transplant. 2015, 30, 781–789. [Google Scholar]
- Andersen, R.F.; Buhl, K.B.; Jensen, B.L.; Svenningsen, P.; Friis, U.G.; Jespersen, B.; Rittig, S. Remission of nephrotic syndrome diminishes urinary plasmin content and abolishes activation of ENaC. Pediatr. Nephrol. 2013, 28, 1227–1234. [Google Scholar]
- Buhl, K.B.; Friis, U.G.; Svenningsen, P.; Gulaveerasingam, A.; Ovesen, P.; Frederiksen-Møller, B.; Jespersen, B.; Bistrup, C.; Jensen, B.L. Urinary plasmin activates collecting duct ENaC current in preeclampsia. Hypertension 2012, 60, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Buhl, K.B.; Oxlund, C.S.; Friis, U.G.; Svenningsen, P.; Bistrup, C.; Jacobsen, I.A.; Jensen, B.L. Plasmin in urine from patients with type 2 diabetes and treatment-resistant hypertension activates ENaC in vitro. J. Hypertens. 2014, 32, 1672–1677; discussion 1677. [Google Scholar] [CrossRef] [PubMed]
- Oxlund, C.S.; Buhl, K.B.; Jacobsen, I.A.; Hansen, M.R.; Gram, J.; Henriksen, J.E.; Schousboe, K.; Tarnow, L.; Jensen, B.L. Amiloride lowers blood pressure and attenuates urine plasminogen activation in patients with treatment-resistant hypertension. J. Am. Soc. Hypertens. 2014, 8, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, X.; Sharma, N.M.; Li, Y.; Pliquett, R.U.; Patel, K.P. Urinary Proteolytic Activation of Renal Epithelial Na+ Channels in Chronic Heart Failure. Hypertension 2016, 67, 197–205. [Google Scholar] [CrossRef]
- Hinrichs, G.R.; Michelsen, J.S.; Zachar, R.; Friis, U.G.; Svenningsen, P.; Birn, H.; Bistrup, C.; Jensen, B.L. Albuminuria in kidney transplant recipients is associated with increased urinary serine proteases and activation of the epithelial sodium channel. Am. J. Physiol. Renal Physiol. 2018, 315, F151–F160. [Google Scholar] [CrossRef]
- Zachar, R.; Al-Mashhadi, A.; Dimke, H.; Svenningsen, P.; Jensen, B.L.; Carlström, M. Hydronephrosis is associated with elevated plasmin in urine in pediatric patients and rats and changes in NCC and γ-ENaC abundance in rat kidney. Am. J. Physiol. Renal Physiol. 2018, 315, F547–F557. [Google Scholar] [CrossRef]
- Schork, A.; Woern, M.; Kalbacher, H.; Voelter, W.; Nacken, R.; Bertog, M.; Haerteis, S.; Korbmacher, C.; Heyne, N.; Peter, A.; et al. Association of Plasminuria with Overhydration in Patients with CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 761–769. [Google Scholar] [CrossRef]
- Chen, J.L.; Wang, L.; Yao, X.M.; Zang, Y.J.; Wang, Y.; Li, Z.J.; Pearce, D.; Wang, H. Association of Urinary Plasminogen-Plasmin with Edema and Epithelial Sodium Channel Activation in Patients with Nephrotic Syndrome. Am. J. Nephrol. 2019, 50, 92–104. [Google Scholar] [CrossRef]
- Bohnert, B.N.; Daiminger, S.; Wörn, M.; Sure, F.; Staudner, T.; Ilyaskin, A.V.; Batbouta, F.; Janessa, A.; Schneider, J.C.; Essigke, D.; et al. Urokinase-type plasminogen activator (uPA) is not essential for epithelial sodium channel (ENaC)-mediated sodium retention in experimental nephrotic syndrome. Acta Physiol. 2019, 227, e13286. [Google Scholar] [CrossRef]
- Xiao, M.; Bohnert, B.N.; Aypek, H.; Kretz, O.; Grahammer, F.; Aukschun, U.; Wörn, M.; Janessa, A.; Essigke, D.; Daniel, C.; et al. Plasminogen deficiency does not prevent sodium retention in a genetic mouse model of experimental nephrotic syndrome. Acta Physiol. 2021, 231, e13512. [Google Scholar] [CrossRef]
- Hinrichs, G.R.; Weyer, K.; Friis, U.G.; Svenningsen, P.; Lund, I.K.; Nielsen, R.; Mollet, G.; Antignac, C.; Bistrup, C.; Jensen, B.L.; et al. Urokinase-type plasminogen activator contributes to amiloride-sensitive sodium retention in nephrotic range glomerular proteinuria in mice. Acta Physiol. 2019, 227, e13362. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.; Hansen, M.H.; Buhl, K.B.; Stæhr, M.; Friis, U.G.; Enggaard, C.; Supramaniyam, S.; Lund, I.K.; Svenningsen, P.; Hansen, P.B.L.; et al. Plasminogen Deficiency and Amiloride Mitigate Angiotensin II-Induced Hypertension in Type 1 Diabetic Mice Suggesting Effects Through the Epithelial Sodium Channel. J. Am. Heart Assoc. 2020, 9, e016387. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, B.N.; Menacher, M.; Janessa, A.; Wörn, M.; Schork, A.; Daiminger, S.; Kalbacher, H.; Häring, H.U.; Daniel, C.; Amann, K.; et al. Aprotinin prevents proteolytic epithelial sodium channel (ENaC) activation and volume retention in nephrotic syndrome. Kidney Int. 2018, 93, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, L.M.; Lin, C.Y.; Chai, K.X. The epidermal growth factor receptor (EGFR) is proteolytically modified by the Matriptase-Prostasin serine protease cascade in cultured epithelial cells. Biochim. Biophys. Acta 2008, 1783, 896–903. [Google Scholar] [CrossRef][Green Version]
- Aggarwal, S.; Dabla, P.K.; Arora, S. Prostasin: An Epithelial Sodium Channel Regulator. J. Biomark. 2013, 2013, 179864. [Google Scholar] [CrossRef]
- Moons, L.; Shi, C.; Ploplis, V.; Plow, E.; Haber, E.; Collen, D.; Carmeliet, P. Reduced transplant arteriosclerosis in plasminogen-deficient mice. J. Clin. Investig. 1998, 102, 1788–1797. [Google Scholar] [CrossRef]
- Syrovets, T.; Lunov, O.; Simmet, T. Plasmin as a proinflammatory cell activator. J. Leukoc. Biol. 2012, 92, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, P.; Uhrenholt, T.R.; Palarasah, Y.; Skjødt, K.; Jensen, B.L.; Skøtt, O. Prostasin-dependent activation of epithelial Na+ channels by low plasmin concentrations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1733–R1741. [Google Scholar] [CrossRef]
- Stæhr, M.; Buhl, K.B.; Andersen, R.F.; Svenningsen, P.; Nielsen, F.; Hinrichs, G.R.; Bistrup, C.; Jensen, B.L. Aberrant glomerular filtration of urokinase-type plasminogen activator in nephrotic syndrome leads to amiloride-sensitive plasminogen activation in urine. Am. J. Physiol. Renal Physiol. 2015, 309, F235–F241. [Google Scholar] [CrossRef]
- Haerteis, S.; Schork, A.; Dörffel, T.; Bohnert, B.N.; Nacken, R.; Wörn, M.; Xiao, M.; Essigke, D.; Janessa, A.; Schmaier, A.H.; et al. Plasma kallikrein activates the epithelial sodium channel in vitro but is not essential for volume retention in nephrotic mice. Acta Physiol. 2018, 224, e13060. [Google Scholar] [CrossRef]
- Bohovyk, R.; Khedr, S.; Levchenko, V.; Stefanenko, M.; Semenikhina, M.; Kravtsova, O.; Isaeva, E.; Geurts, A.M.; Klemens, C.A.; Palygin, O.; et al. Protease-Activated Receptor 1 Mediated Damage of Podocytes in Diabetic Nephropathy. Diabetes 2023, db230032. [Google Scholar] [CrossRef] [PubMed]
- Oe, Y.; Hayashi, S.; Fushima, T.; Sato, E.; Kisu, K.; Sato, H.; Ito, S.; Takahashi, N. Coagulation Factor Xa and Protease-Activated Receptor 2 as Novel Therapeutic Targets for Diabetic Nephropathy. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Hou, Q.; Cao, K.; Sun, X.; Liang, Y.; Gu, M.; Xue, X.; Zhao, A.Z.; Dai, C. Complement factor B in high glucose-induced podocyte injury and diabetic kidney disease. JCI Insight 2021, 6, e147716. [Google Scholar] [CrossRef] [PubMed]
- Asami, T.; Tomisawa, S.; Uchiyama, M. Effect of oral camostat mesilate on hematuria and/or proteinuria in children. Pediatr. Nephrol. 2004, 19, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Ito, H.; Hashimoto, K. Effect of camostat mesilate on urinary protein excretion in three patients with advanced diabetic nephropathy. J. Diabetes Complicat. 1999, 13, 56–58. [Google Scholar] [CrossRef]
- Matsubara, M.; Taguma, Y.; Kurosawa, K.; Hotta, O.; Suzuki, K.; Ishizaki, M. Effect of camostat mesilate for the treatment of advanced diabetic nephropathy. J. Lab. Clin. Med. 1990, 116, 206–210. [Google Scholar]
- Onbe, T.; Makino, H.; Kumagai, I.; Haramoto, T.; Murakami, K.; Ota, Z. Effect of proteinase inhibitor camostat mesilate on nephrotic syndrome with diabetic nephropathy. J. Diabet. Complicat. 1991, 5, 167–168. [Google Scholar] [CrossRef]
- Katunuma, N.; Le, Q.T.; Miyauchi, R.; Hirose, S. Double-layer fluorescent zymography for processing protease detection. Anal. Biochem. 2005, 347, 208–212. [Google Scholar] [CrossRef]





| Group | Control | HS | HS+CM |
|---|---|---|---|
| Relative organ weight | |||
| Heart Wt/BW (g) | 3.7 ± 0.2 | 5.3 ± 0.6 *** | 4.6 ± 0.3 *,‡ |
| Kidney Wt/BW (g) | 8.1 ± 0.5 | 11.8 ± 0.7 **** | 10.9 ± 0.8 ****,# |
| Blood test | |||
| TP (mg/dL) | 6.0 ± 0.7 | 6.0 ± 0.5 | 5.6 ± 0.7 |
| Cr (mg/dL) | 0.27 ± 0.04 | 0.40 ± 0.08 * | 0.30 ± 0.03 # |
| Na (mEq/L) | 143.0 ± 1.4 | 144.8 ± 1.5 † | 140.8 ± 1.9 ## |
| Cl (mEq/L) | 99.0 ± 1.4 | 100.7 ± 2.4 | 97.3 ± 2.0 # |
| K (mEq/L) | 4.4 ± 0.2 | 4.3 ± 0.4 | 4.2 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwata, Y.; Deng, Q.; Kakizoe, Y.; Nakagawa, T.; Miyasato, Y.; Nakagawa, M.; Nishiguchi, K.; Nagayoshi, Y.; Narita, Y.; Izumi, Y.; et al. A Serine Protease Inhibitor, Camostat Mesilate, Suppresses Urinary Plasmin Activity and Alleviates Hypertension and Podocyte Injury in Dahl Salt-Sensitive Rats. Int. J. Mol. Sci. 2023, 24, 15743. https://doi.org/10.3390/ijms242115743
Iwata Y, Deng Q, Kakizoe Y, Nakagawa T, Miyasato Y, Nakagawa M, Nishiguchi K, Nagayoshi Y, Narita Y, Izumi Y, et al. A Serine Protease Inhibitor, Camostat Mesilate, Suppresses Urinary Plasmin Activity and Alleviates Hypertension and Podocyte Injury in Dahl Salt-Sensitive Rats. International Journal of Molecular Sciences. 2023; 24(21):15743. https://doi.org/10.3390/ijms242115743
Chicago/Turabian StyleIwata, Yasunobu, Qinyuan Deng, Yutaka Kakizoe, Terumasa Nakagawa, Yoshikazu Miyasato, Miyuki Nakagawa, Kayo Nishiguchi, Yu Nagayoshi, Yuki Narita, Yuichiro Izumi, and et al. 2023. "A Serine Protease Inhibitor, Camostat Mesilate, Suppresses Urinary Plasmin Activity and Alleviates Hypertension and Podocyte Injury in Dahl Salt-Sensitive Rats" International Journal of Molecular Sciences 24, no. 21: 15743. https://doi.org/10.3390/ijms242115743
APA StyleIwata, Y., Deng, Q., Kakizoe, Y., Nakagawa, T., Miyasato, Y., Nakagawa, M., Nishiguchi, K., Nagayoshi, Y., Narita, Y., Izumi, Y., Kuwabara, T., Adachi, M., & Mukoyama, M. (2023). A Serine Protease Inhibitor, Camostat Mesilate, Suppresses Urinary Plasmin Activity and Alleviates Hypertension and Podocyte Injury in Dahl Salt-Sensitive Rats. International Journal of Molecular Sciences, 24(21), 15743. https://doi.org/10.3390/ijms242115743





