Unveiling the Potent Fibrino(geno)lytic, Anticoagulant, and Antithrombotic Effects of Papain, a Cysteine Protease from Carica papaya Latex Using κ-Carrageenan Rat Tail Thrombosis Model
Abstract
1. Introduction
2. Results
2.1. SDS-PAGE Profile of Papain
2.2. Effect of Temperature and pH on Protease Activity and Stability
2.3. Fibrinolytic Activity of Papain
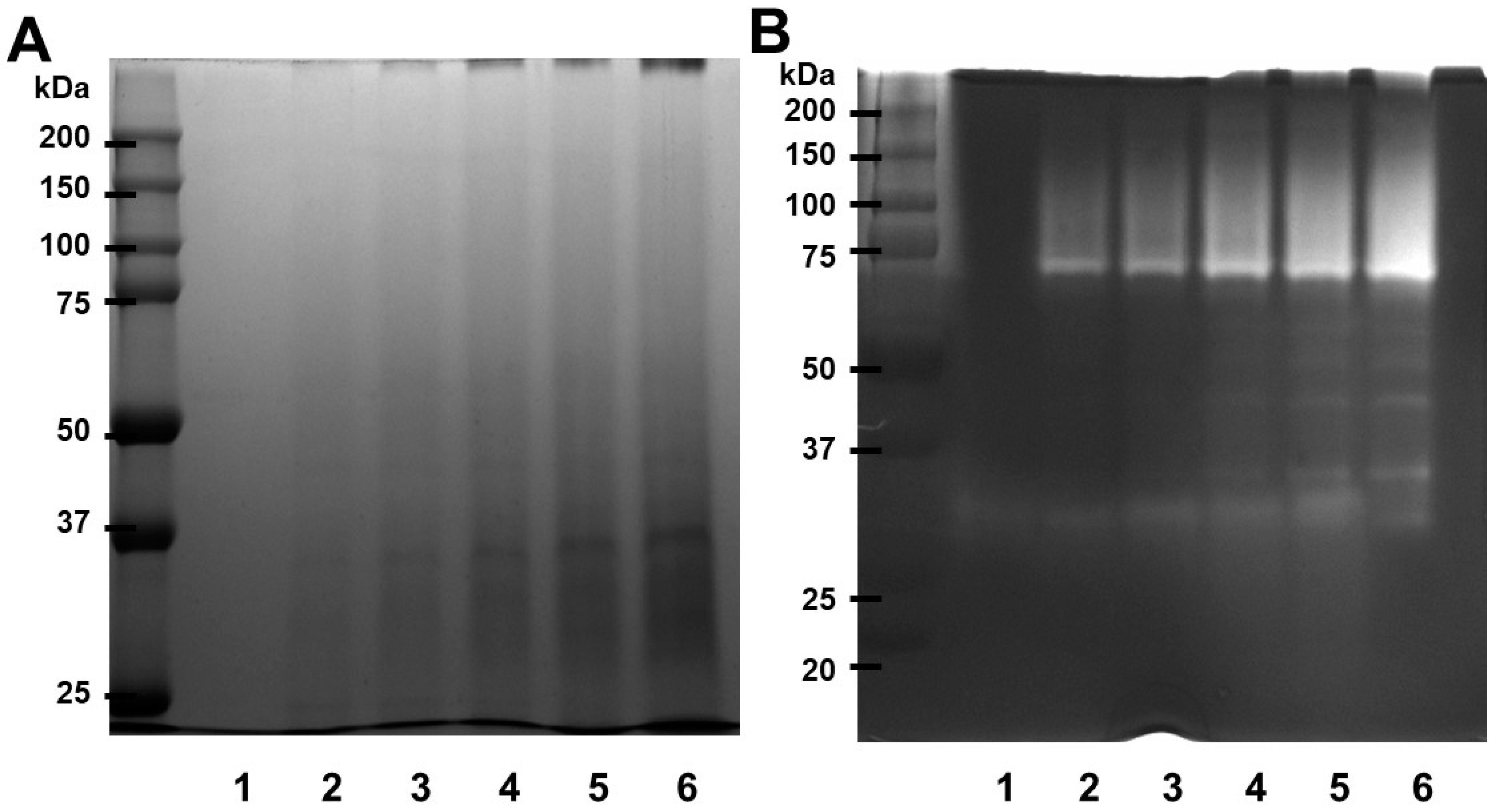
2.4. Fibrinogenolytic Activity of Papain
2.5. Blood Clot Lysis
2.6. Anti-Coagulation Effect of Papain
2.7. κ-Carrageenan-Induced Rat Tail Thrombosis Model
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.3. Proteolytic Activity Assay
4.4. Effect of Temperature and pH on Protease Activity and Stability
4.5. Fibrin Plate Assay
4.6. Examination of Fibrinolytic Activity Using Fibrin Zymography
4.7. Fibrinogenolytic Activity
4.8. Blood Clot Lysis Assay
4.9. PT/aPTT Measurement
4.10. Experimental Animals
4.11. κ-Carrageenan-Induced Rat Tail Thrombosis Model
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wadekar, A.B.; Nimbalwar, M.G.; Panchale, W.A.; Gudalwar, B.R.; Manwar, J.V.; Bakal, R.L.; Wadekar, A.B.; Nimbalwar, M.G.; Panchale, W.A.; Gudalwar, B.R.; et al. Morphology, Phytochemistry and Pharmacological Aspects of Carica Papaya, an Review. GSC Biol. Pharm. Sci. 2021, 14, 234–248. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumar, S.; Mathan, S.V.; Tomar, M.S.; Singh, R.K.; Verma, P.K.; Kumar, A.; Kumar, S.; Singh, R.P.; Acharya, A. Therapeutic Application of Carica Papaya Leaf Extract in the Management of Human Diseases. DARU J. Pharm. Sci. 2020, 28, 735. [Google Scholar] [CrossRef]
- Gracz-bernaciak, J.; Mazur, O.; Nawrot, R. Functional Studies of Plant Latex as a Rich Source of Bioactive Compounds: Focus on Proteins and Alkaloids. Int. J. Mol. Sci. 2021, 22, 12427. [Google Scholar] [CrossRef]
- Mótyán, J.A.; Tóth, F.; Tőzsér, J. Research Applications of Proteolytic Enzymes in Molecular Biology. Biomolecules 2013, 3, 923. [Google Scholar] [CrossRef]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial Proteases Applications. Front. Bioeng. Biotechnol. 2019, 7, 451237. [Google Scholar] [CrossRef]
- Ajlia, S.A.S.H.; Majid, F.A.A.; Suvik, A.; Effendy, M.A.W.; Serati Nouri, H. Efficacy of Papain-Based Wound Cleanser in Promoting Wound Regeneration. Pak. J. Biol. Sci. 2010, 13, 596–603. [Google Scholar] [CrossRef][Green Version]
- Chen, C.; Yang, F.Q.; Zhang, Q.; Wang, F.Q.; Hu, Y.J.; Xia, Z.N. Natural Products for Antithrombosis. Evid.-Based Complement. Altern. Med. 2015, 2015, 876426. [Google Scholar] [CrossRef]
- Subramani, B.; Sathiyarajeswaran, P. Current Update on Herbal Sources of Antithrombotic Activity—A Comprehensive Review. Egypt. J. Intern. Med. 2022, 34, 26. [Google Scholar] [CrossRef]
- Kubatka, P.; Mazurakova, A.; Koklesova, L.; Samec, M.; Sokol, J.; Samuel, S.M.; Kudela, E.; Biringer, K.; Bugos, O.; Pec, M.; et al. Antithrombotic and Antiplatelet Effects of Plant-Derived Compounds: A Great Utility Potential for Primary, Secondary, and Tertiary Care in the Framework of 3P Medicine. EPMA J. 2022, 13, 407–431. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Schaff, M.; Peter, K. Current and Future Antiplatelet Therapies: Emphasis on Preserving Haemostasis. Nat. Rev. Cardiol. 2018, 15, 181–191. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Khan, H.; Ahmad, I.; Lobine, D.; Mahomoodally, F.; Suroowan, S.; Hassan, S.T.S.; Xu, S.; Patel, S.; Daglia, M.; et al. Bioactive Peptides and Proteins as Alternative Antiplatelet Drugs. Med. Res. Rev. 2019, 39, 2153–2171. [Google Scholar] [CrossRef]
- Seyoum, M.; Enawgaw, B.; Melku, M. Human Blood Platelets and Viruses: Defense Mechanism and Role in the Removal of Viral Pathogens. Thromb. J. 2018, 16, 1–6. [Google Scholar] [CrossRef]
- Periayah, M.H.; Halim, A.S.; Saad, A.Z.M. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int. J. Hematol. Stem Cell Res. 2017, 11, 319. [Google Scholar]
- Dahlbäck, B. Blood Coagulation and Its Regulation by Anticoagulant Pathways: Genetic Pathogenesis of Bleeding and Thrombotic Diseases. J. Intern. Med. 2005, 257, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Binder, N.B.; Depasse, F.; Mueller, J.; Wissel, T.; Schwers, S.; Germer, M.; Hermes, B.; Turecek, P.L. Clinical Use of Thrombin Generation Assays. J. Thromb. Haemost. 2021, 19, 2918–2929. [Google Scholar] [CrossRef]
- Gallus, A.S.; Hirsh, J. Antithrombotic Drugs: Part I. Drugs 1976, 12, 41–68. [Google Scholar] [CrossRef]
- Phillips, D.R.; Conley, P.B.; Sinha, U.; Andre, P. Therapeutic Approaches in Arterial Thrombosis. J. Thromb. Haemost. 2005, 3, 1577–1589. [Google Scholar] [CrossRef]
- Renner, E.; Barnes, G.D. Antithrombotic Management of Venous Thromboembolism: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 76, 2142–2154. [Google Scholar] [CrossRef]
- Lin, L.; Zhao, L.; Gao, N.; Yin, R.; Li, S.; Sun, H.; Zhou, L.; Zhao, G.; Purcell, S.W.; Zhao, J. From Multi-Target Anticoagulants to DOACs, and Intrinsic Coagulation Factor Inhibitors. Blood Rev. 2020, 39, 100615. [Google Scholar] [CrossRef]
- Małek, Ł.A.; Bilińska, Z.T.; Sitkiewicz, D.; Kłopotowski, M.; Witkowski, A.; Ruzyłło, W. Platelet Reactivity on Aspirin, Clopidogrel and Abciximab in Patients with Acute Coronary Syndromes and Reduced Estimated Glomerular Filtration Rate. Thromb. Res. 2010, 125, 67–71. [Google Scholar] [CrossRef]
- Jneid, H.; Bhatt, D.L.; Corti, R.; Badimon, J.J.; Fuster, V.; Francis, G.S. Aspirin and Clopidogrel in Acute Coronary Syndromes: Therapeutic Insights From the CURE Study. Arch. Intern. Med. 2003, 163, 1145–1153. [Google Scholar] [CrossRef]
- Butcher, K.; Shuaib, A.; Saver, J.; Donnan, G.; Davis, S.M.; Norrving, B.; Wong, K.S.L.; Abd-Allah, F.; Bhatia, R.; Khan, A. Thrombolysis in the Developing World: Is There a Role for Streptokinase? Int. J. Stroke 2013, 8, 560–565. [Google Scholar] [CrossRef]
- Baig, M.U.; Bodle, J. Thrombolytic Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Nazari, J.; Davison, R.; Kaplan, K.; Fintel, D. Adverse Reactions to Thrombolytic Agents: Implications for Coronary Reperfusion Following Myocardial Infarction. Med. Toxicol. Advers. Drug Exp. 1987, 2, 274–286. [Google Scholar] [CrossRef]
- Yang, H.R.; Hwang, D.H.; Prakash, R.L.M.; Kim, J.H.; Hong, I.H.; Kim, S.; Kim, E.; Kang, C. Exploring the Fibrin(Ogen)Olytic, Anticoagulant, and Antithrombotic Activities of Natural Cysteine Protease (Ficin) with the κ-Carrageenan-Induced Rat Tail Thrombosis Model. Nutrients 2022, 14, 3552. [Google Scholar] [CrossRef] [PubMed]
- Nurhayati, T.; Nurjanah, N.; Astiana, I. Characteristics of Papain Soluble Collagen from Redbelly Yellowtail Fusilier (Caesio Cuning). IOP Conf. Ser. Earth Environ. Sci. 2018, 196, 012034. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Salvesen, G. Handbook of Proteolytic Enzymes. In Handbook of Proteolytic Enzymes; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1. [Google Scholar]
- Grzonka, Z.; Kasprzykowski, F.; Wiczk, W. Cysteine Proteases. In Industrial Enzymes; Springer: Berlin/Heidelberg, Germany, 2007; pp. 181–195. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.; Sachan, S. Enzymes Used in the Food Industry: Friends or Foes? In Enzymes in Food Biotechnology Production, Applications, and Future Prospects; Elsevier: Amsterdam, The Netherlands, 2018; pp. 827–843. [Google Scholar] [CrossRef]
- Musa, H.H.; Musa, T.H.; Oderinde, O.; Musa, I.H.; Shonekan, O.O.; Akintunde, T.Y.; Onasanya, A.K. Traditional Herbal Medicine: Overview of Research Indexed in the Scopus Database. Adv. Tradit. Med. 2022, 23, 1173–1183. [Google Scholar] [CrossRef]
- Kang, Y.M.; Kang, H.A.; Cominguez, D.C.; Kim, S.H.; An, H.J. Papain Ameliorates Lipid Accumulation and Inflammation in High-Fat Diet-Induced Obesity Mice and 3t3-L1 Adipocytes via Ampk Activation. Int. J. Mol. Sci. 2021, 22, 9885. [Google Scholar] [CrossRef] [PubMed]
- Amri, E.; Mamboya, F. Papain, a Plant Enzyme of Biological Importance: A Review. Am. J. Biochem. Biotechnol. 2012, 8, 99–104. [Google Scholar] [CrossRef]
- Yap, J.Y.; Hii, C.L.; Ong, S.P.; Lim, K.H.; Abas, F.; Pin, K.Y. Effects of Drying on Total Polyphenols Content and Antioxidant Properties of Carica Papaya Leaves. J. Sci. Food Agric. 2020, 100, 2932–2937. [Google Scholar] [CrossRef]
- Ayodipupo Babalola, B.; Ifeolu Akinwande, A.; Otunba, A.A.; Ebenezer Adebami, G.; Babalola, O.; Nwuofo, C. Therapeutic Benefits of Carica Papaya: A Review on Its Pharmacological Activities and Characterization of Papain. Arab. J. Chem. 2024, 17, 105369. [Google Scholar] [CrossRef]
- Eagle, H.; Harris, T.N. Studies in Blood Coagulation v. the Coagulation of Blood by Proteolytic Enzymes (Trypsin, Papain). J. Gen. Physiol. 1937, 20, 543–560. [Google Scholar] [CrossRef][Green Version]
- Lin, M.; Yu, L.; Xiao, L.; Fan, J. A Cysteine Enzyme Hemostat for Efficient Heparin-Tolerant Blood Coagulation. J. Mater. Chem. B 2023, 11, 1079–1089. [Google Scholar] [CrossRef]
- Fei, X.; Yuan, W.; Zhao, Y.; Wang, H.; Bai, S.; Huang, Q. Papain Ameliorates the MPAs Formation-Mediated Activation of Monocytes by Inhibiting Cox-2 Expression via Regulating the MAPKs and PI3K/Akt Signal Pathway. Biomed Res. Int. 2018, 2018, 3632084. [Google Scholar] [CrossRef]
- Kim, K.; Park, K.I. A Review of Antiplatelet Activity of Traditional Medicinal Herbs on Integrative Medicine Studies. Evid.-Based Complement. Altern. Med. 2019, 2019, 7125162. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, H.R.; Collen, D. Impaired Fibrinolysis and the Risk for Coronary Heart Disease. Circulation 1996, 94, 2052–2054. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Johnson, T.A.; Duru, N.; Buzza, M.S.; Pawar, N.R.; Sarkar, R.; Antalis, T.M. Fibrinolysis and Inflammation in Venous Thrombus Resolution. Front. Immunol. 2019, 10, 461233. [Google Scholar] [CrossRef]
- Laurens, N.; Koolwijk, P.; de Maat, M.P. Fibrin Structure and Wound Healing. J. Thromb. Haemost. 2006, 4, 932–939. [Google Scholar] [CrossRef]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the Control of Blood Coagulation. Blood Rev. 2015, 29, 17. [Google Scholar] [CrossRef]
- Berntorp, E.; Salvagno, G.L. Standardization and Clinical Utility of Thrombin-Generation Assays. Semin. Thromb. Hemost. 2008, 34, 670–682. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, N.; Li, Y.; Wu, J.; Zheng, J.; Li, X.; Zeng, M. Coagulation Dysfunction in Patients with Liver Cirrhosis and Splenomegaly and Its Countermeasures: A Retrospective Study of 1522 Patients. Dis. Markers 2023, 2023, 5560560. [Google Scholar] [CrossRef]
- Abdul Rahim, P.; Rengaswamy, D. Fibrinolytic Enzyme—An Overview. Curr. Pharm. Biotechnol. 2022, 23, 1336–1345. [Google Scholar] [CrossRef]
- Rahman, A.; Hasan, K.A.M.M.; Hanufa, M.U.; Rahman, A.; Hasan, K.A.M.M.; Hanufa, M.U. Study of Adverse Events of Streptokinase Therapy in Patients with Acute ST Elevation Myocardial Infarction. World J. Cardiovasc. Dis. 2020, 10, 500–508. [Google Scholar] [CrossRef]
- Myers, M.J.; Deaver, C.M.; Lewandowski, A.J. Molecular Mechanism of Action Responsible for Carrageenan-Induced Inflammatory Response. Mol. Immunol. 2019, 109, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Albrecht, S.; Märki, C. Proteolytic Processing of Chemokines: Implications in Physiological and Pathological Conditions. Int. J. Biochem. Cell Biol. 2008, 40, 1185–1198. [Google Scholar] [CrossRef]
- Repnik, U.; Starr, A.E.; Overall, C.M.; Turk, B. Cysteine Cathepsins Activate ELR Chemokines and Inactivate Non-ELR Chemokines. J. Biol. Chem. 2015, 290, 13800. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Segers, R.; Butt, T.M.; Kerry, B.R.; Peberdy, J.F. The Nematophagous Fungus Verticillium Chlamydosporium Produces a Chymoelastase-like Protease Which Hydrolyses Host Nematode Proteins in Situ. Microbiology 1994, 140, 2715–2723. [Google Scholar] [CrossRef]
- Aissaoui, N.; Marzouki, M.N.; Abidi, F. Purification and Biochemical Characterization of a Novel Intestinal Protease from Scorpaena Notata. Int. J. Food Prop. 2017, 20, 2151–2165. [Google Scholar] [CrossRef]
- Astrup, T.; Müllertz, S. The Fibrin Plate Method for Estimating Fibrinolytic Activity. Arch. Biochem. Biophys. 1952, 40, 346–351. [Google Scholar] [CrossRef]
- Matsubara, K.; Hori, K.; Matsuura, Y.; Miyazawa, K. Purification and Characterization of a Fibrinolytic Enzyme and Identification of Fibrinogen Clotting Enzyme in a Marine Green Alga, Codium Divaricatum. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 125, 137–143. [Google Scholar] [CrossRef]





| Papain | PT (s) | aPTT (s) |
|---|---|---|
| Control | 17.5 ± 1.8 | 93.9 ± 4.1 |
| 4 U/mL | 18.2 ± 1.6 | 100.4 ± 5.8 |
| 8 U/mL | 35< | 400< |
| Normal range | 14–19 | 75–105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.R.; Zahan, M.N.; Yoon, Y.; Kim, K.; Hwang, D.H.; Kim, W.H.; Rho, I.R.; Kim, E.; Kang, C. Unveiling the Potent Fibrino(geno)lytic, Anticoagulant, and Antithrombotic Effects of Papain, a Cysteine Protease from Carica papaya Latex Using κ-Carrageenan Rat Tail Thrombosis Model. Int. J. Mol. Sci. 2023, 24, 16770. https://doi.org/10.3390/ijms242316770
Yang HR, Zahan MN, Yoon Y, Kim K, Hwang DH, Kim WH, Rho IR, Kim E, Kang C. Unveiling the Potent Fibrino(geno)lytic, Anticoagulant, and Antithrombotic Effects of Papain, a Cysteine Protease from Carica papaya Latex Using κ-Carrageenan Rat Tail Thrombosis Model. International Journal of Molecular Sciences. 2023; 24(23):16770. https://doi.org/10.3390/ijms242316770
Chicago/Turabian StyleYang, Hye Ryeon, Most Nusrat Zahan, Yewon Yoon, Kyuri Kim, Du Hyeon Hwang, Woo Hyun Kim, Il Rae Rho, Euikyung Kim, and Changkeun Kang. 2023. "Unveiling the Potent Fibrino(geno)lytic, Anticoagulant, and Antithrombotic Effects of Papain, a Cysteine Protease from Carica papaya Latex Using κ-Carrageenan Rat Tail Thrombosis Model" International Journal of Molecular Sciences 24, no. 23: 16770. https://doi.org/10.3390/ijms242316770
APA StyleYang, H. R., Zahan, M. N., Yoon, Y., Kim, K., Hwang, D. H., Kim, W. H., Rho, I. R., Kim, E., & Kang, C. (2023). Unveiling the Potent Fibrino(geno)lytic, Anticoagulant, and Antithrombotic Effects of Papain, a Cysteine Protease from Carica papaya Latex Using κ-Carrageenan Rat Tail Thrombosis Model. International Journal of Molecular Sciences, 24(23), 16770. https://doi.org/10.3390/ijms242316770






