Dendritic Cell Subpopulations Are Associated with Prognostic Characteristics of Breast Cancer after Neoadjuvant Chemotherapy—An Observational Study
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemical Techniques
4.3. Histologic DC Scoring and Analysis
4.4. Statistical Elaboration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APCs | antigen presenting cells |
| BC | breast cancer |
| BDCA | blood dendritic cell antigens |
| cDCs | conventional dendritic cells |
| CMF | cyclophosphamide/methotrexate/5-fluorouracil |
| DCIS | ductal carcinoma in situ |
| DCs | dendritic cells |
| DC-LAMP | dendritic-cell-lysosome-associated membrane glycoprotein |
| DC-SIGN | dendritic-cell-specific intercellular-adhesion-molecule-3-grabbing non-integrin |
| ER | estrogen receptor |
| FISH | fluorescence in situ hybridization |
| Flt3L | FMS-like tyrosine kinase 3 ligand |
| HER2 | human epidermal growth factor receptor 2 |
| HIF-1α | hypoxia inducible factor-1α |
| ICOSL | inducible costimulatory ligand |
| IDO | indoleamine 2,3-dioxygenaze |
| IFN | interferon |
| MHC | major histocompatibility complex |
| NAC | neoadjuvant chemotherapy |
| NK cells | natural killer cells |
| pCR | pathological complete response |
| pDCs | plasmacytoid dendritic cells |
| PD-L1 | programmed death-ligand 1 |
| PR | progesterone receptor |
| PRRs | pattern recognition receptors |
| RCB | residual cancer burden |
| SLNs | sentinel lymph nodes |
| TGF-β | transforming growth factor-β |
| TLR | toll-like receptor |
| TNBC | triple-negative breast cancer |
| TNF-α | tumor necrosis factor-α |
| VEGF | vascular endothelial growth factor |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; Volume 2. [Google Scholar]
- Gralow, J.R.; Burstein, H.J.; Wood, W.; Hortobagyi, G.N.; Gianni, L.; von Minckwitz, G.; Buzdar, A.U.; Smith, I.E.; Symmans, W.F.; Singh, B.; et al. Preoperative therapy in invasive breast cancer: Pathologic assessment and systemic therapy issues in operable disease. J. Clin. Oncol. 2008, 26, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, M.; Hortobagyi, G.N.; Goldhirsch, A.; Scholl, S.; Makris, A.; Valagussa, P.; Blohmer, J.U.; Eiermann, W.; Jackesz, R.; Jonat, W.; et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: An update. J. Clin. Oncol. 2006, 24, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.F.; Hortobagyi, G.N. Proceedings of the consensus conference on neoadjuvant chemotherapy in carcinoma of the breast, April 26–28, 2003, Philadelphia, Pennsylvania. Cancer 2004, 100, 2512–2532. [Google Scholar] [CrossRef]
- Shannon, C.; Smith, I. Is there still a role for neoadjuvant therapy in breast cancer? Crit. Rev. Oncol. Hematol. 2003, 45, 77–90. [Google Scholar] [CrossRef]
- Mamtani, A.; Barrio, A.V.; King, T.A.; Van Zee, K.J.; Plitas, G.; Pilewskie, M.; El-Tamer, M.; Gemignani, M.L.; Heerdt, A.S.; Sclafani, L.M.; et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients with Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann. Surg. Oncol. 2016, 23, 3467–3474. [Google Scholar] [CrossRef]
- Woeste, M.R.; Bhutiani, N.; Donaldson, M.; McMasters, K.M.; Ajkay, N. Evaluating the effect of neoadjuvant chemotherapy on surgical outcomes after breast conserving surgery. J. Surg. Oncol. 2021, 123, 439–445. [Google Scholar] [CrossRef]
- Daniel, F.H. Targeting adjuvant chemotherapy: A good idea that needs to be proven! J. Clin. Oncol. 2012, 30, 1264–1267. [Google Scholar]
- Coates, A.S.; Colleoni, M.; Goldhirsch, A. Is adjuvant chemotherapy useful for women with luminal a breast cancer? J. Clin. Oncol. 2012, 30, 1260–1263. [Google Scholar] [CrossRef]
- Schott, A.F.; Hayes, D.F. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J. Clin. Oncol. 2012, 30, 1747–1749. [Google Scholar] [CrossRef]
- Buzdar, A.U.; Valero, V.; Theriault, R.L.; Frye, D.; Green, M.; Booser, D.; Guerra, L.; Sahin, A.; Ames, F.C.; Smith, T.; et al. Pathological complete response to chemotherapy is related to hormone receptor status. Breast Cancer Res. Treat. 2003, 88, 302. [Google Scholar]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; on behalf of the ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Panelists of the St Gallen Consensus Conference; et al. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef] [PubMed]
- Yau, C.; Osdoit, M.; van der Noordaa, M.; Shad, S.; Wei, J.; de Croze, D.; Hamy, A.S.; Laé, M.; Reyal, F.; Sonke, G.S.; et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: A multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022, 23, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Symmans, W.F.; Wei, C.; Gould, R.; Yu, X.; Zhang, Y.; Liu, M.; Walls, A.; Bousamra, A.; Ramineni, M.; Sinn, B.; et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated with Residual Cancer Burden and Breast Cancer Subtype. J. Clin. Oncol. 2017, 35, 1049–1060. [Google Scholar] [CrossRef]
- Galati, D.; Zanotta, S. Dendritic Cell and Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 4253. [Google Scholar] [CrossRef]
- Li, R.-J.E.; Hogervorst, T.P.; Achilli, S.; Bruijns, S.C.; Arnoldus, T.; Vivès, C.; Wong, C.C.; Thépaut, M.; Meeuwenoord, N.J.; van den Elst, H.; et al. Systematic Dual Targeting of Dendritic Cell C-Type Lectin Receptor DC-SIGN and TLR7 Using a Trifunctional Mannosylated Antigen. Front. Chem. 2019, 7, 650. [Google Scholar] [CrossRef]
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic cell subsets and locations. Int. Rev. Cell Mol. Biol. 2019, 348, 1–68. [Google Scholar]
- Ladányi, A.; Kiss, J.; Somlai, B.; Gilde, K.; Fejos, Z.; Mohos, A.; Gaudi, I.; Tímár, J. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol. Immunother. 2007, 56, 1459–1469. [Google Scholar] [CrossRef]
- Gelao, L.; Criscitiello, C.; Esposito, A.; De Laurentiis, M.; Fumagalli, L.; Locatelli, M.A.; Minchella, I.; Santangelo, M.; De Placido, S.; Goldhirsch, A.; et al. Dendritic cell-based vaccines: Clinical applications in breast cancer. Immunotherapy 2014, 6, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell. Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Reichert, T.E.; Scheuer, C.; Day, R.; Wagner, W.; Whiteside, T.L. The number of intratumoral dendritic cells and zeta-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer 2001, 91, 2136–2147. [Google Scholar] [CrossRef]
- Goldman, S.A.; Baker, E.; Weyant, R.J.; Clarke, M.R.; Myers, J.N.; Lotze, M.T. Peritumoral CD1a-positive dendritic cells are associated with improved survival in patients with tongue carcinoma. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 641–646. [Google Scholar] [CrossRef]
- La Rocca, G.; Anzalone, R.; Corrao, S.; Magno, F.; Rappa, F.; Marasà, S.; Czarnecka, A.M.; Marasà, L.; Sergi, C.; Zummo, G.; et al. CD1a down-regulation in primary invasive ductal breast carcinoma may predict regional lymph node invasion and patient outcome. Histopathology 2007, 52, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Hillenbrand, E.E.; Neville, A.M.; Coventry, B.J. Immunohistochemical localization of CD1a-positive putative dendritic cells in human breast tumours. Br. J. Cancer 1999, 79, 940–944. [Google Scholar] [CrossRef]
- Treilleux, I.; Blay, J.-Y.; Bendriss-Vermare, N.; Ray-Coquard, I.; Bachelot, T.; Guastalla, J.-P.; Bremond, A.; Goddard, S.; Pin, J.-J.; Barthelemy-Dubois, C.; et al. Dendritic Cell Infiltration and Prognosis of Early Stage Breast Cancer. Clin. Cancer Res. 2004, 10, 7466–7474. [Google Scholar] [CrossRef]
- Sisirak, V.; Faget, J.; Gobert, M.; Goutagny, N.; Vey, N.; Treilleux, I.; Renaudineau, S.; Poyet, G.; Labidi-Galy, I.; Goddard-Leon, S.; et al. Impaired IFN-α production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012, 72, 5188–5197. [Google Scholar] [CrossRef]
- Takagi, S.; Miyagawa, S.; Ichikawa, E.; Soeda, J.; Miwa, S.; Miyagawa, Y.; Iijima, S.; Noike, T.; Kobayashi, A.; Kawasaki, S. Dendritic cells, T-cell infiltration, and Grp94 expression in cholangiocellular carcinoma. Hum. Pathol. 2004, 35, 881–886. [Google Scholar] [CrossRef]
- Miyagawa, S.; Soeda, J.; Takagi, S.; Miwa, S.; Ichikawa, E.; Noike, T. Prognostic significance of mature dendritic cells and factors associated with their accumulation in metastatic liver tumors from colorectal cancer. Hum. Pathol. 2004, 35, 1392–1396. [Google Scholar] [CrossRef]
- Coventry, B.; Heinzel, S. CD1a in human cancers: A new role for an old molecule. Trends Immunol. 2004, 25, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Chomarat, P.; Broyles, D.; Netto, G.; Harb, G.M.; Lebecque, S.; Valladeau, J.; Davoust, J.; Palucka, K.A.; Banchereau, J. In Breast Carcinoma Tissue, Immature Dendritic Cells Reside within the Tumor, Whereas Mature Dendritic Cells Are Located in Peritumoral Areas. J. Exp. Med. 1999, 190, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Donkor, M.; Scholar, E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007, 26, 373–400. [Google Scholar] [CrossRef]
- Karthaus, N.; Torensma, R.; Tel, J. Deciphering the Message Broadcast by Tumor-Infiltrating Dendritic Cells. Am. J. Pathol. 2012, 181, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Szpor, J.; Streb, J.; Glajcar, A.; Streb-Smoleń, A.; Łazarczyk, A.; Korta, P.; Brzuszkiewicz, K.; Jach, R.; Hodorowicz-Zaniewska, D. Dendritic Cell Subpopulations Are Associated with Morphological Features of Breast Ductal Carcinoma In Situ. Int. J. Mol. Sci. 2023, 24, 9918. [Google Scholar] [CrossRef]
- Szpor, J.; Streb, J.; Glajcar, A.; Frączek, P.; Winiarska, A.; Tyrak, K.E.; Basta, P.; Okoń, K.; Jach, R.; Hodorowicz-Zaniewska, D. Dendritic Cells Are Associated with Prognosis and Survival in Breast Cancer. Diagnostics 2021, 11, 702. [Google Scholar] [CrossRef]
- Szpor, J.; Streb, J.; Glajcar, A.; Sadowski, P.; Streb-Smoleń, A.; Jach, R.; Hodorowicz-Zaniewska, D. Presence of Dendritic Cell Subsets in Sentinel Nodes of Breast Cancer Patients Is Related to Nodal Burden. Int. J. Mol. Sci. 2022, 23, 8461. [Google Scholar] [CrossRef]
- Hännikäinen, E.N.; Mattson, J.; Karihtala, P. Predictors of successful neoadjuvant treatment in HER2-positive breast cancer. Oncol. Lett. 2023, 26, 434. [Google Scholar] [CrossRef]
- Huang, M.; O’Shaughnessy, J.; Zhao, J.; Haiderali, A.; Cortes, J.; Ramsey, S.; Briggs, A.; Karantza, V.; Aktan, G.; Qi, C.Z.; et al. Evaluation of Pathologic Complete Response as a Surrogate for Long-Term Survival Outcomes in Triple-Negative Breast Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 1096–1104. [Google Scholar] [CrossRef]
- Joo, S.; Ko, E.S.; Kwon, S.; Jeon, E.; Jung, H.; Kim, J.Y.; Chung, M.J.; Im, Y.H. Multimodal deep learning models for the prediction of pathologic response to neoadjuvant chemotherapy in breast cancer. Sci. Rep. 2021, 11, 18800. [Google Scholar] [CrossRef]
- Davey, M.G.; Ryan, É.J.; Boland, M.R.; Barry, M.K.; Lowery, A.J.; Kerin, M.J. Clinical utility of the 21-gene assay in predicting response to neoadjuvant endocrine therapy in breast cancer: A systematic review and meta-analysis. Breast 2021, 58, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Bulut, G.; Atilgan, H.I.; Çınarer, G.; Kılıç, K.; Yıkar, D.; Parlar, T. Prediction of pathological complete response to neoadjuvant chemotherapy in locally advanced breast cancer by using a deep learning model with 18F-FDG PET/CT. PLoS ONE 2023, 18, e0290543. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, T.; Heidemann, L.N.; Möller, S.; Bille, C. Impact of neoadjuvant chemotherapy on surgical complications in breast cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2022, 48, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Sabitovic, A.; Trøstrup, H.; Damsgaard, T.E. The impact of neoadjuvant chemotherapy on surgical outcomes following autologous and implant-based immediate breast reconstruction: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthet. Surg. 2023, 87, 7–23. [Google Scholar] [CrossRef]
- Ahmed, S.H. Safety of neoadjuvant chemotherapy for the treatment of breast cancer. Expert Opin. Drug Saf. 2019, 18, 817–827. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, E.; Castelli, G.; Testa, U. CD123 a Therapeutic Target for Acute Myeloid Leukemia and Blastic Plasmocytoid Dendritic Neoplasm. Int. J. Mol. Sci. 2023, 24, 2718. [Google Scholar] [CrossRef]
- Broughton, S.E.; Dhagat, U.; Hercus, T.R.; Nero, T.L.; Grimbaldeston, M.A.; Bonder, C.S.; Lopez, A.F.; Parker, M.W. The GM-CSF/IL-3/IL-5 cytokine receptor family: From ligand recognition to initiation of signaling. Immunol. Rev. 2012, 250, 277–302. [Google Scholar] [CrossRef]
- Hercus, T.R.; Dhagat, U.; Kan, W.L.; Broughton, S.E.; Nero, T.L.; Perugini, M.; Sandow, J.J.; D’Andrea, R.J.; Ekert, P.G.; Hughes, T.; et al. Signalling by the βc family of cytokines. Cytokine Growth Factor Rev. 2013, 24, 189–201. [Google Scholar] [CrossRef]
- El Achi, H.; Dupont, E.; Paul, S.; Khoury, J.D. CD123 as a Biomarker in Hematolymphoid Malignancies: Principles of Detection and Targeted Therapies. Cancers 2020, 12, 3087. [Google Scholar] [CrossRef]
- Tandon, A.; Zhang, Y.; Sokol, L. Tagraxofusp, a novel CD123-directed cytotoxin to treat blastic plasmacytoid dendritic cell neoplasm. Drugs. Today 2019, 55, 735–742. [Google Scholar] [CrossRef]
- Khoury, J.D. Blastic Plasmacytoid Dendritic Cell Neoplasm. Curr. Hematol. Malig. Rep. 2018, 13, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Bardawil, T.; Khalil, S.; Kurban, M.; Abbas, O. Diagnostic utility of plasmacytoid dendritic cells in dermatopathology. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.; Chintala, S.; Dey, M. Plasmacytoid dendritic cell in immunity and cancer. J. Neuroimmunol. 2018, 322, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhou, L.; Mi, Q.S.; Jiang, A. Plasmacytoid Dendritic Cells and Cancer Immunotherapy. Cells 2022, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.D.; Baban, B.; Chandler, P.; Hou, D.Y.; Singh, N.; Yagita, H.; Azuma, M.; Blazar, B.R.; Mellor, A.L.; Munn, D.H. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Investig. 2007, 117, 2570–2582. [Google Scholar] [CrossRef]
- Koucký, V.; Bouček, J.; Fialová, A. Immunology of Plasmacytoid Dendritic Cells in Solid Tumors: A Brief Review. Cancers 2019, 11, 470. [Google Scholar] [CrossRef]
- Fu, C.; Peng, P.; Loschko, J.; Feng, L.; Pham, P.; Cui, W.; Lee, K.P.; Krug, A.B.; Jiang, A. Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes. Proc. Natl. Acad. Sci. USA 2020, 117, 23730–23741. [Google Scholar] [CrossRef]
- Demoulin, S.; Herfs, M.; Delvenne, P.; Hubert, P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: Insight into the molecular mechanisms. J. Leukoc. Biol. 2013, 93, 343–352. [Google Scholar] [CrossRef]
- Aspord, C.; Leccia, M.T.; Charles, J.; Plumas, J. Melanoma hijacks plasmacytoid dendritic cells to promote its own progression. Oncoimmunology 2014, 3, e27402. [Google Scholar] [CrossRef]
- Aspord, C.; Leccia, M.T.; Charles, J.; Plumas, J. Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL. Cancer Immunol. Res. 2013, 1, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Kiessler, M.; Plesca, I.; Sommer, U.; Wehner, R.; Wilczkowski, F.; Muller, L.; Tunger, A.; Lai, X.; Rentsch, A.; Peuker, K.; et al. Tumor-infiltrating plasmacytoid dendritic cells are associated with survival in human colon cancer. J. Immunother. Cancer 2021, 9, e001813. [Google Scholar] [CrossRef] [PubMed]
- Tel, J.; Aarntzen, E.H.; Baba, T.; Schreibelt, G.; Schulte, B.M.; Benitez-Ribas, D.; Boerman, O.C.; Croockewit, S.; Oyen, W.J.; van Rossum, M.; et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013, 73, 1063–1075. [Google Scholar] [CrossRef]
- Hernández, S.S.; Jakobsen, M.R.; Bak, R.O. Plasmacytoid Dendritic Cells as a Novel Cell-Based Cancer Immunotherapy. Int. J. Mol. Sci. 2022, 23, 11397. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.; Di Domizio, J.; Salameire, D.; Bendriss-Vermare, N.; Aspord, C.; Muhammad, R.; Lefebvre, C.; Plumas, J.; Leccia, M.T.; Chaperot, L. Characterization of circulating dendritic cells in melanoma: Role of CCR6 in plasmacytoid dendritic cell recruitment to the tumor. J. Investig. Dermatol. 2010, 130, 1646–1656. [Google Scholar] [CrossRef]
- Pang, L.; Ng, K.T.; Liu, J.; Yeung, W.O.; Zhu, J.; Chiu, T.S.; Liu, H.; Chen, Z.; Lo, C.M.; Man, K. Plasmacytoid dendritic cells recruited by HIF-1alpha/eADO/ADORA1 signaling induce immunosuppression in hepatocellular carcinoma. Cancer Lett. 2021, 522, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Sawant, A.; Hensel, J.A.; Chanda, D.; Harris, B.A.; Siegal, G.P.; Maheshwari, A.; Ponnazhagan, S. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J. Immunol. 2012, 189, 4258–4265. [Google Scholar] [CrossRef]
- Gadalla, R.; Hassan, H.; Ibrahim, S.A.; Abdullah, M.S.; Gaballah, A.; Greve, B.; El Deeb, S.; El-Shinawi, M.; Mohamed, M.M. Tumor Microenvironmental Plasmacytoid Dendritic Cells Contribute to Breast Cancer Lymph Node Metastasis via CXCR4/SDF-1 Axis. Breast Cancer Res. Treat. 2019, 174, 679–691. [Google Scholar] [CrossRef]
- Kini Bailur, J.; Gueckel, B.; Pawelec, G. Prognostic impact of high levels of circulating plasmacytoid dendritic cells in breast cancer. J. Transl. Med. 2016, 14, 151. [Google Scholar] [CrossRef]
- Wagner, F.; Hölig, U.; Wilczkowski, F.; Plesca, I.; Sommer, U.; Wehner, R.; Kießler, M.; Jarosch, A.; Flecke, K.; Arsova, M.; et al. Neoadjuvant Radiochemotherapy Significantly Alters the Phenotype of Plasmacytoid Dendritic Cells and 6-Sulfo LacNAc+ Monocytes in Rectal Cancer. Int. J. Cancer 2019, 10, 602. [Google Scholar] [CrossRef]
- Sisirak, V.; Vey, N.; Goutagny, N.; Renaudineau, S.; Malfroy, M.; Thys, S.; Treilleux, I.; Labidi-Galy, S.I.; Bachelot, T.; Dezutter-Dambuyant, C.; et al. Breast cancer-derived transforming growth factor-beta and tumor necrosis factor-alpha compromise interferon-alpha production by tumor-associated plasmacytoid dendritic cells. Int. J. Cancer 2013, 133, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Bembenek, A.; Li, J.; Loddenkemper, C.; Kemmner, W.; Stein, H.; Wernecke, K.D.; Schlag, P.M. Presence of mature DC-Lamp+ dendritic cells in sentinel and non-sentinel lymph nodes of breast cancer patients. Eur. J. Surg. Oncol. 2008, 34, 514–518. [Google Scholar] [CrossRef] [PubMed]
- El Deeb, N.M.F.; Mehanna, R.A. Assessment of Maturation Status of Tumor-Infiltrating Dendritic Cells in Invasive Ductal Carcinoma of the Breast: Relation with Vascular Endothelial Growth Factor Expression. Turk. J. Pathol. 2013, 29, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Gianni, L. Her2-Positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.B.; Im, Y.H.; Im, S.A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. APHINITY Steering Committee and Investigators. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Griguolo, G.; Pascual, T.; Dieci, M.V.; Guarneri, V.; Prat, A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. J. Immunother. Cancer 2019, 7, 90. [Google Scholar] [CrossRef]
- Tóth, G.; Szöőr, Á.; Simon, L.; Yarden, Y.; Szöllősi, J.; Vereb, G. The combination of trastuzumab and pertuzumab administered at approved doses may delay development of trastuzumab resistance by additively enhancing antibody-dependent cell-mediated cytotoxicity. MAbs 2016, 8, 1361–1370. [Google Scholar] [CrossRef]
- Kono, K.; Sato, E.; Naganuma, H.; Takahashi, A.; Mimura, K.; Nukui, H.; Fujii, H. Trastuzumab (Herceptin) enhances class I-restricted antigen presentation recognized by HER-2/neu-specific T cytotoxic lymphocytes. Clin. Cancer Res. 2004, 10, 2538–2544. [Google Scholar] [CrossRef]
- Gall, V.A.; Philips, A.V.; Qiao, N.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Ma, Q.; Reddy, S.M.; et al. Trastuzumab increases HER2 uptake and cross-presentation by dendritic cells. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef]
- Honkanen, T.J.; Moilanen, T.; Karihtala, P.; Tiainen, S.; Auvinen, P.; Väyrynen, J.P.; Mäkinen, M.; Koivunen, J.P. Prognostic and predictive role of spatially positioned tumour infiltrating lymphocytes in metastatic HER2 positive breast cancer treated with trastuzumab. Sci. Rep. 2017, 7, 18027. [Google Scholar] [CrossRef] [PubMed]
- Varadan, V.; Gilmore, H.; Miskimen, K.L.; Tuck, D.; Parsai, S.; Awadallah, A.; Krop, I.E.; Winer, E.P.; Bossuyt, V.; Somlo, G.; et al. Immune signatures following single dose trastuzumab predict pathologic response to preoperative trastuzumab and chemotherapy in HER2-positive early breast cancer. Clin. Cancer Res. 2016, 22, 3249–3259. [Google Scholar] [CrossRef] [PubMed]
- Esteva, F.J.; Hubbard-Lucey, V.M.; Tang, J.; Pusztai, L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 2019, 20, e175–e186. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Rosemblit, C.; Berk, E.; Showalter, L.; Namjoshi, P.; Mick, R.; Lee, K.P.; Brod, A.M.; Yang, R.L.; Kelz, R.R.; et al. Progressive loss of anti-HER2 CD4+ T-helper type 1 response in breast tumorigenesis and the potential for immune restoration. Oncoimmunology 2015, 4, e1022301. [Google Scholar] [CrossRef]
- Datta, J.; Berk, E.; Xu, S.; Fitzpatrick, E.; Rosemblit, C.; Lowenfeld, L.; Goodman, N.; Lewis, D.A.; Zhang, P.J.; Fisher, C.; et al. Anti-HER2 CD4(+) T-helper type 1 response is a novel immune correlate to pathologic response following neoadjuvant therapy in HER2-positive breast cancer. Breast Cancer Res. 2015, 17, 71. [Google Scholar] [CrossRef]
- Kodumudi, K.N.; Ramamoorthi, G.; Snyder, C.; Basu, A.; Jia, Y.; Awshah, S.; Beyer, A.P.; Wiener, D.; Lam, L.; Zhang, H.; et al. Sequential anti-PD1 therapy following dendritic cell vaccination improves survival in a HER2 mammary carcinoma model and identifies a critical role for CD4 T cells in mediating the response. Front. Immunol. 2019, 10, 1939. [Google Scholar] [CrossRef]
- Kolstad, A.; Kumari, S.; Walczak, M.; Madsbu, U.; Hagtvedt, T.; Bogsrud, T.V.; Kvalheim, G.; Holte, H.; Aurlien, E.; Delabie, J.; et al. Sequential intranodal immunotherapy induces antitumor immunity and correlated regression of disseminated follicular lymphoma. Blood 2015, 125, 82–89. [Google Scholar] [CrossRef]
- Cox, M.C.; Castiello, L.; Mattei, M.; Santodonato, L.; D’Agostino, G.; Muraro, E.; Martorelli, D.; Lapenta, C.; Di Napoli, A.; Di Landro, F.; et al. Clinical and antitumor immune responses in relapsed/refractory follicular lymphoma patients after intranodal injections of IFNα-Dendritic cells and rituximab: A phase I clinical trial. Clin. Cancer Res. 2019, 25, 5231–5241. [Google Scholar] [CrossRef]
- Ramamoorthi, G.; Kodumudi, K.; Snyder, C.; Grover, P.; Zhang, H.; Greene, M.I.; Basu, A.; Gallen, C.; Wiener, D.; Costa, R.L.B.; et al. Intratumoral delivery of dendritic cells plus anti-HER2 therapy triggers both robust systemic antitumor immunity and complete regression in HER2 mammary carcinoma. J. Immunother. Cancer 2022, 10, e004841. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Residual Cancer Burden Calculaor. Available online: https://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3&fbclid=IwAR0rYfvtKBhhAiR5lHIkQ4uylVKSuUmxWUS_M_HFdVToaSLaj2kj1uZv-gI (accessed on 1 September 2023).
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Colleoni, M.; Buyse, M.; Hortobagyi, G.; Gianni, L.; Winer, E.; Loibl, S.; et al. Surrogacy of Pathologic Complete Response in Trials of Neoadjuvant Therapy for Early Breast Cancer. JAMA. Oncol. 2022, 8, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
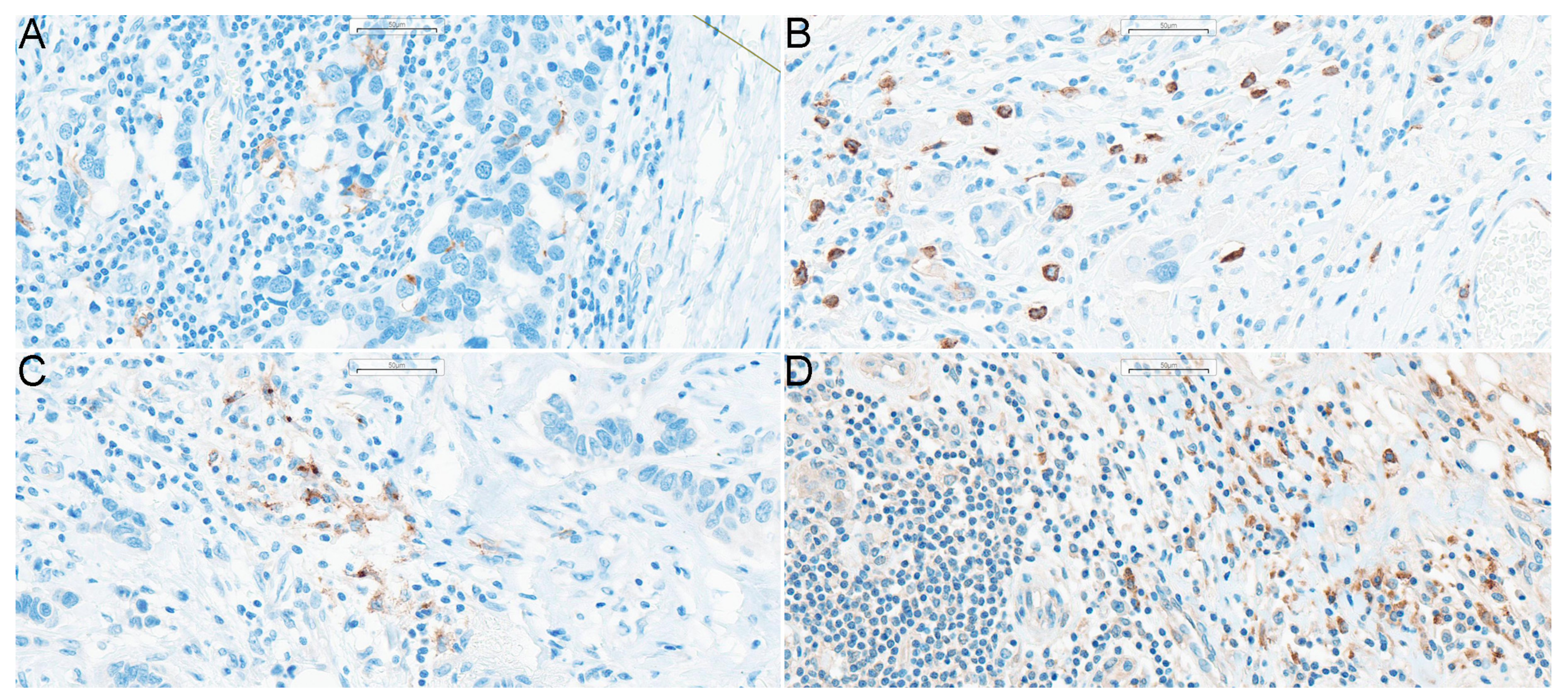

| Characteristic | Value | |
|---|---|---|
| Clinical Data | ||
| Age at diagnosis (years) | 51.5 (28–85) | |
| Menopausal status | premenopausal | 54 (49%) |
| postmenopausal | 57 (51%) | |
| Time from diagnosis to surgical treatment (months) | 6 (3–9) | |
| Surgery type | ||
| BCT | 60 (53%) | |
| Mastectomy | 54 (47%) | |
| Lymph nodes surgery | ||
| SLNB | 64 (56%) | |
| ALND | 50 (44%) | |
| cT stage | ||
| cT1 | 9 (8%) | |
| cT2 | 61 (54%) | |
| cT3 | 28 (25%) | |
| cT4 | 15 (13%) | |
| cN stage | ||
| cN0 | 40 (35%) | |
| cN1 | 61 (54%) | |
| cN2 | 4 (4%) | |
| cN3 | 8 (7%) | |
| NAC cycles (number) | 8 (4–18) | |
| Chemotherapeutics used in NAC | ||
| Anthracyclines | 105 (92%) | |
| Taxanes | 107 (94%) | |
| Platinum (IV) derivatives | 33 (29%) | |
| Cyclophosphamide | 101 (87%) | |
| Immune therapy | ||
| Trastuzumab | 21 (18%) | |
| Trastuzumab + pertuzumab | 17 (15%) | |
| Histological data | ||
| TIL (%) | 5 (1–60) | |
| Histological type | ||
| NST | 95 (83%) | |
| ILC | 6 (5%) | |
| other | 13 (12%) | |
| Molecular subtype | ||
| luminal A | 4 (4%) | |
| luminal B HER2- | 42 (37%) | |
| luminal B HER2+ | 26 (23%) | |
| non-luminal HER2+ | 15 (13%) | |
| TNBC | 27 (24%) | |
| Ki-67 (percent in core-needle biopsy) | 49.0 ± 23.0 | |
| RCB score | 1.7 (0–5.2) | |
| RCB class | ||
| 0 | 40 (35%) | |
| I | 11 (10%) | |
| II | 37 (32%) | |
| III | 26 (23%) | |
| Pathological response | ||
| pCR | 40 (35%) | |
| pPR | 62 (54%) | |
| pNR | 12 (11%) | |
| Nuclear grade (before NAC) | ||
| G1 | 7 (6%) | |
| G2 | 51 (45%) | |
| G3 | 56 (49%) | |
| Nuclear grade (after NAC) | ||
| G0 | 40 (39%) | |
| G1 | 9 (9%) | |
| G2 | 39 (39%) | |
| G3 | 13 (13%) | |
| HER2 status | ||
| (+) | 42 (37%) | |
| (−) | 71 (63%) | |
| ER (%) | 50 (0–100) | |
| PR (%) | 1 (0–98) | |
| ypT stage | ||
| ypT0 | 34 (30%) | |
| DCIS | 8 (7%) | |
| ypT 1 | 43 (37%) | |
| ypT 2 | 22 (19%) | |
| ypT 3 | 4 (4%) | |
| ypT 4 | 3 (2%) | |
| ypN stage | ||
| ypN0 | 70 (61%) | |
| ypN1mi | 2 (2%) | |
| ypN1 | 19 (17%) | |
| ypN2 | 16 (14%) | |
| ypN3 | 7 (6%) | |
| Vascular invasion (post-NAC), n (%) | 45 (39%) | |
| Characteristic | CD123+ | CD1a+ | DC-LAMP+ | DC-SIGN+ |
|---|---|---|---|---|
| Age (years) | −0.07 0.4/- | −0.22 0.015/0.09 | 0.02 0.9/- | 0.03 0.8/- |
| Number of chemotherapy cycles | −0.14 0.1/- | −0.04 0.7/- | 0.08 0.4/- | −0.09 0.3/- |
| TIL (%) | 0.17 0.08/- | −0.08 0.4/- | 0.28 0.003/0.021 | 0.14 0.1/- |
| Ki-67 before chemotherapy | −0.02 0.9/- | 0.23 0.016/0.09 | 0.24 0.01/0.04 | −0.03 0.8/- |
| Estrogen receptor expression | 0.14 0.1/- | −0.08 0.4/- | −0.24 0.01/0.04 | 0.02 0.8/- |
| Progesterone receptor expression | 0.07 0.5/- | 0.00 1.0/- | −0.22 0.02/0.04 | 0.00 1.0/- |
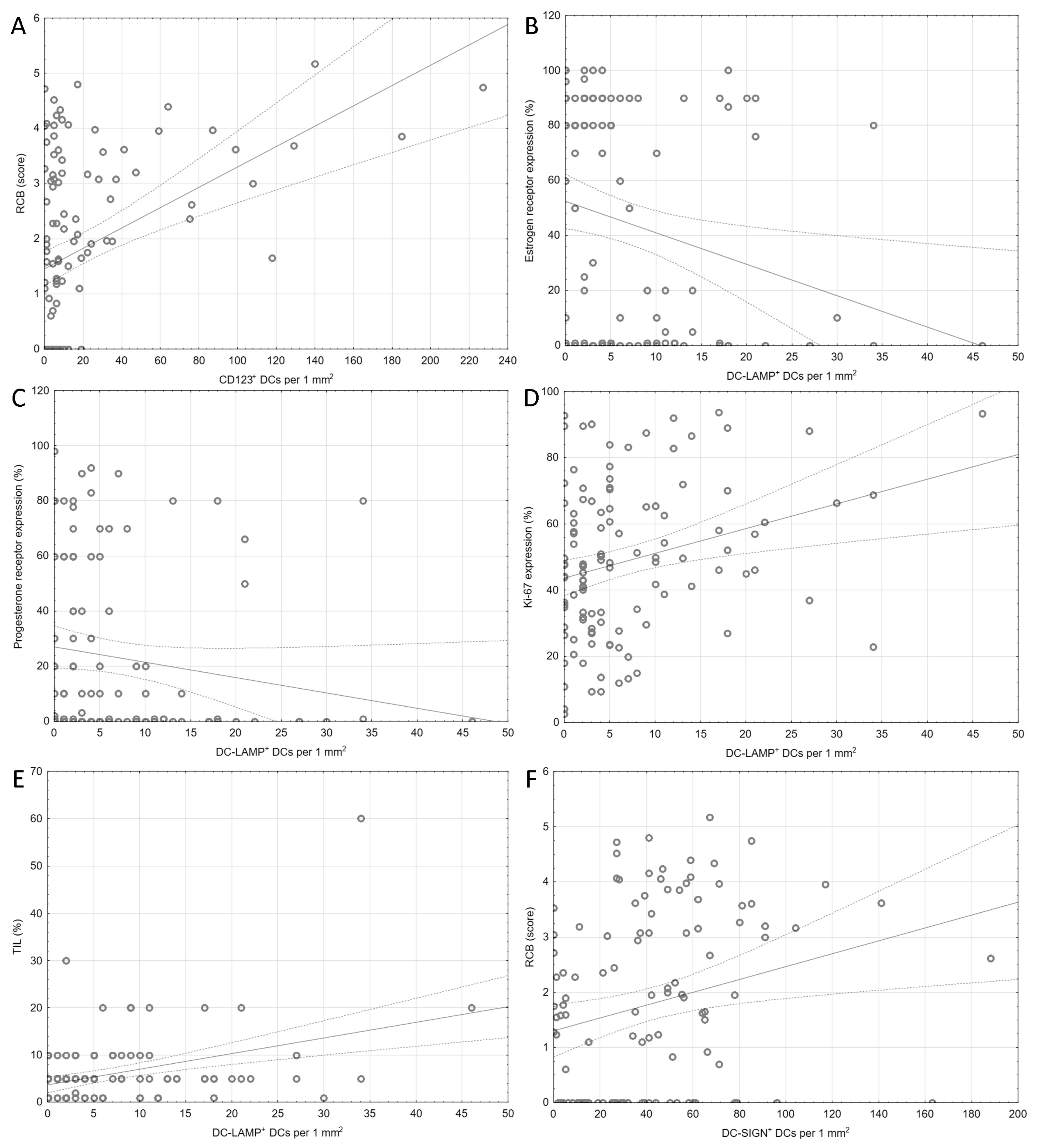
| RCB | 0.38 <0.001/<0.001 | −0.03 0.8/- | −0.10 0.3/- | 0.28 0.003/0.021 |
| Characteristic | CD123+ | p/pBH-Value | CD1a+ | p/pBH-Value | DC-LAMP+ | p/pBH-Value | DC-SIGN+ | p/pBH-Value | |
|---|---|---|---|---|---|---|---|---|---|
| HER2-negative HER2-positive | 8 (0–227) 5 (0–140) | 0.004/0.022 | 6 (0–42) 5 (0–33) | 0.3/- | 4 (0–34) 4 (0–46) | 0.8/- | 41 (0–188) 33 (0–163) | 0.3/- | |
| ypT0-is ypT1–4 | 5 (0–19) 9 (0–227) | 0.0002/0.0022 | 5 (0–33) 5.5 (0–42) | 0.6/- | 5.5 (0–46) 3 (0–34) | 0.003/0.03 | 28 (0–163) 43.5 (0–188) | 0.04/- | |
| ypN0 ypN1–3 | 5 (0–118) 9 (0–227) | 0.008/0.03 | 5 (0–34) 5 (0–42) | 0.8/- | 4.5 (0–46) 3 (0–34) | 1.0/- | 29.5 (0–188) 48 (0–141) | 0.009/0.1 | |
| vascular invasion | no yes | 5 (0–227) 9 (0–185) | 0.014/0.04 | 6 (0–42) 5 (0–23) | 0.5/- | 5 (0–46) 3 (0–34) | 0.2/- | 29.5 (0–188) 46 (0–141) | 0.08/- |
| Characteristic | CD123+ | p/pBH-Value | CD1a+ | p/pBH-Value | DC-LAMP+ | p/pBH-Value | DC-SIGN+ | p/pBH-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Histological type | NST lobular others | 6 (0–185) 5.5 (0–17) 16 (1–227) | 0.06/- | 5 (0–42) 5 (1–10) 8 (0–16) | 0.3/- | 4 (0–46) 1.5 (0–8) 3 (0–27) | 0.2/- | 38 (0–141) 34 (0–80) 49 (0–188) | 0.7/- |
| Molecular subtype | luminal A luminal B HER2- luminal B HER2+ non-luminal HER2+ TNBC | 3 (0–6) 10 (0–185) 5 (0–140) * 3 (0–87) * 6 (0–227) | 0.024/0.03 | 1.5 (0–5) 7 (0–23) 4.5 (0–16) 5 (0–33) 5.5 (0–42) | 0.2/- | 2 (0–7) 2.5 (0–34) 4 (0–18) 4 (0–46) 5 (0–34) | 0.2/- | 46.5 (28–59) 41.5 (0–188) 32.5 (0–163) 32 (4–91) 29 (1–117) | 0.7/- |
| anty-HER2 treatment | no treatment trastuzumab trastuzumab + pertuzumab | 9 (0–227) *,# 3 (0–22) * 5 (0–47) # | 0.0001/0.0005 | 5.5 (0–42) 5 (0–16) 5 (0–33) | 1.0/- | 5 (0–34) 2 (0–10) 6 (0–46) | 0.04/0.08 | 42 (0–188) 15 (0–71) 29 (6–163) | 0.008/0.08 |
| grading after chemotherapy | 0 1 2 3 | 5 (0–19) * 15 (0–26) 6 (0–227) # 59 (6–185) *,# | <0.0001/<0.001 | 5 (0–33) 5 (0–18) 5 (0–42) 7 (1–23) | 0.8/- | 5 (0–46) * 2 (0–8) # 2 (0–34) *,† 11 (2–22) #,† | 0.0004/0.002 | 29 (2–163) 28 (0–104) 41 (0–141) 57 (0–188) | 0.039/0.1 |
| cT | 1 2 3 4 | 3 (0–108) 7 (0–227) 6 (0–118) 12 (0–140) | 0.2/- | 6 (0–42) 5 (0–33) 7 (0–14) 5 (0–34) | 0.9/- | 4 (1–34) 4 (0–46) 3 (0–21) 5 (0–30) | 0.3/- | 39 (0–91) 37.5 (0–163) 34 (0–141) 49 (1–188) | 0.5/- |
| cN | 0 1 2 3 | 4 (0–118) * 8 (0–227) * 0.5 (0–59) 5 (1–140) | 0.0174/0.023 | 5 (0–34) 6 (0–42) 2 (1–3) 3.5 (0–15) | 0.3/- | 4 (0–46) 4 (0–34) 2 (0–22) 12.5 (1–30) | 0.2/- | 27 (0–188) 41 (0–163) 69.5 (34–117) 51 (13–81) | 0.07/- |
| ypT | 0 is 1 2 3 4 | 5 (0–12) * 3.5 (0–19) 7 (0–118) 15.5 (0–227) * 12.5 (5–76) 6 (5–140) | 0.011/0.018 | 5 (0–33) 7.5 (2–23) 6 (0–42) 4 (0–23) 10.5 (3–11) 8 (0–14) | 0.4/- | 5 (0–46) 5.5 (2–17) 2 (0–34) 4.5 (0–34) 3 (0–11) 7 (3–14) | 0.012/0.03 | 27.5 (2–96) 34 (0–163) 41 (0–141) 45.5 (0–117) 55 (27–188) 49 (47–67) | 0.2/- |
| ypN | 0 mi 1 2 3 | 5 (0–118) * 17 (6–28) 34 (1–227) * 6.5 (0–87) 5 (0–140) | 0.0023/0.005 | 5 (0–34) 15.5 (8–23) 7 (0–42) 3.5 (0–14) 5 (1–15) | 0.1/- | 4.5 (0–46) 14.5 (12–17) 4 (0–34) 2.5 (0–18) 1 (0–14) | 0.2/- | 29.5 (0–188) 54 (51–57) 41 (0–141) 53 (11–85) 41 (0–80) | 0.8/- |
| pathological response | pCR pPR pNR | 5 (0–19) *,# 9 (0–227) * 8.5 (0–140) # | 0.0011/0.003 | 5 (0–33) 6 (0–42) 5 (0–11) | 0.4/- | 5.5 (0–46) * 2.5 (0–34) * 7.5 (0–34) | 0.007/0.02 | 28 (2–163) 41.5 (0–188) 53 (0–141) | 0.1/- |
| RCB class | 0 1 2 3 | 5 (0–19) *,# 6 (0–18) 10 (0–118) * 10.5 (0–227) # | 0.0003/0.001 | 5 (0–33) 6 (1–23) 7 (0–42) 4 (0–23) | 0.2/- | 5.5 (0–46) *,# 1 (0–17) *,† 2 (0–34) # 6.5 (0–34) † | 0.0003/0.003 | 28 (2–163) 38 (0–71) 41 (0–188) 51.5 (0–141) | 0.028/0.1 |
| Characteristic | OR (95%CI) | p-Value | |
|---|---|---|---|
| Histological type | NST other | 2.08 (0.34–12.65) reference | 0.4 |
| Molecular subtype | TNBC other | 9.45 (0.95–94.27) reference | 0.056 |
| Grading before chemotherapy | 2.99 (0.94–9.49) | 0.063 | |
| Ki-67 before chemotherapy | 1.01 (0.98–1.04) | 0.5 | |
| TIL (%) | 1.11 (0.96–1.30) | 0.2 | |
| HER2 status | (+) (−) | 3.30 (0.67–16.21) reference | 0.1 |
| menopausal status | (+) (−) | 0.11 (0.02–0.53) reference | 0.006 |
| Estrogen receptor expression | 0.99 (0.97–1.02) | 0.5 | |
| Progesterone receptor expression | 0.98 (0.95–1.02) | 0.4 | |
| Number of chemotherapy cycles | 0.91 (0.75–1.10) | 0.3 | |
| CD123+ cells/mm2 | 0.87 (0.79–0.96) | 0.006 | |
| Antibody | Clone | Dilution | Antigen Retrieval | Incubation Time | Manufacturer | Detection System |
|---|---|---|---|---|---|---|
| CD1a | polyclonal | 8:100 | Citrate | 36 min | Novocastra | ultraView Universal DAB Detection Kit (Roche Ventana) |
| CD123 | polyclonal | 1:100 | EDTA | 60 min | Novocastra | OptiView DAB IHC Detection Kit (Roche Ventana) |
| DC-LAMP | polyclonal | 2:100 | ULTRA CC1 (Roche Ventana) | 72 min | NovusBio | ultraView Universal DAB Detection Kit (Roche Ventana) |
| DC-SIGN | 5D7 | 2:100 | ULTRA CC1 (Roche Ventana) | 72 min | Abcam | ultraView Universal DAB Detection Kit (Roche Ventana) |
| ER | SP1 | RTU | EDTA | 16 min | Roche | ultraView Universal DAB Detection Kit (Roche Ventana) |
| PR | 1E2 | RTU | EDTA | 16 min | Roche | ultraView Universal DAB Detection Kit (Roche Ventana) |
| Ki67 | MIB-1 | RTU | EDTA | 20 min | Dako, USA | ultraView Universal DAB Detection Kit (Roche Ventana) |
| HER2/neu | 4B5 | RTU | Citrate | 16 min | Roche | ultraView Universal DAB Detection Kit (Roche Ventana) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łazarczyk, A.; Streb, J.; Glajcar, A.; Streb-Smoleń, A.; Hałubiec, P.; Wcisło, K.; Laskowicz, Ł.; Hodorowicz-Zaniewska, D.; Szpor, J. Dendritic Cell Subpopulations Are Associated with Prognostic Characteristics of Breast Cancer after Neoadjuvant Chemotherapy—An Observational Study. Int. J. Mol. Sci. 2023, 24, 15817. https://doi.org/10.3390/ijms242115817
Łazarczyk A, Streb J, Glajcar A, Streb-Smoleń A, Hałubiec P, Wcisło K, Laskowicz Ł, Hodorowicz-Zaniewska D, Szpor J. Dendritic Cell Subpopulations Are Associated with Prognostic Characteristics of Breast Cancer after Neoadjuvant Chemotherapy—An Observational Study. International Journal of Molecular Sciences. 2023; 24(21):15817. https://doi.org/10.3390/ijms242115817
Chicago/Turabian StyleŁazarczyk, Agnieszka, Joanna Streb, Anna Glajcar, Anna Streb-Smoleń, Przemysław Hałubiec, Kacper Wcisło, Łukasz Laskowicz, Diana Hodorowicz-Zaniewska, and Joanna Szpor. 2023. "Dendritic Cell Subpopulations Are Associated with Prognostic Characteristics of Breast Cancer after Neoadjuvant Chemotherapy—An Observational Study" International Journal of Molecular Sciences 24, no. 21: 15817. https://doi.org/10.3390/ijms242115817





