Effects and Mechanisms of Non-Thermal Plasma-Mediated ROS and Its Applications in Animal Husbandry and Biomedicine
Abstract
:1. Introduction
2. Effects of ROS on Animal Health
2.1. Mechanism of ROS Generation and Removal
2.2. ROS Homeostasis and Animal Health
2.3. Excessive ROS and Animal Disease
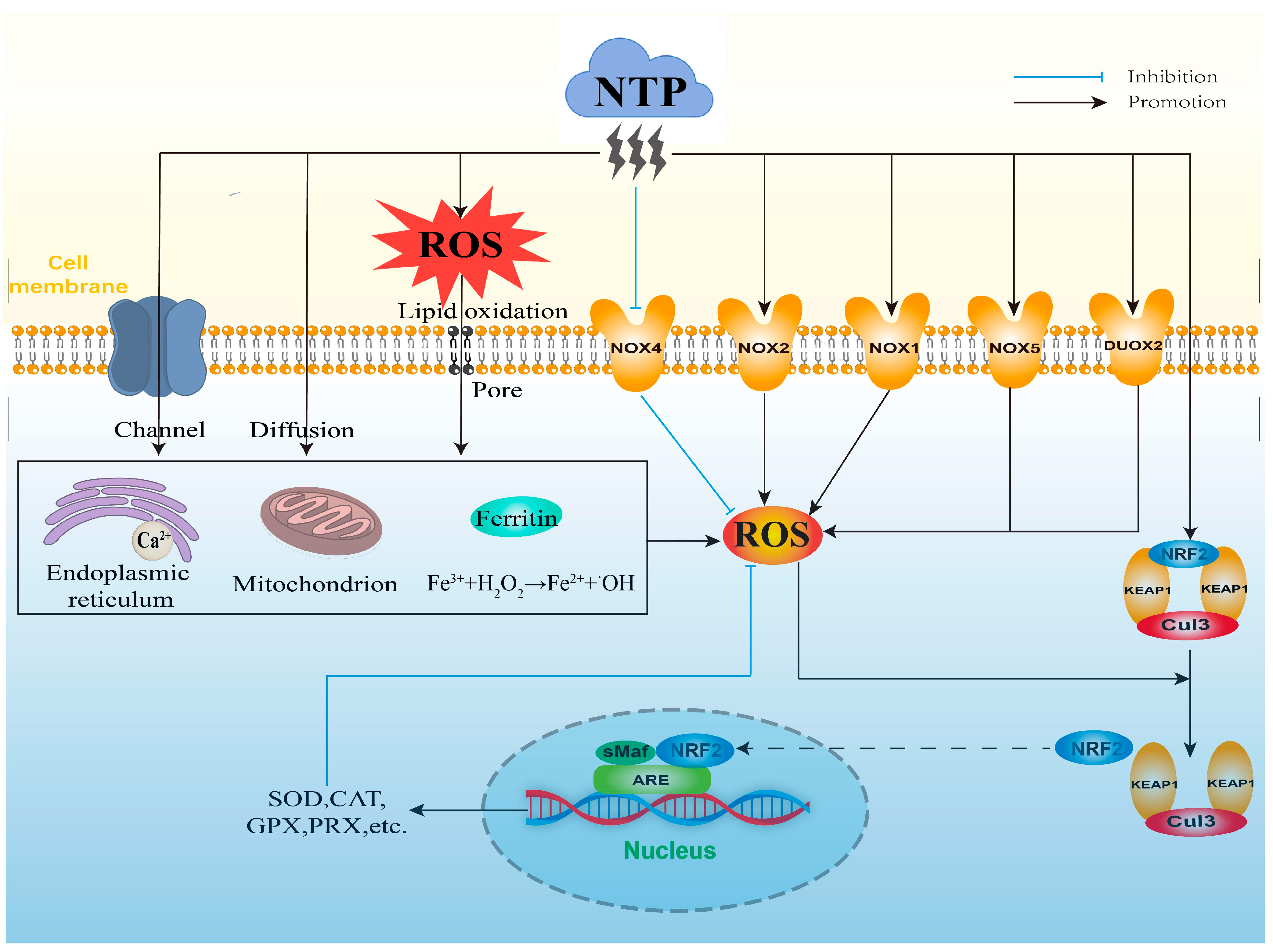
3. Mechanisms of NTP-Mediated ROS
3.1. Direct Generation of Exogenous ROS by NTP
3.2. Stimulatory Generation of Intracellular ROS by NTP
3.3. Indirect Regulation of Intracellular ROS by NTP
4. Applications of NTP-Mediated ROS
4.1. Application of NTP in Animal Husbandry
4.1.1. NTP Improves the Growth and Breeding Ability of Animals
4.1.2. NTP Improves the Animal Health
4.1.3. NTP Ensures the Safety of Animal-Derived Foods
| Bacterium | Treated Sample | Plasma Generating Source | Treatment Parameters | Inhibition Rate | |||
|---|---|---|---|---|---|---|---|
| Frequency | Voltage | Time | Gas | ||||
| Staphylococcus aureus | Suspension on Petri dish [72] | DBD | 27 kHz | 900 V | 3 min | air | 99% |
| Biofilm in plates containing TSB medium [89] | DBD | 22 kHz | 7 kV | 4 min | air | 70% | |
| Sample solution in Petri dish (combination with ultrasound) [90] | DBD | 10 kHz | 14 kV | NTP 5 minUS 20 min | air | 100% | |
| Pseudomonas aeruginosa | Suspension on Luria–Bertani agar plates [91] | plasma jet | * | 10 kV | 10 min | air | more than 88% |
| Suspension on Mueller–Hinton agar [92] | plasma jet | 20 kHz | 6 kV | 2 min | helium: oxygen (99.5%: 0.5%) | more than 75% | |
| Suspension on Luria–Bertani agar plate [93] | plasma jet | 20 kHz | 14.7 kV | 60 s | air | more than 40% | |
| Escherichia coli | Suspension in a glass container [94] | DBD | 25 kHz | 8.5 kV | 15 s | air | 28% |
| Suspension in filtered PBS [95] | DBD microfluidic plasma reactor | 17 kHz | 10 kV | 5.3 s | air | 100% | |
| Exists in vacuum packaging of pork butt [86] | DBD | 30 kHz | 3 kV | 10 min | nitrogen: oxygen (99.9%: 0.1%) | 43% | |
| Suspension into sterile milk in a sterile Petri dish [84] | corona discharge | 50 kHz | 9 kV | 3 min | air | 54% | |
| Salmonella | Suspension in sterile saline solution [96] | DBD | 2.5 kHz | 3 kV | 5 min | air | more than 70% |
| Inoculated on shell eggs [85] | plasma jet | 16 kHz | 3 kV | 90 s | air | more than 60% | |
| Exists in vacuum packaging of pork butt [86] | DBD | 30 kHz | 3 kV | 10 min | nitrogen: oxygen (99.9%: 0.1%) | 49% | |
4.2. Application of NTP in Biomedicine
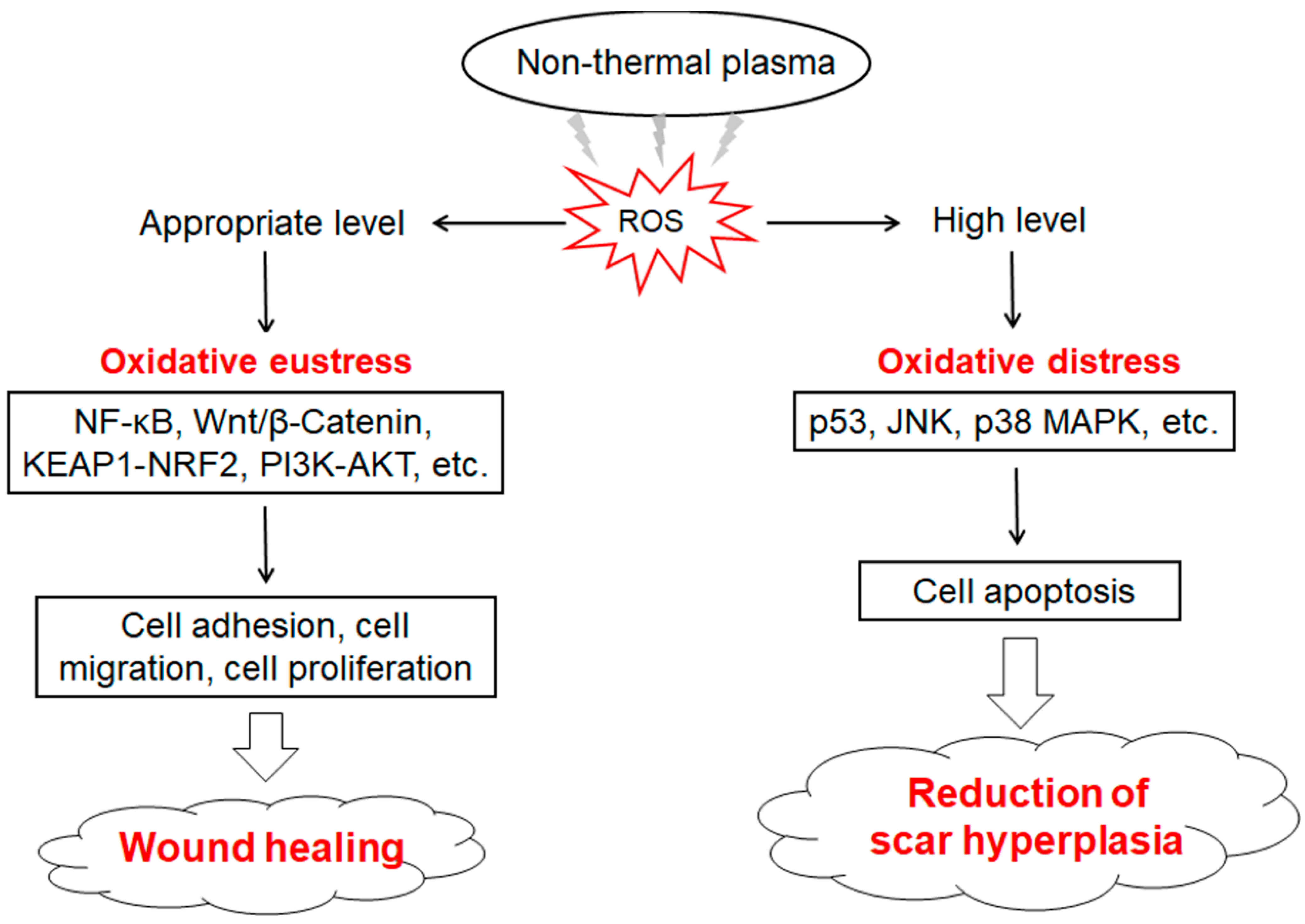
4.2.1. NTP Promotes Wound Healing
4.2.2. NTP Used in Oral Treatment
4.2.3. NTP Contributes to Cancer Therapy
Application of NTP Alone in Cancer Therapy
Application of NTP Combination in Cancer Therapy
| Cancer Category | Method | Cell Lines or Models | Result |
|---|---|---|---|
| Lung cancer | In vitro | CALU-1, SPC-A1 | Inhibit cancer cell proliferation and migration, induce cancer cell apoptosis and necrosis [123] |
| Glioblastoma | In vitro In vivo | U-87 MG, LN-229, T98G SB28 (subcutaneous injection into female C57BL/6J mice) | Inhibit the growth and proliferation of glioblastoma cells, induce cancer cell apoptosis, decrease tumor volume, increase the survival rates of GBM-bearing mice [136] |
| Pancreatic cancer | In vitro | Aspc1 | Inhibit cancer cell metastasis and proliferation, induce cancer cell apoptosis and autophagy [124] |
| Thyroid cancer | In vitro In vivo | BCPAP, HTh7, KTC2, 8505C, FRO-Luc FRO-Luc (subcutaneous injection into BALB/c-nude mice) | Cause mitochondrial dysfunction in cancer cells, induce cancer cell death [125] |
| Human colon cancer | In vitro | HCT116 | Increase DNA damage, cause cancer cell death [126] |
| Anaplastic squamous cell carcinoma | In vitro | VX2 | Decrease cell viability in a time-dependent manner, result in cell death [127] |
| Ovarian carcinoma | In vitro In vivo | ES2, SKOV3, OV90, OVCAR3, CAOV3 ES2 (intraperitoneal injection into female BALB/C mice) | Inhibit cancer cell growth and viability, induce cancer cell apoptosis, inhibit the intraperitoneal metastasis of cancer cells, improve the survival rates of cancer mouse [128] |
| Melanoma cancer | In vitro In vivo | B16F10 B16F10 (subcutaneous injection into C57BL/6 mice) | Cause cancer cell death, induce immunogenic cancer cell death, reduce tumor mass, increase leukocyte tumor infiltrates in vivo [129] |
| Breast cancer | In vivo | MCF7, AMJ13, AMN3, HBL AMN3 (intraperitoneal injection into female albino Swiss mice) | Reduce tumor volume, inhibit tumor growth, improve mice weight [130] |
4.2.4. NTP Improves the Performance of Biomedical Materials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arjunan, K.P.; Sharma, V.K.; Ptasinska, S. Effects of atmospheric pressure plasmas on isolated and cellular DNA—A Review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef] [PubMed]
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Pipliya, S.; Kumar, S.; Babar, N.; Srivastav, P.P. Recent trends in non-thermal plasma and plasma activated water: Effect on quality attributes, mechanism of interaction and potential application in food & agriculture. Food Chem. Adv. 2023, 2, 100249. [Google Scholar]
- Mohseni, P.; Ghorbani, A.; Fariborzi, N. Exploring the potential of cold plasma therapy in treating bacterial infections in veterinary medicine: Opportunities and challenges. Front. Vet. Sci. 2023, 10, 1240596. [Google Scholar] [CrossRef] [PubMed]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold atmospheric plasma: A powerful tool for modern medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef]
- Liu, P.; Wang, G.; Ruan, Q.; Tang, K.; Chu, P.K. Plasma-activated interfaces for biomedical engineering. Bioact. Mater. 2021, 6, 2134–2143. [Google Scholar] [CrossRef]
- Mumtaz, S.; Khan, R.; Rana, J.N.; Javed, R.; Iqbal, M.; Choi, E.H.; Han, I. Review on the biomedical and environmental applications of nonthermal plasma. Catalysts 2023, 13, 685. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Lushchak, O. Interplay between reactive oxygen and nitrogen species in living organisms. Chem.-Biol. Interact. 2021, 349, 109680. [Google Scholar] [CrossRef]
- Chen, Z.; Simonyan, H.; Cheng, X.; Gjika, E.; Lin, L.; Canady, J.; Sherman, J.H.; Young, C.; Keidar, M. A novel micro cold atmospheric plasma device for glioblastoma both in vitro and in vivo. Cancers 2017, 9, 61. [Google Scholar] [CrossRef]
- Szili, E.J.; Hong, S.-H.; Oh, J.-S.; Gaur, N.; Short, R.D. Tracking the penetration of plasma reactive species in tissue models. Trends Biotechnol. 2018, 36, 594–602. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wang, X.Z.; Kwon, T.; Huynh, D.L.; Chandimali, N.; Kim, N.; Kang, T.Y.; Ghosh, M.; Gera, M.; Lee, S.B.; et al. Innovative approach of non-thermal plasma application for improving the growth rate in chickens. Int. J. Mol. Sci. 2018, 19, 2301. [Google Scholar] [CrossRef] [PubMed]
- Zhunussova, A.; Vitol, E.A.; Polyak, B.; Tuleukhanov, S.; Brooks, A.D.; Sensenig, R.; Friedman, G.; Orynbayeva, Z. Mitochondria-mediated anticancer effects of non-thermal atmospheric plasma. PLoS ONE 2016, 11, e0156818. [Google Scholar] [CrossRef]
- Furuta, T.; Shi, L.; Toyokuni, S. Non-thermal plasma as a simple ferroptosis inducer in cancer cells: A possible role of ferritin. Pathol. Int. 2018, 68, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.-O.; Lee, M.-H.; Kim, G.-H.; Kim, E.-H. Quantitation of the ROS production in plasma and radiation treatments of biotargets. Sci. Rep. 2019, 9, 19837. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.-S. Targeting cancer cells with reactive oxygen and nitrogen species generated by atmospheric-pressure air plasma. PLoS ONE 2014, 9, e86173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Chandimali, N.; Kim, N.; Kang, T.Y.; Kim, S.B.; Kim, J.S.; Wang, X.Z.; Kwon, T.; Jeong, D.K. Demethylation and microRNA differential expression regulate plasma-induced improvement of chicken sperm quality. Sci. Rep. 2019, 9, 8865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Huynh, D.L.; Chandimali, N.; Kang, T.Y.; Kim, N.; Mok, Y.S.; Kwon, T.; Jeong, D.K. Growth and male reproduction improvement of non-thermal dielectric barrier discharge plasma treatment on chickens. J. Phys. D Appl. Phys. 2018, 51, 205201. [Google Scholar] [CrossRef]
- Schmidt, A.; Dietrich, S.; Steuer, A.; Weltmann, K.-D.; von Woedtke, T.; Masur, K.; Wende, K. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J. Biol. Chem. 2015, 290, 6731–6750. [Google Scholar] [CrossRef]
- Ishaq, M.; Evans, M.D.M.; Ostrikov, K. Atmospheric pressure gas plasma-induced colorectal cancer cell death is mediated by Nox2–ASK1 apoptosis pathways and oxidative stress is mitigated by Srx–Nrf2 anti-oxidant system. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 2827–2837. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Eom, S.; Yoon, S.Y.; Ryu, S.; Kim, S.B.; Yi, J.M.; Hyun, J.W. Non-thermal dielectric-barrier discharge plasma induces reactive oxygen species by epigenetically modifying the expression of NADPH oxidase family genes in keratinocytes. Redox Biol. 2020, 37, 101698. [Google Scholar] [CrossRef]
- Mitra, S.; Nguyen, L.N.; Akter, M.; Park, G.; Choi, E.H.; Kaushik, N.K. Impact of ROS generated by chemical, physical, and plasma techniques on cancer attenuation. Cancers 2019, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free radicals as a double-edged sword: The cancer preventive and therapeutic roles of curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, V.; Vaňková, E.; Kašparová, P.; Premanath, R.; Karunasagar, I.; Julák, J. Non-thermal plasma treatment of ESKAPE pathogens: A Review. Front. Microbiol. 2021, 12, 737635. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Parab, S.; Alexander, A.; Agrawal, M.; Achalla, V.P.K.; Pal, U.N.; Pandey, M.M.; Kesharwani, P. Cold atmospheric plasma therapy in wound healing. Process Biochem. 2022, 112, 112–123. [Google Scholar] [CrossRef]
- Xie, N.; Liu, F.; Li, Z.; Tang, C.; Yun, N.; Ji, X.; Wang, D. Application of atmospheric-pressure low-temperature plasma in stomatology: A review. Chin. J. Med. Phys. 2021, 38, 245–249. [Google Scholar]
- Lin, L.; Wang, L.; Liu, Y.; Xu, C.; Tu, Y.; Zhou, J. Non-thermal plasma inhibits tumor growth and proliferation and enhances the sensitivity to radiation in vitro and in vivo. Oncol. Rep. 2018, 40, 3405–3415. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Linh, N.N.; Ghimire, B.; Pengkit, A.; Sornsakdanuphap, J.; Lee, S.-J.; Choi, E.H. Plasma and nanomaterials: Fabrication and biomedical applications. Nanomaterials 2019, 9, 98. [Google Scholar] [CrossRef]
- Warne, G.R.; Williams, P.M.; Pho, H.Q.; Tran, N.N.; Hessel, V.; Fisk, I.D. Impact of cold plasma on the biomolecules and organoleptic properties of foods: A review. J. Food Sci. 2021, 86, 3762–3777. [Google Scholar] [CrossRef]
- Ahmadi, M.; Nasri, Z.; von Woedtke, T.; Wende, K. d-Glucose oxidation by cold atmospheric plasma-induced reactive species. ACS Omega 2022, 7, 31983–31998. [Google Scholar] [CrossRef]
- Dharini, M.; Jaspin, S.; Mahendran, R. Cold plasma reactive species: Generation, properties, and interaction with food biomolecules. Food Chem. 2023, 405, 134746. [Google Scholar] [CrossRef]
- Ekezie, F.C.; Sun, D.W.; Cheng, J.H. Altering the IgE binding capacity of king prawn (Litopenaeus Vannamei) tropomyosin through conformational changes induced by cold argon-plasma jet. Food Chem. 2019, 300, 125143. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Wang, Z.; Yan, W.; Zhuang, H.; Zhang, J. Impact of dielectric barrier discharge cold plasma on the lipid oxidation, color stability, and protein structures of myoglobin-added washed pork muscle. Front. Nutr. 2023, 10, 1137457. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, H.B.; Annapure, U. Consequences of non-thermal cold plasma treatment on meat and dairy lipids—A review. Future Foods 2021, 4, 100095. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 system: A mediator between oxidative stress and aging. Oxid. Med. Cell Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Lenaz, G. Molecular and supramolecular structure of the mitochondrial oxidative phosphorylation system: Implications for pathology. Life 2021, 11, 242. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Orr, A.L.; Perevoshchikova, I.V.; Treberg, J.R.; Ackrell, B.A.; Brand, M.D. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 2012, 287, 27255–27264. [Google Scholar] [CrossRef]
- de Almeida, A.; de Oliveira, J.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic concepts, sources, cellular signaling, and its implications in aging pathways. Oxid. Med. Cell Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Magnani, F.; Mattevi, A. Structure and mechanisms of ROS generation by NADPH oxidases. Curr. Opin. Struct. Biol. 2019, 59, 91–97. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, Y.; Fang, S.; Kim, W.; Kim, H.J.; Kim, J.W. GPx7 ameliorates non-alcoholic steatohepatitis by regulating oxidative stress. BMB Rep. 2020, 53, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Glasauer, A.; Hoover, P.; Yang, S.; Blatt, H.; Mullen, A.R.; Getsios, S.; Gottardi, C.J.; Deberardinis, R.J.; Lavker, R.M. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Cell. Logist. 2013, 6, ra8. [Google Scholar] [CrossRef]
- Dong, S.H.; Liang, S.; Cheng, Z.Q.; Zhang, X.; Luo, L.; Li, L.; Zhang, W.J.; Li, S.H.; Xu, Q.; Zhong, M.W.; et al. ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 41, 15. [Google Scholar] [CrossRef]
- Mungai, P.T.; Waypa, G.B.; Jairaman, A.; Prakriya, M.; Dokic, D.; Ball, M.K.; Schumacker, P.T. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol. Cell. Biol. 2011, 31, 3531–3545. [Google Scholar] [CrossRef]
- Kotsafti, A.; Scarpa, M.; Castagliuolo, I.; Scarpa, M. Reactive oxygen species and antitumor immunity-from surveillance to evasion. Cancers 2020, 12, 1748. [Google Scholar] [CrossRef]
- Yarosz, E.L.; Chang, C.H. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw. 2018, 18, e14. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, M. The role of reactive oxygen species in immune response. Chin. J. Immunol. 2015, 31, 855–858. [Google Scholar]
- Black, H.S. A Synopsis of the associations of oxidative stress, ROS, and antioxidants with diabetes mellitus. Antioxidants 2022, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Chlopicki, S. Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: Evidence for redox-based therapies. Free Radic. Biol. Med. 2020, 157, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Cross-talk between NADPH oxidase and mitochondria: Role in ROS signaling and angiogenesis. Cells 2020, 9, 1849. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Ja Kim, S.; Min Joh, H.; Chung, T.H. Production of intracellular reactive oxygen species and change of cell viability induced by atmospheric pressure plasma in normal and cancer cells. Appl. Phys. Lett. 2013, 103, 153705. [Google Scholar] [CrossRef]
- Shen, J.; Cheng, C.; Xu, Z.; Lan, Y.; Ni, G.; Sui, S. Principles and characteristics of cold plasma at gas phase and gas-liquid phase. Appl. Cold Plasma Food Saf. 2021. [Google Scholar] [CrossRef]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer 2012, 130, 2185–2194. [Google Scholar] [CrossRef]
- Adhikari, B.C.; Lamichhane, P.; Lim, J.S.; Nguyen, L.N.; Choi, E.H. Generation of reactive species by naturally sucked air in the Ar plasma jet. Results Phys. 2021, 30, 104863. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl. Env. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef]
- Bekeschus, S.; Kolata, J.; Winterbourn, C.; Kramer, A.; Turner, R.; Weltmann, K.D.; Bröker, B.; Masur, K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free Radic. Res. 2014, 48, 542–549. [Google Scholar] [CrossRef]
- Schröder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 Is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Do, H.L.; Chandimali, N.; Lee, S.B.; Mok, Y.S.; Kim, N.; Kim, S.B.; Kwon, T.; Jeong, D.K. Non-thermal plasma treatment improves chicken sperm motility via the regulation of demethylation levels. Sci. Rep. 2018, 8, 7576. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cheng, L.; Wu, H.; He, P.; Zhang, Y.; Yang, Y.; Chen, J.; Chen, M. Activation of the KEAP1-NRF2-ARE signaling pathway reduces oxidative stress in Hep2 cells. Mol. Med. Rep. 2018, 18, 2541–2550. [Google Scholar] [CrossRef]
- Uruno, A.; Motohashi, H. The Keap1–Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide 2011, 25, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.W.; Liu, J.; Kong, X.Z.; Zhang, S.G.; Wang, X.H.; Yu, M.; Zhan, Y.Q.; Li, W.; Xu, W.X.; Tang, L.J.; et al. Induction of activation of the antioxidant response element and stabilization of Nrf2 by 3-(3-pyridylmethylidene)-2-indolinone (PMID) confers protection against oxidative stress-induced cell death. Toxicol. Appl. Pharmacol. 2012, 259, 227–235. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and oxidative stress: A general overview of mechanisms and implications in human disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. The Keap1-Nrf2-antioxidant response element pathway: A review of its regulation by melatonin and the proteasome. Mol. Cell. Endocrinol. 2015, 401, 213–220. [Google Scholar] [CrossRef]
- Khor, T.O.; Fuentes, F.; Shu, L.; Paredes-Gonzalez, X.; Kong, A.N.T. Epigenetic DNA methylation of anti-oxidative stress regulator NRF2 in human prostate cancer. Cancer Prev. Res. 2014, 7, 1186–1197. [Google Scholar] [CrossRef]
- Zhang, J.J.; Jo, J.O.; Huynh, D.L.; Ghosh, M.; Kim, N.; Lee, S.B.; Lee, H.K.; Mok, Y.S.; Kwon, T.; Jeong, D.K. Lethality of inappropriate plasma exposure on chicken embryonic development. Oncotarget 2017, 8, 85642–85654. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Effects of reactive oxygen species on sperm function. Theriogenology 2012, 78, 1700–1708. [Google Scholar] [CrossRef]
- Hernández-Torres, C.J.; Reyes-Acosta, Y.K.; Chávez-González, M.L.; Dávila-Medina, M.D.; Kumar Verma, D.; Martínez-Hernández, J.L.; Narro-Céspedes, R.I.; Aguilar, C.N. Recent trends and technological development in plasma as an emerging and promising technology for food biosystems. Saudi J. Biol. Sci. 2022, 29, 1957–1980. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, A.G.; Okuyama, T.; Kristof, J.; Blajan, M.G.; Shimizu, K. Direct and indirect bactericidal effects of cold atmospheric-pressure microplasma and plasma jet. Molecules 2021, 26, 2523. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Dielectric barrier discharge cold atmospheric plasma: Bacterial inactivation mechanism. J. Food Saf. 2019, 39, e12705. [Google Scholar] [CrossRef]
- Huang, M.; Zhuang, H.; Zhao, J.; Wang, J.; Yan, W.; Zhang, J. Differences in cellular damage induced by dielectric barrier discharge plasma between Salmonella Typhimurium and Staphylococcus aureus. Bioelectrochemistry 2020, 132, 107445. [Google Scholar] [CrossRef]
- Czapka, T.; Maliszewska, I.; Olesiak-Bańska, J. Influence of atmospheric pressure non-thermal plasma on inactivation of biofilm cells. Plasma Chem. Plasma Process. 2018, 38, 1181–1197. [Google Scholar] [CrossRef]
- Asilevi, P.J.; Boakye, P.; Oduro-Kwarteng, S.; Fei-Baffoe, B.; Sokama-Neuyam, Y.A. Indoor air quality improvement and purification by atmospheric pressure Non-Thermal Plasma (NTP). Sci. Rep. 2021, 11, 22830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.J.; Li, K.; Lin, Q.; Ning, P.; Tang, L.H.; Wang, F.; Yuan, Q. Research progress of removing atmospheric pollutants by non-thermal plasma technology. Mater. Rev. 2015, 29, 137–142. [Google Scholar]
- Li, X.Y. Research progress of plasma technology in wastewater treatment. Mod. Salt Chem. Ind. 2020, 47, 3–4. [Google Scholar]
- Li, W.S.; Zhou, R.W.; Zhou, R.S.; Weerasinghe, J.; Zhang, T.Q.; Gissibl, A.; Cullen, P.J.; Speight, R.; Ostrikov, K. Insights into amoxicillin degradation in water by non-thermal plasmas. Chemosphere 2022, 291, 132757. [Google Scholar] [CrossRef]
- Misra, N.N.; Jo, C. Applications of cold plasma technology for microbiological safety in meat industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Moutiq, R.; Misra, N.N.; Mendonça, A.; Keener, K. In-package decontamination of chicken breast using cold plasma technology: Microbial, quality and storage studies. Meat Sci. 2020, 159, 107942. [Google Scholar] [CrossRef] [PubMed]
- Varilla, C.; Marcone, M.; Annor, G.A. Potential of cold plasma technology in ensuring the safety of foods and agricultural produce: A Review. Foods 2020, 9, 1435. [Google Scholar] [CrossRef]
- Wu, X.; Luo, Y.; Zhao, F.; Murad, M.S.; Mu, G. Influence of dielectric barrier discharge cold plasma on physicochemical property of milk for sterilization. Plasma Process. Polym. 2021, 18, 1900219. [Google Scholar] [CrossRef]
- Korachi, M.; Ozen, F.; Aslan, N.; Vannini, L.; Guerzoni, M.E.; Gottardi, D.; Ekinci, F.Y. Biochemical changes to milk following treatment by a novel, cold atmospheric plasma system. Int. Dairy J. 2015, 42, 64–69. [Google Scholar] [CrossRef]
- Lin, C.-M.; Hsiao, C.-P.; Lin, H.-S.; Liou, J.S.; Hsieh, C.-W.; Wu, J.-S.; Hou, C.-Y. The antibacterial efficacy and mechanism of plasma-activated water against salmonella enteritidis (ATCC 13076) on shell eggs. Foods 2020, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, D.D.; Kim, H.J.; Yong, H.I.; Park, S.; Kim, K.; Choe, W.; Jo, C. Flexible thin-layer dielectric barrier discharge plasma treatment of pork butt and beef loin: Effects on pathogen inactivation and meat-quality attributes. Food Microbiol. 2015, 46, 51–57. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, Q.; Shi, W.; Yu, X.; Wang, S. Food preservation by cold plasma from dielectric barrier discharges in agri-food industries. Front. Nutr. 2022, 9, 1015980. [Google Scholar] [CrossRef]
- Chacha, J.S.; Zhang, L.; Ofoedu, C.E.; Suleiman, R.A.; Dotto, J.M.; Roobab, U.; Agunbiade, A.O.; Duguma, H.T.; Mkojera, B.T.; Hossaini, S.M.; et al. Revisiting non-thermal food processing and preservation methods—Action mechanisms, pros and cons: A technological update (2016–2021). Foods 2021, 10, 1430. [Google Scholar] [CrossRef]
- Khosravi, S.; Jafari, S.; Zamani, H.; Nilkar, M. Inactivation of staphylococcus aureus and escherichia coli biofilms by air-based atmospheric-pressure DBD plasma. Appl. Biochem. Biotechnol. 2021, 193, 3641–3650. [Google Scholar] [CrossRef]
- Liao, X.; Li, J.; Muhammad, A.I.; Suo, Y.; Ahn, J.; Liu, D.; Chen, S.; Hu, Y.; Ye, X.; Ding, T. Preceding treatment of non-thermal plasma (NTP) assisted the bactericidal effect of ultrasound on Staphylococcus aureus. Food Control 2018, 90, 241–248. [Google Scholar] [CrossRef]
- Wang, L.; Xia, C.; Guo, Y.; Yang, C.; Cheng, C.; Zhao, J.; Yang, X.; Cao, Z. Bactericidal efficacy of cold atmospheric plasma treatment against multidrug-resistant Pseudomonas aeruginosa. Future Microbiol. 2020, 15, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Maybin, J.-A.; Thompson, T.P.; Flynn, P.B.; Skvortsov, T.; Hickok, N.J.; Freeman, T.A.; Gilmore, B.F. Cold atmospheric pressure plasma-antibiotic synergy in Pseudomonas aeruginosa biofilms is mediated via oxidative stress response. Biofilm 2023, 5, 100122. [Google Scholar] [CrossRef] [PubMed]
- Labadie, M.; Marchal, F.; Merbahi, N.; Girbal-Neuhauser, E.; Fontagné-Faucher, C.; Marcato-Romain, C.-E. Response of controlled cell load biofilms to cold atmospheric plasma jet: Evidence of extracellular matrix contribution. Life 2021, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Usta, Y.H.; Çukur, E.; Yıldırım, Ç.; Ercan, U.K. Design of a portable, battery-powered non-thermal atmospheric plasma device and characterization of its antibacterial efficacies. J. Electrost. 2019, 99, 1–8. [Google Scholar] [CrossRef]
- Patinglag, L.; Melling, L.M.; Whitehead, K.A.; Sawtell, D.; Iles, A.; Shaw, K.J. Non-thermal plasma-based inactivation of bacteria in water using a microfluidic reactor. Water Res. 2021, 201, 117321. [Google Scholar] [CrossRef]
- Baek, K.H.; Heo, Y.S.; Park, J.Y.; Kang, T.; Lee, Y.E.; Lim, J.; Kim, S.B.; Jo, C. Inactivation of salmonella typhimurium by non-thermal plasma bubbles: Exploring the key reactive species and the influence of organic matter. Foods 2020, 9, 1689. [Google Scholar] [CrossRef]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine-current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 14031. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma medicine: Applications of cold atmospheric pressure plasma in dermatology. Oxid. Med. Cell Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and sterilization using plasma technology: Fundamentals and future perspectives for biological applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef]
- von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.-D. Plasma medicine: A field of applied redox biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Poschkamp, B.; van der Linde, J. Medical gas plasma promotes blood coagulation via platelet activation. Biomaterials 2021, 278, 120433. [Google Scholar] [CrossRef] [PubMed]
- Moszczyńska, J.; Roszek, K.; Wiśniewski, M. Non-thermal plasma application in medicine—focus on reactive species involvement. Int. J. Mol. Sci. 2023, 24, 12667. [Google Scholar]
- Striesow, J.; Wesche, J.; McKitterick, N.; Busch, L.M.; von Woedtke, T.; Greinacher, A.; Bekeschus, S.; Wende, K. Gas plasma-induced platelet activation corresponds to reactive species profiles and lipid oxidation. Free Radic. Biol. Med. 2023, 207, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Brüggemeier, J.; Hackbarth, C.; Weltmann, K.-D.; von Woedtke, T.; Partecke, L.-I.; van der Linde, J. The feed gas composition determines the degree of physical plasma-induced platelet activation for blood coagulation. Plasma Sources Sci. Technol. 2018, 27, 034001. [Google Scholar] [CrossRef]
- Martusevich, A.K.; Surovegina, A.V.; Bocharin, I.V.; Nazarov, V.V.; Minenko, I.A.; Artamonov, M.Y. Cold argon athmospheric plasma for biomedicine: Biological effects, applications and possibilities. Antioxidants 2022, 11, 1262. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Sahun, M.; Smits, E.; Bogaerts, A.; Privat-Maldonado, A. Modulating the antioxidant response for better oxidative stress-inducing therapies: How to take advantage of two sides of the same medal? Biomedicines 2022, 10, 823. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S. Redox for repair: Cold physical plasmas and Nrf2 signaling promoting wound healing. Antioxidants 2018, 7, 146. [Google Scholar] [CrossRef]
- Schmidt, A.; von Woedtke, T.; Vollmar, B.; Hasse, S.; Bekeschus, S. Nrf2 signaling and inflammation are key events in physical plasma-spurred wound healing. Theranostics 2019, 9, 1066–1084. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Zhou, Y.Y.; Yan, Z.N.; Chen, J.W.; Lu, T.T.; Song, W.C. Low-dose non-thermal atmospheric plasma promotes the proliferation and migration of human normal skin cells. Appl. Sci. 2023, 13, 2866. [Google Scholar] [CrossRef]
- Liu, J.R.; Xu, G.M.; Shi, X.M.; Zhang, G.J. Low temperature plasma promoting fibroblast proliferation by activating the NF-κB pathway and increasing cyclinD1 expression. Sci. Rep. 2017, 7, 11698. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.M.; Xu, G.M.; Zhang, G.J.; Liu, J.R.; Wu, Y.M.; Gao, L.G.; Yang, Y.; Chang, Z.S.; Yao, C.W. Low-temperature plasma promotes fibroblast proliferation in wound healing by ROS-activated NF-κB signaling pathway. Curr. Med. Sci. 2018, 38, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Heinlin, J.; Zimmermann, J.L.; Zeman, F.; Bunk, W.; Isbary, G.; Landthaler, M.; Maisch, T.; Monetti, R.; Morfill, G.; Shimizu, T.; et al. Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites: Cold plasma improves wound healing. Wound Repair. Regen. 2013, 21, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Landscheidt, K.; Engelhardt, C.; Hernekamp, J.-F.; Goertz, O. Use of cold plasma in wound healing: A case report. Adv. Ski. Wound Care 2022, 35, 1–3. [Google Scholar] [CrossRef]
- Mirpour, S.; Fathollah, S.; Mansouri, P.; Larijani, B.; Ghoranneviss, M.; Mohajeri Tehrani, M.; Amini, M.R. Cold atmospheric plasma as an effective method to treat diabetic foot ulcers: A randomized clinical trial. Sci. Rep. 2020, 10, 10440. [Google Scholar] [CrossRef]
- Bolgeo, T.; Maconi, A.; Gardalini, M.; Gatti, D.; Di Matteo, R.; Lapidari, M.; Longhitano, Y.; Savioli, G.; Piccioni, A.; Zanza, C. The role of cold atmospheric plasma in wound healing processes in critically Ill patients. J. Pers. Med. 2023, 13, 736. [Google Scholar] [CrossRef]
- Seo, S.-H.; Han, I.; Lee, H.S.; Choi, J.J.; Choi, E.H.; Kim, K.-N.; Park, G.; Kim, K.-M. Antibacterial activity and effect on gingival cells of microwave-pulsed non-thermal atmospheric pressure plasma in artificial saliva. Sci. Rep. 2017, 7, 8395. [Google Scholar] [CrossRef]
- Zhong, R.Q.; Zhu, M.Q.; Li, Y.L.; Pan, J. Antibacterial effect of low-temperature plasma on Enterococcus faecalis in dentinal tubules in vitro. Beijing Da Xue Xue Bao Yi Xue Ban 2023, 55, 38–43. [Google Scholar]
- Flynn, P.B.; Higginbotham, S.; Alshraiedeh, N.H.; Gorman, S.P.; Graham, W.G.; Gilmore, B.F. Bactericidal efficacy of atmospheric pressure non-thermal plasma (APNTP) against the ESKAPE pathogens. Int. J. Antimicrob. Agents 2015, 46, 101–107. [Google Scholar] [CrossRef]
- Hong, Q.; Dong, X.; Chen, M.; Sun, H.; Hong, L.; Wang, Y.; Li, H.; Yu, Q. An in vitro and in vivo study of plasma treatment effects on oral biofilms. J. Oral. Microbiol. 2019, 11, 1603524. [Google Scholar] [CrossRef]
- Jablonowski, L.; Fricke, K.; Matthes, R.; Holtfreter, B.; Schlüter, R.; Woedtke, T.; Weltmann, K.D.; Kocher, T. Removal of naturally grown human biofilm with an atmospheric pressure plasma jet: An in-vitro study. J. Biophotonics 2017, 10, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Kumar Dubey, S.; Dabholkar, N.; Narayan Pal, U.; Singhvi, G.; Kumar Sharma, N.; Puri, A.; Kesharwani, P. Emerging innovations in cold plasma therapy against cancer: A paradigm shift. Drug Discov. Today 2022, 27, 2425–2439. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Bao, J.; Chen, J.; Song, W. Low temperature plasma suppresses lung cancer cells growth via VEGF/VEGFR2/RAS/ERK axis. Molecules 2022, 27, 5934. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Sun, H.N.; Liu, R.; Choi, H.S.; Lee, D.S. Non-thermal plasma-activated medium induces apoptosis of Aspc1 cells through the ROS-dependent autophagy pathway. In Vivo 2020, 34, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.N.; Oh, C.; Chang, J.W.; Liu, L.; Lim, M.A.; Jin, Y.L.; Piao, Y.; Kim, H.J.; Won, H.R.; Lee, S.E.; et al. EGR1/GADD45alpha activation by ROS of non-thermal plasma mediates cell death in thyroid carcinoma. Cancers 2021, 13, 351. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.; Hong, Y.J.; Bae, W.Y.; Choi, E.H.; Jeong, J.W.; Park, H.K. Evaluation of non-thermal plasma-induced anticancer effects on human colon cancer cells. Biomed. Opt. Express 2017, 8, 2649–2659. [Google Scholar] [CrossRef]
- Bai, F.; Lu, Y.; Zhi, Y.; Huang, Y.; Li, L.; Luo, J.; Razzokov, J.; Koval, O.; Yusupov, M.; Chen, G.; et al. Air cold atmospheric plasma with patterns for anaplastic squamous cell carcinoma treatment. arXiv 2023, arXiv:2304.10380. [Google Scholar]
- Nakamura, K.; Yoshikawa, N.; Mizuno, Y.; Ito, M.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F.; Kajiyama, H. Preclinical verification of the efficacy and safety of aqueous plasma for ovarian cancer therapy. Cancers 2021, 13, 1141. [Google Scholar] [CrossRef]
- Bekeschus, S.; Clemen, R.; Nießner, F.; Sagwal, S.K.; Freund, E.; Schmidt, A. Medical gas plasma jet technology targets murine melanoma in an immunogenic fashion. Adv. Sci. 2020, 7, 1903438. [Google Scholar] [CrossRef]
- Adil, B.H.; Al-Shammari, A.M.; Murbat, H.H. Breast cancer treatment using cold atmospheric plasma generated by the FE-DBD scheme. Clin. Plasma Med. 2020, 19–20, 100103. [Google Scholar]
- Nasri, Z.; Memari, S.; Wenske, S.; Clemen, R.; Martens, U.; Delcea, M.; Bekeschus, S.; Weltmann, K.D.; von Woedtke, T.; Wende, K. Singlet-oxygen-induced phospholipase A(2) inhibition: A major role for interfacial tryptophan dioxidation. Chemistry 2021, 27, 14702–14710. [Google Scholar] [CrossRef]
- Bekeschus, S. Medical gas plasma technology: Roadmap on cancer treatment and immunotherapy. Redox Biol. 2023, 65, 102798. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S. Immunostimulation in experimental gas plasma therapy for breast cancer. Trends Biotechnol. 2022, 40, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.; Adhikari, M.; Simonyan, H.; Lin, L.; Sherman, J.H.; Young, C.N.; Keidar, M. In vitro and in vivo enhancement of temozolomide effect in human glioblastoma by non-invasive application of cold atmospheric plasma. Cancers 2021, 13, 4485. [Google Scholar] [CrossRef] [PubMed]
- Kniazeva, V.; Tzerkovsky, D.; Baysal, Ö.; Kornev, A.; Roslyakov, E.; Kostevitch, S. Adjuvant composite cold atmospheric plasma therapy increases antitumoral effect of doxorubicin hydrochloride. Front. Oncol. 2023, 13, 1171042. [Google Scholar] [CrossRef] [PubMed]
- Van Loenhout, J.; Freire Boullosa, L.; Quatannens, D.; De Waele, J.; Merlin, C.; Lambrechts, H.; Lau, H.W.; Hermans, C.; Lin, A.; Lardon, F.; et al. Auranofin and cold atmospheric plasma synergize to trigger distinct cell death mechanisms and immunogenic responses in glioblastoma. Cells 2021, 10, 2936. [Google Scholar] [CrossRef]
- Boeckmann, L.; Berner, J.; Kordt, M.; Lenz, E.; Schäfer, M.; Semmler, M.L.; Frey, A.; Sagwal, S.K.; Rebl, H.; Miebach, L.; et al. Synergistic effect of cold gas plasma and experimental drug exposure exhibits skin cancer toxicity in vitro and in vivo. J. Adv. Res. 2023. [Google Scholar] [CrossRef]
- Kenari, A.J.; Siadati, S.N.; Abedian, Z.; Sohbatzadeh, F.; Amiri, M.; Gorji, K.E.; Babapour, H.; Zabihi, E.; Ghoreishi, S.M.; Mehraeen, R.; et al. Therapeutic effect of cold atmospheric plasma and its combination with radiation as a novel approach on inhibiting cervical cancer cell growth (HeLa cells). Bioorganic Chem. 2021, 111, 104892. [Google Scholar] [CrossRef]
- Ahmadi, M.; Potlitz, F.; Link, A.; von Woedtke, T.; Nasri, Z.; Wende, K. Flucytosine-based prodrug activation by cold physical plasma. Arch. Pharm. 2022, 355, 2200061. [Google Scholar] [CrossRef]
- Herrlinger, E.M.; Hau, M.; Redhaber, D.M.; Greve, G.; Willmann, D.; Steimle, S.; Müller, M.; Lübbert, M.; Miething, C.C.; Schüle, R.; et al. Nitroreductase-mediated release of inhibitors of lysine-specific demethylase 1 (LSD1) from prodrugs in transfected acute myeloid leukaemia cells. Chembiochem 2020, 21, 2329–2347. [Google Scholar] [CrossRef]
- Peiró Cadahía, J.; Previtali, V.; Troelsen, N.S.; Clausen, M.H. Prodrug strategies for targeted therapy triggered by reactive oxygen species. MedChemComm 2019, 10, 1531–1549. [Google Scholar] [CrossRef]
- Ahmadi, M.; Singer, D.; Potlitz, F.; Nasri, Z.; von Woedtke, T.; Link, A.; Bekeschus, S.; Wende, K. Cold physical plasma-mediated fenretinide prodrug activation confers additive cytotoxicity in epithelial cells. Antioxidants 2023, 12, 1271. [Google Scholar] [CrossRef] [PubMed]
- Saxon, E.; Peng, X. Recent advances in hydrogen peroxide responsive organoborons for biological and biomedical applications. ChemBioChem 2022, 23, e202100366. [Google Scholar] [CrossRef] [PubMed]
- Berner, J.; Miebach, L.; Kordt, M.; Seebauer, C.; Schmidt, A.; Lalk, M.; Vollmar, B.; Metelmann, H.-R.; Bekeschus, S. Chronic oxidative stress adaptation in head and neck cancer cells generates slow-cyclers with decreased tumour growth in vivo. Br. J. Cancer 2023, 129, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Freund, E.; Miebach, L.; Clemen, R.; Schmidt, M.; Heidecke, A.; von Woedtke, T.; Weltmann, K.-D.; Kersting, S.; Bekeschus, S. Large volume spark discharge and plasma jet-technology for generating plasma-oxidized saline targeting colon cancer in vitro and in vivo. J. Appl. Phys. 2021, 129, 053301. [Google Scholar] [CrossRef]
- Gugin, P.P.; Zakrevskii, D.É.; Milakhina, E.V.; Biryukov, M.M.; Koval’, O.A.; Patrakova, E.A.; Shveigert, I.V. Optimization of the parameters of a cold plasma jet produced by sinusoidal voltage excitation for effective suppression of cancer cell viability. Biomed. Eng. 2023, 56, 409–413. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.; Wang, Z.; Obenchain, R.; Wen, D.; Li, H.; Wirz, R.E.; Gu, Z. Portable air-fed cold atmospheric plasma device for postsurgical cancer treatment. Sci. Adv. 2021, 7, eabg5686. [Google Scholar] [CrossRef] [PubMed]
- Ercan, U.K.; Ibis, F.; Dikyol, C.; Horzum, N.; Karaman, O.; Yildirim, C.; Cukur, E.; Demirci, E.A. Prevention of bacterial colonization on non-thermal atmospheric plasma treated surgical sutures for control and prevention of surgical site infections. PLoS ONE 2018, 13, e0202703. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.; Yost, A.; Goren, R.; Fridman, G.; Lowman, A. Nonthermal atmospheric pressure plasma decontamination of protein-loaded biodegradable nanoparticles for nervous tissue repair. Plasma Med. 2011, 1, 215–230. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Lee, D.S.; Chijimatsu, R.; Thamina, K.; Masuda, K.; Itsuki, D.; Yoshikawa, H.; Hamaguchi, S.; Myoui, A. Impact of non-thermal plasma surface modification on porous calcium hydroxyapatite ceramics for bone regeneration. PLoS ONE 2018, 13, e0194303. [Google Scholar] [CrossRef]
- Wang, S.; Deng, Y.; Yang, L.; Shi, X.; Yang, W.; Chen, Z.G. Enhanced antibacterial property and osteo-differentiation activity on plasma treated porous polyetheretherketone with hierarchical micro/nano-topography. J. Biomater. Sci. Polym. Ed. 2018, 29, 520–542. [Google Scholar] [CrossRef]
- Guo, L.; Smeets, R.; Kluwe, L.; Hartjen, P.; Barbeck, M.; Cacaci, C.; Gosau, M.; Henningsen, A. Cytocompatibility of titanium, zirconia and modified PEEK after surface treatment using UV light or non-thermal plasma. Int. J. Mol. Sci. 2019, 20, 5596. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, Y.; Wei, S.; Wang, X.; Zhang, J. Effects and Mechanisms of Non-Thermal Plasma-Mediated ROS and Its Applications in Animal Husbandry and Biomedicine. Int. J. Mol. Sci. 2023, 24, 15889. https://doi.org/10.3390/ijms242115889
Yang Y, Wang Y, Wei S, Wang X, Zhang J. Effects and Mechanisms of Non-Thermal Plasma-Mediated ROS and Its Applications in Animal Husbandry and Biomedicine. International Journal of Molecular Sciences. 2023; 24(21):15889. https://doi.org/10.3390/ijms242115889
Chicago/Turabian StyleYang, Yuhan, Yuan Wang, Shang Wei, Xianzhong Wang, and Jiaojiao Zhang. 2023. "Effects and Mechanisms of Non-Thermal Plasma-Mediated ROS and Its Applications in Animal Husbandry and Biomedicine" International Journal of Molecular Sciences 24, no. 21: 15889. https://doi.org/10.3390/ijms242115889





