Abstract
A previous study found that a crude Perilla seed polysaccharide (PFSP) fraction exhibited obviously antitumor activity; however, the structural characterization and antitumor properties of this polysaccharide remain unclear. In this study, the PFSP was extracted and purified via combined column chromatography, and the structure of a single polysaccharide fraction was characterized by methylation, IC, GC-MS, NMR, and AFM. The results demonstrated that the efficient antitumor polysaccharide fraction PFSP-2-1 was screened from PFSP with a relative molecular weight of 8.81 × 106 Da. The primary structure of the PFSP main chain was →1)-Araf-(5→, →1,3)-Galp-(6→, →1)-Galp-(6→, →1,3)-Araf-(5→ and →1)-Xylp-(4→, and that of the side chains was →1)-Arap, →1,3)-Galp-(6→, →1)-Araf and →1)-Glcp-(4→, →1)-Galp-(3→ and →1)-Glcp, leading to a three-dimensional helical structure. CCK-8 experiments revealed that PFSP-2-1 significantly inhibited the growth of human hepatocellular carcinoma cells in vitro (p < 0.05), and its inhibitory effect positively correlation with the concentration of PFSP-2-1, and when the concentration of PFSP-2-1 was 1600 µg/mL, it showed the highest inhabitation rate on three hepatocellular carcinoma cells (HepG-2, Hep3b, and SK-Hep-1), for which the survival rates of HepG-2, Hep3b, and SK-Hep-1 were 53.34%, 70.33%, and 71.06%. This study clearly elucidated the structure and antitumor activity of PFSP-2-1, which lays a theoretical foundation for revealing the molecular mechanism of antitumor activity of Perilla seed polysaccharides and provides an important theoretical basis for the development of high-value Perilla.
1. Introduction
Perilla frutescens is an annual herb of the Labiatae family. It is the first medicinal and edible plant recognized by the Ministry of Health of China [1]. Perilla is undemanding and has good survival, and it is widely grown in Asian countries such as China, Korea, Japan, India, and Vietnam [2]. Perilla is rich in a variety of bioactive components, such as polysaccharides, flavonoids, phenolic acids, pigments, terpenoids, and fatty acids [3]. Some studies found that total flavonoids extracted from Perilla leaves can reduce blood lipid levels and lipid accumulation in adipose tissue, inhibit the formation of lipid peroxidation products, regulate the metabolic disorders of lipoproteins, increase the activity of antioxidant enzymes, and inhibit hyperlipidemia [4]. Moreover, anthocyanins from Perilla frutescens have been shown to induce apoptosis in Hela cells and inhibit tumor growth [5]. In addition, rosemarinic acid extracted from Perilla can inhibit epidermal inflammatory responses [6]. However, there are few studies of the structure and function of Perilla polysaccharides.
Polysaccharides are a kind of natural biological macromolecules formed by connecting 10 or more monosaccharides through glycosidic bonds; they exist in a wide range of animals, plants, algae, and microorganisms [7]. Plant polysaccharides demonstrated good biological activity and application value in terms of antitumor [8,9], antioxidation [10], hypolipidemic [11], hypoglycemic [12], and immunomodulation [13,14,15] activities. In terms of antitumor activity, plant polysaccharides have good biocompatibility and biodegradability and low toxicity, showing great application potential. The biological activities of plant polysaccharides are determined by the structure of the polysaccharide, including factors such as the molecular weight, monosaccharide composition, and type of glycosidic bond and higher structure [16]. It was found that five polysaccharide fractions with antitumor activity were isolated and purified from ginger, and structural characterization revealed that the five polysaccharides differed in terms of their type of glycosidic bond [17]. Small-molecule polysaccharides extracted from the root of Platycodon grandiflorum clearly inhibit the proliferation of lung cancer cell SPC-A-1 [18]. In addition, the inhibitory effect of Lentinan on sarcoma S-180 cells was found, and the inhibitory rate of Lentinan with a three-dimensional triple-helix structure was significantly higher than those of polysaccharides without a triple-helix structure [19]. Based on these findings, we can see that the structural composition of plant polysaccharides is complex, and the biological activities of different polysaccharide structural components are also significantly different. Therefore, the biological activities of different structures of Perilla polysaccharides may also change significantly. Unfortunately, so far, the structure of Perilla polysaccharides is not clear, and research into their anticancer activity has not been carried out. This creates a limitation to the development and utilization of Perilla polysaccharides.
In our previous study, crude polysaccharides of Perilla frutescens (PFSP) were extracted from Perilla frutescens seeds, and three main polysaccharide fractions (PFSP-1, PFSP-2, and PFSP-3) were obtained from Perilla frutescens after isolation and purification. Animal experiments demonstrated that the three polysaccharide fractions had a significant inhibitory effect on the growth of tumor cells in the H22-loaded mice, and the highest tumor inhibition rate was found in PFSP-2. In this study, PFSP-2 was further isolated and purified to obtain two fractions, PFSP-2-1 and PFSP-2-2, which were initially screened via in vitro antitumor experiments. Based on the viewpoint of inhibitory effect on hepatocellular carcinoma cells, PFSP-2-1 was more effective than PFSP-2-2. Therefore, this experiment was finalized to structurally characterize PFSP-2-1, and the structural composition and properties of this polysaccharide fraction were analyzed via methylation, IC, GC-MS, NMR, and AFM. This study provides a theoretical basis for the conformational relationship with the antitumor activity of Perilla polysaccharides and provides an important theoretical basis for the high-value development and application of Perilla.
2. Results and Discussion
2.1. Extraction and Purification of PFSP-2-1
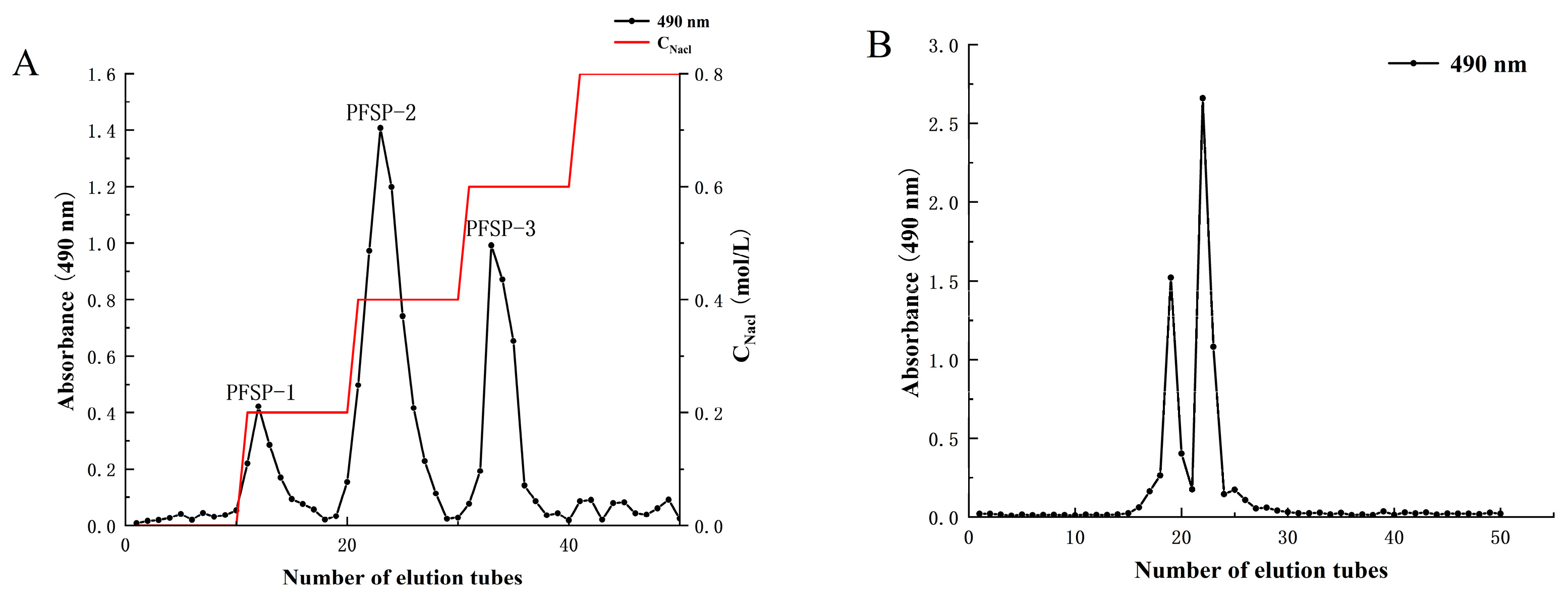
The yield of crude polysaccharides from Perilla seed (PFSP) was 3.42% ± 1.97%. After separation in a DEAE-52 cellulose column (Figure 1A), three polysaccharide fractions were obtained, namely PFSP-1, PFSP-2, and PFSP-3. PFSP-2 had the highest polysaccharide content, with a yield of 11.25% ± 2.17%. PFSP-2 was further purified using a Sephacryl S-500 HR column (Figure 1B), and two elution peaks were obtained. The two components corresponding to the two peaks were collected separately, dialyzed, desalted, and freeze-dried. The calculated yields of the two components, namely PFSP-2-1 and PFSP-2-2, were 28.40% ± 2.36% and 36.3% ± 3.22%, respectively. In this study, we focused on PFSP-2-1 and analyzed its structure.
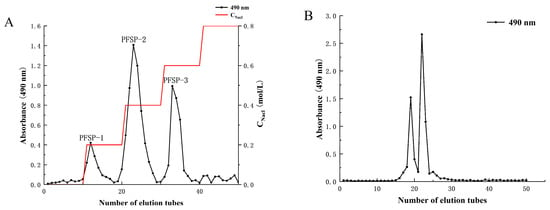
Figure 1.
(A) Column elution profile obtained via ion-exchange chromatography using a DEAE-52 cellulose column; (B) column elution profile obtained using a Sephacryl S-500 HR gel filtration column.
2.2. Characterization of PFSP-2-1
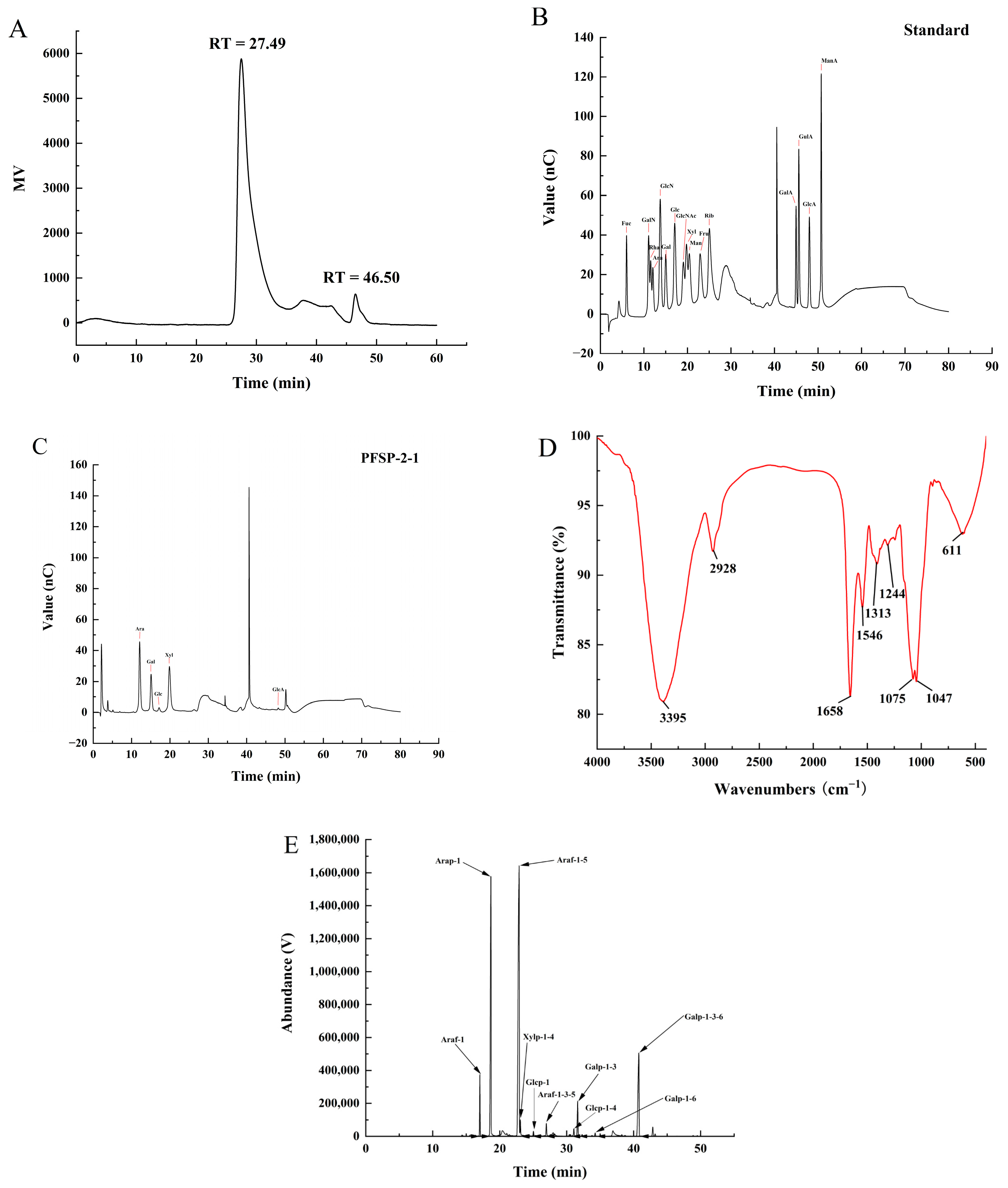
The structure of plant polysaccharides is complex, and studies have shown that their monosaccharide composition, molecular weight, type of glycosidic bond, and high-order structure are closely related to their biological activities. The purity and molecular weight of PFSP-2-1 were analyzed using HPGPC. A single peak was obtained in the HPGPC spectrum (Figure 2A), indicating that PFSP-2-1 was a homogeneous polysaccharide. The average molecular weight of PFSP-2-1 was calculated to be 8.81 × 106 Da, primarily on the basis of a log Mw–RT calibration curve. The fitted curve of the glucuronide standard equation was y = 1.4186x + 0.47791, and the glucuronide content of PFSP-2-1 was found to be 16.22% ± 0.15% of the total. Qualitative and quantitative analyses of the monosaccharide composition of PFSP-2-1 via ion chromatography showed that PFSP-2-1 was mainly composed of arabinose, galactose, glucose, xylose, and glucuronic acid (Figure 2B,C), having a molar ratio of 20.207:11.223:1.228:18.232:0.331 in terms of the contents of each element.
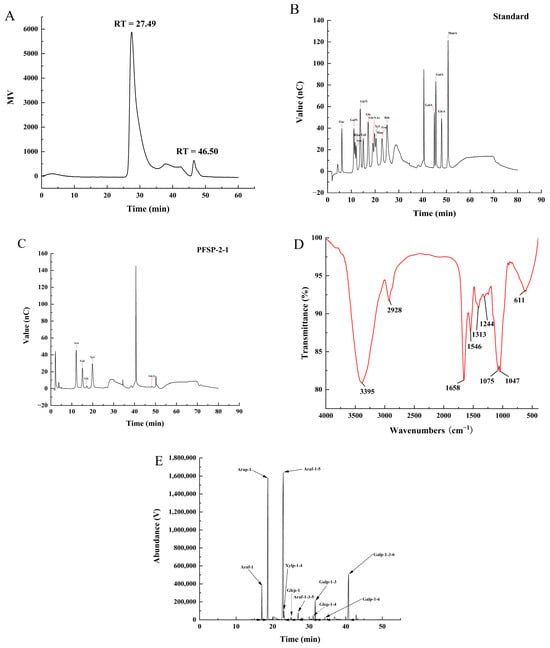
Figure 2.
(A) The molecular weight and purity of PFSP-2-1; (B) standard chromatogram of mixed monosaccharides; (C) ion chromatogram of PFSP-2-1; (D) FT-IR spectrum; (E) GC–MS chromatogram of PFSP-2-1 (PMAA).
The IR spectrum of PFSP-2-1 (Figure 2D) confirmed a wide and strong absorption peak at 3395 cm−1, caused by O–H stretching vibrations [20]. The absorption peak at 2928 cm−1 was associated with the bending vibration of the methyl C–H in the sugar ring. The absorption peak at 1658 cm−1 was assigned to the stretching vibration of COO− [21], confirming the presence of glyoxalate. The weak absorption peak at 1546 cm−1 was attributed to –CO–NH–, indicating that PFSP-2-1 contained a small amount of protein [22]. The absorption band at 1244 cm−1 was considered to correspond to the C–H bending vibration [23]. The signal at 1313 cm−1 corresponded to the in-plane bending vibration of a saturated C–H. The two absorption peaks at 1075 cm−1 and 1047 cm−1 indicated that PFSP-2-1 contained pyranose rings [24].
The types of glycosidic bonds between the monosaccharide residues in PFSP-2-1 were investigated via methylation analysis (Figure 2E), and they were identified on the basis of the mass spectra of the ion fragments (Table 1). They mainly included →5)-Araf-(1→ (50.3%), Arap-(1→ (23.8%), and →3,6)-Galp-(1→ (13.2%). Based on the linkage structures of →3,5)-Araf-(1→ and →3,6)-Galp-(1, it was clear that PFSP-2-1 was a polysaccharide with a branched structure.

Table 1.
Results of PFSP-2-1 methylated sugar alcohol acetyl ester (PMAA) analysis.
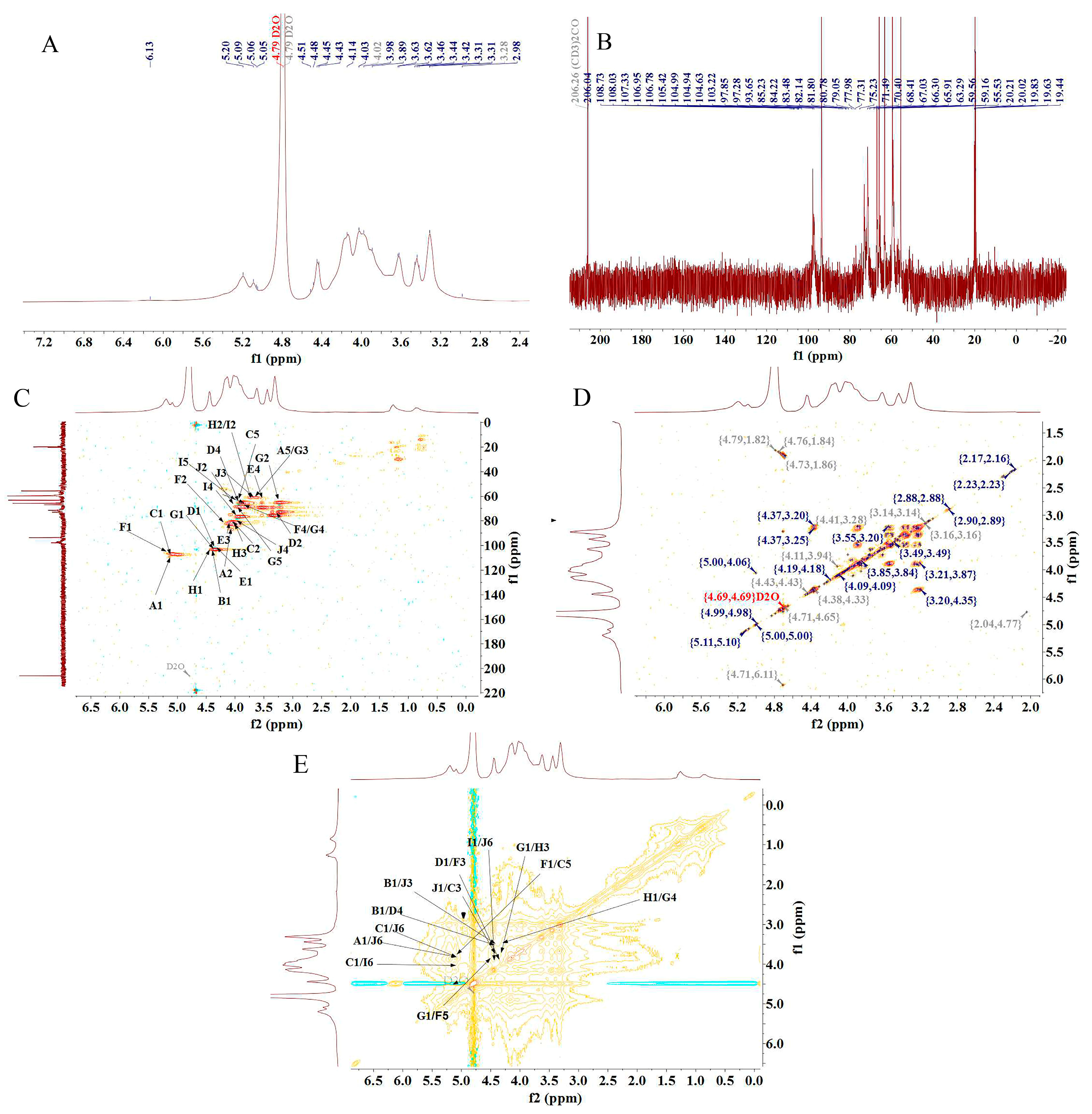
The structure of PFSP-2-1 was further analyzed via NMR spectroscopy. In the 1H-NMR spectrum (Figure 3A), a massive number of proton resonance signals were concentrated in the δ 3.0–5.5 ppm region, with severe signal overlap. Multiple hetero-head proton coupling signals were discovered in the δ 4.5–5.5 ppm region [25]. In the 13C-NMR spectrum (Figure 3B), two hetero-head carbons were present in the δ 90–110 ppm region of PFSP-2-1, indicating that PFSP-2-1 contained both α- and β-glycosidic bonds. In particular, the signal at 108.73 ppm was characteristic of the arabinose end group, and that at 101.91 ppm corresponded to the xylose end group [26]. The presence of many signals in the δ 80–90 ppm range indicated the presence of furanose in PFSP-2-1. The chemical shift of the hetero-head carbon in furanose is often in the δ 105–110 ppm region, and the peak observed in this region was attributed to the hetero-head carbon of α-L-Araf [27].
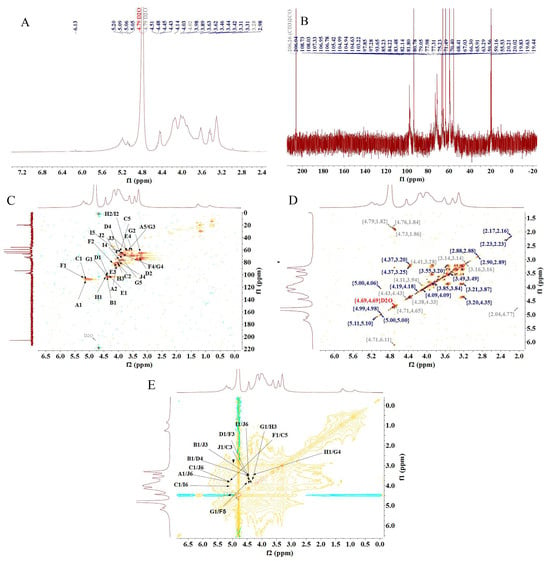
Figure 3.
(A) 1H-NMR spectrum; (B) 13C-NMR spectrum; (C) HSQC NMR spectrum; (D) 1H–1H COSY NMR spectrum; (E) NOESY NMR spectrum.
There were 10 relatively significant hetero-head hydrogen peaks in the hydrogen region and hetero-head carbon peaks in the carbon region in the NMR spectrum. The chemical shift signals of the hetero-head region were found at δ 108.73 ppm, δ 105.42 ppm, δ 106.95 ppm, δ 101.91 ppm, δ 103.22 pm, δ 106.78, ppm, δ 104.94 ppm, δ 104.99 ppm, δ 104.63 ppm, and δ 103.22 ppm, sequentially named A, B, C, D, E, F, G, H, I, and J. After the attribution of heterodimeric signals, the results obtained via HSQC (Figure 3C), 1H–1H COSY (Figure 3D), and NOESY (Figure 3E) were combined with those from the 1H-NMR and 13C-NMR spectra, as well as the methylation results and the literature data. The 1H and 13C chemical shift signals of the main types of sugar residues in PFSP-2-1 were assigned based on the chemical shift data of similar sugar residue substitutions reported in the literature [28,29,30,31,32,33,34]. The results are reported in Table 2.

Table 2.
1H-NMR and 13C-NMR spectral assignments for PFSP-2-1 (ppm).
The NOESY spectrum provided information on intra- and inter-residue linkages totally on the basis of dipole correlation. In accordance with the NOESY spectrum (Figure 3E), a correlation peak was found between the heterodimeric proton signals of →5)-Araf-(1→ (δ 5.09) and H6 of →3,6)-Galp-(1→ (δ 3.85), as well as that of H6 of →6)-Galp-(1→ (δ 4.09), indicating the presence of →5)-Araf-(1→3,6)-Galp-(1→ and →5)-Araf-(1→6)-Galp-(1→ linkage fragments. A correlation peak between the heterodimeric proton signals of Arap-(1→ (δ 4.45) and H3 of →3,6)-Galp-(1→ (δ 3.63), as well as that of H4 of →4)-Xylp-(1→ (δ 3.83), indicated the presence of the linkage fragments of Arap-(1→3,6)-Galp-(1→ and Arap-(1→4)-Xylp-(1→. A correlation peak between the hetero-head proton signals of →3,6)-Galp-(1→ (δ 4.45) and its own H3 (δ 3.63), as well as the H5 of →5)-Araf-(1→ (δ 3.83), indicated the presence of →3,6)-Galp-(1→3,6)-Galp-(1→ and →3,6)-Galp-(1→5)-Araf-(1→. A correlation peak was observed between the heterodimeric proton signal of Araf-1→ (δ 5.09) and the H6 of →3,6)-Galp-(1→ (δ 3.85), indicating the presence of the linkage fragment of Araf-1→6,3)-Galp-(1→. The correlation peak between the heterodimeric proton signal of →4)-Xylp-(1→ (δ 4.40) and the H3 of →3,5)-Araf-(1→ (δ 4.04) indicated the presence of the linker fragment →4)-Xylp-(1→3,5)-Araf-(1→. The correlation peak between the heterodimeric proton signal of →4)-Glcp-(1→ (δ 4.44) and the H3 of →3)-Galp-(1→ (δ 3.98) indicated the presence of the linker fragment →4)-Glcp-(1→3)-Galp-(1→. The correlation peak between the heterodimeric proton signal of →3,5)-Araf-(1→ (δ 5.10) and the H5 of→5)-Araf-(1→ (δ 3.83) indicated the presence of the linker fragment →3,5)-Araf-(1→5)-Araf-(1→. The correlation peak between the hetero-head proton signal of →4)-Glcp-(1→ (δ 4.44) and the H5 of →3,5)-Araf-(1→ (δ 3.54) indicated the presence of the linker fragment →4)-Glcp-(1→5,3)-Araf-(1→. The correlation peak between the heterodimeric proton signal of →3)-Galp-(1→ (δ 4.46) and the H4 of →4)-Glcp-(1→ (δ 3.80) indicated the presence of the linker fragment →3)-Galp-(1→4)-Glcp-(1→. The correlation peak between the heterodimeric proton signal of →6)-Galp-(1→ (δ 4.46) and the H6 of →3,6)-Galp-(1→ (δ 3.85) indicated the presence of the linker fragment →6)-Galp-(1→6,3)-Galp-(1→.
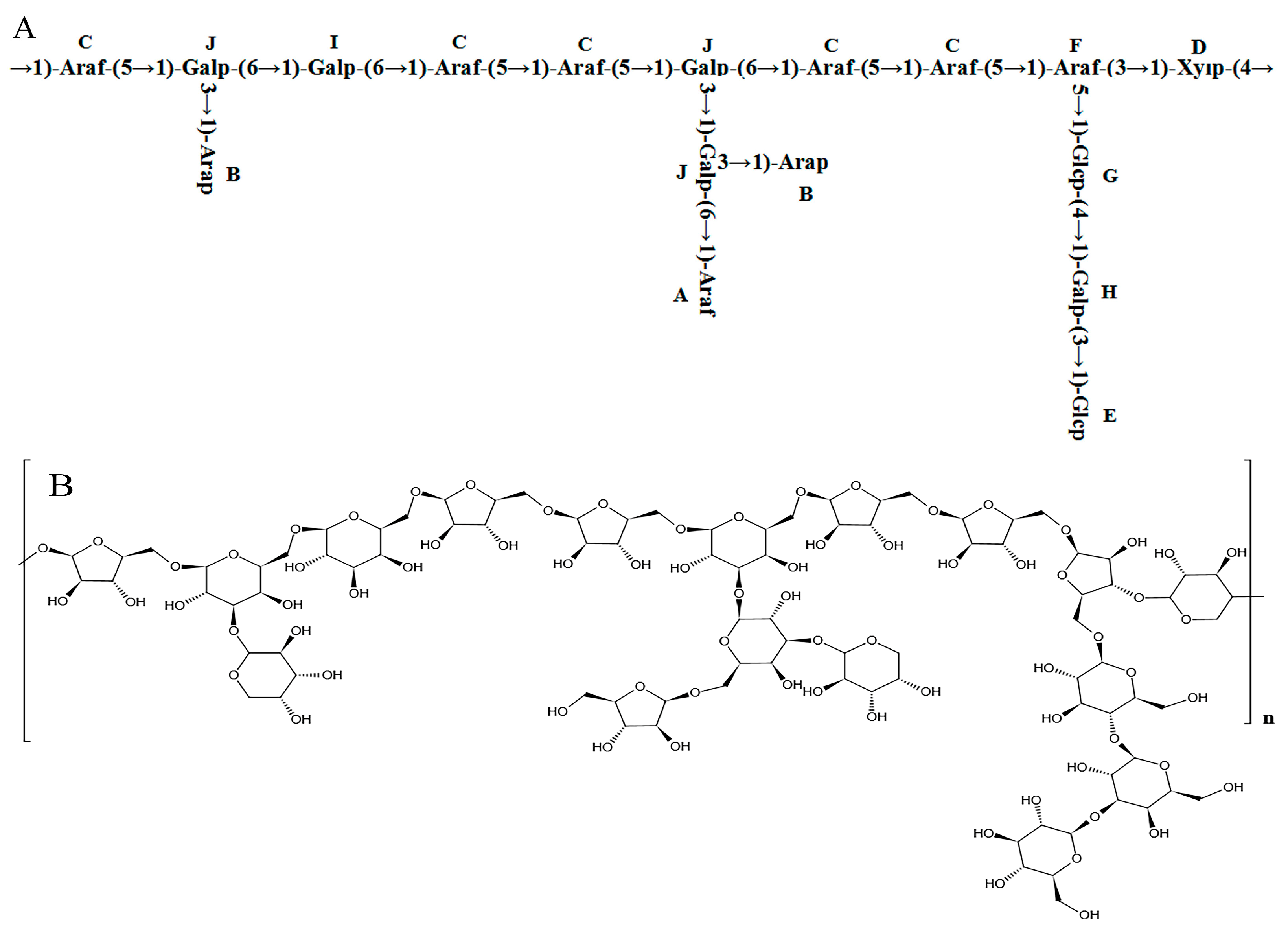
Combining the 1H-NMR, 13C-NMR, COSY, HSQC, NOESY, and methylation analysis results, it was speculated that the main chain structure of PFSP-2-1 was →1)-Araf-(5→1)-Galp-(6→1)-Galp-(6→1)-Araf-(5→1)-Araf-(5→1)-Galp-(6→1)-Araf- (5→1)-Araf-(5→1)-Araf-(3→1)-Xylp-(4→, along with side-chain structures of →1,6)-Galp-(3→1)-Arap, →1,6)-Galp-(3→1,3)-Galp-(6→1)-Araf, →1,6)-Galp-(3→1)-Arap, and →1,3)-Araf-(5→1)-Glcp-(4→1)-Galp-(3→1)-Glcp. The major glycosidic bond of PFSP-2-1 consisted of →5)-Araf-(1→, Arap-(1→ and →3,6)-Galp-(1→, while the linkage structures of →3,5)-Araf-(1→ and →3,6)-Galp-(1 PFSP-2-1 indicated a polysaccharide with a branching structure [35]. We predicted the repetitive structural unit and planar structure of PFSP-2-1, and the results were shown in (Figure 4A,B).
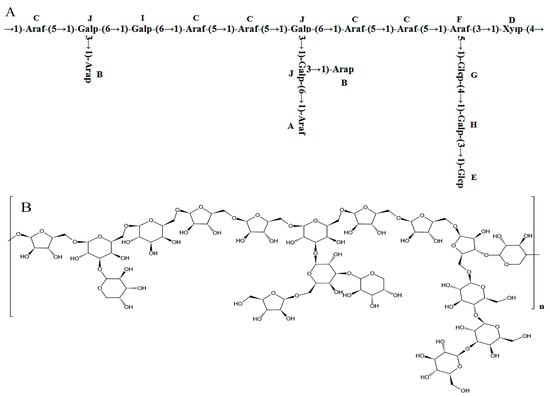
Figure 4.
(A) Structure of PFSP-2-1 repeat unit; (B) diagram of the planar structure of PFSP-2-1.
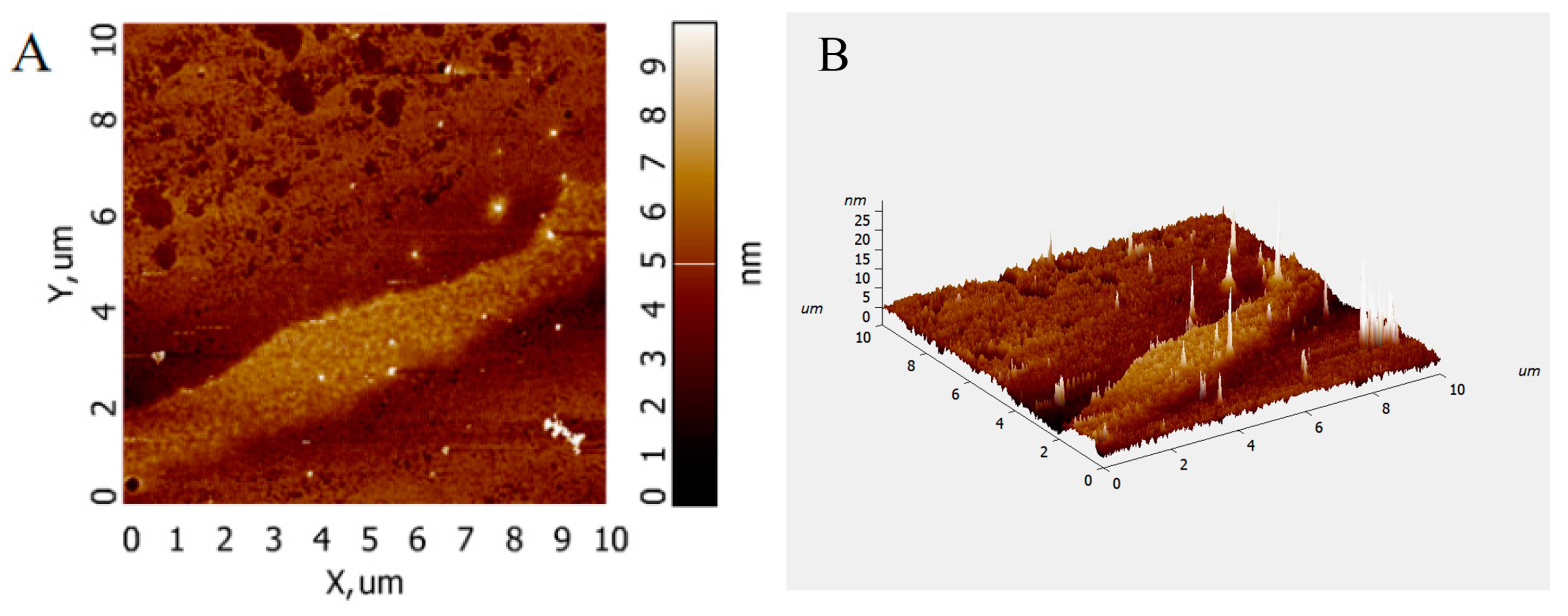
Atomic force microscopy (AFM) analysis allows the observation of the surface morphology of biomolecules and the conformation of glycan chains without sample pretreatment. The AFM images of PFSP-2-1 (Figure 5A,B) show that PFSP-2-1 presented an irregular spherical shape with a molecular diameter in the range of 0–25 nm, which is significantly larger than the average molecular diameters of individual polysaccharide chains (0.1–1.0 nm) [21], located at 15–50 nm in the polysaccharide triple-helix structure. This indicates that PFSP-2-1 had a branching structure, and the chains were intertwined with each other, probably forming a triple-helix structure.
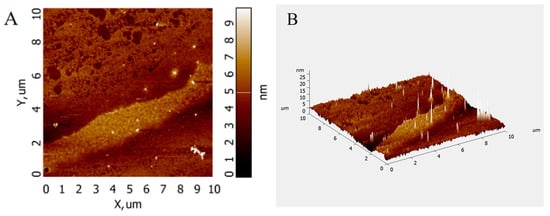
Figure 5.
AFM scanning diagram of PFSP-2-1: (A) two-dimensional diagram; (B) three-dimensional diagram.
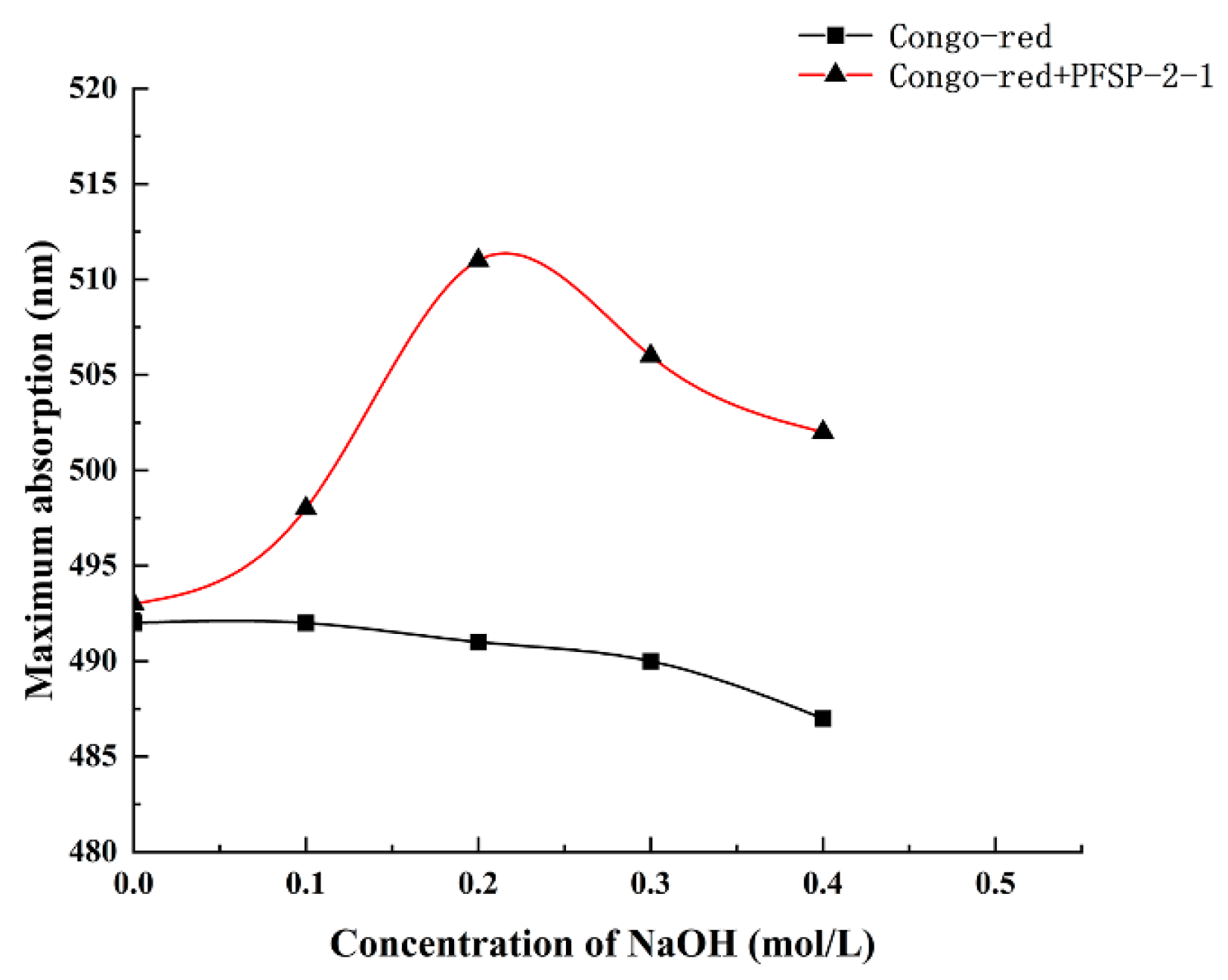
The maximum absorption wavelengths of PFSP-2-1–Congo red complexes were determined in the presence of a NaOH solution at a concentration of 0–0.5 M (Figure 6). It was observed that with the increase in NaOH solution concentration, the maximum absorption wavelength of the control gradually decreased, whereas the maximum absorption wavelength of the polysaccharide–Congo red complex first increased and then decreased, reaching its highest value at the NaOH concentration of 0.2 mol/L. This indicates that under low-alkali conditions, the triple-helix structure of the polysaccharide formed a complex with the Congo red solution, whose maximum absorption wavelength was red-shifted compared to that of the control; however, when the NaOH concentration was increased beyond a certain value, the structure of the triple-helix was destroyed, as shown by the decrease in the maximum absorption wavelength. This indicates that PFSP-2-1 had a triple-helix spatial structure, consistent with the AFM results.
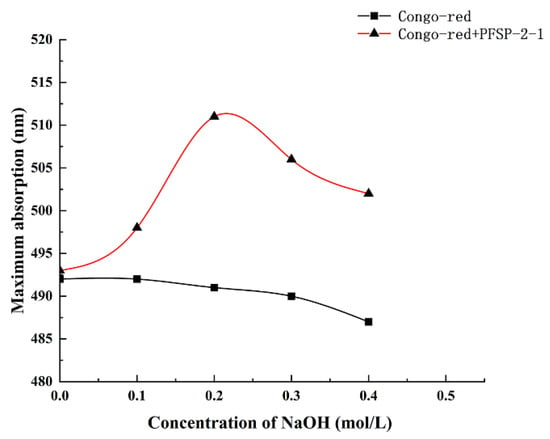
Figure 6.
Different concentrations of NaOH affect the maximum absorption wavelength of the PFSP-2-1–Congo red complex.
2.3. Antitumor Activity In Vitro
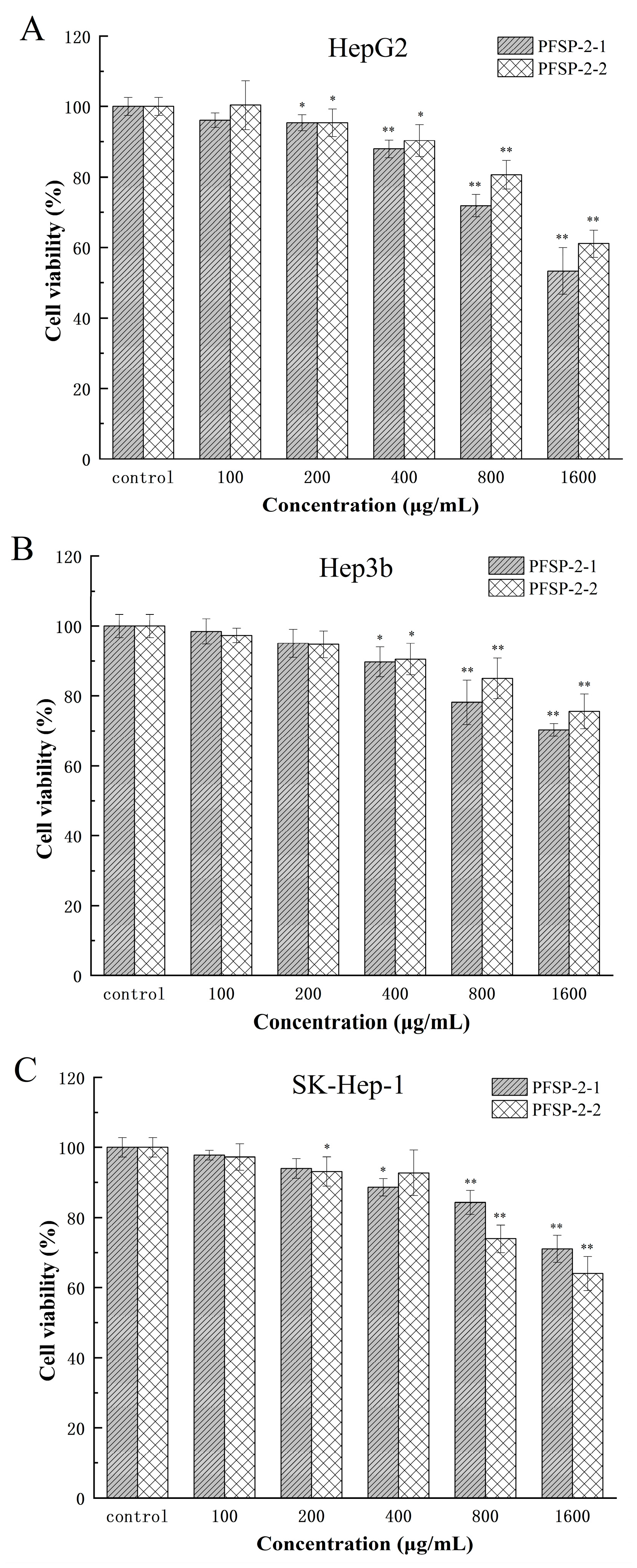
A CCK-8 experiments were used to determine the inhibitory effect of polysaccharide components on HepG2, Hep3b, and SK-Hep-1 cells, and the results are shown in Figure 7 and Table 3. Using the sample without polysaccharides as the control group, two kinds of polysaccharides (PFSP-2-1 and PFSP-2-2) had significant inhibitory effects on HepG2 cells (Figure 7A, p < 0.05). With the increase in polysaccharide concentration, the inhibition of PFSP-2-1 on HepG2 cells gradually increased. When the concentration was 100 µg/mL, PFSP-2-1 was not significant compared to CK (p > 0.05), and the cell survival rate was 96.12%. However, at the concentration of 200–1600 µg/mL, the inhibitory effect of PFSP-2-1 gradually increased, reaching its maximum effect at the a concentration of 1600 µg/mL, and the cell survival rate was 53.34%. The inhibitory effect of PFSP-2-2 was consistent with that of PFSP-2-1.
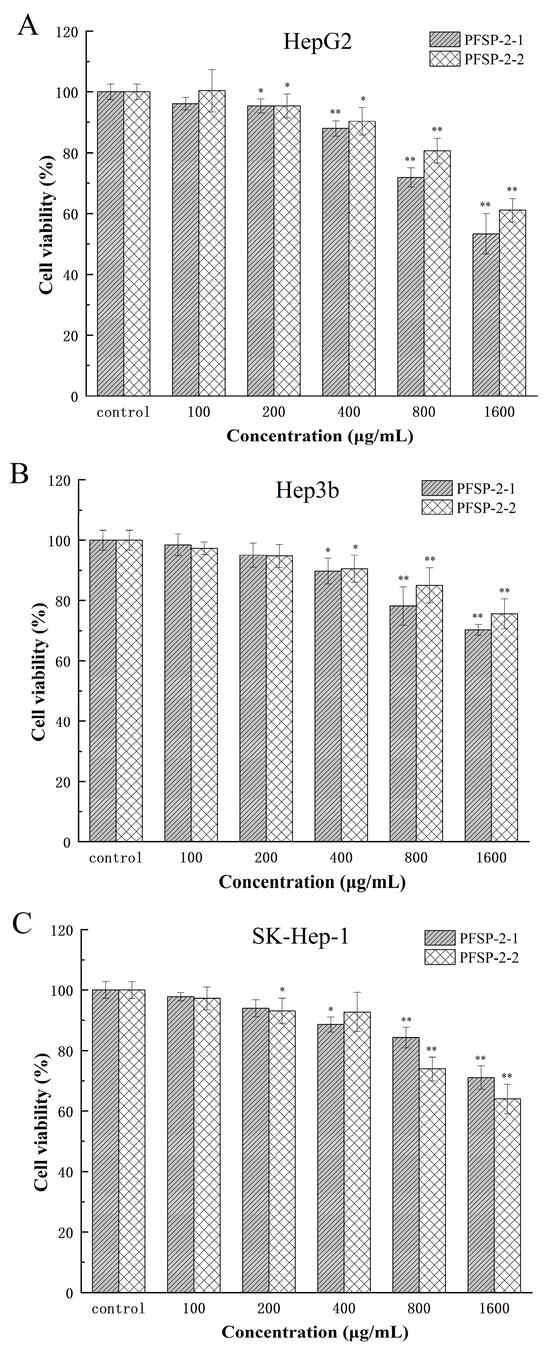
Figure 7.
The effect of PFSP-2-1 on the viability of different cells: (A) HepG2 cells; (B) Hep3b cells; (C) SK-Hep-1 cells. The differences were statistically significant at * p < 0.05 and ** p < 0.01.

Table 3.
Difference analysis of cell survival rates of two polysaccharides at different concentrations.
Compared to the control group, two kinds of polysaccharides (PFSP-2-1 and PFSP-2-2) had significant inhibitory effects on Hep3b cells (Figure 7B, p < 0.05). With the increase in polysaccharide concentration, the inhibition of PFSP-2-1 on Hep3b cells gradually increased. When the concentration was 100 µg/mL and 200 µg/mL, the effect of PFSP-2-1 was not significant compared to that of CK (p > 0.05), and the cell survival rates were 98.41% and 95.04%, respectively. However, at the concentration of 400–1600 µg/mL, the inhibitory effect of PFSP-2-1 gradually increased, reaching its maximum effect at a concentration of 1600 µg/mL, and the cell survival rate was 70.33%. The inhibitory effect of PFSP-2-2 was consistent with that of PFSP-2-1.
Compared to the control group, the two kinds of polysaccharides (PFSP-2-1 and PFSP-2-2) had significant inhibitory effects on SK-Hep-1 cells (Figure 7C, p < 0.05). Based on the inhibitory results of PFSP-2-1 on SK-Hep-1 cells, the inhibitory effects of 100 µg/mL and 200 µg/mL on SK-Hep-1 cells were not significant (p > 0.05), but the inhibitory effects of 400–1600 µg/mL on SK-Hep-1 cells was significant (p < 0.05). The inhibition results of PFSP-2-2 on SK-Hep-1 cells were different to those of PFSP-2-1, in which the inhibitory effect on SK-Hep-1 cells was not significant (p > 0.05) at the concentrations of 100 µg/mL and 400 µg/mL; however, at concentrations of 200 µg/mL, 800 µg/mL, and 1600 µg/mL, it had a significant inhibition effect (p < 0.05), reaching its maximum effect at a concentration of 1600 µg/mL, and the cell survival rate was 64.02%. In summary, the inhibitory effects of the polysaccharide fractions on different tumor cells were different. At the same concentration, the highest inhibition was observed in HepG2 cells, which are often used to screen for drugs, and the inhibitory effect of PFSP-2-1 was found to be more significant than that of PFSP-2-2 (p < 0.05).
2.4. Discussion
As natural biomacromolecules, polysaccharides’ structures are the key factors affecting their biological activity and physical and chemical properties. In this study, it was found that both polysaccharides (PFSP-2-1 and PFSP-2-2) had inhibitory effects on HepG2, Hep3b, and SK-Hep-1 cells, and the inhibitory effects gradually increased with the increase in the concentration (Figure 7). The monosaccharide composition of Perilla polysaccharide PFSP-2-1 is mostly arabinose and xylose, which is quite different from the previous report stating that glucose is the main monosaccharide composition in Perilla polysaccharides, which may be related to the variety of Perilla used and the methods for the extraction and purification the polysaccharides. At present, it has been found that hemicellulose in Astragalus residue mainly contains monosaccharides such as arabinose and xylose, which have an inhibitory effect on the proliferation of human lung adenocarcinoma cells and human normal lung epithelial cells [36]. In addition, arabinoxylan in wheat bran can significantly inhibit the growth of transplanted tumors in mice (p < 0.05) [37]. Therefore, it is speculated that arabinose and xylose in Perilla polysaccharides are some of the factors inhibiting the growth of liver cancer cells.
In addition to monosaccharide composition, molecular weight and glycosidic bond can be considered important structural features of polysaccharides that affect their biological activity. Studies have shown that polysaccharides with high molecular weights tend to have greater biological activity [38,39]. For example, researchers have studied the inhibitory effect of Grifola frondosa polysaccharides with different molecular weights on human lung cancer cells. The inhibitory rates of Grifola frondosa polysaccharides with high molecular weights on human lung cancer cells were significantly higher than those of other low-molecular-weight polysaccharides [40]. The reason for this may be that there are (1→3) α-D-Galp glycosidic bonds and a high content of (1→3,4) α-D-Galp in high-molecular-weight Grifola frondosa polysaccharides, and special glycosidic bonds are the key to the high antitumor activity of Grifola frondosa polysaccharides. We obtained high-molecular-weight Perilla PFSP-2-1 through separation and purification, and the glycosidic bonds were mainly →5)-Araf-(1→(50.3%) and Arap-(1→(23.8%). Previous studies found that the Arachis polysaccharide ALP-3 with the →5)-Araf-(1→, Araf-(1→, and →3,5)-Araf-(1→ branching structure was able to stimulate the immune activity of RAW264.7 cells better than the branched polysaccharide ALP-1 [34]. Therefore, it can be determined that the molecular weights and glycosidic bonds of Perilla polysaccharides have a certain influence on their antitumor activity.
Through NMR and methylation analysis, the main chain structure, side-chain structure, and glycosidic linkage mode of Perilla polysaccharide PFSP-2-1 were inferred, and it was determined that PFSP-2-1 was a polysaccharide with a branching structure. An AFM experiment and a Congo red experiment confirmed the triple-helix spatial structure of Perilla polysaccharides. It is generally believed that the higher structure of polysaccharides has a greater influence on the polysaccharides’ activity than the primary structure. At present, there are many studies of the triple-helix structure of Lentinan. For example, Lentinan with a triple-helix structure has a higher tumor inhibition rate in mice, and after being treated with DMSO, it becomes a single, flexible chain, which destroys its spatial configuration and greatly reduces its antitumor activity [41]. The antitumor activities of 10 kinds of Lentinan degraded via ultrasonic wave were investigated. Among them, the Lentinan with a single-stranded structure had no obvious antitumor activity, and the Lentinan with a triple-helix structure had the strongest antitumor activity. It can be seen that the advanced structure of polysaccharides has a greater influence on tumor activity than the primary structure [19]. At present, the research into the advanced structure of Perilla polysaccharides is insufficient, which limits the research into their antitumor activity.
3. Materials and Methods
3.1. Materials
Perilla meal was purchased from Heilongjiang Huainan Nongshengyuan Food Co., Ltd. (Jiamusi, China). HepG2, Hep3b, and SK-Hep-1 cells were donated by the Institute of Nature and Ecology, Heilongjiang Provincial Academy of Sciences. Phenol, sulfuric acid, chloroform, and n-butanol were purchased from Tianli Chemical Reagent Co., Ltd. (Tianjin, China). Petroleum ether, polyamide powder, anhydrous ethanol, acetic anhydride, perchloric acid, acetic acid, glacial acetic acid, methanol, sodium hydroxide, toluene, potassium bromide, and Congo red were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Trifluoroacetic acid, methyl iodide, sodium borohydride, ethyl acetate, dimethyl sulfoxide, and sodium hydride were purchased from Shanghai Adamas Reagent Co., Ltd. (Shanghai, China). DEAE-52 cellulose and Sephacryl S-500 HR propylene dextran gel columns were supplied by Beijing BoaoTuoDa Technology Co., Ltd. (Beijing, China). Monosaccharide standards (rhamnose, fucose, galactose, arabinose, xylose, glucose, mannose, ribose, fructose, glucuronic acid, galacturonic acid, amino galactose hydrochloride, N-acetyl-glucosamine, glucosamine hydrochloride, mannuronic acid, and gulo-glucuronic acid) and methylation kits were supplied by Borealis Biotechnology (Yangzhou, China). Sigma (St. Louis, MO, USA) supplied the dextran standards. High-sugar DMEM, MEM, and RPMI-1640 media were supplied by Solarbio (Beijing, China). Zhejiang Tianhang Bio (Zhejiang, China) supplied the fetal bovine serum. The CCK-8 kit was supplied by Glpbio (Shanghai, China).
3.2. Extraction and Purification of PFSP-2-1
After crushing, the Perilla seeds were sieved through 60-mesh screens. They were defatted with petroleum ether (1:20, m/v) for 6 h, followed by extraction with distilled water (1:20, m/v) at 85 °C for 2.5 h. The extraction was repeated three times, and then the extracts of the polysaccharides were combined. After evaporation and concentration under reduced pressure, anhydrous ethanol at the final concentration of 75% was added for alcohol precipitation, and the precipitate was centrifuged overnight at 4 °C and frozen, obtaining the crude polysaccharide of the Perilla seeds. After dissolving the crude polysaccharide in deionized water, free protein was precipitated using the Sevage method, and the supernatant was collected and freeze-dried. Decolorization was performed using the polyamide chromatography column method [42], and the eluate was collected, concentrated through evaporation under reduced pressure, and freeze-dried, yielding the Perilla seed polysaccharide (PFSP).
After dissolving 50 mg of PFSP in 5 mL of water, the solution was added to an activated equilibrated DEAE-52 cellulose column, which was sequentially eluted with a NaCl gradient solution (0–0.8 M). An eluate of the Perilla seeds was collected, concentrated, dialyzed, and lyophilized to obtain a polysaccharide fraction. According to previously obtained experimental results revealing the antitumor activity of the Perilla seed polysaccharide, Sephacryl S-500 HR column chromatography was selected for the further purification of PFSP-2. In this study, we selected the fraction PFSP-2-1 for structural characterization and verified its antitumor activity using the CCK-8 cell viability assay.
3.3. Structural Characterization of PFSP-2-1
3.3.1. Physicochemical Characterization of PFSP-2-1
The phenol–sulfuric acid method was used to determine the polysaccharide content of PFSP-2-1, using d-glucose as a standard. Using d-glucuronic acid as the standard, uronic acid was detected via a carbazole reaction [43].
3.3.2. Relative Molecular Weight (Mw) of PFSP-2-1
Dextran strands with different relative molecular weights and the polysaccharide PFSP-2-1 were weighed precisely, dissolved to obtain 5 mg/mL solutions, and centrifuged for 10 min at 12,000× g. The supernatant was filtered and transferred to the injection vial. The purity and the molecular weight of PFSP-2-1 were analyzed using a GPC system equipped with a Shimadzu RID-10A differential refractive index detector and connected to a BRT 105-104-102 tandem gel column (8 × 300 mm). The column temperature was maintained at 40 °C, the flow rate was maintained at 0.6 mL/min, and the injection volume was 20 µL [44].
3.3.3. Monosaccharide Composition Analysis
To detect the monosaccharide composition of PFSP-2-1, an ICS5000 ion chromatograph (Billerica, MA, USA) equipped with a Dionex Carbopac TMPA 20 column (3 × 150) was used. Then, 10.0 mg of the polysaccharide PFSP-2-1 and 16 monosaccharide standards (rhamnose, fucose, galactose, arabinose, xylose, glucose, mannose, ribose, fructose, glucuronic acid, galacturonic acid, amino galactose hydrochloride, N-acetyl-glucosamine, glucosamine hydrochloride, mannuronic acid, and gulo-glucuronic acid) were precisely weighed in ampoules, before adding 10 mL of 3 M TFA. The ampoules were sealed under nitrogen purge, and the samples were hydrolyzed at 120 °C for 3 h. The acid hydrolysis solution was transferred to a glass tube through aspiration, and 10 mL of deionized water was added before mixing; then, 100 µL of the solution was aspirated, and 900 µL of deionized water was added. The supernatant was centrifuged at 12,000× g for 5 min, and the monosaccharide composition of PFSP-2-1 was determined using an ion chromatograph equipped with a Dionex Carbopac TMPA20 (3 × 150) column [45].
3.3.4. Methylation Analysis
The methylation analysis of PFSP-2-1 was performed according to the method of Xie et al. [46]. Firstly, 2.0–3.0 mg of the polysaccharide PFSP-2-1 was added to a glass reaction flask, along with 1 mL of anhydrous DMSO, before quickly adding the methylation reagent A solution (anhydrous base solution). The flask was closed, the sample was dissolved using ultrasonic waves, and the methylation reagent B solution (iodomethane solution) was added. The mixture was stirred in a water bath at 30 °C for 60 min, before 2 mL of ultrapure water was added to stop the methylation reaction. At this point, 1 mL of 2 M trifluoroacetic acid (TFA) was added to the methylated polysaccharide to hydrolyze it, letting the reaction proceed for 90 min, and then the sample was concentrated through rotary evaporation. The residue was reduced by adding 2 mL of double-distilled water and 60 mg of sodium borohydride, and it was left to react for 8 h. Glacial acetic acid was added to neutralize the residue; the sample was evaporated, concentrated, and dried in a drying oven at 101 °C, and 1 mL of acetic anhydride was added to react at 100 °C for 1 h. The sample was then cooled. After adding 3 mL of toluene, the sample was concentrated under reduced pressure, and the excess acetic anhydride was removed through evaporation. The procedure was repeated 4–5 times. The acetylated product was dissolved with chloroform to obtain derivatives, which were analyzed using GCMS-QP 2010 gas chromatography/mass spectrometry (Kyoto, Japan) with an RXI-5 SIL MS column (30 m × 0.25 mm × 0.25 µm).
3.3.5. FT-IR and NMR Spectroscopy
The dried PFSP-2-1 (1.0 mg) was combined with KBr to obtain clear, pressed tablets, which were then scanned in the range of 4000–400 cm−1 using FT-IR (Waltham, MA, USA) to analyze the organic functional groups of PFSP-2-1 [47].
PFSP-2-1 (50 mg) was weighed, dissolved in 0.5 mL of D2O, and freeze-dried, and we repeated the procedure twice. The samples were then dissolved in 0.5 mL of D2O, and 1H-NMR, 13C-NMR, chemical shift correlation (COSY), heteronuclear single quantum coherence (HSQC), and two-dimensional NOE spectra (NOESY) were obtained at 25 °C using a Bruker 600 NMR instrument (Billerica, MA, USA).
3.3.6. Microscopic Analysis
A suitable quantity of polysaccharide powder was dissolved in ultrapure water. The sample was then diluted and dispersed well, dropped onto a smooth mica sheet, dried at room temperature, and tested [48]. Scanning was performed using atomic-pressure microscopy (Moscow, Russia); the probe was mounted, the laser was adjusted, and the sample stage and probe were moved such that the sample was located directly under the probe until the surface of the sample was visible. The Nanoscope software was used for the analysis.
3.3.7. Triple-Helix Structure Determination
The triple-helix structure of PFSP-2-1 was determined using Congo red. Firstly, 5 mg of PFSP-2-1 was dissolved in 2 mL of a Congo red (80 μmol/L) solution. Six experimental groups were set up, to which 1 mol/L of NaOH was added, obtaining final NaOH concentrations of 0, 0.1, 0.2, 0.3, 0.4, and 0.5 mol/L. After mixing well, the control group was left to stand for 10 min. The control group was treated as the experimental group, with the exception that PFSP-2-1 was not added. A full-wavelength scan was performed in the 300–700 nm range, using a UV–visible spectrophotometer (Waltham, MA, USA) [49].
3.4. Evaluation of Antitumor Activity
3.4.1. Cell Culture
HepG2 cells were cultured in high-sugar DMEM containing 10% fetal bovine serum and 1% double antibody. Hep3b cells were cultured in MEM containing 10% fetal bovine serum and 1% double antibody. SK-Hep-1 cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum and 1% double antibody. The cell cultures were maintained at 37 °C in a 5% CO2 atmosphere.
3.4.2. Cytotoxicity Experiment
The toxicity of PFSP-2-1 and PFSP-2-2 in the three tumor cell lines (HepG2, Hep3b, and SK-Hep-1) was assessed using a CCK-8 assay. In brief, the tumor cells were seeded in a 96-well plate at a density of 1 × 104 cells/mL. The medium without polysaccharides was used as the control group. The polysaccharide samples PFSP-2-1 and PFSP-2-2 at five concentrations (100, 200, 400, 800, and 1600 µg/mL) were added to the cells on the 96-well plate. Using the CCK-8 kit [50], cell viability was calculated by measuring each well’s absorbance (OD) at 450 nm.
3.5. Statistical Analysis
SPSS 27.0 software was used to analyze the experimental data. In this paper, the results are expressed as the mean ± standard deviation, and the difference between different treatments was compared via one-way ANOVA, and multiple comparisons between each treatment were performed using the Tukey test method at p < 0.05.
4. Conclusions
PFSP-2-1 was isolated from Perilla meal as an effective antitumor polysaccharide fraction. The composition and structure of PFSP-2-1 were analyzed through FT-IR, GC–MS, IC, NMR, and AFM, and its antitumor activity was determined. The results showed that PFSP-2-1 is an acidic polysaccharide with a glyoxylate content of 16.22% ± 0.15% and a relative molecular weight of 8.81 × 106 Da. Its composition was found to consist of arabinose, galactose, glucose, xylose, and glucuronide. Its main chain structure was →1)-Araf-(5→1)-Galp-(6→1)-Galp-(6→1)-Araf-(5→1)-Araf-(5→1)-Galp-(6→1)-Araf-(5→1)-Araf-(5→1)-Araf-(3→1)-Xylp-(4→, and its side-chain structures were →1,6)-Galp-(3→1)-Arap, →1,6)-Galp-(3→1,3)-Galp-(6→1)-Araf, →1,6)-Galp-(3→1)-Arap, and →1,3)-Araf-(5→1)-Glcp-(4→1)-Galp-(3→1)-Glcp, suggesting a three-dimensional helical structure. In vitro, we observed that PFSP-2-1 exerted proliferation-inhibitory effects on HepG2, Hep3b, and SK-Hep-1 cells in a dose-dependent manner. Thus, the results of our study lay a theoretical foundation for the elucidation of the molecular mechanism of the antitumor activity of Perilla seed polysaccharides and provide theoretical support for the development of Perilla seed polysaccharides as antitumor drugs.
5. Limitation
The limitations of this experiment are as follows:
- (1)
- We did not compare the structure of polysaccharides via different extraction methods and, thus, could not possibly understand the entire structure of polysaccharides;
- (2)
- This study only investigated the antitumor activity of polysaccharides from Perilla frutescens, but the mechanism of antitumor activity will need to be explored in the further.
Author Contributions
H.L., conceptualization, methodology, investigation, formal analysis, validation, resources, writing—original draft, and visualization; M.L. (Ming Liu), conceptualization, methodology, investigation, data curation, and writing—original draft; Z.L., methodology and resources; L.C., methodology; M.L. (Mengsha Li), validation and writing—review and editing; C.L., conceptualization, formal analysis, writing—review and editing, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Heilongjiang Province of China (TD2019C002) and the Provincial Scientific Research Institutions in Heilongjiang Province of 2023 (ZNNQ2023ZR03).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Xin Sui, who is from Heilongjiang University for data analysis and preparing and revising this manuscript during the whole manuscript process. We also thank the Institute of Natural Ecology, Heilongjiang Academy of Sciences for providing experimental materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barbieri, C.; Ferrazzi, P. Perilla frutescens: Interesting New Medicinal and Melliferous Plant in Italy. Nat. Prod. Commun. 2011, 6, 1934578X1100601. [Google Scholar] [CrossRef]
- Chen, F.; Liu, S.; Zhao, Z.; Gao, W.; Ma, Y.; Wang, X.; Yan, S.; Luo, D. Ultrasound Pre-Treatment Combined with Microwave-Assisted Hydrodistillation of Essential Oils from Perilla frutescens (L.) Britt. Leaves and Its Chemical Composition and Biological Activity. Ind. Crops Prod. 2020, 143, 111908. [Google Scholar] [CrossRef]
- Hou, T.; Netala, V.R.; Zhang, H.; Xing, Y.; Li, H.; Zhang, Z. Perilla frutescens: A Rich Source of Pharmacological Active Compounds. Molecules 2022, 27, 3578. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.-J.; Yu, C.-H.; Ying, K.-J.; Hua, J.; Dai, X.-Y. Hypolipidemic and Antioxidant Effects of Total Flavonoids of Perilla frutescens Leaves in Hyperlipidemia Rats Induced by High-Fat Diet. Food Res. Int. 2011, 44, 404–409. [Google Scholar] [CrossRef]
- He, Y.-K.; Yao, Y.-Y.; Chang, Y.-N. Characterization of Anthocyanins in Perilla frutescens Var. Acuta Extract by Advanced UPLC-ESI-IT-TOF-MSn Method and Their Anticancer Bioactivity. Molecules 2015, 20, 9155–9169. [Google Scholar] [CrossRef]
- Osakabe, N. Rosmarinic Acid Inhibits Epidermal Inflammatory Responses: Anticarcinogenic Effect of Perilla frutescens Extract in the Murine Two-Stage Skin Model. Carcinogenesis 2003, 25, 549–557. [Google Scholar] [CrossRef]
- Simayi, Z.; Rozi, P.; Yang, X.; Ababaikeri, G.; Maimaitituoheti, W.; Bao, X.; Ma, S.; Askar, G.; Yadikar, N. Isolation, Structural Characterization, Biological Activity, and Application of Glycyrrhiza Polysaccharides: Systematic Review. Int. J. Biol. Macromol. 2021, 183, 387–398. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Niu, Y.; Ju, H.; Chen, R.; Li, B.; Song, X.; Song, L. Chemical Characterization, Antitumor, and Immune-Enhancing Activities of Polysaccharide from Sargassum pallidum. Molecules 2021, 26, 7559. [Google Scholar] [CrossRef]
- Wang, Q.; Niu, L.-L.; Liu, H.-P.; Wu, Y.-R.; Li, M.-Y.; Jia, Q. Structural Characterization of a Novel Polysaccharide from Pleurotus citrinopileatus and Its Antitumor Activity on H22 Tumor-Bearing Mice. Int. J. Biol. Macromol. 2021, 168, 251–260. [Google Scholar] [CrossRef]
- Ji, D.; You, L.; Ren, Y.; Wen, L.; Zheng, G.; Li, C. Protective Effect of Polysaccharides from Sargassum fusiforme against UVB-Induced Oxidative Stress in HaCaT Human Keratinocytes. J. Funct. Foods 2017, 36, 332–340. [Google Scholar] [CrossRef]
- Yang, X.; Lin, P.; Wang, J.; Liu, N.; Yin, F.; Shen, N.; Guo, S. Purification, Characterization and Anti-Atherosclerotic Effects of the Polysaccharides from the Fruiting Body of Cordyceps militaris. Int. J. Biol. Macromol. 2021, 181, 890–904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, Y.; Wei, X.; Yang, Z.; Xiao, J.; Jin, Z. Sulfation of Tea Polysaccharides: Synthesis, Characterization and Hypoglycemic Activity. Int. J. Biol. Macromol. 2010, 46, 270–274. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fang, J.; Ruan, Y.; Wang, X.; Sun, Y.; Wu, N.; Zhao, Z.; Chang, Y.; Ning, N.; Guo, H.; et al. Structures, Bioactivities and Future Prospective of Polysaccharides from Morus alba (White Mulberry): A Review. Food Chem. 2018, 245, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhao, L.; Li, S.; Zhao, X.; Zhang, Q.; Xiong, Q. Preliminary Characterization and Immunostimulatory Activity of Polysaccharides from Glossaulax didyma. Food Chem. Toxicol. 2013, 62, 226–230. [Google Scholar] [CrossRef]
- Li, S.; Dai, S.; Shah, N.P. Sulfonation and Antioxidative Evaluation of Polysaccharides from Pleurotus Mushroom and Streptococcus thermophilus Bacteria: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 282–294. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated Modification of Polysaccharides: Synthesis, Characterization and Bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Liao, D.; Cheng, C.; Liu, J.; Zhao, L.; Huang, D.-C.; Chen, G. Characterization and Antitumor Activities of Polysaccharides Obtained from Ginger (Zingiber Officinale) by Different Extraction Methods. Int. J. Biol. Macromol. 2020, 152, 894–903. [Google Scholar] [CrossRef]
- Sheng, Y.; Liu, G.; Wang, M.; Lv, Z.; Du, P. A Selenium Polysaccharide from Platycodon Grandiflorum rescues PC12 Cell Death Caused by H2O2 via Inhibiting Oxidative Stress. Int. J. Biol. Macromol. 2017, 104, 393–399. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Xu, X.; Zeng, F. Correlation between Antitumor Activity, Molecular Weight, and Conformation of Lentinan. Carbohydr. Res. 2005, 340, 1515–1521. [Google Scholar] [CrossRef]
- Zhan, Q.; Wang, Q.; Lin, R.; He, P.; Lai, F.; Zhang, M.; Wu, H. Structural Characterization and Immunomodulatory Activity of a Novel Acid Polysaccharide Isolated from the Pulp of Rosa laevigata Michx Fruit. Int. J. Biol. Macromol. 2020, 145, 1080–1090. [Google Scholar] [CrossRef]
- Li, F.; Wei, Y.; Liang, L.; Huang, L.; Yu, G.; Li, Q. A Novel Low-Molecular-Mass Pumpkin Polysaccharide: Structural Characterization, Antioxidant Activity, and Hypoglycemic Potential. Carbohydr. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from Fruit Bodies of Cultivated Mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and Potential Prebiotic Activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, R.; Li, H.; Wang, Y.; Luo, Y.; Zou, J.; Zhao, B.; Chen, S. New Insight into the Joint Significance of Dietary Jujube Polysaccharides and 6-Gingerol in Antioxidant and Antitumor Activities. RSC Adv. 2021, 11, 33219–33234. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Huang, F.; Yang, C.; Huang, Q. In Vitro Digestion and Fermentation by Human Fecal Microbiota of Polysaccharides from Flaxseed. Molecules 2020, 25, 4354. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, S.; Feng, W.; Zhang, Z.; Li, H. Structural Characterization and Immunomodulatory Activities of Two Polysaccharides from Rehmanniae Radix Praeparata. Int. J. Biol. Macromol. 2021, 186, 385–395. [Google Scholar] [CrossRef]
- Xiong, Q.; Luo, G.; Zheng, F.; Wu, K.; Yang, H.; Chen, L.; Tian, W. Structural Characterization and Evaluation the Elicitors Activity of Polysaccharides from Chrysanthemum indicum. Carbohydr. Polym. 2021, 263, 117994. [Google Scholar] [CrossRef]
- Dourado, F.; Cardoso, S.; Silva, A.; Gama, F.; Coimbra, M. NMR Structural Elucidation of the Arabinan from Prunus dulcis Immunobiological Active Pectic Polysaccharides. Carbohydr. Polym. 2006, 66, 27–33. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Yu, J.; Liu, Y.; Lu, W.; Chai, Y.; Liu, C.; Pan, C.; Yao, W.; Gao, X. Structure, Chain Conformation, and Immunomodulatory Activity of the Polysaccharide Purified from Bacillus Calmette Guerin Formulation. Carbohydr. Polym. 2016, 150, 149–158. [Google Scholar] [CrossRef]
- Fu, Z.; Xia, L.; De, J.; Zhu, M.; Li, H.; Lu, Y.; Chen, D. Beneficial Effects on H1N1-Induced Acute Lung Injury and Structure Characterization of Anti-Complementary Acidic Polysaccharides from Juniperus pingii Var. Wilsonii. Int. J. Biol. Macromol. 2019, 129, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Gao, X.; Cheng, C.; Zhao, H.; Wang, Z.; Yi, J. Hepatoprotection Mechanism against Alcohol-Induced Liver Injury in Vivo and Structural Characterization of Pinus koraiensis Pine Nut Polysaccharide. Int. J. Biol. Macromol. 2020, 154, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, Q.; Pang, Z.; Xie, J.; Lin, L.; Yao, Q. Optimization Extraction, Characterization and Anticancer Activities of Polysaccharides from Mango Pomace. Int. J. Biol. Macromol. 2018, 117, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Nai, J.; Zhang, C.; Shao, H.; Li, B.; Li, H.; Gao, L.; Dai, M.; Zhu, L.; Sheng, H. Extraction, Structure, Pharmacological Activities and Drug Carrier Applications of Angelica sinensis Polysaccharide. Int. J. Biol. Macromol. 2021, 183, 2337–2353. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, X.; Ma, J.; Zhang, M.; Wu, H. Structural Elucidation of a Novel Pectin-Polysaccharide from the Petal of Saussurea laniceps and the Mechanism of Its Anti-HBV Activity. Carbohydr. Polym. 2019, 223, 115077. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, Y.; Hu, X.; Wang, J. Structural Characterization and Anti-Inflammatory Activity of a Polysaccharide from the Lignified okra. Carbohydr. Polym. 2021, 265, 118081. [Google Scholar] [CrossRef]
- Shang, X.-L.; Liu, C.-Y.; Dong, H.-Y.; Peng, H.-H.; Zhu, Z.-Y. Extraction, Purification, Structural Characterization, and Antioxidant Activity of Polysaccharides from Wheat Bran. J. Mol. Struct. 2021, 1233, 130096. [Google Scholar] [CrossRef]
- Cao, L.; Liu, X.; Qian, T.; Sun, G.; Guo, Y.; Chang, F.; Zhou, S.; Sun, X. Antitumor and Immunomodulatory Activity of Arabinoxylans: A Major Constituent of Wheat Bran. Int. J. Biol. Macromol. 2011, 48, 160–164. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Wang, D.; Li, X.; Wu, X.; Liu, X.; Du, G.; Li, X.; Qin, X.; Du, Y. Extraction, Characterization, Antitumor and Immunological Activities of Hemicellulose Polysaccharide from Astragalus Radix Herb Residue. Molecules 2019, 24, 3644. [Google Scholar] [CrossRef]
- Hu, J.; Yao, W.; Chang, S.; You, L.; Zhao, M.; Chi-Keung Cheung, P.; Hileuskaya, K. Structural Characterization and Anti-Photoaging Activity of a Polysaccharide from Sargassum fusiforme. Food Res. Int. 2022, 157, 111267. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Siu, K.-C.; Geng, P. Bioactive Ingredients and Medicinal Values of Grifola frondosa (Maitake). Foods 2021, 10, 95. [Google Scholar] [CrossRef]
- Li, X.-Q.; Liu, A.-J. Relationship between Heat Treatment on Structural Properties and Antitumor Activity of the Cold-Water Soluble Polysaccharides from Grifola frondosa. Glycoconj J 2020, 37, 107–117. [Google Scholar] [CrossRef]
- Surenjav, U.; Zhang, L.; Xu, X.; Zhang, X.; Zeng, F. Effects of Molecular Structure on Antitumor Activities of (1→3)-β-d-Glucans from Different Lentinus Edodes. Carbohydr. Polym. 2006, 63, 97–104. [Google Scholar] [CrossRef]
- Chang, X.; Chen, X.; Gong, P.; Yang, W.; Wang, L.; Liu, N.; Su, Y.; Zhao, Y. Anti-oxidant and anti-fatigue properties of apple pomace polysaccharides by acid or alkali extraction. Int. J. Food Sci. Technol. 2021, 57, 78–91. [Google Scholar] [CrossRef]
- Felz, S.; Vermeulen, P.; van Loosdrecht, M.C.; Lin, Y.M. Chemical characterization methods for the analysis of structural extracellular polymeric substances (EPS). Water Res. 2019, 157, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Xie, F.; Yaseen, A.; Chen, B.; Zhang, G.-L.; Wang, M.-K.; Shen, X.-F.; Li, F. A polysaccharide TKP-2-1 from Tamarindus indica L: Purification, structural characterization and immunomodulating activity. J. Funct. Foods 2021, 78, 104384. [Google Scholar] [CrossRef]
- Niu, L.; Wu, Y.; Liu, H.; Wang, Q.; Li, M.; Jia, Q. The Structural Characterization of a Novel Water-Soluble Polysaccharide from Edible Mushroom Leucopaxillus giganteus and Its Antitumor Activity on H22 Tumor-Bearing Mice. Chem. Biodivers. 2021, 18, e2001010. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Shen, W.; Zhou, Y.; Ma, L.; Xu, D.; Ding, J.; He, L.; Shen, B.; Zhou, C. Characterization of a polysaccharide from Eupolyphaga sinensis walker and its effective antitumor activity via lymphocyte activation. Int. J. Biol. Macromol. 2020, 162, 31–42. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, P.; Zhao, H.; Qiu, J.; Mac Regenstein, J.; Yang, X. Isolation, purification, structure and antioxidant activity of polysaccharide from pinecones of Pinus koraiensis. Carbohydr. Polym. 2020, 251, 117078. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.; Ren, P.; Zhang, Y.; Onyango, S.O. Ultrasound irradiation alters the spatial structure and improves the antioxidant activity of the yellow tea polysaccharide. Ultrason. Sonochemistry 2020, 70, 105355. [Google Scholar] [CrossRef]
- Xiang, B.; Yu, X.; Li, B.; Xiong, Y.; Long, M.; He, Q. Characterization, antioxidant, and anticancer activities of a neutral polysaccharide from Duchesnea indica (Andr.) Focke. J. Food Biochem. 2019, 43, e12899. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, X.; Lu, X.-T.; Cui, C.-B.; Gao, X.-F. Study on hypoglycemic effects of irradiated ginseng adventitious roots. Food Chem. X 2022, 13, 100234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).