Downregulation of the INDEHISCENT Gene by RNAi Resulted in Desired Pod Shatter Reduction of Lepidium campestre in Subsequent Generations
Abstract
1. Introduction
2. Results and Discussion
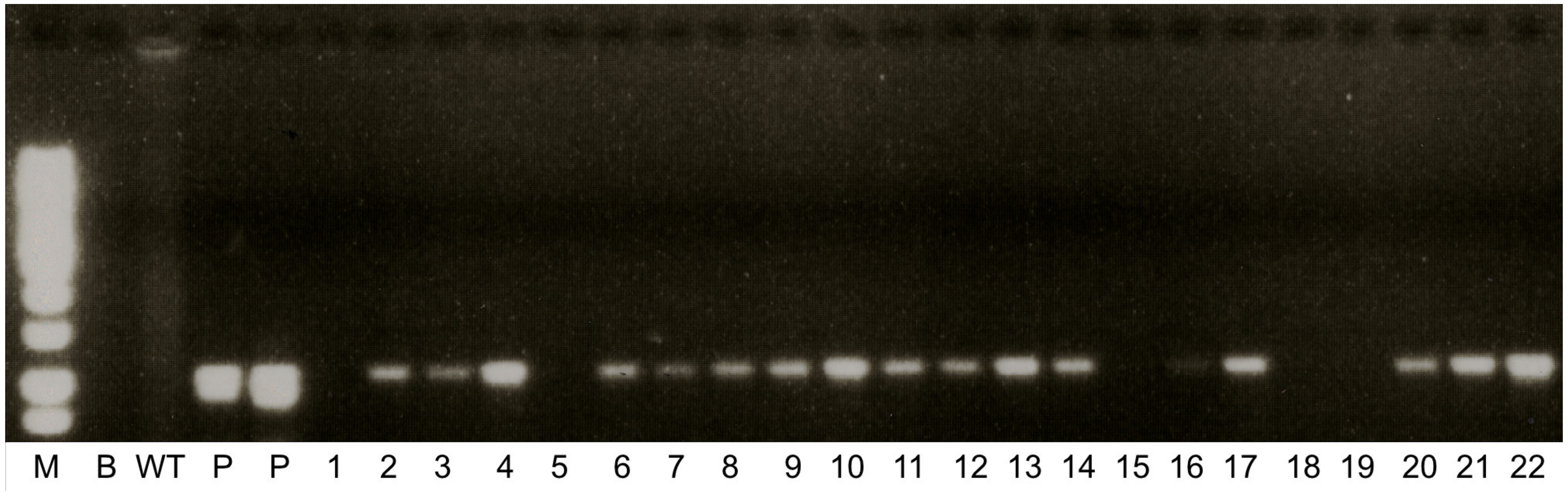
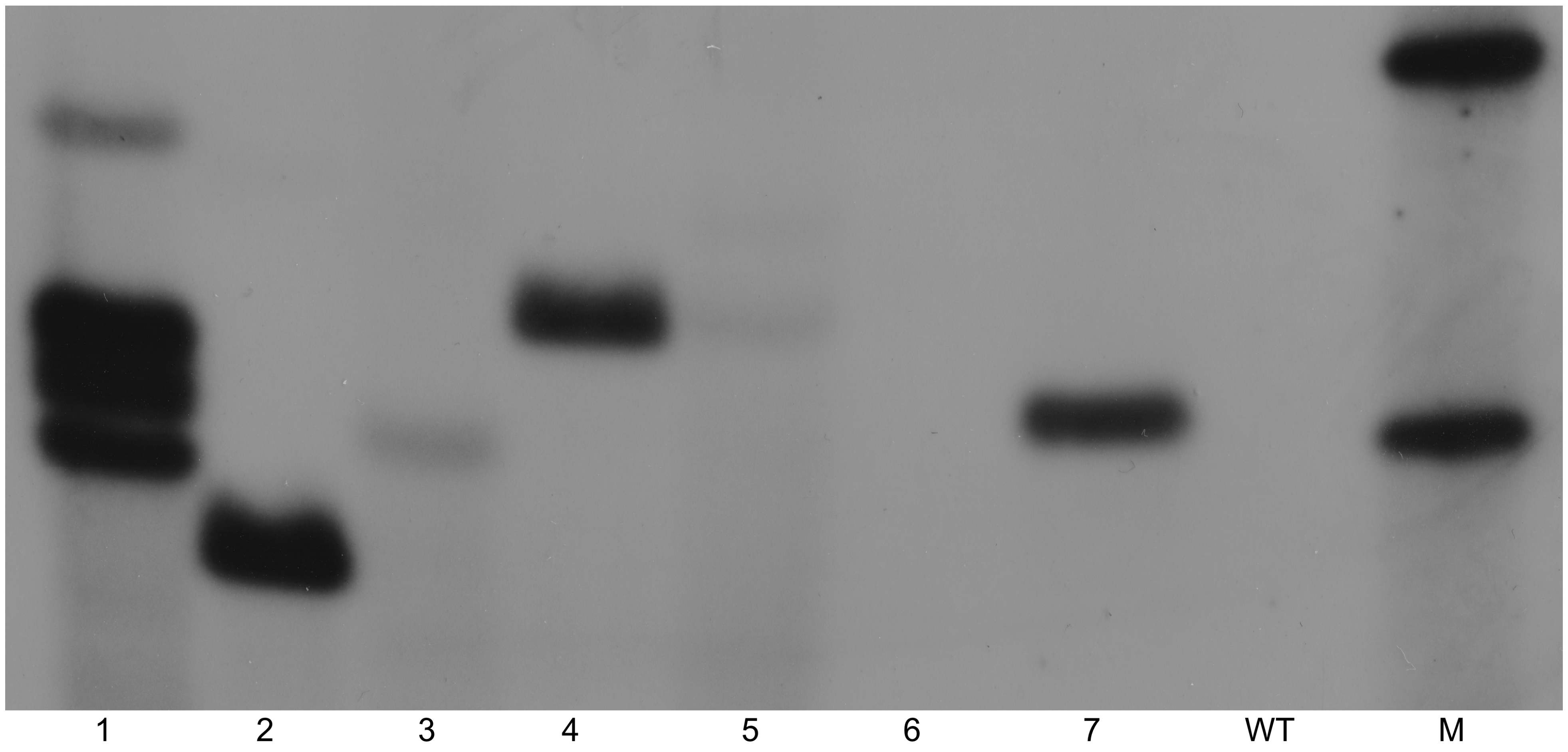
2.1. Confirmation of Transgenic Lines
2.2. Gene Expression Level
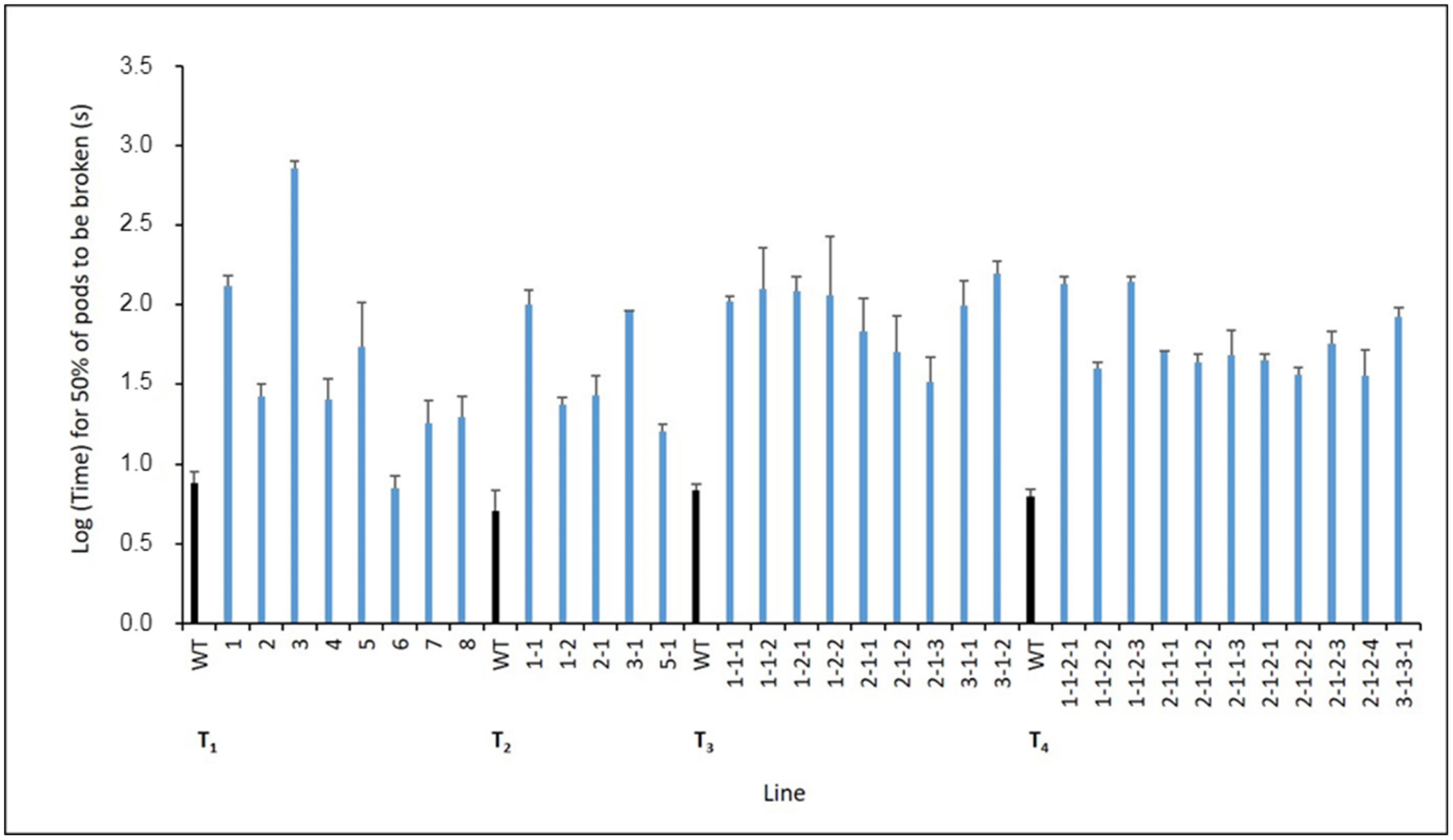
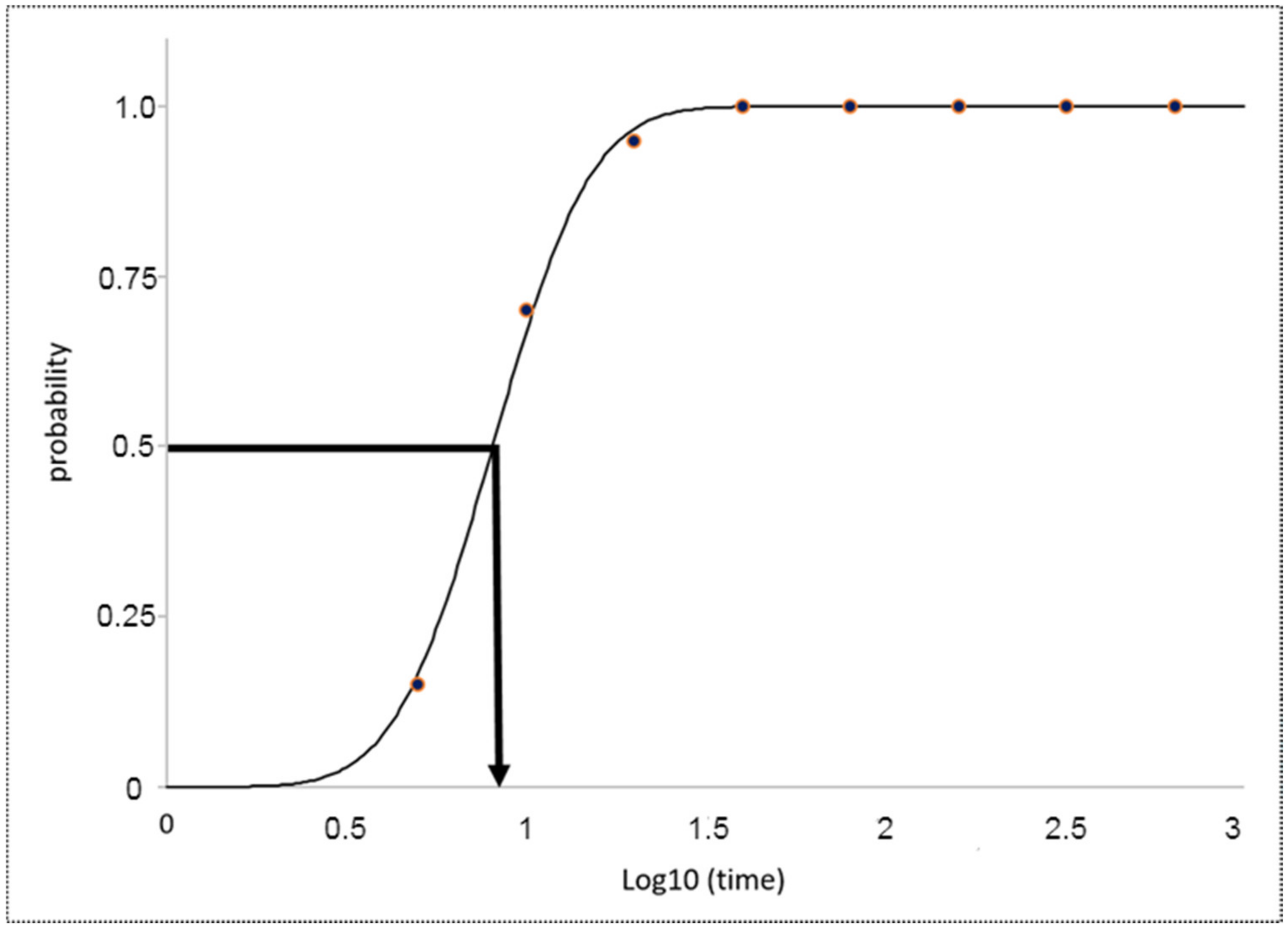
2.3. Pod Shatter Resistance
3. Materials and Methods
3.1. Plant Material
3.2. Transformation Vectors
3.3. Transformation
3.4. In Vitro Culture, Plant Growth Conditions and Management
3.5. Pod Shatter Resistance Analysis
3.6. Half-Life of Dehiscence
3.7. PCR Analysis
3.8. Southern Blot Analysis
3.9. Quantitative Reverse Transcription PCR Analysis
3.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, J.; Hobson, R.; Neale, M.; Bruce, D. Seed losses in commercial harvesting of oilseed rape. J. Agric. Eng. Res. 1996, 65, 183–191. [Google Scholar] [CrossRef]
- Summers, J.; Bruce, D.; Vancanneyt, G.; Redig, P.; Werner, C.; Morgan, C.; Child, R. Pod shatter resistance in the resynthesized Brassica napus line DK142. J. Agric. Sci. 2003, 140, 43–52. [Google Scholar] [CrossRef]
- Ostergaard, L.; Kempin, S.A.; Bies, D.; Klee, H.J.; Yanofsky, M.F. Pod shatter-resistant Brassica fruit produced by ectopic expression of the FRUITFULL gene. Plant Biotechnol. J. 2006, 4, 45–51. [Google Scholar] [CrossRef]
- Rajani, S.; Sundaresan, V. The Arabidopsis myc/bHLH gene ALCATRAZ enables cell separation in fruit dehiscence. Curr. Biol. 2001, 11, 1914–1922. [Google Scholar] [CrossRef]
- Meakin, P.J.; Roberts, J.A. Dehiscence of fruit in oilseed rape (Brassica napus L.): II. The role of cell wall degrading enzymes and ethylene. J. Exp. Bot. 1990, 41, 1003–1011. [Google Scholar] [CrossRef]
- Spence, J.; Vercher, Y.; Gates, P.; Harris, N. ‘Pod shatter’ in Arabidopsis thaliana Brassica napus and B. juncea. J. Microsc. 1996, 181, 195–203. [Google Scholar] [CrossRef]
- Ferrándiz, C.; Liljegren, S.J.; Yanofsky, M.F. Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science 2000, 289, 436–438. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Ditta, G.S.; Eshed, Y.; Savidge, B.; Bowman, J.L.; Yanofsky, M.F. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 2000, 404, 766–770. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Roeder, A.H.; Kempin, S.A.; Gremski, K.; Østergaard, L.; Guimil, S.; Reyes, D.K.; Yanofsky, M.F. Control of fruit patterning in Arabidopsis by INDEHISCENT. Cell 2004, 116, 843–853. [Google Scholar] [CrossRef]
- Gu, Q.; Ferrandiz, C.; Yanofsky, M.F.; Martienssen, R. The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development 1998, 125, 1509–1517. [Google Scholar] [CrossRef]
- Roeder, A.H.; Ferrandiz, C.; Yanofsky, M.F. The role of the REPLUMLESS homeodomain protein in patterning the Arabidopsis fruit. Curr. Biol. 2003, 13, 1630–1635. [Google Scholar] [CrossRef]
- Girin, T.; Stephenson, P.; Goldsack, C.M.P.; Kempin, S.A.; Perez, A.; Pires, N.; Sparrow, P.A.; Wood, T.A.; Yanofsky, M.F.; Østergaard, L. Brassicaceae INDEHISCENT genes specify valve margin cell fate and repress replum formation. Plant J. 2010, 63, 329–338. [Google Scholar] [CrossRef]
- Sorefan, K.; Girin, T.; Liljegren, S.J.; Ljung, K.; Robles, P.; Galván-Ampudia, C.S.; Offringa, R.; Friml, J.; Yanofsky, M.F.; Østergaard, L. A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature 2009, 459, 583–586. [Google Scholar] [CrossRef]
- Arnaud, N.; Girin, T.; Sorefan, K.; Fuentes, S.; Wood, T.A.; Lawrenson, T.; Sablowski, R.; Østergaard, L. Gibberellins control fruit patterning in Arabidopsis thaliana. Genes Dev. 2010, 24, 2127–2132. [Google Scholar] [CrossRef] [PubMed]
- Kord, H.; Shakib, A.M.; Daneshvar, M.H.; Azadi, P.; Bayat, V.; Mashayekhi, M.; Zarea, M.; Seifi, A.; Ahmad-Raji, M. RNAi-mediated down-regulation of SHATTERPROOF gene in transgenic oilseed rape. 3 Biotech 2014, 5, 271–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chandler, J.; Corbesier, L.; Spielmann, P.; Dettendorfer, J.; Stahl, D.; Apel, K.; Melzer, S. Modulating flowering time and prevention of pod shatter in oilseed rape. Mol. Breed. 2005, 15, 87–94. [Google Scholar] [CrossRef]
- Lenser, T.; Theissen, G. Conservation of fruit dehiscence pathways between Lepidium campestre and Arabidopsis thaliana sheds light on the regulation of INDEHISCENT. Plant J. 2013, 76, 545–556. [Google Scholar] [CrossRef]
- Stephenson, P.; Stacey, N.; Brüser, M.; Pullen, N.; Ilyas, M.; O’Neill, C.; Wells, R.; Østergaard, L. The power of model-to-crop translation illustrated by reducing seed loss from pod shatter in oilseed rape. Plant Reprod. 2019, 32, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Mummenhoff, K.; Polster, A.; Mühlhausen, A.; Theißen, G. Lepidium as a model system for studying the evolution of fruit development in Brassicaceae. J. Exp. Bot. 2009, 60, 1503–1513. [Google Scholar] [CrossRef]
- Petersen, M.; Sander, L.; Child, R.; van Onckelen, H.; Ulvskov, P.; Borkhardt, B. Isolation and characterisation of a pod dehiscence zone-specific polygalacturonase from Brassica napus. Plant Mol. Biol. 1996, 31, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Bruce, D.; Farrent, J.; Morgan, C.; Child, R. PA—Precision agriculture: Determining the oilseed rape pod strength needed to reduce seed loss due to pod shatter. Biosyst. Eng. 2002, 81, 179–184. [Google Scholar] [CrossRef]
- Ivarson, E.; Ahlman, A.; Li, X.; Zhu, L.-H. Development of an efficient regeneration and transformation method for the new potential oilseed crop Lepidium campestre. BMC Plant Biol. 2013, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Li, X.; Ahlman, A.; Yan, X.; Lindgren, H.; Zhu, L.-H. Genetic transformation of the oilseed crop Crambe abyssinica. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 100, 149–156. [Google Scholar] [CrossRef]
- Li, X.; Ahlman, A.; Lindgren, H.; Zhu, L.-H. Highly efficient in vitro regeneration of the industrial oilseed crop Crambe abyssinica. Ind. Crops Prod. 2011, 33, 170–175. [Google Scholar] [CrossRef]
- Smyth, D.R.; Bowman, J.L.; Meyerowitz, E.M. Early flower development in Arabidopsis. Plant Cell 1990, 2, 755–767. [Google Scholar] [PubMed]
- Aldrich, J.; Cullis, C. RAPD analysis in flax: Optimization of yield and reproducibility using klenTaq1 DNA polymerase, chelex 100, and gel purification of genomic DNA. Plant Mol. Biol. Rep. 1993, 11, 128–141. [Google Scholar] [CrossRef]
- Zhu, L.; Welander, M. Adventitious shoot regeneration of two dwarfing pear rootstocks and the development of a transformation protocol. J. Hortic. Sci. Biotechnol. 2000, 75, 745–752. [Google Scholar] [CrossRef]
- Zhu, L.H.; Li, X.Y.; Welander, M. Overexpression of the Arabidopsis gai gene in apple significantly reduces plant size. Plant Cell Rep. 2008, 27, 289–296. [Google Scholar] [CrossRef]
- Muhlhausen, A.; Lenser, T.; Mummenhoff, K.; Theissen, G. Evidence that an evolutionary transition from dehiscent to indehiscent fruits in Lepidium (Brassicaceae) was caused by a change in the control of valve margin identity genes. Plant J. 2013, 73, 824–835. [Google Scholar] [CrossRef]
- Ivarson, E.; Ahlman, A.; Lager, I.; Zhu, L.H. Significant increase of oleic acid level in the wild species Lepidium campestre through direct gene silencing. Plant Cell Rep. 2016, 35, 2055–2063. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivarson, E.; Ahlman, A.; Englund, J.-E.; Lager, I.; Zhu, L.-H. Downregulation of the INDEHISCENT Gene by RNAi Resulted in Desired Pod Shatter Reduction of Lepidium campestre in Subsequent Generations. Int. J. Mol. Sci. 2023, 24, 15943. https://doi.org/10.3390/ijms242115943
Ivarson E, Ahlman A, Englund J-E, Lager I, Zhu L-H. Downregulation of the INDEHISCENT Gene by RNAi Resulted in Desired Pod Shatter Reduction of Lepidium campestre in Subsequent Generations. International Journal of Molecular Sciences. 2023; 24(21):15943. https://doi.org/10.3390/ijms242115943
Chicago/Turabian StyleIvarson, Emelie, Annelie Ahlman, Jan-Eric Englund, Ida Lager, and Li-Hua Zhu. 2023. "Downregulation of the INDEHISCENT Gene by RNAi Resulted in Desired Pod Shatter Reduction of Lepidium campestre in Subsequent Generations" International Journal of Molecular Sciences 24, no. 21: 15943. https://doi.org/10.3390/ijms242115943
APA StyleIvarson, E., Ahlman, A., Englund, J.-E., Lager, I., & Zhu, L.-H. (2023). Downregulation of the INDEHISCENT Gene by RNAi Resulted in Desired Pod Shatter Reduction of Lepidium campestre in Subsequent Generations. International Journal of Molecular Sciences, 24(21), 15943. https://doi.org/10.3390/ijms242115943





