Regulatory Roles of Histone Deacetylation in Metabolic Stress-Induced Expression of Caspase Recruitment Domain-Containing Protein 9 (CARD9) in Pancreatic β-Cells
Abstract
1. Introduction
2. Results
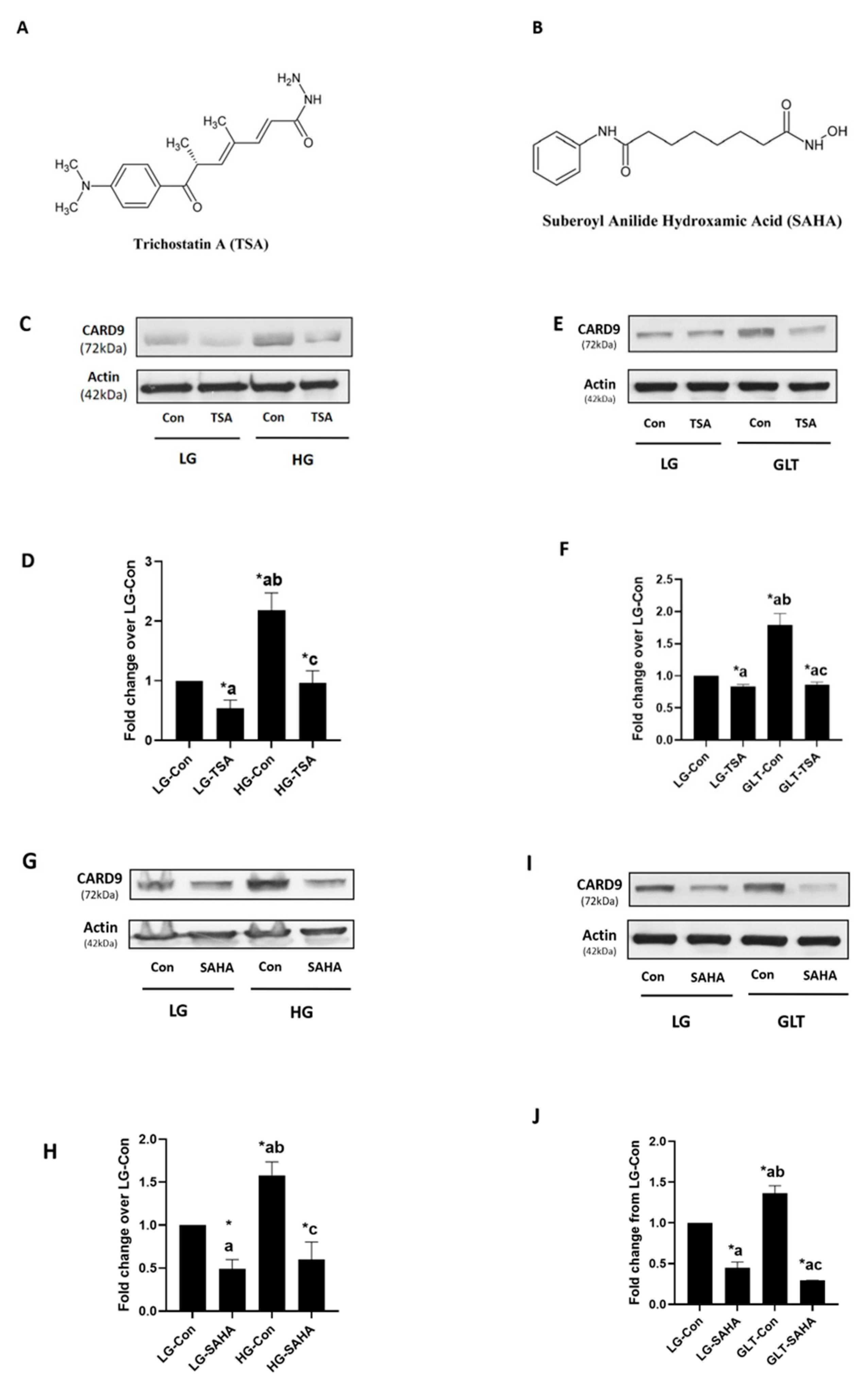
2.1. TSA and SAHA Inhibit Metabolic Stress-Induced CARD9 Expression in INS-1 832/13 Cells
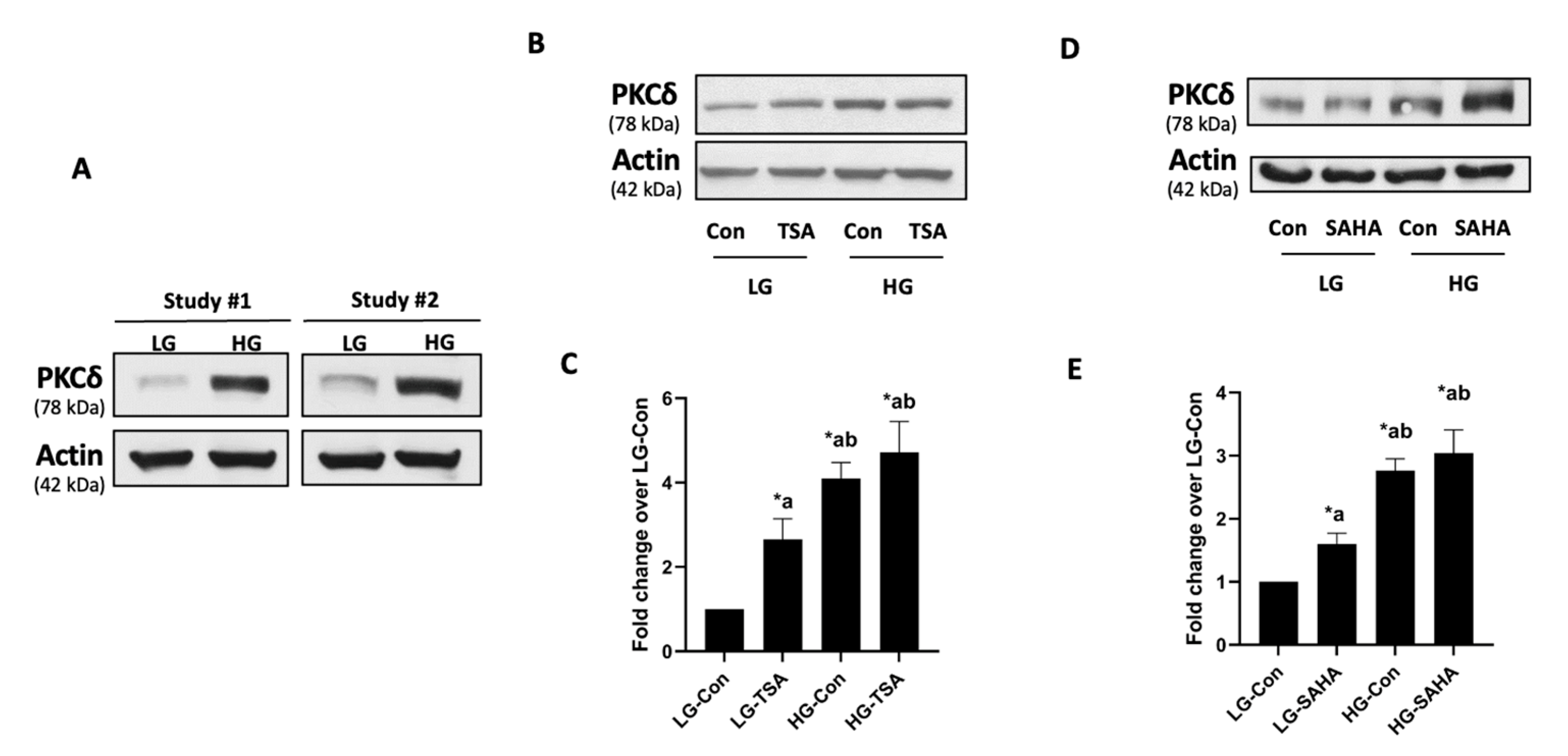
2.2. Hyperglycemic Conditions Significantly Increase the Expression of PKCδ in an HDAC-Independent Manner
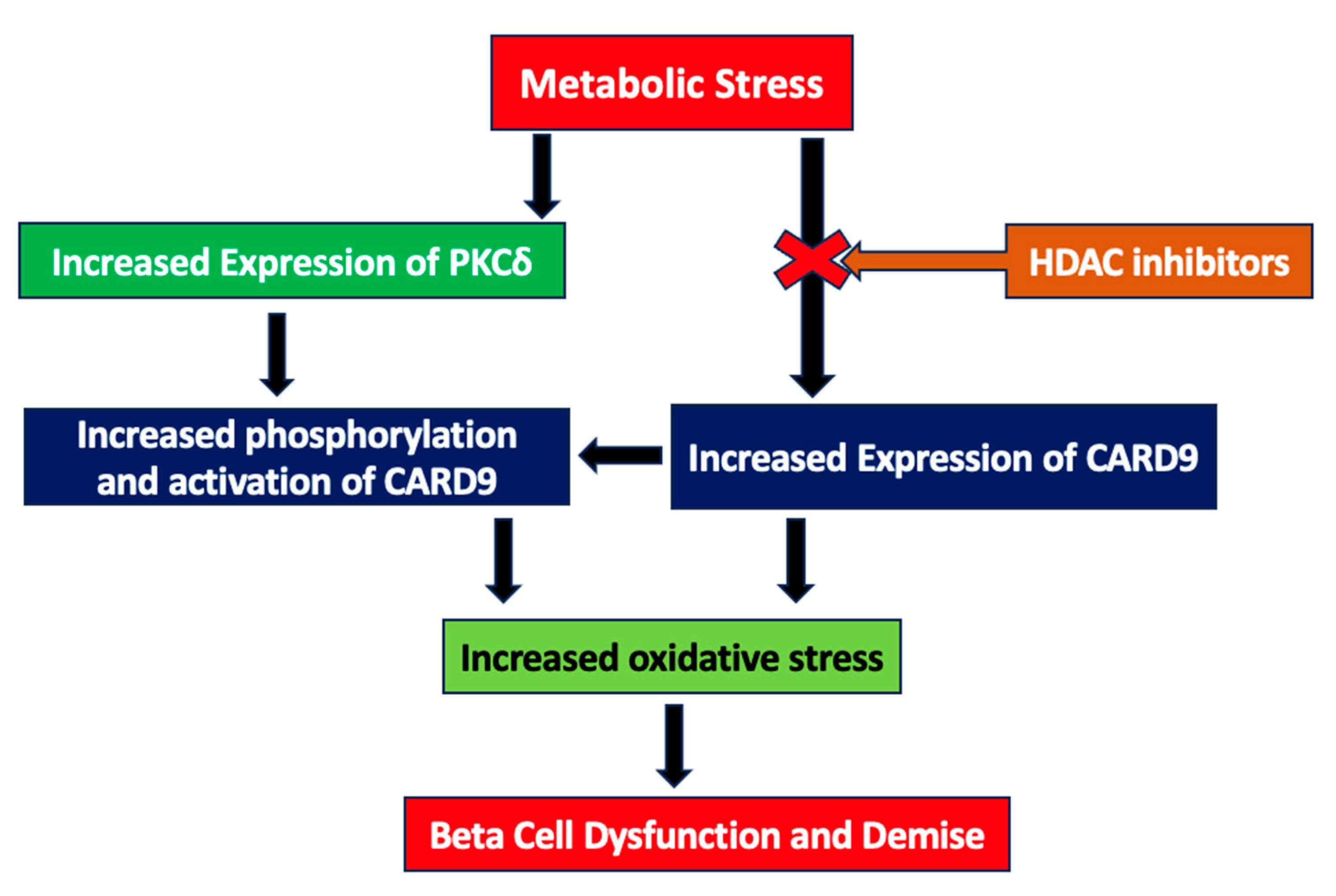
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Culture of Insulin-Secreting INS-1 832/13 Cells
4.3. Western Blotting
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milazzo, G.; Mercatelli, D.; Di Muzio, G.; Triboli, L.; De Rosa, P.; Perini, G.; Giorgi, F.M. Histone Deacetylases (HDACs): Evolution, Specificity, Role in Transcriptional Complexes, and Pharmacological Actionability. Genes 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Patel, M.; Chandrasekaran, S. Metabolism, HDACs, and HDAC Inhibitors: A Systems Biology Perspective. Metabolites 2021, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Psilopatis, I.; Garmpis, N.; Garmpi, A.; Vrettou, K.; Sarantis, P.; Koustas, E.; Antoniou, E.A.; Dimitroulis, D.; Kouraklis, G.; Karamouzis, M.V.; et al. The Emerging Role of Histone Deacetylase Inhibitors in Cervical Cancer Therapy. Cancers 2023, 15, 2222. [Google Scholar] [CrossRef] [PubMed]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef]
- Bocchi, L.; Motta, B.M.; Savi, M.; Vilella, R.; Meraviglia, V.; Rizzi, F.; Galati, S.; Buschini, A.; Lazzaretti, M.; Pramstaller, P.P.; et al. The Histone Deacetylase Inhibitor Suberoylanilide Hydroxamic Acid (SAHA) Restores Cardiomyocyte Contractility in a Rat Model of Early Diabetes. Int. J. Mol. Sci. 2019, 20, 1873. [Google Scholar] [CrossRef]
- Garmpi, A.; Damaskos, C.; Garmpis, N.; Kaminiotis, V.V.; Georgakopoulou, V.E.; Spandidos, D.A.; Papalexis, P.; Diamantis, E.; Patsouras, A.; Kyriakos, G.; et al. Role of histone deacetylase inhibitors in diabetic cardiomyopathy in experimental models (Review). Med. Int. 2022, 2, 26. [Google Scholar] [CrossRef]
- Hadden, M.J.; Advani, A. Histone Deacetylase Inhibitors and Diabetic Kidney Disease. Int. J. Mol. Sci. 2018, 19, 2630. [Google Scholar] [CrossRef]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; Chakraborty, P.; Gangopadhyay, M.; Sahu, R.; Medala, V.; John, A.; Reddy, P.H.; De Feo, V.; et al. The Emerging Role of HDACs: Pathology and Therapeutic Targets in Diabetes Mellitus. Cells 2021, 10, 1340. [Google Scholar] [CrossRef]
- Yoon, S.; Kang, G.; Eom, G.H. HDAC Inhibitors: Therapeutic Potential in Fibrosis-Associated Human Diseases. Int. J. Mol. Sci. 2019, 20, 1329. [Google Scholar] [CrossRef]
- Lamas, B.; Michel, M.L.; Waldschmitt, N.; Pham, H.P.; Zacharioudaki, V.; Dupraz, L.; Delacre, M.; Natividad, J.M.; Costa, G.D.; Planchais, J.; et al. Card9 mediates susceptibility to intestinal pathogens through microbiota modulation and control of bacterial virulence. Gut 2018, 67, 1836–1844. [Google Scholar] [CrossRef]
- Jia, X.M.; Tang, B.; Zhu, L.L.; Liu, Y.H.; Zhao, X.Q.; Gorjestani, S.; Hsu, Y.M.; Yang, L.; Guan, J.H.; Xu, G.T.; et al. CARD9 mediates Dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity. J. Exp. Med. 2014, 211, 2307–2321. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hsu, Y.M.; Bi, L.; Songyang, Z.; Lin, X. CARD9 facilitates microbe-elicited production of reactive oxygen species by regulating the LyGDI-Rac1 complex. Nat. Immunol. 2009, 10, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Ruland, J.; Hartjes, L. CARD-BCL-10-MALT1 signalling in protective and pathological immunity. Nat. Rev. Immunol. 2019, 19, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Ruland, J. CARD9 signaling in the innate immune response. Ann. N. Y. Acad. Sci. 2008, 1143, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Vornholz, L.; Ruland, J. Physiological and Pathological Functions of CARD9 Signaling in the Innate Immune System. Curr. Top. Microbiol. Immunol. 2020, 429, 177–203. [Google Scholar]
- Jiang, C.; Lin, X. Regulation of NF-κB by the CARD proteins. Immunol. Rev. 2012, 246, 141–153. [Google Scholar] [CrossRef]
- Roth, S.; Ruland, J. Caspase recruitment domain-containing protein 9 signaling in innate immunity and inflammation. Trends Immunol. 2013, 34, 243–250. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, B.; Hao, H.; Liu, Z. CARD9 Signaling, Inflammation, and Diseases. Front. Immunol. 2022, 13, 880879. [Google Scholar] [CrossRef]
- Tian, C.; Tuo, Y.L.; Lu, Y.; Xu, C.R.; Xiang, M. The Role of CARD9 in Metabolic Diseases. Curr. Med. Sci. 2020, 40, 199–205. [Google Scholar] [CrossRef]
- Cao, L.; Qin, X.; Peterson, M.R.; Haller, S.E.; Wilson, K.A.; Hu, N.; Lin, X.; Nair, S.; Ren, J.; He, G. CARD9 knockout ameliorates myocardial dysfunction associated with high fat diet-induced obesity. J. Mol. Cell Cardiol. 2016, 92, 185–195. [Google Scholar] [CrossRef]
- Zeng, X.; Du, X.; Zhang, J.; Jiang, S.; Liu, J.; Xie, Y.; Shan, W.; He, G.; Sun, Q.; Zhao, J. The essential function of CARD9 in diet-induced inflammation and metabolic disorders in mice. J. Cell Mol. Med. 2018, 22, 2993–3004. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.R.; Haller, S.E.; Ren, J.; Nair, S.; He, G. CARD9 as a potential target in cardiovascular disease. Drug Des. Dev. Ther. 2016, 10, 3799–3804. [Google Scholar] [CrossRef]
- Gamage, S.; Hali, M.; Chen, F.; Kowluru, A. CARD9 Mediates Pancreatic Islet Beta-Cell Dysfunction under the Duress of Hyperglycemic Stress. Cell Physiol. Biochem. 2022, 56, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, L.; Cordery, D.; Biden, T.J. Protein kinase Cdelta activation by interleukin-1beta stabilizes inducible nitric-oxide synthase mRNA in pancreatic beta-cells. J. Biol. Chem. 2001, 276, 5368–5374. [Google Scholar] [CrossRef] [PubMed]
- Eitel, K.; Staiger, H.; Rieger, J.; Mischak, H.; Brandhorst, H.; Brendel, M.D.; Bretzel, R.G.; Häring, H.U.; Kellerer, M. Protein kinase C delta activation and translocation to the nucleus are required for fatty acid-induced apoptosis of insulin-secreting cells. Diabetes 2003, 52, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Hennige, A.M.; Ranta, F.; Heinzelmann, I.; Düfer, M.; Michael, D.; Braumüller, H.; Lutz, S.Z.; Lammers, R.; Drews, G.; Bosch, F.; et al. Overexpression of kinase-negative protein kinase Cdelta in pancreatic beta-cells protects mice from diet-induced glucose intolerance and beta-cell dysfunction. Diabetes 2010, 59, 119–127. [Google Scholar] [CrossRef]
- Cantley, J.; Boslem, E.; Laybutt, D.R.; Cordery, D.V.; Pearson, G.; Carpenter, L.; Leitges, M.; Biden, T.J. Deletion of protein kinase Cδ in mice modulates stability of inflammatory genes and protects against cytokine-stimulated beta cell death in vitro and in vivo. Diabetologia 2011, 54, 380–389. [Google Scholar] [CrossRef]
- Li, D.D.; Jawale, C.V.; Zhou, C.; Lin, L.; Trevejo-Nunez, G.J.; Rahman, S.A.; Mullet, S.J.; Das, J.; Wendell, S.G.; Delgoffe, G.M.; et al. Fungal sensing enhances neutrophil metabolic fitness by regulating antifungal Glut1 activity. Cell Host Microbe 2022, 30, 530–544.e6. [Google Scholar] [CrossRef]
- Staal, J.; Driege, Y.; Haegman, M.; Kreike, M.; Iliaki, S.; Vanneste, D.; Lork, M.; Afonina, I.S.; Braun, H.; Beyaert, R. Defining the combinatorial space of PKC::CARD-CC signal transduction nodes. FEBS J. 2021, 288, 1630–1647. [Google Scholar] [CrossRef]
- Pandori, W.J.; Lima, T.S.; Mallya, S.; Kao, T.H.; Gov, L.; Lodoen, M.B. Toxoplasma gondii activates a Syk-CARD9-NF-κB signaling axis and gasdermin D-independent release of IL-1β during infection of primary human monocytes. PLoS Pathog. 2019, 15, e1007923. [Google Scholar] [CrossRef]
- Strasser, D.; Neumann, K.; Bergmann, H.; Marakalala, M.J.; Guler, R.; Rojowska, A.; Hopfner, K.P.; Brombacher, F.; Urlaub, H.; Baier, G.; et al. Syk kinase-coupled C-type lectin receptors engage protein kinase C-δ to elicit Card9 adaptor-mediated innate immunity. Immunity 2012, 36, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Gamage, S.; Hali, M.; Kowluru, A. CARD9 mediates glucose-stimulated insulin secretion in pancreatic beta cells. Biochem. Pharmacol. 2021, 192, 114670. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, V.; Rolland, L.; Gmyr, V.; Annicotte, J.S.; Kerr-Conte, J.; Pattou, F.; Abderrahmani, A. The class I histone deacetylase inhibitor MS-275 prevents pancreatic beta cell death induced by palmitate. J. Diabetes Res. 2014, 2014, 195739. [Google Scholar] [CrossRef]
- Remsberg, J.R.; Ediger, B.N.; Ho, W.Y.; Damle, M.; Li, Z.; Teng, C.; Lanzillotta, C.; Stoffers, D.A.; Lazar, M.A. Deletion of histone deacetylase 3 in adult beta cells improves glucose tolerance via increased insulin secretion. Mol. Metab. 2017, 6, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Daneshpajooh, M.; Bacos, K.; Bysani, M.; Bagge, A.; Laakso, E.O.; Vikman, P.; Eliasson, L.; Mulder, H.; Ling, C. HDAC7 is overexpressed in human diabetic islets and impairs insulin secretion in rat islets and clonal beta cells. Diabetologia 2017, 60, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Sonthalia, M.; Roy, B.S.; Chandrawanshi, D.; Ganesh, G.V.; Jayasuriya, R.; Mohandas, S.; Rajagopal, S.; Ramkumar, K.M. Histone deacetylase inhibitors as antidiabetic agents: Advances and opportunities. Eur. J. Pharmacol. 2022, 935, 175328. [Google Scholar] [CrossRef]
- Mócsai, A.; Ruland, J.; Tybulewicz, V.L. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat. Rev. Immunol. 2010, 10, 387–402. [Google Scholar] [CrossRef]
- Jin, H.; Kanthasamy, A.; Harischandra, D.S.; Kondru, N.; Ghosh, A.; Panicker, N.; Anantharam, V.; Rana, A.; Kanthasamy, A.G. Histone hyperacetylation up-regulates protein kinase Cδ in dopaminergic neurons to induce cell death: Relevance to epigenetic mechanisms of neurodegeneration in Parkinson disease. J. Biol. Chem. 2014, 289, 34743–34767. [Google Scholar] [CrossRef]
- Wang, L.T.; Liou, J.P.; Li, Y.H.; Liu, Y.M.; Pan, S.L.; Teng, C.M. A novel class I HDAC inhibitor, MPT0G030, induces cell apoptosis and differentiation in human colorectal cancer cells via HDAC1/PKCδ and E-cadherin. Oncotarget 2014, 5, 5651–5662. [Google Scholar] [CrossRef][Green Version]
- Kaur, S.; Mirza, A.H.; Overgaard, A.J.; Pociot, F.; Størling, J. A Dual Systems Genetics Approach Identifies Common Genes, Networks, and Pathways for Type 1 and 2 Diabetes in Human Islets. Front. Genet. 2021, 12, 630109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hali, M.; Pinto, N.; Gleason, N.; Kowluru, A. Regulatory Roles of Histone Deacetylation in Metabolic Stress-Induced Expression of Caspase Recruitment Domain-Containing Protein 9 (CARD9) in Pancreatic β-Cells. Int. J. Mol. Sci. 2023, 24, 15994. https://doi.org/10.3390/ijms242115994
Hali M, Pinto N, Gleason N, Kowluru A. Regulatory Roles of Histone Deacetylation in Metabolic Stress-Induced Expression of Caspase Recruitment Domain-Containing Protein 9 (CARD9) in Pancreatic β-Cells. International Journal of Molecular Sciences. 2023; 24(21):15994. https://doi.org/10.3390/ijms242115994
Chicago/Turabian StyleHali, Mirabela, Nelson Pinto, Noah Gleason, and Anjaneyulu Kowluru. 2023. "Regulatory Roles of Histone Deacetylation in Metabolic Stress-Induced Expression of Caspase Recruitment Domain-Containing Protein 9 (CARD9) in Pancreatic β-Cells" International Journal of Molecular Sciences 24, no. 21: 15994. https://doi.org/10.3390/ijms242115994
APA StyleHali, M., Pinto, N., Gleason, N., & Kowluru, A. (2023). Regulatory Roles of Histone Deacetylation in Metabolic Stress-Induced Expression of Caspase Recruitment Domain-Containing Protein 9 (CARD9) in Pancreatic β-Cells. International Journal of Molecular Sciences, 24(21), 15994. https://doi.org/10.3390/ijms242115994




