Stimulation of the Pro-Resolving Receptor Fpr2 Reverses Inflammatory Microglial Activity by Suppressing NFκB Activity
Abstract
1. Introduction
2. Results
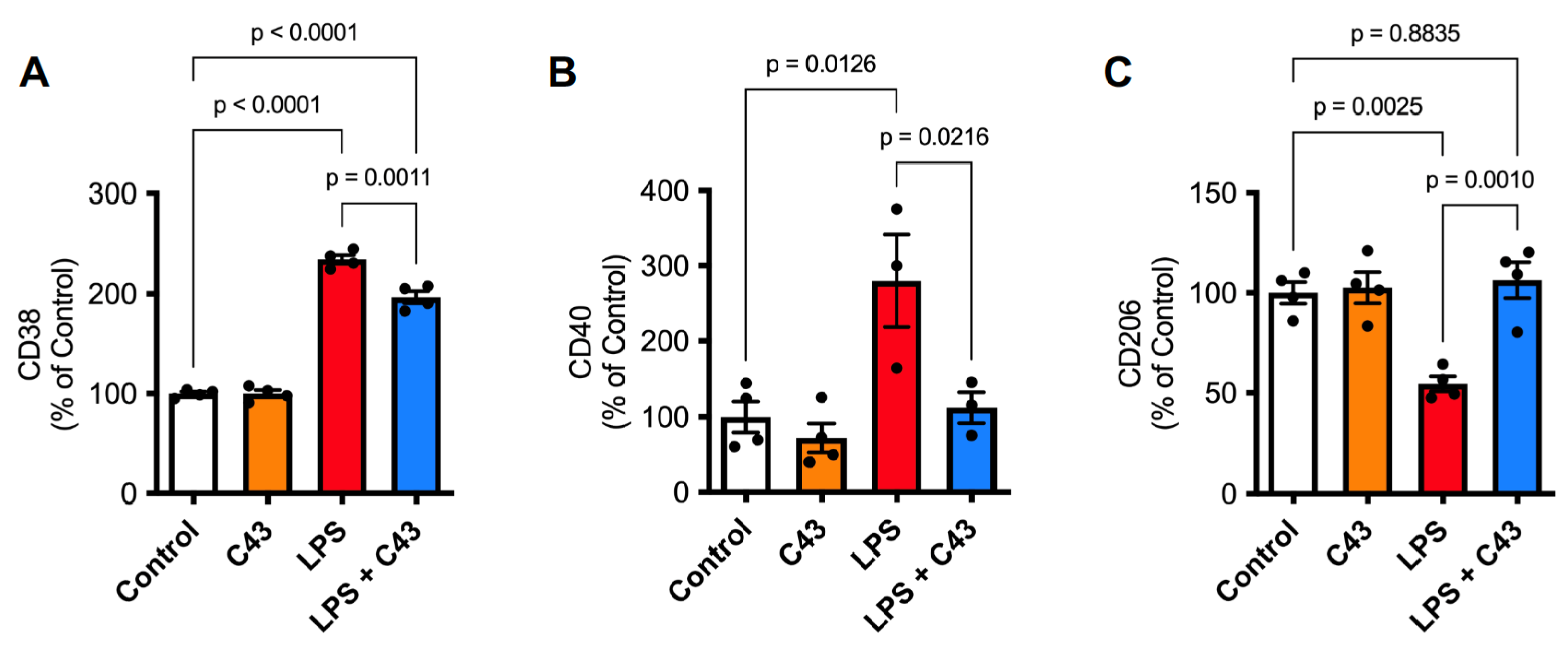
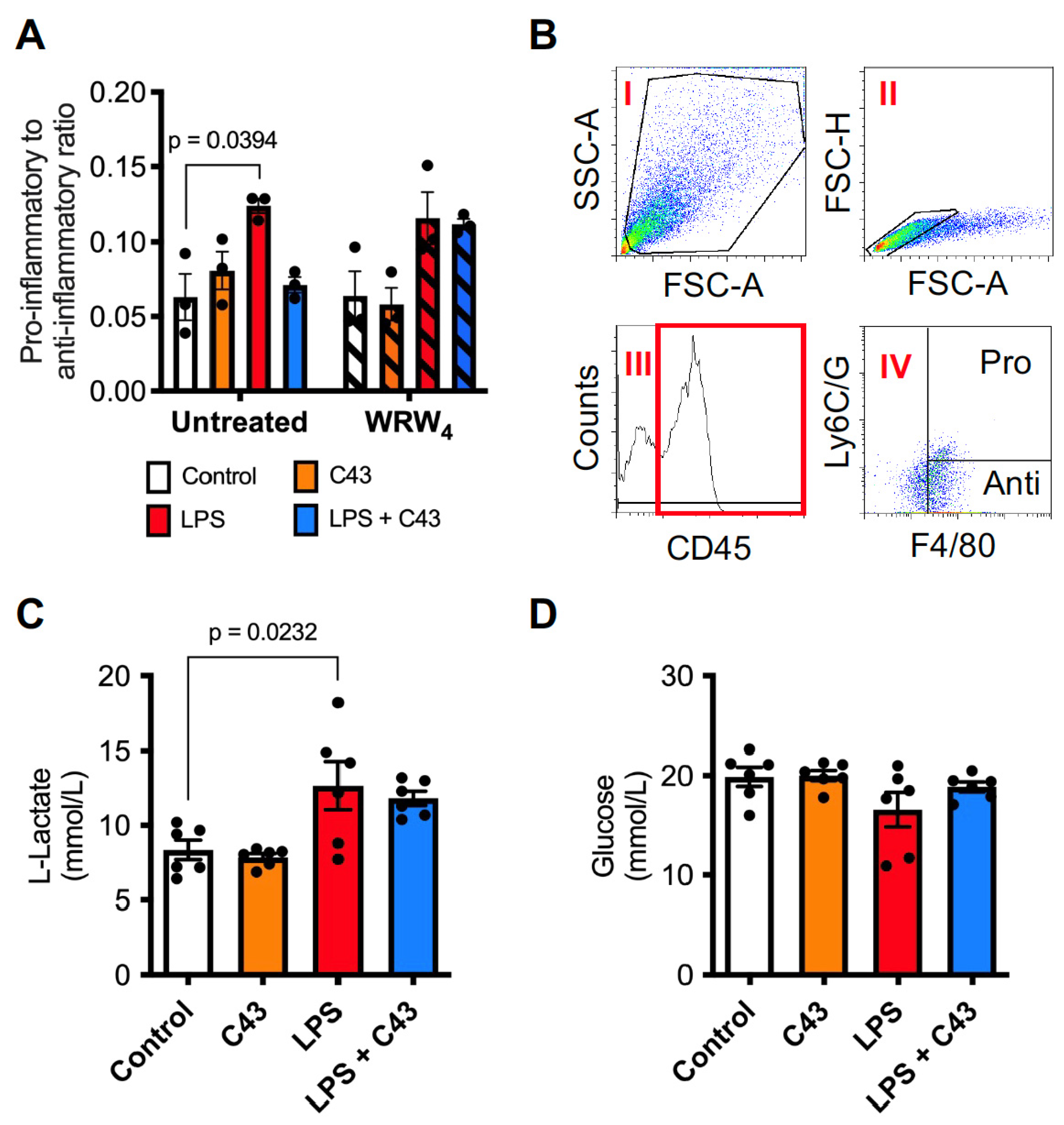
2.1. Fpr2 Stimulation Reverses the Pro-Inflammatory Effects of LPS upon Microglia
2.2. LPS Induces ROS Production through Both the Mitochondria and NADPH Oxidase Activation, a Response Reversed by Fpr2/3 Agonist C43
2.3. C43 Signals through p38 MAPK but Not ERK1/2
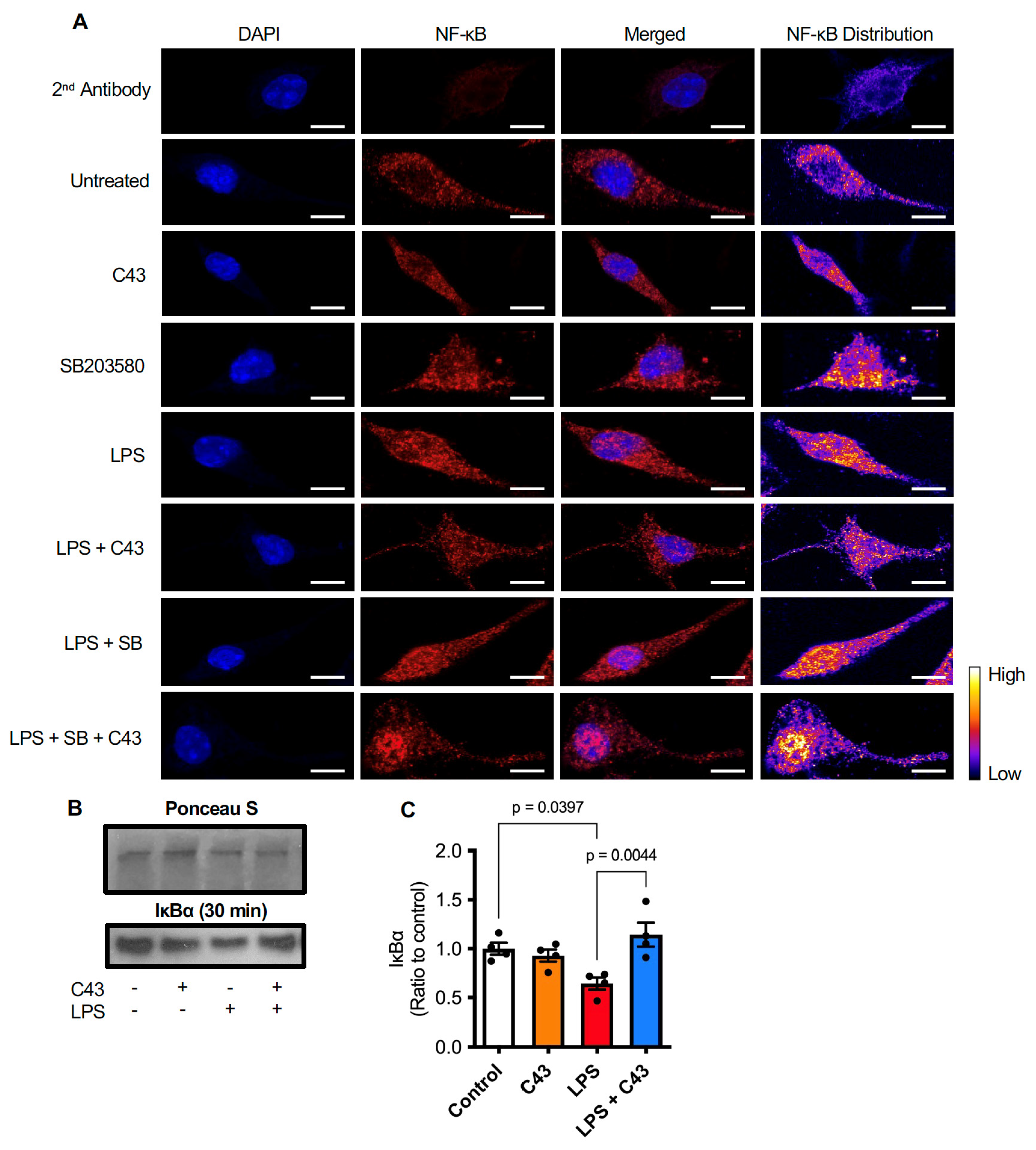
2.4. C43 Reduces NFκB Activation by Decreasing LPS-Induced IκBα Degradation
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Cell Culture
4.3. Primary Microglial Culture
4.4. PrestoBlue Cell Proliferation Assay
4.5. Griess Assay
4.6. Cytokine ELISA
4.7. p38 MAPK ELISA
4.8. Phenotypic Marker Expression
4.9. PC12 Cell Phagocytosis
4.10. Lactate & Glucose Determination
4.11. Reactive Oxygen Species (ROS) Assays
4.12. Western Blot Analysis
4.13. Immunofluorescence & Confocal Microscopy
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heckmann, B.L.; Teubner, B.J.W.; Tummers, B.; Boada-Romero, E.; Harris, L.; Yang, M.; Guy, C.S.; Zakharenko, S.S.; Green, D.R. LC3-Associated Endocytosis Facilitates β-Amyloid Clearance and Mitigates Neurodegeneration in Murine Alzheimer’s Disease. Cell 2019, 178, 536–551.e14. [Google Scholar] [CrossRef] [PubMed]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.E.; Vernoux, N.; Tremblay, M.È.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia Are an Essential Component of the Neuroprotective Scar That Forms after Spinal Cord Injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef] [PubMed]
- Zabala, A.; Vazquez-Villoldo, N.; Rissiek, B.; Gejo, J.; Martin, A.; Palomino, A.; Perez-Samartín, A.; Pulagam, K.R.; Lukowiak, M.; Capetillo-Zarate, E.; et al. P2X4 Receptor Controls Microglia Activation and Favors Remyelination in Autoimmune Encephalitis. EMBO Mol. Med. 2018, 10, e8743. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in Acute Stroke: Targeting Excitotoxicity, Oxidative and Nitrosative Stress, and Inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Gyoneva, S.; Ransohoff, R.M. Inflammatory Reaction after Traumatic Brain Injury: Therapeutic Potential of Targeting Cell-Cell Communication by Chemokines. Trends Pharmacol. Sci. 2015, 36, 471–480. [Google Scholar] [CrossRef]
- Serhan, C.N. Treating Inflammation and Infection in the 21st Century: New Hints from Decoding Resolution Mediators and Mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef]
- Dokalis, N.; Prinz, M. Resolution of Neuroinflammation: Mechanisms and Potential Therapeutic Option. Semin. Immunopathol. 2019, 41, 699–709. [Google Scholar] [CrossRef]
- McArthur, S.; Gobbetti, T.; Kusters, D.H.M.; Reutelingsperger, C.P.; Flower, R.J.; Perretti, M. Definition of a Novel Pathway Centered on Lysophosphatidic Acid To Recruit Monocytes during the Resolution Phase of Tissue Inflammation. J. Immunol. 2015, 195, 1139–1151. [Google Scholar] [CrossRef]
- Gobbetti, T.; Coldewey, S.M.; Chen, J.; McArthur, S.; Le Faouder, P.; Cenac, N.; Flower, R.J.; Thiemermann, C.; Perretti, M. Nonredundant Protective Properties of FPR2/ALX in Polymicrobial Murine Sepsis. Proc. Natl. Acad. Sci. USA 2014, 111, 18685–18690. [Google Scholar] [CrossRef]
- Dufton, N.; Hannon, R.; Brancaleone, V.; Dalli, J.; Patel, H.B.; Gray, M.; D’Acquisto, F.; Buckingham, J.C.; Perretti, M.; Flower, R.J. Anti-Inflammatory Role of the Murine Formyl-Peptide Receptor 2: Ligand-Specific Effects on Leukocyte Responses and Experimental Inflammation. J. Immunol. 2010, 184, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Stempel, H.; Jung, M.; Pérez-Gómez, A.; Leinders-Zufall, T.; Zufall, F.; Bufe, B. Strain-Specific Loss of Formyl Peptide Receptor 3 in the Murine Vomeronasal and Immune Systems. J. Biol. Chem. 2016, 291, 9762–9775. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, M.; De Jong, R.; Rossaint, J.; Viola, J.R.; Leoni, G.; Wang, J.M.; Grommes, J.; Hinkel, R.; Kupatt, C.; Weber, C.; et al. Annexin A1 Counteracts Chemokine-Induced Arterial Myeloid Cell Recruitment. Circ. Res. 2015, 116, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Scannell, M.; Flanagan, M.B.; deStefani, A.; Wynne, K.J.; Cagney, G.; Godson, C.; Maderna, P. Annexin-1 and Peptide Derivatives Are Released by Apoptotic Cells and Stimulate Phagocytosis of Apoptotic Neutrophils by Macrophages. J. Immunol. 2007, 178, 4595–4605. [Google Scholar] [CrossRef]
- McArthur, S.; Juban, G.; Gobbetti, T.; Desgeorges, T.; Theret, M.; Gondin, J.; Toller-Kawahisa, J.E.; Reutelingsperger, C.P.; Chazaud, B.; Perretti, M.; et al. Annexin A1 Drives Macrophage Skewing to Accelerate Muscle Regeneration through AMPK Activation. J. Clin. Investig. 2020, 130, 1156–1167. [Google Scholar] [CrossRef]
- de Araújo, S.; de Melo Costa, V.R.; Santos, F.M.; de Sousa, C.D.F.; Moreira, T.P.; Gonçalves, M.R.; Félix, F.B.; Queiroz-Junior, C.M.; Campolina-Silva, G.H.; Nogueira, M.L.; et al. Annexin A1-FPR2/ALX Signaling Axis Regulates Acute Inflammation during Chikungunya Virus Infection. Cells 2022, 11, 2717. [Google Scholar] [CrossRef]
- Birkl, D.; O’Leary, M.N.; Quiros, M.; Azcutia, V.; Schaller, M.; Reed, M.; Nishio, H.; Keeney, J.; Neish, A.S.; Lukacs, N.W.; et al. Formyl Peptide Receptor 2 Regulates Monocyte Recruitment to Promote Intestinal Mucosal Wound Repair. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 13632–13643. [Google Scholar] [CrossRef]
- Kain, V.; Jadapalli, J.K.; Tourki, B.; Halade, G.V. Inhibition of FPR2 Impaired Leukocytes Recruitment and Elicited Non-Resolving Inflammation in Acute Heart Failure. Pharmacol. Res. 2019, 146, 104295. [Google Scholar] [CrossRef]
- Wickstead, E.S.; Solito, E.; McArthur, S. Promiscuous Receptors and Neuroinflammation: The Formyl Peptide Class. Life 2022, 12, 2009. [Google Scholar] [CrossRef]
- Cooray, S.N.; Gobbetti, T.; Montero-Melendez, T.; McArthur, S.; Thompson, D.; Clark, A.J.L.; Flower, R.J.; Perretti, M. Ligand-Specific Conformational Change of the G-Protein-Coupled Receptor ALX/FPR2 Determines Proresolving Functional Responses. Proc. Natl. Acad. Sci. USA 2013, 110, 18232–18237. [Google Scholar] [CrossRef]
- Cui, Y.H.; Le, Y.; Zhang, X.; Gong, W.; Abe, K.; Sun, R.; Van Damme, J.; Proost, P.; Wang, J.M. Up-Regulation of FPR2, a Chemotactic Receptor for Amyloid β 1-42 (Aβ42), in Murine Microglial Cells by TNFα1. Neurobiol. Dis. 2002, 10, 366–377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, K.; Iribarren, P.; Hu, J.; Chen, J.; Gong, W.; Cho, E.H.; Lockett, S.; Dunlop, N.M.; Ji, M.W. Activation of Toll-like Receptor 2 on Microglia Promotes Cell Uptake of Alzheimer Disease-Associated Amyloid β Peptide. J. Biol. Chem. 2006, 281, 3651–3659. [Google Scholar] [CrossRef] [PubMed]
- Pourbadie, H.G.; Sayyah, M.; Khoshkholgh-Sima, B.; Choopani, S.; Nategh, M.; Motamedi, F.; Shokrgozar, M.A. Early Minor Stimulation of Microglial TLR2 and TLR4 Receptors Attenuates Alzheimer’s Disease–Related Cognitive Deficit in Rats: Behavioral, Molecular, and Electrophysiological Evidence. Neurobiol. Aging 2018, 70, 203–216. [Google Scholar] [CrossRef]
- Ries, M.; Loiola, R.; Shah, U.N.; Gentleman, S.M.; Solito, E.; Sastre, M. The Anti-Inflammatory Annexin A1 Induces the Clearance and Degradation of the Amyloid- β Peptide. J. Neuroinflamm. 2016, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Wickstead, E.S.; Karim, H.A.; Manuel, R.E.; Biggs, C.S.; Getting, S.J.; McArthur, S. Reversal of β-Amyloid-Induced Microglial Toxicity In Vitro by Activation of Fpr2/3. Oxidative Med. Cell. Longev. 2020, 2020, 2139192. [Google Scholar] [CrossRef]
- Slowik, A.; Merres, J.; Elfgen, A.; Jansen, S.; Mohr, F.; Wruck, C.J.; Pufe, T.; Brandenburg, L.O. Involvement of Formyl Peptide Receptors in Receptor for Advanced Glycation End Products (RAGE)—And Amyloid Beta 1-42-Induced Signal Transduction in Glial Cells. Mol. Neurodegener. 2012, 7, 1. [Google Scholar] [CrossRef]
- McArthur, S.; Cristante, E.; Paterno, M.; Christian, H.; Roncaroli, F.; Gillies, G.E.; Solito, E. Annexin A1: A Central Player in the Anti-Inflammatory and Neuroprotective Role of Microglia. J. Immunol. 2010, 185, 6317–6328. [Google Scholar] [CrossRef]
- Brown, G.C.; Neher, J.J. Eaten Alive! Cell Death by Primary Phagocytosis: “Phagoptosis”. Trends Biochem. Sci. 2012, 37, 325–332. [Google Scholar] [CrossRef]
- Kelly, B.; O’Neill, L.A.J. Metabolic Reprogramming in Macrophages and Dendritic Cells in Innate Immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Forsman, H.; Önnheim, K.; Andreasson, E.; Dahlgren, C. What Formyl Peptide Receptors, If Any, Are Triggered by Compound 43 and Lipoxin A4? Scand. J. Immunol. 2011, 74, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Loiola, R.A.; Wickstead, E.S.; Solito, E.; McArthur, S. Estrogen Promotes Pro-Resolving Microglial Behavior and Phagocytic Cell Clearance through the Actions of Annexin A1. Front. Endocrinol. 2019, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, J.G.; Cho, W.S.; Cho, K.H.; Sakong, J.; Kim, J.R.; Chin, B.R.; Baek, S.H. Role of NADPH Oxidase-2 in Lipopolysaccharide-Induced Matrix Metalloproteinase Expression and Cell Migration. Immunol. Cell Biol. 2010, 88, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.A.; Cole, M.P. Oxidative and Nitrative Stress in Neurodegeneration. Neurobiol. Dis. 2015, 84, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Lin, S.; Chen, H.Y.; Chen, Y.P.; Chen, T.Y.; Hsu, K.S.; Wu, H.M. NADPH Oxidases as Potential Pharmacological Targets against Increased Seizure Susceptibility after Systemic Inflammation. J. Neuroinflamm. 2018, 15, 140. [Google Scholar] [CrossRef]
- Haslund-Vinding, J.; McBean, G.; Jaquet, V.; Vilhardt, F. NADPH Oxidases in Oxidant Production by Microglia: Activating Receptors, Pharmacology and Association with Disease. Br. J. Pharmacol. 2017, 174, 1733–1749. [Google Scholar] [CrossRef]
- Youssef, M.; Ibrahim, A.; Akashi, K.; Hossain, M.S. PUFA-Plasmalogens Attenuate the LPS-Induced Nitric Oxide Production by Inhibiting the NF-kB, P38 MAPK and JNK Pathways in Microglial Cells. Neuroscience 2019, 397, 18–30. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Aslanidis, A.; Karlstetter, M.; Scholz, R.; Fauser, S.; Neumann, H.; Fried, C.; Pietsch, M.; Langmann, T. Activated Microglia/Macrophage Whey Acidic Protein (AMWAP) Inhibits NFΚB Signaling and Induces a Neuroprotective Phenotype in Microglia. J. Neuroinflamm. 2015, 12, 77. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Bar, E.; Barak, B. Microglia Roles in Synaptic Plasticity and Myelination in Homeostatic Conditions and Neurodevelopmental Disorders. Glia 2019, 67, 2125–2141. [Google Scholar] [CrossRef] [PubMed]
- Kurematsu, C.; Sawada, M.; Ohmuraya, M.; Tanaka, M.; Kuboyama, K.; Ogino, T.; Matsumoto, M.; Oishi, H.; Inada, H.; Ishido, Y.; et al. Synaptic Pruning of Murine Adult-Born Neurons by Microglia Depends on Phosphatidylserine. J. Exp. Med. 2022, 219, e20202304. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; El Khoury, J. Microglia in Health and Disease. Cold Spring Harb. Perspect. 2015, 8, a020560. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in Neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Stevens, B. Microglia Emerge as Central Players in Brain Disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Meng, H.; Zhao, H.; Cao, X.; Hao, J.; Zhang, H.; Liu, Y.; Zhu, M.S.; Fan, L.; Weng, L.; Qian, L.; et al. Double-Negative T Cells Remarkably Promote Neuroinflammation after Ischemic Stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 5558–5563. [Google Scholar] [CrossRef]
- Schattling, B.; Engler, J.B.; Volkmann, C.; Rothammer, N.; Woo, M.S.; Petersen, M.; Winkler, I.; Kaufmann, M.; Rosenkranz, S.C.; Fejtova, A.; et al. Bassoon Proteinopathy Drives Neurodegeneration in Multiple Sclerosis. Nat. Neurosci. 2019, 22, 887–896. [Google Scholar] [CrossRef]
- Felsky, D.; Roostaei, T.; Nho, K.; Risacher, S.L.; Bradshaw, E.M.; Petyuk, V.; Schneider, J.A.; Saykin, A.; Bennett, D.A.; De Jager, P.L. Neuropathological Correlates and Genetic Architecture of Microglial Activation in Elderly Human Brain. Nat. Commun. 2019, 10, 409. [Google Scholar] [CrossRef]
- Smith, C.J.; Hulme, S.; Vail, A.; Heal, C.; Parry-Jones, A.R.; Scarth, S.; Hopkins, K.; Hoadley, M.; Allan, S.M.; Rothwell, N.J.; et al. SCIL-STROKE (Subcutaneous Interleukin-1 Receptor Antagonist in Ischemic Stroke): A Randomized Controlled Phase 2 Trial. Stroke 2018, 49, 1210–1216. [Google Scholar] [CrossRef]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Cox, L.M.; Tjon, E.; Kivisakk, P.; Vanande, I.P.; Cook, S.; Gandhi, R.; Glanz, B.; et al. Investigation of Probiotics in Multiple Sclerosis. Mult. Scler. 2018, 24, 58–63. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Chen, Y.; Han, X.; Li, L.; Yang, Z.; Duan, L.; Lu, H.; He, Q. The Protective Effect of WKYMVm Peptide on Inflammatory Osteolysis through Regulating NF-κB and CD9/Gp130/STAT3 Signalling Pathway. J. Cell. Mol. Med. 2020, 24, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Kirkley, K.S.; Popichak, K.A.; Afzali, M.F.; Legare, M.E.; Tjalkens, R.B. Microglia Amplify Inflammatory Activation of Astrocytes in Manganese Neurotoxicity. J. Neuroinflamm. 2017, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Savill, J. Resolution of Inflammation: The Beginning Programs the End. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Malik, A.; Choi, H.B.; Ko, R.W.Y.; Dissing-Olesen, L.; MacVicar, B.A. Microglial CR3 Activation Triggers Long-Term Synaptic Depression in the Hippocampus via NADPH Oxidase. Neuron 2014, 82, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Sobotka, K.S.; Joshi, P.; Gressens, P.; Fleiss, B.; Thornton, C.; Mallard, C.; Hagberg, H. Lipopolysaccharide-Induced Alteration of Mitochondrial Morphology Induces a Metabolic Shift in Microglia Modulating the Inflammatory Response in Vitro and in Vivo. Glia 2019, 67, 1047–1061. [Google Scholar] [CrossRef]
- Park, J.; Min, J.S.; Kim, B.; Chae, U.B.; Yun, J.W.; Choi, M.S.; Kong, I.K.; Chang, K.T.; Lee, D.S. Mitochondrial ROS Govern the LPS-Induced pro-Inflammatory Response in Microglia Cells by Regulating MAPK and NF-κB Pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef]
- Ye, J.; Jiang, Z.; Chen, X.; Liu, M.; Li, J.; Liu, N. The Role of Autophagy in Pro-Inflammatory Responses of Microglia Activation via Mitochondrial Reactive Oxygen Species in Vitro. J. Neurochem. 2017, 142, 215–230. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, L.; Wei, X.; Wang, Y.; Ren, Z.; Zeng, S.; Zhang, X.; Wen, H.; Gao, C.; Liu, H. NOD2 Promotes Dopaminergic Degeneration Regulated by NADPH Oxidase 2 in 6-Hydroxydopamine Model of Parkinson’s Disease. J. Neuroinflamm. 2018, 15, 243. [Google Scholar] [CrossRef]
- Zhou, L.J.; Peng, J.; Xu, Y.N.; Zeng, W.J.; Zhang, J.; Wei, X.; Mai, C.L.; Lin, Z.J.; Liu, Y.; Murugan, M.; et al. Microglia Are Indispensable for Synaptic Plasticity in the Spinal Dorsal Horn and Chronic Pain. Cell Rep. 2019, 27, 3844–3859.e6. [Google Scholar] [CrossRef]
- Bernaus, A.; Blanco, S.; Sevilla, A. Glia Crosstalk in Neuroinflammatory Diseases. Front. Cell. Neurosci. 2020, 14, 209. [Google Scholar] [CrossRef]
- Walker, J.M.; Kruger, N.J. The Bradford Method for Protein Quantitation. In Basic Protein and Peptide Protocols; Humana Press: Totowa, NJ, USA, 2003; Volume 32, pp. 9–16. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wickstead, E.S.; Elliott, B.T.; Pokorny, S.; Biggs, C.; Getting, S.J.; McArthur, S. Stimulation of the Pro-Resolving Receptor Fpr2 Reverses Inflammatory Microglial Activity by Suppressing NFκB Activity. Int. J. Mol. Sci. 2023, 24, 15996. https://doi.org/10.3390/ijms242115996
Wickstead ES, Elliott BT, Pokorny S, Biggs C, Getting SJ, McArthur S. Stimulation of the Pro-Resolving Receptor Fpr2 Reverses Inflammatory Microglial Activity by Suppressing NFκB Activity. International Journal of Molecular Sciences. 2023; 24(21):15996. https://doi.org/10.3390/ijms242115996
Chicago/Turabian StyleWickstead, Edward S., Bradley T. Elliott, Sarah Pokorny, Christopher Biggs, Stephen J. Getting, and Simon McArthur. 2023. "Stimulation of the Pro-Resolving Receptor Fpr2 Reverses Inflammatory Microglial Activity by Suppressing NFκB Activity" International Journal of Molecular Sciences 24, no. 21: 15996. https://doi.org/10.3390/ijms242115996
APA StyleWickstead, E. S., Elliott, B. T., Pokorny, S., Biggs, C., Getting, S. J., & McArthur, S. (2023). Stimulation of the Pro-Resolving Receptor Fpr2 Reverses Inflammatory Microglial Activity by Suppressing NFκB Activity. International Journal of Molecular Sciences, 24(21), 15996. https://doi.org/10.3390/ijms242115996







