Addressing Key Questions in Organoid Models: Who, Where, How, and Why?
Abstract
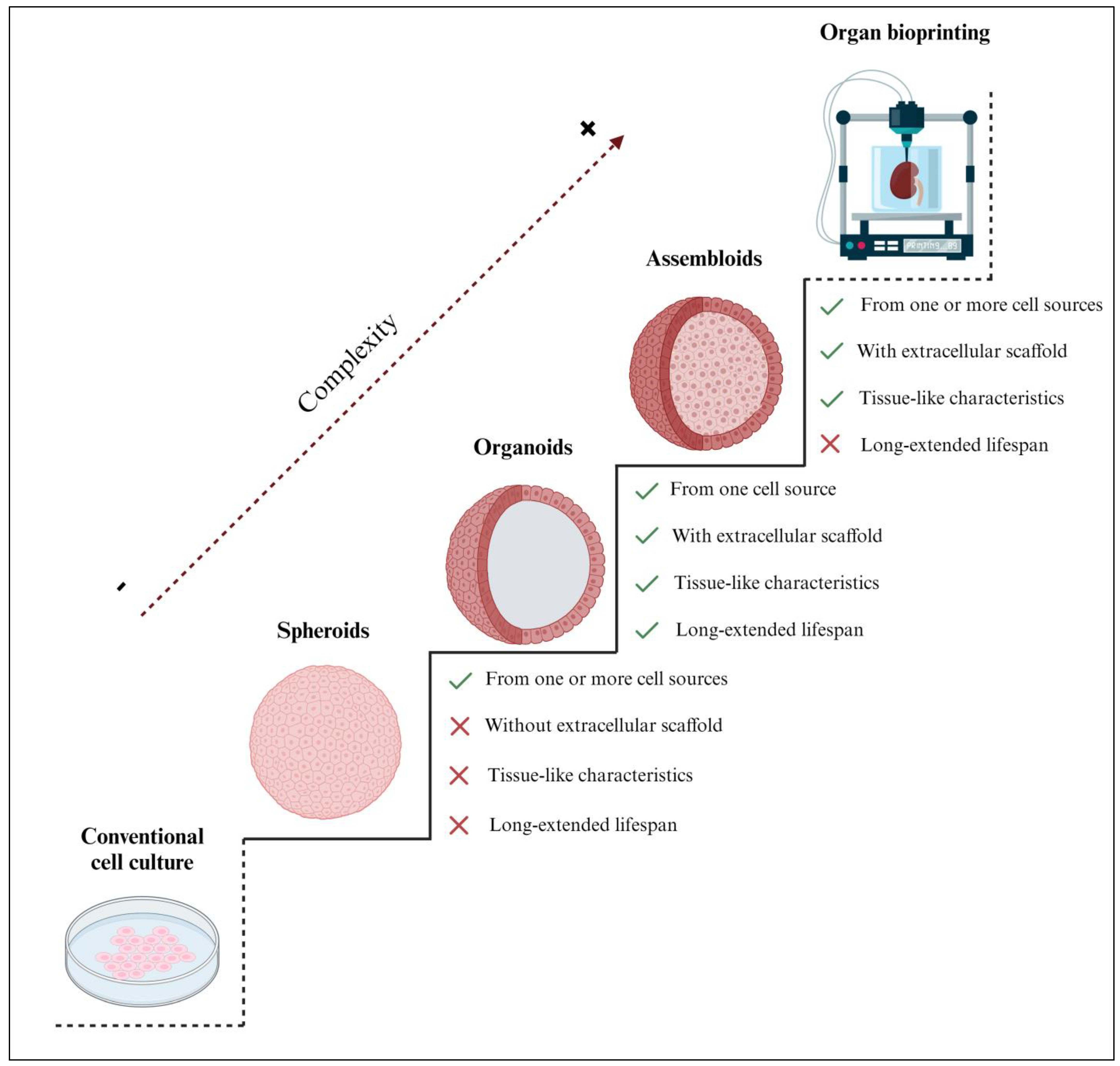
:1. Introduction—Organoid Definition
2. What Constitutes Organoids? The Cellular Material
2.1. Pluripotent Stem Cells
2.2. Primary Cells Isolated from Original Tissues
2.3. Cell Lines
3. Where Do Organoids Grow? The Extracellular Scaffold
3.1. Natural Hydrogels
3.1.1. Protein-Based Hydrogels
3.1.2. Polysaccharide-Based Hydrogels
3.1.3. Decellularized Extracellular Matrix Hydrogels
3.2. Synthetic Hydrogels
4. How Are Organoids Maintained In Vitro?—The Culture Medium
4.1. Basal Medium
4.2. Serum
4.3. Antibiotics and Antimycotics
4.4. Soluble Factors

5. Why Are Organoids Suitable In Vitro Models?—General Biomedical Applications
5.1. Biobanking
5.2. Disease Modeling
5.2.1. Infectious Diseases
5.2.2. Genetic Diseases
5.2.3. Cancer
5.3. Drug Discovery and Toxicology Studies
5.4. Personalized Medicine
5.4.1. Transplantation Therapy
5.4.2. Immunotherapy
5.4.3. Gene Repairing
6. Applications of Organoids Modeling the Female Reproductive Tract
6.1. Ovary
6.2. Fallopian Tubes
6.3. Endometrium
6.4. Cervix
6.5. Vagina
6.6. Future Challenges in Modeling the Female Reproductive Tract
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Prim. 2022, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Heydari, Z.; Moeinvaziri, F.; Agarwal, T.; Pooyan, P.; Shpichka, A.; Maiti, T.K.; Timashev, P.; Baharvand, H.; Vosough, M. Organoids: A Novel Modality in Disease Modeling. Bio-Des. Manuf. 2021, 4, 689–716. [Google Scholar] [CrossRef] [PubMed]
- Mu, P.; Zhou, S.; Lv, T.; Xia, F.; Shen, L.; Wan, J.; Wang, Y.; Zhang, H.; Cai, S.; Peng, J.; et al. Newly Developed 3D in Vitro Models to Study Tumor–Immune Interaction. J. Exp. Clin. Cancer Res. 2023, 42, 81. [Google Scholar] [CrossRef]
- Gjorevski, N.; Nikolaev, M.; Brown, T.E.; Mitrofanova, O.; Brandenberg, N.; DelRio, F.W.; Yavitt, F.M.; Liberali, P.; Anseth, K.S.; Lutolf, M.P. Tissue Geometry Drives Deterministic Organoid Patterning. Science 2022, 375, eaaw9021. [Google Scholar] [CrossRef]
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and Potential in Organoid Research. Nat. Rev. Genet. 2018, 19, 671–687. [Google Scholar] [CrossRef]
- Petrovic, V.; Fallon, J.; Kuester, F. Visualizing Whole-Brain DTI Tractography with GPU-Based Tuboids and LoD Management. IEEE Trans. Vis. Comput. Graph. 2007, 13, 1488–1495. [Google Scholar] [CrossRef]
- Sanaki-Matsumiya, M.; Matsuda, M.; Gritti, N.; Nakaki, F.; Sharpe, J.; Trivedi, V.; Ebisuya, M. Periodic Formation of Epithelial Somites from Human Pluripotent Stem Cells. Nat. Commun. 2022, 13, 2325. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering Organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Mathivanan, S.; Ji, H.; Simpson, R.J. Two Distinct Populations of Exosomes Are Released from LIM1863 Colon Carcinoma Cell-Derived Organoids. Mol. Cell. Proteom. 2013, 12, 587–598. [Google Scholar] [CrossRef]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; De Boer, J. Spheroid Culture as a Tool for Creating 3D Complex Tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, M.; Hagel, G.; Glenthoj, A.; Vainer, B.; Ibsen, P.; Harling, H.; Thastrup, O.; Jørgensen, L.N.; Thastrup, J. Short-Term Spheroid Culture of Primary Colorectal Cancer Cells as an in Vitro Model for Personalizing Cancer Medicine. PLoS ONE 2017, 12, e0183074. [Google Scholar] [CrossRef] [PubMed]
- Luan, Q.; Becker, J.H.; Macaraniag, C.; Massad, M.G.; Zhou, J.; Shimamura, T.; Papautsky, I. Non-Small Cell Lung Carcinoma Spheroid Models in Agarose Microwells for Drug Response Studies. Lab Chip 2022, 22, 2364–2375. [Google Scholar] [CrossRef]
- Kopper, O.; de Witte, C.J.; Lõhmussaar, K.; Valle-Inclan, J.E.; Hami, N.; Kester, L.; Balgobind, A.V.; Korving, J.; Proost, N.; Begthel, H.; et al. An Organoid Platform for Ovarian Cancer Captures Intra- and Interpatient Heterogeneity. Nat. Med. 2019, 25, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; Van Zundert, I.; et al. Patient-Derived Organoids from Endometrial Disease Capture Clinical Heterogeneity and Are Amenable to Drug Screening. Nat. Cell Biol. 2019, 21, 1041–1051. [Google Scholar] [CrossRef]
- Xie, Y.; Park, E.S.; Xiang, D.; Li, Z. Long-Term Organoid Culture Reveals Enrichment of Organoid-Forming Epithelial Cells in the Fimbrial Portion of Mouse Fallopian Tube. Stem Cell Res. 2018, 32, 51–60. [Google Scholar] [CrossRef]
- Schutgens, F.; Clevers, H. Human Organoids: Tools for Understanding Biology and Treating Diseases. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 211–234. [Google Scholar] [CrossRef]
- Kim, E.; Choi, S.; Kang, B.; Kong, J.H.; Kim, Y.; Yoon, W.H.; Lee, H.R.; Kim, S.E.; Kim, H.M.; Lee, H.S.; et al. Creation of Bladder Assembloids Mimicking Tissue Regeneration and Cancer. Nature 2020, 588, 664–669. [Google Scholar] [CrossRef]
- Rawlings, T.M.; Makwana, K.; Taylor, D.M.; Molè, M.A.; Fishwick, K.J.; Tryfonos, M.; Odendaal, J.; Hawkes, A.; Zernicka-Goetz, M.; Hartshorne, G.M.; et al. Modelling the Impact of Decidual Senescence on Embryo Implantation in Human Endometrial Assembloids. eLife 2021, 10, e69603. [Google Scholar] [CrossRef]
- Xue, J.; Sun, N.; Liu, Y. Self-Assembled Nano-Peptide Hydrogels with Human Umbilical Cord Mesenchymal Stem Cell Spheroids Accelerate Diabetic Skin Wound Healing by Inhibiting Inflammation and Promoting Angiogenesis. Int. J. Nanomed. 2022, 17, 3057–3058. [Google Scholar] [CrossRef]
- Moroni, L.; Elisseeff, J.H. Biomaterials Engineered for Integration. Mater. Today 2008, 11, 44–51. [Google Scholar] [CrossRef]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrova-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-Term, Hormone-Responsive Organoid Cultures of Human Endometrium in a Chemically Defined Medium. Nat. Cell Biol. 2017, 19, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Francés-Herrero, E.; Juárez-Barber, E.; Campo, H.; López-Martínez, S.; de Miguel-Gómez, L.; Faus, A.; Pellicer, A.; Ferrero, H.; Cervelló, I. Improved Models of Human Endometrial Organoids Based on Hydrogels from Decellularized Endometrium. J. Pers. Med. 2021, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zang, Z.; Luo, J.; Liu, T.; Yang, L.; Cai, Y.; Wang, L.; Zhang, D.; Zhao, J.; Gao, J.; et al. Chronic Exposure to (2R,6R)-Hydroxynorketamine Induces Developmental Neurotoxicity in HESC-Derived Cerebral Organoids. J. Hazard. Mater. 2023, 453, 131379. [Google Scholar] [CrossRef]
- Pitstick, A.L.; Poling, H.M.; Sundaram, N.; Lewis, P.L.; Kechele, D.O.; Sanchez, J.G.; Scott, M.A.; Broda, T.R.; Helmrath, M.A.; Wells, J.M.; et al. Aggregation of Cryopreserved Mid-Hindgut Endoderm for More Reliable and Reproducible HPSC-Derived Small Intestinal Organoid Generation. Stem Cell Rep. 2022, 17, 1889–1902. [Google Scholar] [CrossRef]
- Deng, H. Derivation of Pluripotent Stem Cells with in Vivo Embryonic and Extraembryonic Potency. Cell 2015, 27, 215–225. [Google Scholar] [CrossRef]
- Tsuruta, S.; Uchida, H.; Akutsu, H. Intestinal Organoids Generated from Human Pluripotent Stem Cells. JMA J. 2020, 3, 9–19. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-eldor, J.; Shapiro, S.S.; Michelle, A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M.; Thomson, J.A.; Itskovitz-eldor, J.; Shapiro, S.S.; et al. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Kakni, P.; López-Iglesias, C.; Truckenmüller, R.; Habibović, P.; Giselbrecht, S. Reversing Epithelial Polarity in Pluripotent Stem Cell-Derived Intestinal Organoids. Front. Bioeng. Biotechnol. 2022, 10, 879024. [Google Scholar] [CrossRef]
- Rempel, S.K.; Welch, M.J.; Ludwig, A.L.; Phillips, M.J.; Kancherla, Y.; Zack, D.J.; Gamm, D.M.; Gómez, T.M. Human photoreceptors switch from autonomous axon extension to cell-mediated process pulling during synaptic marker redistribution. Cell Rep. 2022, 39, 110827. [Google Scholar] [CrossRef] [PubMed]
- Suhito, I.R.; Kim, J.W.; Koo, K.M.; Nam, S.A.; Kim, Y.K.; Kim, T.H. In Situ Detection of Kidney Organoid Generation From Stem Cells Using a Simple Electrochemical Method. Adv. Sci. 2022, 9, e2200074. [Google Scholar] [CrossRef] [PubMed]
- Van Lent, J.; Vendredy, L.; Adriaenssens, E.; Da Silva Authier, T.; Asselbergh, B.; Kaji, M.; Weckhuysen, S.; Van Den Bosch, L.; Baets, J.; Timmerman, V. Downregulation of PMP22 Ameliorates Myelin Defects in IPSC-Derived Human Organoid Cultures of CMT1A. Brain 2022, 146, 2885–2896. [Google Scholar] [CrossRef]
- Ning, R.; Zheng, D.; Xie, B.; Gao, G.; Xu, J.; Xu, P.; Wang, Y.; Peng, F.; Jiang, B.; Ge, J.; et al. Spatial and Temporal Development of Müller Glial Cells in HiPSC-Derived Retinal Organoids Facilitates the Cell Enrichment and Transcriptome Analysis. Front. Cell. Neurosci. 2022, 16, 820396. [Google Scholar] [CrossRef] [PubMed]
- Shinozawa, T.; Kimura, M.; Cai, Y.; Saiki, N.; Yoneyama, Y.; Ouchi, R.; Koike, H.; Maezawa, M.; Zhang, R.R.; Dunn, A.; et al. High-Fidelity Drug-Induced Liver Injury Screen Using Human Pluripotent Stem Cell–Derived Organoids. Gastroenterology 2021, 160, 831–846.e10. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Gong, S.; Lees, J.G.; Yin, J.; Yap, L.W.; Kong, A.M.; Shi, Q.; Fu, R.; Zhu, Q.; Dyer, A.; et al. A Soft and Ultrasensitive Force Sensing Diaphragm for Probing Cardiac Organoids Instantaneously and Wirelessly. Nat. Commun. 2022, 13, 7259. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, N.Y.; Jagani, R.; Wang, H.S. Proarrhythmic Toxicity of Low Dose Bisphenol A and Its Analogs in Human IPSC-Derived Cardiomyocytes and Human Cardiac Organoids through Delay of Cardiac Repolarization. Chemosphere 2023, 328, 138562. [Google Scholar] [CrossRef]
- Hayal, T.B.; Doğan, A. Feeder-Free Human Embryonic Stem Cell Culture Under Defined Culture Conditions. Methods Mol. Biol. 2022, 2520, 25–35. [Google Scholar] [CrossRef]
- Kurosawa, H. Methods for Inducing Embryoid Body Formation: In Vitro Differentiation System of Embryonic Stem Cells. J. Biosci. Bioeng. 2007, 103, 389–398. [Google Scholar] [CrossRef]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.I.; Muguruma, K.; et al. A ROCK Inhibitor Permits Survival of Dissociated Human Embryonic Stem Cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef]
- Atanasova, V.S.; de Jesus Cardona, C.; Hejret, V.; Tiefenbacher, A.; Mair, T.; Tran, L.; Pfneissl, J.; Draganić, K.; Binder, C.; Kabiljo, J.; et al. Mimicking Tumor Cell Heterogeneity of Colorectal Cancer in a Patient-Derived Organoid-Fibroblast Model. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 1391–1419. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Miyata, H.; Seo, J.; Nanmo, A.; Fukuda, J. In Vitro Hair Follicle Growth Model for Drug Testing. Sci. Rep. 2023, 13, 4847. [Google Scholar] [CrossRef] [PubMed]
- Minoli, M.; Cantore, T.; Hanhart, D.; Kiener, M.; Fedrizzi, T.; La Manna, F.; Karkampouna, S.; Chouvardas, P.; Genitsch, V.; Rodriguez-Calero, A.; et al. Bladder Cancer Organoids as a Functional System to Model Different Disease Stages and Therapy Response. Nat. Commun. 2023, 14, 2214. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Xiao, T.; Xu, L.; Xie, Y.; Ge, W. UTP18-Mediated P21 MRNA Instability Drives Adenoma-Carcinoma Progression in Colorectal Cancer. Cell Rep. 2023, 42, 112423. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Q.; Huang, W.; Zhou, S.; Wang, Y.; Zeng, X.; Wang, H.; Xie, W.; Kong, H. NLRP3 Inflammasome Mediates Silica-Induced Lung Epithelial Injury and Aberrant Regeneration in Lung Stem/Progenitor Cell-Derived Organotypic Models. Int. J. Biol. Sci. 2023, 19, 1875–1893. [Google Scholar] [CrossRef]
- D’Imprima, E.; Garcia Montero, M.; Gawrzak, S.; Ronchi, P.; Zagoriy, I.; Schwab, Y.; Jechlinger, M.; Mahamid, J. Light and Electron Microscopy Continuum-Resolution Imaging of 3D Cell Cultures. Dev. Cell 2023, 58, 616. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Xin, H.; Deng, G. Single Cell Isolation and Analysis. Front. Cell Dev. Biol. 2016, 4, 116. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus Enzymatic Isolation of Stromal Vascular Fraction Cells from Adipose Tissue. SpringerPlus 2015, 4, 713. [Google Scholar] [CrossRef]
- Reddy, P.; Zhao, D.; Ravikumar, V.; Lauder, E.; Li, L.; Sun, Y.; Oravecz-Wilson, K.; Brooks, M.; Keller, E.; Chen, F.; et al. Inflammatory Memory Restrains Intestinal Stem Cell Regeneration. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Arutyunyan, A.; Roberts, K.; Troulé, K.; Wong, F.C.K.; Sheridan, M.A.; Kats, I.; Garcia-Alonso, L.; Velten, B.; Hoo, R.; Ruiz-Morales, E.R.; et al. Spatial Multiomics Map of Trophoblast Development in Early Pregnancy. Nature 2023, 616, 143–151. [Google Scholar] [CrossRef]
- Wang, Y.; Chiola, S.; Yang, G.; Russell, C.; Armstrong, C.J.; Wu, Y.; Spampanato, J.; Tarboton, P.; Ullah, H.M.A.; Edgar, N.U.; et al. Modeling Human Telencephalic Development and Autism-Associated SHANK3 Deficiency Using Organoids Generated from Single Neural Rosettes. Nat. Commun. 2022, 13, 5688. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, D.; Brouwers, J.F.; Hamer, K.; Geurts, M.H.; Luciana, L.; Massalini, S.; López-Iglesias, C.; Peters, P.J.; Rodríguez-Colman, M.J.; Chuva de Sousa Lopes, S.; et al. Engineered Human Hepatocyte Organoids Enable CRISPR-Based Target Discovery and Drug Screening for Steatosis. Nat. Biotechnol. 2023, 33, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Fernández Ortuño, E.; Marsoner, F.; Artioli, A.; Peters, J.; Namba, T.; Eugster Oegema, C.; Huttner, W.B.; Ladewig, J.; Heide, M. Human-specific ARHGAP11B Ensures Human-like Basal Progenitor Levels in Hominid Cerebral Organoids. EMBO Rep. 2022, 23, e54728. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, D.; Kalamkar, K.; Ahuja, G.; Lackmann, J.W.; Hescheler, J.; Weber, T.; Bazzi, H.; Clamer, M.; Mendjan, S.; Papantonis, A.; et al. MRNA Translational Specialization by RBPMS Presets the Competence for Cardiac Commitment in HESCs. Sci. Adv. 2023, 9, eade1792. [Google Scholar] [CrossRef]
- Kwilas, A.R.; Donahue, R.N.; Tsang, K.Y.; Hodge, J.W. Immune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenviron. 2015, 2, e677. [Google Scholar] [CrossRef]
- Dutta, D.; Heo, I.; Clevers, H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Özmert, E.; Arslan, U. Management of Retinitis Pigmentosa by Wharton’s Jelly-Derived Mesenchymal Stem Cells: Prospective Analysis of 1-Year Results. Stem Cell Res. Ther. 2020, 11, 353. [Google Scholar] [CrossRef]
- Skardal, A.; Shupe, T.; Atala, A. Organoid-on-a-Chip and Body-on-a-Chip Systems for Drug Screening and Disease Modeling. Drug Discov. Today 2016, 21, 1399–1411. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Li, B.; Gu, Y.; Chen, L. Controlled Release of BMP-2 from a Collagen-Mimetic Peptide-Modified Silk Fibroin–Nanohydroxyapatite Scaffold for Bone Regeneration. J. Mater. Chem. B 2017, 5, 8770–8779. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Malmström, H.; Ren, Y.F. Porous Collagen-Hydroxyapatite Scaffolds with Mesenchymal Stem Cells for Bone Regeneration. J. Oral Implantol. 2015, 41, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Wiwatpanit, T.; Murphy, A.R.; Lu, Z.; Urbanek, M.; Burdette, J.E.; Woodruff, T.K.; Kim, J.J. Scaffold-Free Endometrial Organoids Respond to Excess Androgens Associated with Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef]
- Davari, N.; Bakhtiary, N.; Khajehmohammadi, M.; Sarkari, S.; Tolabi, H.; Ghorbani, F.; Ghalandari, B. Protein-Based Hydrogels: Promising Materials for Tissue Engineering. Polymers 2022, 14, 986. [Google Scholar] [CrossRef]
- Passaniti, A.; Kleinman, H.K.; Martin, G.R. Matrigel: History/Background, Uses, and Future Applications. J. Cell Commun. Signal. 2022, 16, 621–626. [Google Scholar] [CrossRef]
- Kashfi, H.; Jinks, N.; Nateri, A.S. Generating and Utilizing Murine Cas9-Expressing Intestinal Organoids for Large-Scale Knockout Genetic Screening. Methods Mol. Biol. 2020, 2171, 257–269. [Google Scholar] [CrossRef]
- Kulkeaw, K.; Tubsuwan, A.; Tongkrajang, N.; Whangviboonkij, N. Generation of Human Liver Organoids from Pluripotent Stem Cell-Derived Hepatic Endoderms. PeerJ 2020, 8, e9968. [Google Scholar] [CrossRef]
- Yu, C.; Kang, R.; Tang, D. Organoids Models of Pancreatic Duct Adenocarcinoma. Methods Mol. Biol. 2023, 2712, 45–60. [Google Scholar] [CrossRef]
- Maru, Y.; Tanaka, N.; Itami, M.; Hippo, Y. Efficient Use of Patient-Derived Organoids as a Preclinical Model for Gynecologic Tumors. Gynecol. Oncol. 2019, 154, 189–198. [Google Scholar] [CrossRef]
- Ma, L.; Li, J.; Nie, Q.; Zhang, Q.; Liu, S.; Ge, D.; You, Z. Organoid Culture of Human Prostate Cancer Cell Lines LNCaP and C4-2B. Am. J. Clin. Exp. Urol. 2017, 5, 25–33. [Google Scholar]
- Drost, J.; Karthaus, W.R.; Gao, D.; Driehuis, E.; Sawyers, C.L.; Chen, Y.; Clevers, H. Organoid Culture Systems for Prostate Epithelial and Cancer Tissue. Nat. Protoc. 2016, 11, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of Organoids from Mouse and Human Endometrium Showing Endometrial Epithelium Physiology and Long-Term Expandability. Development 2017, 144, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in Vitro Model of Human Development and Disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Dorrell, C.; Boj, S.F.; Van Es, J.H.; Li, V.S.W.; Van De Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In Vitro Expansion of Single Lgr5+ Liver Stem Cells Induced by Wnt-Driven Regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Dye, B.R.; Ferrer-Torres, D.; Hill, D.R.; Overeem, A.W.; Shea, L.D.; Spence, J.R. Generation of Lung Organoids from Human Pluripotent Stem Cells in Vitro. Nat. Protoc. 2019, 14, 518–540. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and Functional Human Liver from an IPSC-Derived Organ Bud Transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A Review of Fibrin and Fibrin Composites for Bone Tissue Engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef]
- Tang-Schomer, M.D.; Wu, W.B.; Kaplan, D.L.; Bookland, M.J. In Vitro 3D Regeneration-like Growth of Human Patient Brain Tissue. J. Tissue Eng. Regen. Med. 2018, 12, 1247–1260. [Google Scholar] [CrossRef]
- Cardenas, D.; Bhalchandra, S.; Lamisere, H.; Chen, Y.; Zeng, X.L.; Ramani, S.; Karandikar, U.C.; Kaplan, D.L.; Estes, M.K.; Ward, H.D. Two- and Three-Dimensional Bioengineered Human Intestinal Tissue Models for Cryptosporidium. Methods Mol. Biol. 2020, 2052, 373–402. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sun, S. Silk Fibroin-Based Biomaterials for Tissue Engineering Applications. Molecules 2022, 27, 2757. [Google Scholar] [CrossRef] [PubMed]
- Chooi, W.H.; Ng, C.Y.; Ow, V.; Harley, J.; Ng, W.; Hor, J.H.; Low, K.E.; Malleret, B.; Xue, K.; Ng, S.Y. Defined Alginate Hydrogels Support Spinal Cord Organoid Derivation, Maturation, and Modeling of Spinal Cord Diseases. Adv. Healthc. Mater. 2023, 12, e2202342. [Google Scholar] [CrossRef]
- Zakhem, E.; Raghavan, S.; Gilmont, R.R.; Bitar, K.N. Chitosan-Based Scaffolds for the Support of Smooth Muscle Constructs in Intestinal Tissue Engineering. Biomaterials 2012, 33, 4810–4817. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Oosterhoff, L.A.; van Wolferen, M.E.; Schiele, S.A.; Walther, A.; Geijsen, N.; De Laporte, L.; van der Laan, L.J.W.; Kock, L.M.; Spee, B. Cellulose Nanofibril Hydrogel Promotes Hepatic Differentiation of Human Liver Organoids. Adv. Healthc. Mater. 2020, 9, e1901658. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.S.; et al. Engineering of Human Brain Organoids with a Functional Vascular-like System. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef]
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Gou, D. Chitosan-Based Hydrogel Wound Dressing: From Mechanism to Applications, a Review. Int. J. Biol. Macromol. 2023, 244, 125250. [Google Scholar] [CrossRef]
- Davoudi, Z.; Peroutka-Bigus, N.; Bellaire, B.; Jergens, A.; Wannemuehler, M.; Wang, Q. Gut Organoid as a New Platform to Study Alginate and Chitosan Mediated PLGA Nanoparticles for Drug Delivery. Mar. Drugs 2021, 19, 282. [Google Scholar] [CrossRef]
- Giobbe, G.G.; Crowley, C.; Luni, C.; Campinoti, S.; Khedr, M.; Kretzschmar, K.; De Santis, M.M.; Zambaiti, E.; Michielin, F.; Meran, L.; et al. Extracellular Matrix Hydrogel Derived from Decellularized Tissues Enables Endodermal Organoid Culture. Nat. Commun. 2019, 10, 5658. [Google Scholar] [CrossRef]
- Kim, J.W.; Nam, S.A.; Yi, J.; Kim, J.Y.; Lee, J.Y.; Park, S.Y.; Sen, T.; Choi, Y.M.; Lee, J.Y.; Kim, H.L.; et al. Kidney Decellularized Extracellular Matrix Enhanced the Vascularization and Maturation of Human Kidney Organoids. Adv. Sci. 2022, 9, 2103526. [Google Scholar] [CrossRef] [PubMed]
- De Hilster, R.H.J.; Sharma, P.K.; Jonker, M.R.; White, E.S.; Gercama, E.A.; Roobeek, M.; Timens, W.; Harmsen, M.C.; Hylkema, M.N.; Burgess, J.K. Human Lung Extracellular Matrix Hydrogels Resemble the Stiffness and Viscoelasticity of Native Lung Tissue. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L698–L704. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.H.; Park, K.M.; Yu, L.; Kwak, H.H.; Woo, H.M. Decellularized Hepatic Extracellular Matrix Hydrogel Attenuates Hepatic Stellate Cell Activation and Liver Fibrosis. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111160. [Google Scholar] [CrossRef]
- Simsa, R.; Rothenbücher, T.; Gürbüz, H.; Ghosheh, N.; Emneus, J.; Jenndahl, L.; Kaplan, D.L.; Bergh, N.; Serrano, A.M.; Fogelstrand, P. Brain Organoid Formation on Decellularized Porcine Brain ECM Hydrogels. PLoS ONE 2021, 16, e0245685. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, Y.; Hou, C.; Li, Z.; Yang, S.; Liang, X.; Zhou, L.; Guo, J.; Zhang, J.; Huang, X. Ovary-Derived Decellularized Extracellular Matrix-Based Bioink for Fabricating 3D Primary Ovarian Cells-Laden Structures for Mouse Ovarian Failure Correction. Int. J. Bioprint. 2022, 8, 269–282. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Gazia, C.; Tamburrini, R.; Asthana, A.; Chaimov, D.; Muir, S.M.; Marino, D.I.; Delbono, L.; Villani, V.; Perin, L.; Di Nardo, P.; et al. Extracellular Matrix-Based Hydrogels Obtained from Human Tissues: A Work Still in Progress. Curr. Opin. Organ Transplant. 2019, 24, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.A.; Won, J.E.; Knowles, J.C.; Kim, H.W. Naturally and Synthetic Smart Composite Biomaterials for Tissue Regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in Biomaterials for Tissue Engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Tian, C.-M.; Yang, M.-F.; Xu, H.-M.; Zhu, M.-Z.; Yue, N.-N.; Zhang, Y.; Shi, R.-Y.; Yao, J.; Wang, L.-S.; Liang, Y.-J.; et al. Stem Cell-Derived Intestinal Organoids: A Novel Modality for IBD. Cell Death Discov. 2023, 9, 255. [Google Scholar] [CrossRef]
- Huch, M.; Gehart, H.; Van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.A.; Ellis, E.; Van Wenum, M.; Fuchs, S.A.; De Ligt, J.; et al. Long-Term Culture of Genome-Stable Bipotent Stem Cells from Adult Human Liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Abidian, M.R. Conducting Polymers for Neural Prosthetic and Neural Interface Applications. Adv. Mater. 2015, 27, 7620–7637. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.L.; Swaminathan, G.; Ettayebi, K.; Bomidi, C.; Zeng, X.L.; Blutt, S.E.; Estes, M.K.; Grande-Allen, K.J. Protein-Functionalized Poly(Ethylene Glycol) Hydrogels as Scaffolds for Monolayer Organoid Culture. Tissue Eng. Part C Methods 2021, 27, 12–23. [Google Scholar] [CrossRef] [PubMed]
- KarbalaeiMahdi, A.; Shahrousvand, M.; Javadi, H.R.; Ghollasi, M.; Norouz, F.; Kamali, M.; Salimi, A. Neural Differentiation of Human Induced Pluripotent Stem Cells on Polycaprolactone/Gelatin Bi-Electrospun Nanofibers. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1195–1202. [Google Scholar] [CrossRef]
- Zhang, S. Fabrication of Novel Biomaterials through Molecular Self-Assembly. Nat. Biotechnol. 2003, 21, 1171–1178. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Urbischek, M.; Rannikmae, H.; Foets, T.; Ravn, K.; Hyvönen, M.; de la Roche, M. Organoid Culture Media Formulated with Growth Factors of Defined Cellular Activity. Sci. Rep. 2019, 9, 6193. [Google Scholar] [CrossRef]
- Asnaghi, M.A.; Smith, T.; Martin, I.; Wendt, D. Bioreactors: Enabling Technologies for Research and Manufacturing, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780124201453. [Google Scholar]
- Yao, T.; Asayama, Y. Animal-Cell Culture Media: History, Characteristics, and Current Issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef]
- van der Valk, J.; Bieback, K.; Buta, C.; Cochrane, B.; Dirks, W.G.; Fu, J.; Hickman, J.J.; Hohensee, C.; Kolar, R.; Liebsch, M.; et al. Fetal Bovine Serum (FBS): Past–Present–Future. ALTEX 2018, 35, 99–118. [Google Scholar] [CrossRef]
- Andrée, B.; Bela, K.; Horvath, T.; Lux, M.; Ramm, R.; Venturini, L.; Ciubotaru, A.; Zweigerdt, R.; Haverich, A.; Hilfiker, A. Successful Re-Endothelialization of a Perfusable Biological Vascularized Matrix (BioVaM) for the Generation of 3D Artificial Cardiac Tissue. Basic Res. Cardiol. 2014, 109, 441. [Google Scholar] [CrossRef]
- Kaneko, T.; LePage, G.A.; Shnitka, T.K. KLN205—A Murine Lung Carcinoma Cell Line. In Vitro 1980, 16, 884–892. [Google Scholar] [CrossRef]
- Stacey, G.N. Chapter 7 Cell Culture Contamination. In Cancer Cell Culture. Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 731, pp. 79–91. [Google Scholar] [CrossRef]
- Ryu, A.H.; Eckalbar, W.L.; Kreimer, A.; Yosef, N.; Ahituv, N. Use Antibiotics in Cell Culture with Caution: Genome-Wide Identification of Antibiotic-Induced Changes in Gene Expression and Regulation. Sci. Rep. 2017, 7, 7533. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome®): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Goonoo, N.; Bhaw-Luximon, A. Mimicking Growth Factors: Role of Small Molecule Scaffold Additives in Promoting Tissue Regeneration and Repair. RSC Adv. 2019, 9, 18124–18146. [Google Scholar] [CrossRef]
- Aloia, L.; McKie, M.A.; Vernaz, G.; Cordero-Espinoza, L.; Aleksieva, N.; van den Ameele, J.; Antonica, F.; Font-Cunill, B.; Raven, A.; Aiese Cigliano, R.; et al. Epigenetic Remodelling Licences Adult Cholangiocytes for Organoid Formation and Liver Regeneration. Nat. Cell Biol. 2019, 21, 1321–1333. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Yuan, Q.; Tang, S.; Guo, S.; Zhang, Y.; He, J.; Zhang, X.; Han, M.; Liu, Z.; et al. Integrated Profiling of Human Pancreatic Cancer Organoids Reveals Chromatin Accessibility Features Associated with Drug Sensitivity. Nat. Commun. 2022, 13, 2169. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Swaroop, M.; Papal, S.; Mondal, A.K.; Song, H.B.; Campello, L.; Tawa, G.J.; Regent, F.; Shimada, H.; Nagashima, K.; et al. Reserpine Maintains Photoreceptor Survival in Retinal Ciliopathy by Resolving Proteostasis Imbalance and Ciliogenesis Defects. eLife 2023, 12, e83205. [Google Scholar] [CrossRef]
- Walaas, G.A.; Gopalakrishnan, S.; Bakke, I.; Skovdahl, H.K.; Flatberg, A.; Østvik, A.E.; Sandvik, A.K.; Bruland, T. Physiological Hypoxia Improves Growth and Functional Differentiation of Human Intestinal Epithelial Organoids. Front. Immunol. 2023, 14, 1095812. [Google Scholar] [CrossRef]
- Bunney, P.E.; Zink, A.N.; Holm, A.A.; Billington, C.J.; Kotz, C.M. Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high-fat diet. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Boj, S.F.; Hwang, C.-I.; Baker, L.A.; Chio, I.I.C.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid Model of Human and Mouse Pancreatic Ductal Adenocarcinoma. Cell 2016, 160, 324–338. [Google Scholar] [CrossRef]
- Below, C.R.; Kelly, J.; Brown, A.; Humphries, J.D.; Hutton, C.; Xu, J.; Lee, B.Y.; Cintas, C.; Zhang, X.; Stockdale, L.; et al. A Microenvironment-Inspired Synthetic Three-Dimensional Model for Pancreatic Ductal Adenocarcinoma Organoids. Nat. Mater. 2022, 31, 110–119. [Google Scholar] [CrossRef]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; De Sousa Lopes, S.M.C.; et al. Kidney Organoids from Human IPS Cells Contain Multiple Lineages and Model Human Nephrogenesis. Nature 2015, 526, 564–568. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.M.; Lim, S.; Lee, J.Y.; Choi, S.J.; Yang, S.D.; Yun, M.R.; Kim, C.G.; Gu, S.R.; Park, C.; et al. Modeling Clinical Responses to Targeted Therapies by Patient-Derived Organoids of Advanced Lung Adenocarcinoma. Clin. Cancer Res. 2021, 27, 4397–4409. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Capowski, E.; Zepeda, M.A.F.; Nelson, E.C.; Gamm, D.M.; Sinha, R. The primate fovea: Structure, function and development. Prog. Retin. Eye Res. 2023, 29, 460–471. [Google Scholar] [CrossRef]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.J.; Chun, S.M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-Derived Lung Cancer Organoids as in Vitro Cancer Models for Therapeutic Screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef] [PubMed]
- Baden, P.; Perez, M.J.; Raji, H.; Bertoli, F.; Kalb, S.; Illescas, M.; Spanos, F.; Giuliano, C.; Calogero, A.M.; Oldrati, M.; et al. Glucocerebrosidase Is Imported into Mitochondria and Preserves Complex I Integrity and Energy Metabolism. Nat. Commun. 2023, 14, 1930. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Matano, M.; Toshimitsu, K.; Takano, A.; Mikami, Y.; Nishikori, S.; Sugimoto, S.; Sato, T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 2018, 23, 787–793.e6. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, F.; Jin, M.; Wang, Z.; Xu, X.; Zhou, Y.; Wang, J.; Gu, L.; Fan, H.; Fan, Y.; et al. Development of a High-Throughput Micropatterned Agarose Scaffold for Consistent and Reproducible HPSC-Derived Liver Organoids. Biofabrication 2023, 15, 015006. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Israeli, Y.R.; Wasserman, A.H.; Gabalski, M.A.; Volmert, B.D.; Ming, Y.; Ball, K.A.; Yang, W.; Zou, J.; Ni, G.; Pajares, N.; et al. Self-Assembling Human Heart Organoids for the Modeling of Cardiac Development and Congenital Heart Disease. Nat. Commun. 2021, 12, 5142. [Google Scholar] [CrossRef]
- Finkbeiner, C.; Ortuño-Lizarán, I.; Sridhar, A.; Hooper, M.; Petter, S.; Reh, T.A. Single-Cell ATAC-Seq of Fetal Human Retina and Stem-Cell-Derived Retinal Organoids Shows Changing Chromatin Landscapes during Cell Fate Acquisition. Cell Rep. 2022, 38, 110294. [Google Scholar] [CrossRef]
- Rodriguez-Gatica, J.E.; Iefremova, V.; Sokhranyaeva, L.; Yeung, S.W.C.A.; Breitkreuz, Y.; Brüstle, O.; Schwarz, M.K.; Kubitscheck, U. Imaging Three-Dimensional Brain Organoid Architecture from Meso- to Nanoscale across Development. Development 2022, 149, dev200439. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Zhang, C.Y.; Peng, K.C.; Chen, Z.X.; Su, J.W.; Li, Y.F.; Li, W.F.; Gao, Q.Y.; Zhang, S.L.; Chen, Y.Q.; et al. Using Patient-Derived Organoids to Predict Locally Advanced or Metastatic Lung Cancer Tumor Response: A Real-World Study. Cell Rep. Med. 2023, 4, 100911. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kang, N.; Zhang, W.; Chen, B.; Xu, S.; Wu, L. The Developmental Toxicity of PM2.5 on the Early Stages of Fetal Lung with Human Lung Bud Tip Progenitor Organoids. Environ. Pollut. 2023, 330, 121764. [Google Scholar] [CrossRef]
- Rockel, A.F.; Wagner, N.; Spenger, P.; Ergün, S.; Wörsdörfer, P. Neuro-Mesodermal Assembloids Recapitulate Aspects of Peripheral Nervous System Development in Vitro. Stem Cell Rep. 2023, 18, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Matthys, O.B.; Joy, D.A.; Kauss, M.A.; Natarajan, V.; Lai, M.H.; Turaga, D.; Blair, A.P.; Alexanian, M.; Bruneau, B.G.; et al. Co-Emergence of Cardiac and Gut Tissues Promotes Cardiomyocyte Maturation within Human IPSC-Derived Organoids. Cell Stem Cell 2021, 28, 2137–2152.e6. [Google Scholar] [CrossRef] [PubMed]
- Siller, R.; Greenhough, S.; Naumovska, E.; Sullivan, G.J. Small-Molecule-Driven Hepatocyte Differentiation of Human Pluripotent Stem Cells. Stem Cell Rep. 2015, 4, 939–952. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Huch, M. Disease Modelling in Human Organoids. Dis. Model. Mech. 2019, 12, dmm039347. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human Organoids: Model Systems for Human Biology and Medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Sachs, N.; Clevers, H. Organoid Cultures for the Analysis of Cancer Phenotypes. Curr. Opin. Genet. Dev. 2014, 24, 68–73. [Google Scholar] [CrossRef]
- Ren, X.; Chen, W.; Yang, Q.; Li, X.; Xu, L. Patient-derived cancer organoids for drug screening: Basic technology and clinical application. J. Gastroenterol. Hepatol. 2022, 37, 1446–1454. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in Cancer Research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). Human Cancer Models Initiative. Available online: https://ocg.cancer.gov/programs/hcmi (accessed on 2 November 2023).
- Finkbeiner, S.R.; Zeng, X.L.; Utama, B.; Atmar, R.L.; Shroyer, N.F.; Estesa, M.K. Stem Cell-Derived Human Intestinal Organoids as an Infection Model for Rotaviruses. mBio 2012, 3, e00159-12. [Google Scholar] [CrossRef] [PubMed]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.L.; Qu, L.; et al. Replication of Human Noroviruses in Stem Cell-Derived Human Enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef]
- Dekkers, J.F.; Wiegerinck, C.L.; De Jonge, H.R.; Bronsveld, I.; Janssens, H.M.; De Winter-De Groot, K.M.; Brandsma, A.M.; De Jong, N.W.M.; Bijvelds, M.J.C.; Scholte, B.J.; et al. A Functional CFTR Assay Using Primary Cystic Fibrosis Intestinal Organoids. Nat. Med. 2013, 19, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-Derived Organoids Model Treatment Response of Metastatic Gastrointestinal Cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef]
- Tiriac, H.; Belleau, P.; Engle, D.D.; Plenker, D.; Deschênes, A.; Somerville, T.D.D.; Froeling, F.E.M.; Burkhart, R.A.; Denroche, R.E.; Jang, G.H.; et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov. 2018, 8, 1112–1129. [Google Scholar] [CrossRef]
- Hubert, C.G.; Rivera, M.; Spangler, L.C.; Wu, Q.; Mack, S.C.; Prager, B.C.; Couce, M.; McLendon, R.E.; Sloan, A.E.; Rich, J.N. A Three-Dimensional Organoid Culture System Derived from Human Glioblastomas Recapitulates the Hypoxic Gradients and Cancer Stem Cell Heterogeneity of Tumors Found In Vivo. Cancer Res. 2016, 76, 2465–2477. [Google Scholar] [CrossRef] [PubMed]
- Blomme, E.A.G.; Will, Y. Toxicology Strategies for Drug Discovery: Present and Future. Chem. Res. Toxicol. 2016, 29, 473–504. [Google Scholar] [CrossRef]
- Xu, H.; Jiao, Y.; Qin, S.; Zhao, W.; Chu, Q.; Wu, K. Organoid Technology in Disease Modelling, Drug Development, Personalized Treatment and Regeneration Medicine. Exp. Hematol. Oncol. 2018, 7, 30. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; van de Haar, J.; Fanchi, L.F.; Slagter, M.; van der Velden, D.L.; Kaing, S.; Kelderman, S.; et al. Generation of Tumor-Reactive T Cells by Co-Culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell 2018, 174, 1586–1598.e12. [Google Scholar] [CrossRef]
- Diao, J.; Liu, J.; Wang, S.; Chang, M.; Wang, X.; Guo, B.; Yu, Q.; Yan, F.; Su, Y.; Wang, Y. Sweat Gland Organoids Contribute to Cutaneous Wound Healing and Sweat Gland Regeneration. Cell Death Dis. 2019, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Sağraç, D.; Şişli, H.B.; Şenkal, S.; Hayal, T.B.; Şahin, F.; Doğan, A. Organoids in Tissue Transplantation. Adv. Exp. Med. Biol. 2021, 1347, 45–64. [Google Scholar] [CrossRef]
- Zhou, G.; Lieshout, R.; van Tienderen, G.S.; de Ruiter, V.; van Royen, M.E.; Boor, P.P.C.; Magré, L.; Desai, J.; Köten, K.; Kan, Y.Y.; et al. Modelling Immune Cytotoxicity for Cholangiocarcinoma with Tumour-Derived Organoids and Effector T Cells. Br. J. Cancer 2022, 127, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Mallela, R.K.; Cornuet, P.; Nasonkin, I.O. Derivation of Retinal Cells and Retinal Organoids from Pluripotent Stem Cells for CRISPR-Cas9 Engineering and Retinal Repair. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3591. [Google Scholar]
- Hoffman, B.L.; Schorge, J.O.; Bradshaw, K.D.; Halvorson, L.M.; Schaffer, J.I.; Corton, M.M. Williams Gynecology, 3e; McGraw Hill: Columbus, OH, USA, 2016; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=1758§ionid=118165498 (accessed on 2 November 2023).
- Kwong, J.; Franky, L.C.; Wong, K.K.; Birrer, M.J.; Archibald, K.M.; Balkwill, F.R.; Berkowitz, R.S.; Mok, S.C. Inflammatory Cytokine Tumor Necrosis Factor α Confers Precancerous Phenotype in an Organoid Model of Normal Human Ovarian Surface Epithelial Cells. Neoplasia 2009, 11, 529–541. [Google Scholar] [CrossRef]
- Li, X.; Zheng, M.; Xu, B.; Li, D.; Shen, Y.; Nie, Y.; Ma, L.; Wu, J. Generation of Offspring-Producing 3D Ovarian Organoids Derived from Female Germline Stem Cells and Their Application in Toxicological Detection. Biomaterials 2021, 279, 121213. [Google Scholar] [CrossRef]
- Wang, J.; Du, H.; Ma, L.; Feng, M.; Li, L.; Zhao, X.; Dai, Y. MitoQ Protects Ovarian Organoids against Oxidative Stress during Oogenesis and Folliculogenesis In Vitro. Int. J. Mol. Sci. 2023, 24, 924. [Google Scholar] [CrossRef]
- Zhang, S.; Dolgalev, I.; Zhang, T.; Ran, H.; Levine, D.A.; Neel, B.G. Both Fallopian Tube and Ovarian Surface Epithelium Are Cells-of-Origin for High-Grade Serous Ovarian Carcinoma. Nat. Commun. 2019, 10, 5367. [Google Scholar] [CrossRef]
- Hill, S.J.; Decker, B.; Roberts, E.A.; Horowitz, N.S.; Muto, M.G.; Worley, M.J.; Feltmate, C.M.; Nucci, M.R.; Swisher, E.M.; Nguyen, H.; et al. Prediction of DNA Repair Inhibitor Response in Short Term Patient-Derived Ovarian Cancer Organoids. Cancer Discov. 2018, 8, 1404–1421. [Google Scholar] [CrossRef]
- Hoffmann, K.; Berger, H.; Kulbe, H.; Thillainadarasan, S.; Mollenkopf, H.; Zemojtel, T.; Taube, E.; Darb-Esfahani, S.; Mangler, M.; Sehouli, J.; et al. Stable Expansion of High-Grade Serous Ovarian Cancer Organoids Requires a Low-Wnt Environment. EMBO J. 2020, 39, e104013. [Google Scholar] [CrossRef]
- Maenhoudt, N.; Defraye, C.; Boretto, M.; Jan, Z.; Heremans, R.; Boeckx, B.; Hermans, F.; Arijs, I.; Cox, B.; Van Nieuwenhuysen, E.; et al. Developing Organoids from Ovarian Cancer as Experimental and Preclinical Models. Stem Cell Rep. 2020, 14, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Psilopatis, I.; Sykaras, A.G.; Mandrakis, G.; Vrettou, K.; Theocharis, S. Patient-Derived Organoids: The Beginning of a New Era in Ovarian Cancer Disease Modeling and Drug Sensitivity Testing. Biomedicines 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Spagnol, G.; Sensi, F.; De Tommasi, O.; Marchetti, M.; Bonaldo, G.; Xhindoli, L.; Noventa, M.; Agostini, M.; Tozzi, R.; Saccardi, C. Patient Derived Organoids (PDOs), Extracellular Matrix (ECM), Tumor Microenvironment (TME) and Drug Screening: State of the Art and Clinical Implications of Ovarian Cancer Organoids in the Era of Precision Medicine. Cancers 2023, 15, 2029. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.; Hong, J.J.; Tofig, B.; Mapua, M.; Elashoff, D.; Moatamed, N.A.; Huang, J.; Memarzadeh, S.; Damoiseaux, R.; Soragni, A. A Simple High-Throughput Approach Identifies Actionable Drug Sensitivities in Patient-Derived Tumor Organoids. Commun. Biol. 2019, 2, 78. [Google Scholar] [CrossRef]
- Chen, H.; Gotimer, K.; De Souza, C.; Tepper, C.G.; Karnezis, A.N.; Leiserowitz, G.S.; Chien, J.; Smith, L.H. Short-Term Organoid Culture for Drug Sensitivity Testing of High-Grade Serous Carcinoma. Gynecol. Oncol. 2020, 157, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Lõhmussaar, K.; Kopper, O.; Korving, J.; Begthel, H.; Vreuls, C.P.H.; van Es, J.H.; Clevers, H. Assessing the Origin of High-Grade Serous Ovarian Cancer Using CRISPR-Modification of Mouse Organoids. Nat. Commun. 2020, 11, 2660. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Keany, M.P.; Dong, H.; Al-Alem, L.F.; Pandya, U.M.; Lazo, S.; Boehnke, K.; Lynch, K.N.; Xu, R.; Zarrella, D.T.; et al. Enhanced Efficacy of Simultaneous PD-1 and PD-L1 Immune Checkpoint Blockade in High-Grade Serous Ovarian Cancer. Cancer Res. 2021, 81, 158–173. [Google Scholar] [CrossRef]
- Perets, R.; Wyant, G.A.; Muto, K.W.; Bijron, J.G.; Poole, B.B.; Chin, K.T.; Chen, J.Y.H.; Ohman, A.W.; Stepule, C.D.; Kwak, S.; et al. Transformation of the Fallopian Tube Secretory Epithelium Leads to High-Grade Serous Ovarian Cancer in Brca;Tp53;Pten Models. Cancer Cell 2013, 24, 751–765. [Google Scholar] [CrossRef]
- Kessler, M.; Hoffmann, K.; Brinkmann, V.; Thieck, O.; Jackisch, S.; Toelle, B.; Berger, H.; Mollenkopf, H.J.; Mangler, M.; Sehouli, J.; et al. The Notch and Wnt Pathways Regulate Stemness and Differentiation in Human Fallopian Tube Organoids. Nat. Commun. 2015, 6, 8989. [Google Scholar] [CrossRef]
- Rose, I.M.; Bidarimath, M.; Webster, A.; Godwin, A.K.; Flesken-Nikitin, A.; Nikitin, A.Y. WNT and Inflammatory Signaling Distinguish Human Fallopian Tube Epithelial Cell Populations. Sci. Rep. 2020, 10, 9837. [Google Scholar] [CrossRef]
- Lin, Y.X.; Wei, Y.Z.; Jiang, M.Z.; Tang, X.; Huang, F.; Yang, X.Z. Organoid Culture of Mouse Fallopian Tube Epithelial Stem Cells with a Thermo-Reversible Gelation Polymer. Tissue Cell 2021, 73, 101622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Iyer, S.; Ran, H.; Dolgalev, I.; Gu, S.; Wei, W.; Foster, C.J.R.; Loomis, C.A.; Olvera, N.; Dao, F.; et al. Genetically Defined, Syngeneic Organoid Platform for Developing Combination Therapies for Ovarian Cancer. Cancer Discov. 2021, 11, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Mccartney, S.; Strenk, S.; Valint, D.J.; Haggerty, C.; Fredricks, D. Vaginal Bacteria Elicit Acute Inflammatory Response in Fallopian Tube Organoids: A Model for Pelvic Inflammatory Disease. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chu, T.Y.; Ding, D.C. Human Fallopian Tube Epithelial Cells Exhibit Stemness Features, Self-Renewal Capacity, and Wnt-Related Organoid Formation. J. Biomed. Sci. 2020, 27, 32. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Gamperl, M.; Burkard, T.R.; Kunihs, V.; Kaindl, U.; Junttila, S.; Fiala, C.; Schmidt, K.; Mendjan, S.; Knöfler, M.; et al. Estrogen Signaling Drives Ciliogenesis in Human Endometrial Organoids. Endocrinology 2019, 160, 2282–2297. [Google Scholar] [CrossRef]
- Jamaluddin, M.B.F.F.B.; Ghosh, A.; Ingle, A.; Mohammed, R.; Ali, A.; Bahrami, M.; Kaiko, G.; Gibb, Z.; Filipe, E.C.; Cox, T.R.; et al. Bovine and Human Endometrium-Derived Hydrogels Support Organoid Culture from Healthy and Cancerous Tissues. Proc. Natl. Acad. Sci. USA 2022, 119, e2208040119. [Google Scholar] [CrossRef]
- Filby, C.E.; Wyatt, K.A.; Mortlock, S.; Cousins, F.L.; McKinnon, B.; Tyson, K.E.; Montgomery, G.W.; Gargett, C.E. Comparison of Organoids from Menstrual Fluid and Hormone-Treated Endometrium: Novel Tools for Gynecological Research. J. Pers. Med. 2021, 11, 1314. [Google Scholar] [CrossRef]
- Murphy, A.R.; Campo, H.; Kim, J.J. Strategies for Modelling Endometrial Diseases. Nat. Rev. Endocrinol. 2022, 18, 727–743. [Google Scholar] [CrossRef]
- Juárez-barber, E.; Francés-herrero, E.; Corachán, A.; Vidal, C.; Giles, J.; Alamá, P.; Faus, A.; Pellicer, A.; Cervelló, I.; Ferrero, H. Establishment of Adenomyosis Organoids as a Preclinical Model to Study Infertility. J. Pers. Med. 2022, 12, 219. [Google Scholar] [CrossRef]
- Esfandiari, F.; Favaedi, R.; Heidari-Khoei, H.; Chitsazian, F.; Yari, S.; Piryaei, A.; Ghafari, F.; Baharvand, H.; Shahhoseini, M. Insight into Epigenetics of Human Endometriosis Organoids: DNA Methylation Analysis of HOX Genes and Their Cofactors. Fertil. Steril. 2021, 115, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Girda, E.; Huang, E.C.; Leiserowitz, G.S.; Smith, L.H. The Use of Endometrial Cancer Patient–Derived Organoid Culture for Drug Sensitivity Testing Is Feasible. Int. J. Gynecol. Cancer 2017, 27, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Katcher, A.; Yueh, B.; Ozler, K.; Nizam, A.; Kredentser, A.; Chung, C.; Frimer, M.; Goldberg, G.L.; Beyaz, S. Establishing Patient-Derived Organoids from Human Endometrial Cancer and Normal Endometrium. Front. Endocrinol. 2023, 14, 1059228. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, M.F.B.; Ko, Y.A.; Ghosh, A.; Syed, S.M.; Ius, Y.; O’Sullivan, R.; Netherton, J.K.; Baker, M.A.; Nahar, P.; Jaaback, K.; et al. Proteomic and Functional Characterization of Intra-Tumor Heterogeneity in Human Endometrial Cancer. Cell Rep. Med. 2022, 3, 100738. [Google Scholar] [CrossRef]
- Tamura, H.; Higa, A.; Hoshi, H.; Hiyama, G.; Takahashi, N.; Ryufuku, M.; Morisawa, G.; Yanagisawa, Y.; Ito, E.; Imai, J.I.; et al. Evaluation of Anticancer Agents Using Patient-Derived Tumor Organoids Characteristically Similar to Source Tissues. Oncol. Rep. 2018, 40, 635–646. [Google Scholar] [CrossRef]
- Rawlings, T.M.; Makwana, K.; Tryfonos, M.; Lucas, E.S. Organoids to Model the Endometrium: Implantation and Beyond. Reprod. Fertil. 2021, 2, R85–R101. [Google Scholar] [CrossRef]
- Murphy, A.R.; Wiwatpanit, T.; Lu, Z.; Davaadelger, B.; Kim, J.J. Generation of Multicellular Human Primary Endometrial Organoids. J. Vis. Exp. 2019, 2019, e60384. [Google Scholar] [CrossRef]
- Jones, R.E.; Lopez, K.H. The Female Reproductive System. In Human Reproductive Biology; Academic Press: San Diego, CA, USA, 2014; pp. 23–50. [Google Scholar] [CrossRef]
- Chumduri, C.; Turco, M.Y. Organoids of the Female Reproductive Tract. J. Mol. Med. 2021, 99, 531–553. [Google Scholar] [CrossRef]
- Chumduri, C.; Gurumurthy, R.K.; Berger, H.; Dietrich, O.; Kumar, N.; Koster, S.; Brinkmann, V.; Hoffmann, K.; Drabkina, M.; Arampatzi, P.; et al. Opposing Wnt Signals Regulate Cervical Squamocolumnar Homeostasis and Emergence of Metaplasia. Nat. Cell Biol. 2021, 23, 184–197. [Google Scholar] [CrossRef]
- Lõhmussaar, K.; Oka, R.; Espejo Valle-Inclan, J.; Smits, M.H.H.; Wardak, H.; Korving, J.; Begthel, H.; Proost, N.; van de Ven, M.; Kranenburg, O.W.; et al. Patient-Derived Organoids Model Cervical Tissue Dynamics and Viral Oncogenesis in Cervical Cancer. Cell Stem Cell 2021, 28, 1380–1396.e6. [Google Scholar] [CrossRef]
- Ramirez-Gonzalez, J.A.; Vaamonde-Lemos, R.; Cunha-Filho, J.S.; Varghese, A.C.; Swanson, R.J. Overview of the Female Reproductive System. In Exercise and Human Reproduction; Vaamonde, D., du Plessis, S.S., Agarwal, A., Eds.; Springer: New York, NY, USA, 2016; pp. 19–45. ISBN 9781493934027. [Google Scholar]
- Ali, A.; Syed, S.M.; Jamaluddin, M.F.B.; Colino-Sanguino, Y.; Gallego-Ortega, D.; Tanwar, P.S. Cell Lineage Tracing Identifies Hormone-Regulated and Wnt-Responsive Vaginal Epithelial Stem Cells. Cell Rep. 2020, 30, 1463–1477.e7. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Ni, Z. Microfluidics for Biomedical Applications. Biosensors 2023, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The Present and Future Role of Microfluidics in Biomedical Research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Luni, C.; Elvassore, N. Microfluidics for Secretome Analysis under Enhanced Endogenous Signaling. Biochem. Biophys. Res. Commun. 2018, 497, 480–484. [Google Scholar] [CrossRef]
- Xiao, S.; Coppeta, J.R.; Rogers, H.B.; Isenberg, B.C.; Zhu, J.; Olalekan, S.A.; McKinnon, K.E.; Dokic, D.; Rashedi, A.S.; Haisenleder, D.J.; et al. A Microfluidic Culture Model of the Human Reproductive Tract and 28-Day Menstrual Cycle. Nat. Commun. 2017, 8, 14584. [Google Scholar] [CrossRef]
- Young, R.E.; Huh, D.D. Organ-on-a-Chip Technology for the Study of the Female Reproductive System. Adv. Drug Deliv. Rev. 2021, 173, 461. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Álvarez, M.; Agustina-Hernández, M.; Francés-Herrero, E.; Rodríguez-Eguren, A.; Bueno-Fernandez, C.; Cervelló, I. Addressing Key Questions in Organoid Models: Who, Where, How, and Why? Int. J. Mol. Sci. 2023, 24, 16014. https://doi.org/10.3390/ijms242116014
Gómez-Álvarez M, Agustina-Hernández M, Francés-Herrero E, Rodríguez-Eguren A, Bueno-Fernandez C, Cervelló I. Addressing Key Questions in Organoid Models: Who, Where, How, and Why? International Journal of Molecular Sciences. 2023; 24(21):16014. https://doi.org/10.3390/ijms242116014
Chicago/Turabian StyleGómez-Álvarez, María, Marcos Agustina-Hernández, Emilio Francés-Herrero, Adolfo Rodríguez-Eguren, Clara Bueno-Fernandez, and Irene Cervelló. 2023. "Addressing Key Questions in Organoid Models: Who, Where, How, and Why?" International Journal of Molecular Sciences 24, no. 21: 16014. https://doi.org/10.3390/ijms242116014
APA StyleGómez-Álvarez, M., Agustina-Hernández, M., Francés-Herrero, E., Rodríguez-Eguren, A., Bueno-Fernandez, C., & Cervelló, I. (2023). Addressing Key Questions in Organoid Models: Who, Where, How, and Why? International Journal of Molecular Sciences, 24(21), 16014. https://doi.org/10.3390/ijms242116014







