Synergy of the microRNA Ratio as a Promising Diagnosis Biomarker for Mucinous Borderline and Malignant Ovarian Tumors
Abstract
:1. Introduction
2. Results
2.1. Identification of Differentially Expressed miRNAs between Borderline and Malignant Ovarian Tumors by miRNA Profiling
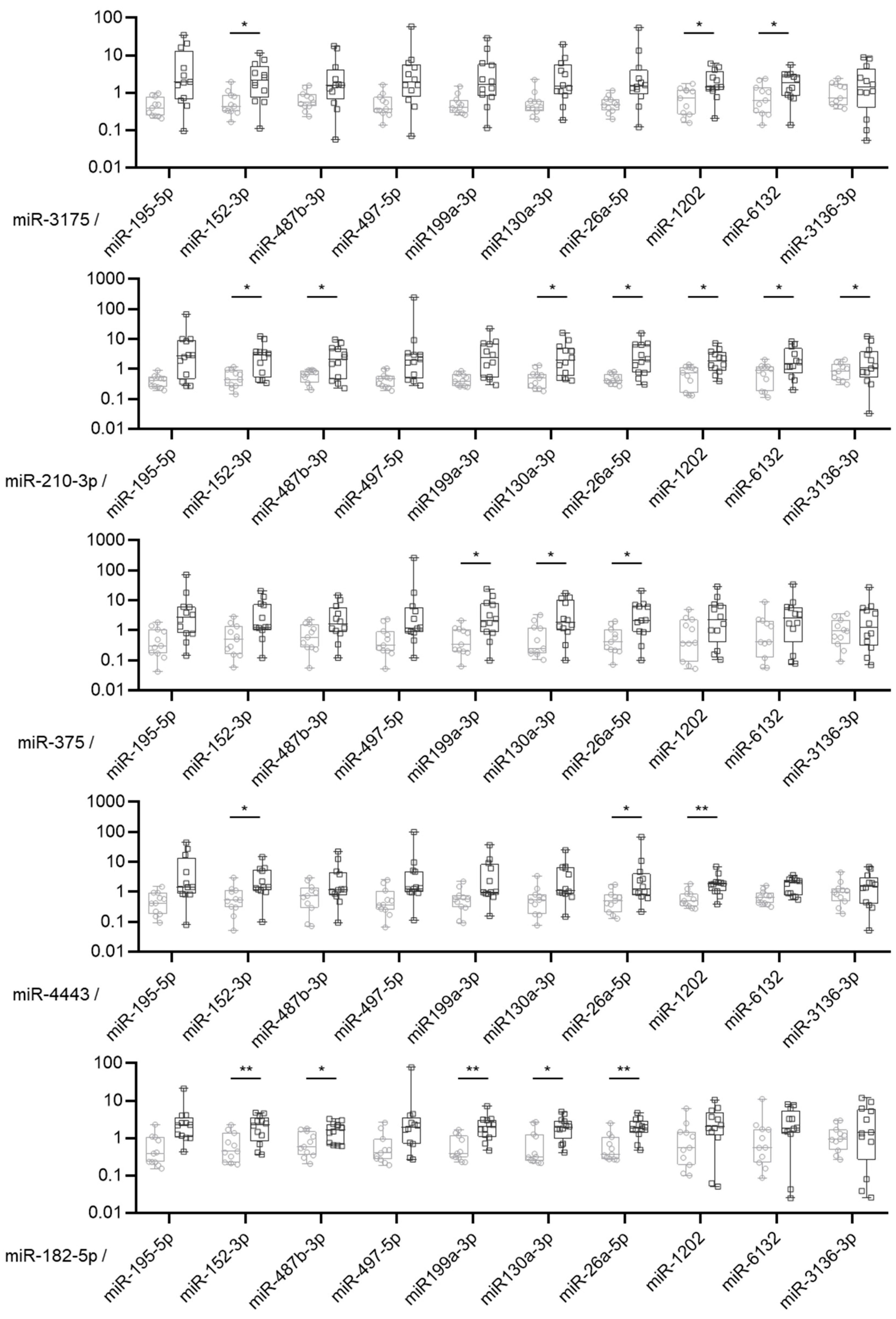
2.2. Validation of Differentially Expressed miRNAs between Borderline and Malignant Ovarian Tumors by RT-qPCR
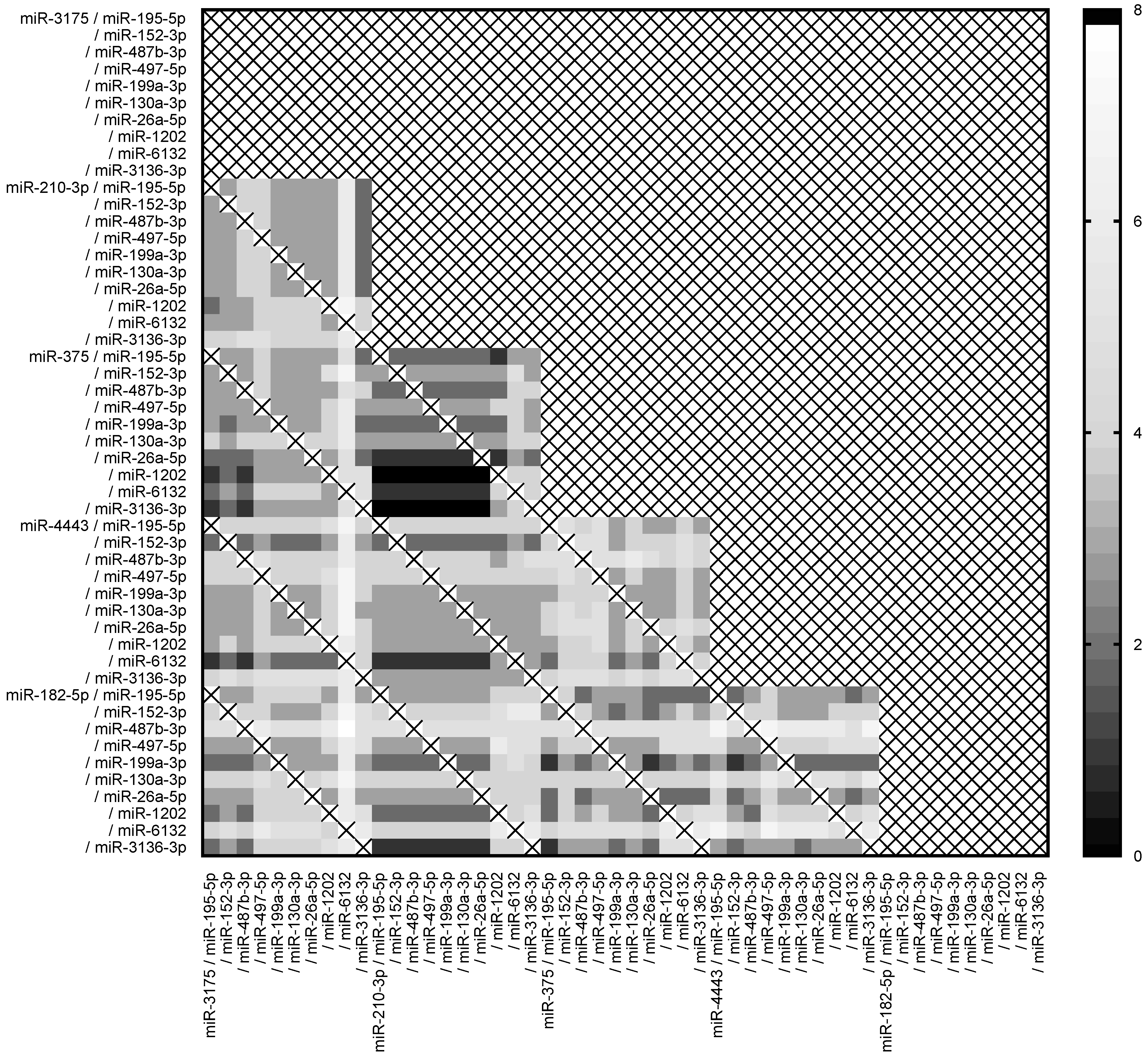
2.3. MiRNA Expression Ratio Allows to Distinguish Borderline and Malignant Tumors but Not Optimally
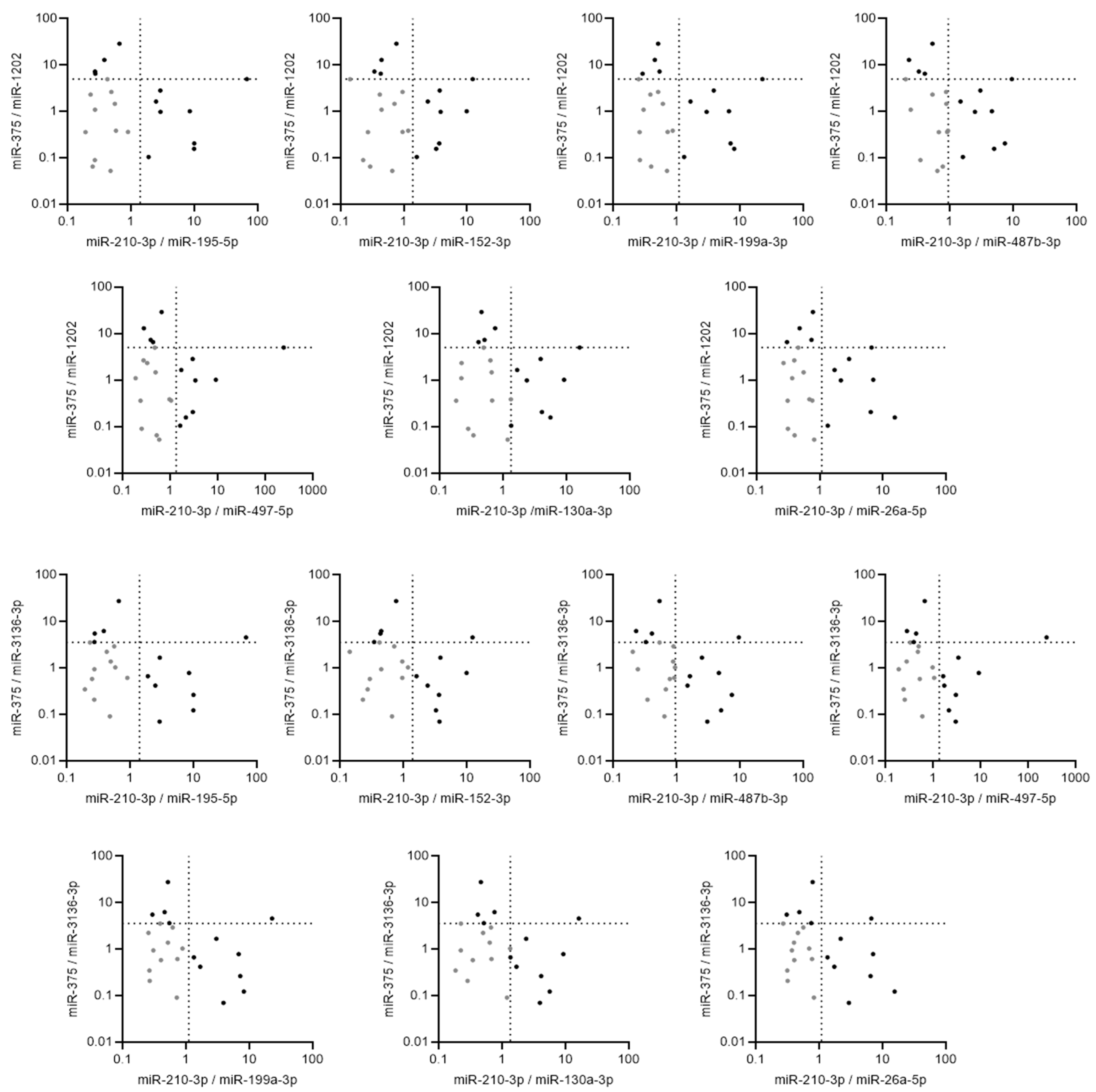
2.4. Pairs of Ratios of miRNAs Demonstrated High Accuracy to Discriminate between Borderline and Malignant Tumors
3. Discussion
4. Materials and Methods
4.1. Clinical Samples
4.2. RNA Extraction
4.3. MicroRNA Profiling by Microarrays
4.4. MiRNA Expression by RT-qPCR
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial Ovarian Cancer: Evolution of Management in the Era of Precision Medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of Ovarian Cancer: A Review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.-J.; Bast, R.C.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A.; et al. Rethinking Ovarian Cancer II: Reducing Mortality from High-Grade Serous Ovarian Cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Gouy, S.; Leary, A. Mucinous Ovarian Carcinoma. N. Engl. J. Med. 2019, 380, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Ezendam, N.; Bulten, J.; Nagtegaal, I.; Massuger, L. Survival of Patients with Mucinous Ovarian Carcinoma and Ovarian Metastases: A Population-Based Cancer Registry Study. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2015, 25, 1208–1215. [Google Scholar] [CrossRef]
- Ricci, F.; Affatato, R.; Carrassa, L.; Damia, G. Recent Insights into Mucinous Ovarian Carcinoma. Int. J. Mol. Sci. 2018, 19, 1569. [Google Scholar] [CrossRef] [PubMed]
- Prat, J.; D’Angelo, E.; Espinosa, I. Ovarian Carcinomas: At Least Five Different Diseases with Distinct Histological Features and Molecular Genetics. Hum. Pathol. 2018, 80, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Marko, J.; Marko, K.I.; Pachigolla, S.L.; Crothers, B.A.; Mattu, R.; Wolfman, D.J. Mucinous Neoplasms of the Ovary: Radiologic-Pathologic Correlation. Radiographics 2019, 39, 982–997. [Google Scholar] [CrossRef]
- Tulpin, L.; Akerman, G.; Morel, O.; Desfeux, P.; Malartic, C.; Barranger, E. Management of Borderline Tumors of the Ovary. J. Gynecol. Obstet. Biol. Reprod. 2008, 37 (Suppl. S2), F69–F75. [Google Scholar] [CrossRef]
- Bourdel, N.; Huchon, C.; Abdel Wahab, C.; Azaïs, H.; Bendifallah, S.; Bolze, P.A.; Brun, J.L.; Canlorbe, G.; Chauvet, P.; Chereau, E.; et al. Borderline Ovarian Tumors: Guidelines from the French National College of Obstetricians and Gynecologists (CNGOF). Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Fauvet, R.; Boccara, J.; Dufournet, C.; David-Montefiore, E.; Poncelet, C.; Daraï, E. Restaging Surgery for Women with Borderline Ovarian Tumors: Results of a French Multicenter Study. Cancer 2004, 100, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Uzan, C.; Fauvet, R.; Gouy, S.; Duvillard, P.; Darai, E. Borderline Ovarian Tumour: Pathological Diagnostic Dilemma and Risk Factors for Invasive or Lethal Recurrence. Lancet Oncol. 2012, 13, e103–e115. [Google Scholar] [CrossRef] [PubMed]
- Bendifallah, S.; Nikpayam, M.; Ballester, M.; Uzan, C.; Fauvet, R.; Morice, P.; Darai, E. New Pointers for Surgical Staging of Borderline Ovarian Tumors. Ann. Surg. Oncol. 2016, 23, 443–449. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA Function in Animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef]
- Mandilaras, V.; Vernon, M.; Meryet-Figuière, M.; Karakasis, K.; Lambert, B.; Poulain, L.; Oza, A.; Denoyelle, C.; Lheureux, S. Updates and Current Challenges in microRNA Research for Personalized Medicine in Ovarian Cancer. Expert Opin. Biol. Ther. 2017, 17, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, Y.K.; Kwon, Y.; Heo, J.H.; Kang, H.; Kim, G.; An, H.J. Deregulation of miR-519a, 153, and 485-5p and Its Clinicopathological Relevance in Ovarian Epithelial Tumours. Histopathology 2010, 57, 734–743. [Google Scholar] [CrossRef]
- Calura, E.; Fruscio, R.; Paracchini, L.; Bignotti, E.; Ravaggi, A.; Martini, P.; Sales, G.; Beltrame, L.; Clivio, L.; Ceppi, L.; et al. miRNA Landscape in Stage I Epithelial Ovarian Cancer Defines the Histotype Specificities. Clin. Cancer Res. 2013, 19, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Brunetti, M.; Davidson, B.; Tropé, C.G.; Eriksson, A.G.Z.; Heim, S.; Panagopoulos, I.; Micci, F. The microRNA miR-192/215 Family Is Upregulated in Mucinous Ovarian Carcinomas. Sci. Rep. 2018, 8, 11069. [Google Scholar] [CrossRef]
- Meng, W.; McElroy, J.P.; Volinia, S.; Palatini, J.; Warner, S.; Ayers, L.W.; Palanichamy, K.; Chakravarti, A.; Lautenschlaeger, T. Comparison of MicroRNA Deep Sequencing of Matched Formalin-Fixed Paraffin-Embedded and Fresh Frozen Cancer Tissues. PLoS ONE 2013, 8, e64393. [Google Scholar] [CrossRef]
- Bucay, N.; Shahryari, V.; Majid, S.; Yamamura, S.; Mitsui, Y.; Tabatabai, Z.L.; Greene, K.; Deng, G.; Dahiya, R.; Tanaka, Y.; et al. miRNA Expression Analyses in Prostate Cancer Clinical Tissues. J. Vis. Exp. 2015, 103, e53123. [Google Scholar] [CrossRef]
- Vojtechova, Z.; Zavadil, J.; Klozar, J.; Grega, M.; Tachezy, R. Comparison of the miRNA Expression Profiles in Fresh Frozen and Formalin-Fixed Paraffin-Embedded Tonsillar Tumors. PLoS ONE 2017, 12, e0179645. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Shah, J.S.; Mackelvie, M.; Gershenson, D.M.; Ramalingam, P.; Kott, M.M.; Brown, J.; Gauthier, P.; Nugent, E.; Ramondetta, L.M.; Frumovitz, M. Accuracy of Intraoperative Frozen Section Diagnosis of Borderline Ovarian Tumors by Hospital Type. J. Minim. Invasive Gynecol. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Tempfer, C.B.; Polterauer, S.; Bentz, E.-K.; Reinthaller, A.; Hefler, L.A. Accuracy of Intraoperative Frozen Section Analysis in Borderline Tumors of the Ovary: A Retrospective Analysis of 96 Cases and Review of the Literature. Gynecol. Oncol. 2007, 107, 248–252. [Google Scholar] [CrossRef]
- Lee, H.; Park, C.S.; Deftereos, G.; Morihara, J.; Stern, J.E.; Hawes, S.E.; Swisher, E.; Kiviat, N.B.; Feng, Q. MicroRNA Expression in Ovarian Carcinoma and Its Correlation with Clinicopathological Features. World J. Surg. Oncol. 2012, 10, 174. [Google Scholar] [CrossRef]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, Normalization, and Summaries of High Density Oligonucleotide Array Probe Level Data. Biostatistics 2003, 4, 249–264. [Google Scholar] [CrossRef]
- Avissar, M.; Christensen, B.C.; Kelsey, K.T.; Marsit, C.J. MicroRNA Expression Ratio Is Predictive of Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 2850–2855. [Google Scholar] [CrossRef]
- Torres, R.; Lang, U.E.; Hejna, M.; Shelton, S.J.; Joseph, N.M.; Shain, A.H.; Yeh, I.; Wei, M.L.; Oldham, M.C.; Bastian, B.C.; et al. MicroRNA Ratios Distinguish Melanomas from Nevi. J. Investig. Dermatol. 2020, 140, 164–173.e7. [Google Scholar] [CrossRef]
- Chiam, K.; Wang, T.; Watson, D.I.; Mayne, G.C.; Irvine, T.S.; Bright, T.; Smith, L.; White, I.A.; Bowen, J.M.; Keefe, D.; et al. Circulating Serum Exosomal miRNAs As Potential Biomarkers for Esophageal Adenocarcinoma. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2015, 19, 1208–1215. [Google Scholar] [CrossRef]
- Gahlawat, A.W.; Witte, T.; Haarhuis, L.; Schott, S. A Novel Circulating miRNA Panel for Non-Invasive Ovarian Cancer Diagnosis and Prognosis. Br. J. Cancer 2022, 127, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liang, X.; Wang, L.; Zhang, X. The Role of miRNA in Ovarian Cancer: An Overview. Reprod. Sci. 2022, 10, 2760–2767. [Google Scholar] [CrossRef] [PubMed]
- Roma, A.A.; Masand, R.P. Different Staining Patterns of Ovarian Brenner Tumor and the Associated Mucinous Tumor. Ann. Diagn. Pathol. 2015, 19, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Simmer, F.; Bulten, J.; Ligtenberg, M.J.; Hollema, H.; Van Vliet, S.; De Voer, R.M.; Kamping, E.J.; Van Essen, D.F.; Ylstra, B.; et al. Two Types of Primary Mucinous Ovarian Tumors Can Be Distinguished Based on Their Origin. Mod. Pathol. 2020, 33, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]


| Malignant Tumor N = 13 | Borderline Tumor N = 11 | |
|---|---|---|
| Age at diagnosis (years), Mean ± SD | 46.9 ± 18.3 | 45.8 ± 20.7 |
| BMI * (kg/m2), Mean ± SD | 25.5 ± 5.0 | 23.8 ± 5.9 |
| Menopaused, n (%) | 4 (30.8) | 5 (45.5) |
| Tumoral markers. Mean ± SD CA 125 (UI/mL) CA 19.9 (UI/mL) | 59.6 ± 77.8 416.5 ± 806.8 | 35.6 ± 30.4 8.3 ± 4.2 |
| FIGO # staging, n (%) I IV | 11 (84.6) 2 (15.4) | 11 (100) 0 |
| Tumor size (cm), Mean ± SD | 18.1 ± 7.5 | 17.8 ± 7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolivet, E.; Gaichies, L.; Jeanne, C.; Bazille, C.; Briand, M.; Vernon, M.; Giffard, F.; Leprêtre, F.; Poulain, L.; Denoyelle, C.; et al. Synergy of the microRNA Ratio as a Promising Diagnosis Biomarker for Mucinous Borderline and Malignant Ovarian Tumors. Int. J. Mol. Sci. 2023, 24, 16016. https://doi.org/10.3390/ijms242116016
Dolivet E, Gaichies L, Jeanne C, Bazille C, Briand M, Vernon M, Giffard F, Leprêtre F, Poulain L, Denoyelle C, et al. Synergy of the microRNA Ratio as a Promising Diagnosis Biomarker for Mucinous Borderline and Malignant Ovarian Tumors. International Journal of Molecular Sciences. 2023; 24(21):16016. https://doi.org/10.3390/ijms242116016
Chicago/Turabian StyleDolivet, Enora, Léopold Gaichies, Corinne Jeanne, Céline Bazille, Mélanie Briand, Mégane Vernon, Florence Giffard, Frédéric Leprêtre, Laurent Poulain, Christophe Denoyelle, and et al. 2023. "Synergy of the microRNA Ratio as a Promising Diagnosis Biomarker for Mucinous Borderline and Malignant Ovarian Tumors" International Journal of Molecular Sciences 24, no. 21: 16016. https://doi.org/10.3390/ijms242116016
APA StyleDolivet, E., Gaichies, L., Jeanne, C., Bazille, C., Briand, M., Vernon, M., Giffard, F., Leprêtre, F., Poulain, L., Denoyelle, C., Vigneron, N., & Fauvet, R. (2023). Synergy of the microRNA Ratio as a Promising Diagnosis Biomarker for Mucinous Borderline and Malignant Ovarian Tumors. International Journal of Molecular Sciences, 24(21), 16016. https://doi.org/10.3390/ijms242116016





