Epigenetic and Hormonal Modulation in Plant–Plant Growth-Promoting Microorganism Symbiosis for Drought-Resilient Agriculture
Abstract
:1. Introduction
2. Impact of PGPMs on Host Plant Gene Expression under Drought Stress
3. Microbial Inoculation and Epigenetic Regulation of Drought-Responsive Genes in Plants
4. Microbial-Mediated Gene Regulation and Hormonal Response in Plants under Drought Stress
5. Challenges and Opportunities for PGPM Application in Drought-Stressed Agriculture
5.1. Challenges
5.2. Opportunities
6. Conclusions
7. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghadirnezhad Shiade, S.R.; Fathi, A.; Taghavi Ghasemkheili, F.; Amiri, E.; Pessarakli, M. Plants’ responses under drought stress conditions: Effects of strategic management approaches—A review. J. Plant Nutr. 2023, 46, 2198–2230. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Park, S.; Mir, R.A.; Mushtaq, M.; Bhat, B.; Bae, H. Deciphering the plant microbiome to improve drought tolerance: Mechanisms and perspectives. Environ. Exp. Bot. 2022, 201, 104933. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Santoyo, G.; Yadav, A.N.; Babalola, O.O. Efforts towards overcoming drought stress in crops: Revisiting the mechanisms employed by plant growth-promoting bacteria. Front. Microbiol. 2022, 13, 962427. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, B.S.; Nivetha, N.; Krishna, G.K.; Elangovan, A.; Pushkar, S.; Chandrashekar, N.; Paul, S. Plant growth-promoting rhizobacteria Shewanella putrefaciens and Cronobacter dublinensis enhance drought tolerance of pearl millet by modulating hormones and stress-responsive genes. Physiol. Plant. 2022, 174, e13676. [Google Scholar] [CrossRef]
- Romero-Munar, A.; Aroca, R.; Zamarreño, A.M.; García-Mina, J.M.; Perez-Hernández, N.; Ruiz-Lozano, J.M. Dual inoculation with Rhizophagus irregularis and Bacillus megaterium improves maize tolerance to combined drought and high temperature stress by enhancing root hydraulics, photosynthesis and hormonal responses. Int. J. Mol. Sci. 2023, 24, 5193. [Google Scholar] [CrossRef] [PubMed]
- Siraj, S.; Khan, M.A.; Hamayun, M.; Ali, S.; Khan, S.A.; Hussain, A.; Lee, I.J. Microbacterium oxydans regulates physio-hormonal and molecular attributes of Solanum lycopersicum under drought stress. Agronomy 2022, 12, 3224. [Google Scholar] [CrossRef]
- Sati, D.; Pande, V.; Pandey, S.C.; Samant, M. Recent advances in PGPR and molecular mechanisms involved in drought stress resistance. J. Soil Sci. Plant Nutr. 2023, 23, 106–124. [Google Scholar] [CrossRef]
- Kour, D.; Yadav, A.N. Bacterial mitigation of drought stress in plants: Current perspectives and future challenges. Curr. Microbiol. 2022, 79, 248. [Google Scholar] [CrossRef]
- Lastochkina, O.; Yakupova, A.; Avtushenko, I.; Lastochkin, A.; Yuldashev, R. Effect of Seed Priming with Endophytic Bacillus subtilis on Some Physio-Biochemical Parameters of Two Wheat Varieties Exposed to Drought after Selective Herbicide Application. Plants 2023, 12, 1724. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, Z.; Wu, H.; Huang, C.; Shuai, P.; Ye, C.-Y.; Tang, S.; Wang, Y.; Yang, L.; Wang, J.; et al. Single-base-resolution methylomes of populus trichocarpa reveal the association between DNA methylation and drought stress. BMC Genet. 2014, 15, S9. [Google Scholar] [CrossRef]
- Da, K.; Nowak, J.; Flinn, B. Potato cytosine methylation and gene expression changes induced by a beneficial bacterial endophyte, Burkholderia phytofirmans strain PsJN. Plant Physiol. Biochem. 2012, 50, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Gagné-Bourque, F.; Mayer, B.F.; Charron, J.B.; Vali, H.; Bertrand, A.; Jabaji, S. Accelerated growth rate and increased drought stress resilience of the model grass Brachypodium distachyon colonized by Bacillus subtilis B26. PLoS ONE 2015, 10, e0130456. [Google Scholar] [CrossRef] [PubMed]
- Dhawi, F. The Role of Plant Growth-Promoting Microorganisms (PGPMs) and Their Feasibility in Hydroponics and Vertical Farming. Metabolites 2023, 13, 247. [Google Scholar] [CrossRef]
- Cantabella, D.; Dolcet-Sanjuan, R.; Teixidó, N. Using plant growth-promoting microorganisms (PGPMs) to improve plant development under in vitro culture conditions. Planta 2022, 255, 117. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Wagner, E.G.H. The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: A possible connection between biotic and abiotic stress responses. Mol. Plant-Microbe Interact. 1999, 12, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sikora, E.; Park, S.W. Plant growth-promoting rhizobacterium, Paenibacillus polymyxa CR1, upregulates dehydration-responsive genes, RD29A and RD29B, during priming drought tolerance in Arabidopsis. Plant Physiol. Biochem. 2020, 156, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Yang, W.; Wang, C.; Gu, C.; Niu, D.D.; Liu, H.X.; Guo, J.H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE 2012, 7, e52565. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Kang, B.R.; Kim, Y.C. Transcriptome analysis of induced systemic drought tolerance elicited by Pseudomonas chlororaphis O6 in Arabidopsis thaliana. Plant Pathol. J. 2013, 29, 209. [Google Scholar] [CrossRef]
- Krishna, R.; Jaiswal, D.K.; Ansari, W.A.; Singh, S.; Soumia, P.S.; Singh, A.K.; Verma, J.P. Potential microbial consortium mitigates drought stress in tomato (Solanum lycopersicum L.) plant by up-regulating stress-responsive genes and improving fruit yield and soil properties. J. Soil Sci. Plant Nutr. 2022, 22, 4598–4615. [Google Scholar] [CrossRef]
- Vaishnav, A.; Choudhary, D.K. Regulation of drought-responsive gene expression in Glycine max l. Merrill is mediated through Pseudomonas simiae strain AU. J. Plant Growth Regul. 2019, 38, 333–342. [Google Scholar] [CrossRef]
- Kaushal, M. Microbes in cahoots with plants: MIST to hit the jackpot of agricultural productivity during drought. Int. J. Mol. Sci. 2019, 20, 1769. [Google Scholar] [CrossRef]
- Wang, D.C.; Jiang, C.H.; Zhang, L.N.; Chen, L.; Zhang, X.Y.; Guo, J.H. Biofilms positively contribute to Bacillus amyloliquefaciens 54-induced drought tolerance in tomato plants. Int. J. Mol. Sci. 2019, 20, 6271. [Google Scholar] [CrossRef] [PubMed]
- Kakar, K.U.; Ren, X.L.; Nawaz, Z.; Cui, Z.Q.; Li, B.; Xie, G.L.; Sun, G.C. A consortium of rhizobacterial strains and biochemical growth elicitors improve cold and drought stress tolerance in rice (Oryza sativa L.). Plant Biol. 2016, 18, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Sarma, R.K.; Saikia, R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil 2014, 377, 111–126. [Google Scholar] [CrossRef]
- Kasim, W.A.; Osman, M.E.; Omar, M.N.; Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Control of drought stress in wheat using plant-growth-promoting bacteria. J. Plant Growth Regul. 2013, 32, 122–130. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C.; Chauhan, P.S.; Nautiyal, C.S. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol. Biochem. 2016, 99, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, S.F.; Yue, L.; Zhao, X.; Zhang, Y.B.; Xie, Z.K.; Wang, R.Y. Epsc involved in the encoding of exopolysaccharides produced by Bacillus amyloliquefaciens FZB42 act to boost the drought tolerance of Arabidopsis thaliana. Int. J. Mol. Sci. 2018, 19, 3795. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Kim, S.B.; Nevo, E.; Abd El Daim, I.; Ek, B.O.; Bergquist, J.; Behers, L. Sfp-type PPTase inactivation promotes bacterial biofilm formation and ability to enhance wheat drought tolerance. Front. Microbiol. 2015, 6, 387. [Google Scholar] [CrossRef]
- Murali, M.; Singh, S.B.; Gowtham, H.G.; Shilpa, N.; Prasad, M.; Aiyaz, M.; Amruthesh, K.N. Induction of drought tolerance in Pennisetum glaucum by ACC deaminase producing PGPR-Bacillus amyloliquefaciens through Antioxidant defense system. Microbiol. Res. 2021, 253, 126891. [Google Scholar] [CrossRef]
- Gontia-Mishra, I.; Sapre, S.; Sharma, A.; Tiwari, S. Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol. 2016, 18, 992–1000. [Google Scholar] [CrossRef]
- Fan, Q.J.; Liu, J.H. Colonization with arbuscular mycorrhizal fungus affects growth, drought tolerance and expression of stress-responsive genes in Poncirus trifoliata. Acta Physiol. Plant. 2021, 33, 1533–1542. [Google Scholar] [CrossRef]
- Jatan, R.; Chauhan, P.S.; Lata, C. Pseudomonas putida modulates the expression of miRNAs and their target genes in response to drought and salt stresses in chickpea (Cicer arietinum L.). Genomics 2019, 111, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Lephatsi, M.; Nephali, L.; Meyer, V.; Piater, L.A.; Buthelezi, N.; Dubery, I.A.; Tugizimana, F. Molecular mechanisms associated with microbial biostimulant-mediated growth enhancement, priming and drought stress tolerance in maize plants. Sci. Rep. 2022, 12, 10450. [Google Scholar] [CrossRef] [PubMed]
- Akhter, Z.; Bi, Z.; Ali, K.; Sun, C.; Fiaz, S.; Haider, F.U.; Bai, J. In response to abiotic stress, DNA methylation confers epigenetic changes in plants. Plants 2021, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- Xuan, A.; Song, Y.; Bu, C.; Chen, P.; El-Kassaby, Y.A.; Zhang, D. Changes in DNA methylation in response to 6-benzylaminopurine affect allele-specific gene expression in Populus tomentosa. Int. J. Mol. Sci. 2020, 21, 2117. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, M.; Germida, J.J.; Vujanovic, V. Fungal endophyte colonization coincides with altered DNA methylation in drought-stressed wheat seedlings. Can. J. Plant Sci. 2014, 94, 223–234. [Google Scholar] [CrossRef]
- Acharjee, S.; Chauhan, S.; Pal, R.; Tomar, R.S. Mechanisms of DNA methylation and histone modifications. Prog. Mol. Biol. Trans. Sci. 2023, 197, 51–92. [Google Scholar]
- Ost, C.; Cao, H.X.; Nguyen, T.L.; Himmelbach, A.; Mascher, M.; Stein, N.; Humbeck, K. Drought-Stress-Related Reprogramming of Gene Expression in Barley Involves Differential Histone Modifications at ABA-Related Genes. Int. J. Mol. Sci. 2023, 24, 12065. [Google Scholar] [CrossRef]
- Guarnizo, Á.L.; Navarro-Ródenas, A.; Calvo-Polanco, M.; Marqués-Gálvez, J.E.; Morte, A. A mycorrhizal helper bacterium alleviates drought stress in mycorrhizal Helianthemum almeriense plants by regulating water relations and plant hormones. Environ. Exp. Bot. 2023, 207, 105228. [Google Scholar] [CrossRef]
- Curá, J.A.; Franz, D.R.; Filosofía, J.E.; Balestrasse, K.B.; Burgueño, L.E. Inoculation with Azospirillum sp. and Herbaspirillum sp. bacteria increases the tolerance of maize to drought stress. Microorganisms 2017, 5, 41. [Google Scholar] [CrossRef]
- Vargas, L.; Santa Brigida, A.B.; Mota Filho, J.P.; de Carvalho, T.G.; Rojas, C.A.; Vaneechoutte, D.; Hemerly, A.S. Drought tolerance conferred to sugarcane by association with Gluconacetobacter diazotrophicus: A transcriptomic view of hormone pathways. PLoS ONE 2014, 9, e114744. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Shahzad, R.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.J. Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: Heavy metals, high temperature and drought stress. Ind. Crops Prod. 2020, 143, 111931. [Google Scholar] [CrossRef]
- Lakshmanan, V.; Ray, P.; Craven, K.D. Rhizosphere sampling protocols for microbiome (16S/18S/ITS rRNA) library preparation and enrichment for the isolation of drought tolerance-promoting microbes. In Plant Stress Tolerance: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2017; pp. 349–362. [Google Scholar]
- Ansari, F.A.; Ahmad, I. Alleviating drought stress of crops through PGPR: Mechanism and application. In Microbial Interventions in Agriculture and Environment: Volume 2: Rhizosphere, Microbiome and Agro-Ecology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 341–358. [Google Scholar]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Smith, D.L. PGPR in agriculture: A sustainable approach to increasing climate change resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Lopes, M.J.D.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful plant growth-promoting microbes: Inoculation methods and abiotic factors. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Imran, A. Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Jambhulkar, P.P.; Sharma, P.; Yadav, R. Delivery systems for introduction of microbial inoculants in the field. In Microbial Inoculants in Sustainable Agricultural Productivity: Volume 2: Functional Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 199–218. [Google Scholar]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Alenezi, F.N.; Belbahri, L. Recent advances in encapsulation techniques of plant growth-promoting microorganisms and their prospects in the sustainable agriculture. Appl. Sci. 2022, 12, 9020. [Google Scholar] [CrossRef]
- Dubey, R.K.; Tripathi, V.; Prabha, R.; Chaurasia, R.; Singh, D.P.; Rao, C.S.; Abhilash, P.C. Unravelling the Soil Microbiome: Perspectives for Environmental Sustainability; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Sharma, V.; Salwan, R.; Al-Ani, L.K.T. (Eds.) Molecular Aspects of Plant Beneficial Microbes in Agriculture; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
| PGPMs | Taxa (Domain, Phylum, Class, Order, Family, and Genus) |
|---|---|
| Paenibacillus polymyxa | Bacteria; Bacillota; Bacilli; Bacillales; Paenibacillaceae; Paenibacillus |
| Pseudomonas chlororaphis | Bacteria; Pseudomonadota; Gammaproteobacteria; Pseudomonadales; Pseudomonadaceae; Pseudomonas |
| Pseudomonas simiae | Bacteria; Pseudomonadota; Gammaproteobacteria; Pseudomonadales; Pseudomonadaceae; Pseudomonas |
| Bacillus amyloliquefaciens | Bacteria; Bacillota; Bacilli; Bacillales; Bacillaceae; Bacillus |
| Pseudomonas aeruginosa | Bacteria; Pseudomonadota; Gammaproteobacteria; Pseudomonadales; Pseudomonadaceae; Pseudomonas |
| Pseudomonas putida | Bacteria; Pseudomonadota; Gammaproteobacteria; Pseudomonadales; Pseudomonadaceae; Pseudomonas |
| Burkholderia phytofirmans | Bacteria; Proteobacteria; Betaproteobacteria; Burkholderiales; Burkholderiaceae; Burkholderia |
| Bacillus subtilis | Bacteria; Bacillota; Bacilli; Bacillales; Bacillaceae; Bacillus |
| Pseudomonas mandelii | Bacteria; Pseudomonadota; Gammaproteobacteria; Pseudomonadales; Pseudomonadaceae; Pseudomonas |
| Terfezia claveryi | Eukaryota; Fungi; Ascomycota; Pezizomycetes; Pezizales; Pezizaceae; Terfezia |
| Azospirillum brasilense | Bacteria; Proteobacteria; Alphaproteobacteria; Rhodospirillales; Rhodospirillaceae; Azospirillum |
| Herbaspirillum seropedicae | Bacteria; Proteobacteria; Betaproteobacteria; Burkholderiales; Oxalobacteraceae; Herbaspirillum |
| Gluconacetobacter diazotrophicus | Bacteria; Proteobacteria; Alphaproteobacteria; Rhodospirillales; Acetobacteraceae; Gluconacetobacter |
| Rhizophagus irregularis | Eukaryota; Fungi; Glomeromycota; Glomeromycetes; Glomerales; Glomeraceae; Rhizophagus |
| Bacillus megaterium | Bacteria; Bacillota; Bacilli; Bacillales; Bacillaceae; Bacillus |
| Paecilomyces formosus | Eukaryota; Fungi; Ascomycota; Eurotiomycetes; Eurotiales; Thermoascaceae; Paecilomyces |
| Microbe | Key Findings | Mechanisms | Key Genes Involved | References |
|---|---|---|---|---|
| Paenibacillus polymyxa | Enhanced drought tolerance in Arabidopsis plants | Induction of drought stress-responsive genes | ERD15, RAB18 | [15] |
| Paenibacillus polymyxa CR1 | Induced drought tolerance in Arabidopsis and soybean | Upregulation of critical drought-responsive genes | RD29A, RD29B | [16] |
| BBS group | Sustained transcriptional levels of key genes in cucumber leaves | Enhancing antioxidant and photosynthetic machinery | cAPX, rbcS, rbcL | [17] |
| Pseudomonas chlororaphis O6 | Induced systemic drought tolerance in Arabidopsis | Modulating gene expression | VSP1, pdf-1.2, PR-1, HEL | [18] |
| Hexa-plant growth-promoting microorganism group | Enhanced tomato plant drought tolerance | Up-regulation of stress-responsive genes | DREB, APX, CAT, SOD, P5CS | [19] |
| Pseudomonas simiae strain AU | Safeguarding soybean plants through modulation of gene expression | Up-regulation of transcription factors, osmoprotectants, and water transporters | DREB/EREB, P5CS, GOLS, PIP & TIP | [20] |
| Microbial-induced systemic tolerance (MIST) | Involvement of microbial communities in gene network orchestration | A complex network of genes including ERD15, RAB18, COX1, and others | ERD15, RAB18, COX1, PKDP, AP2-EREBP, and more | [21] |
| Bacillus amyloliquefaciens 54 | Enhanced drought tolerance in tomato plants | Induction of stress-responsive genes | lea, tdi65, ltpg2 | [22] |
| Rhizobacteria group | Enhanced cold and drought stress tolerance in rice plants | Multiple mechanisms underlying stress tolerance | CAT1 and DREB2A | [23] |
| Pseudomonas aeruginosa GGRJ21 | Upregulated expression of drought-responsive genes in mung bean plants | Upregulation of DREB2A and DHN | DREB2A, DHN | [24] |
| Bacillus amyloliquefaciens 5113 and Azospirillum brasilense NO40 | Upregulation of stress genes including APX1, SAMS1, and HSP17.8 | Enhanced drought tolerance of wheat plants | APX1, SAMS1, HSP17.8 | [25] |
| Pseudomonas putida MTCC5279 | Downregulation of stress-responsive genes including DREB1, NAC1, and ROS scavenging genes | Downregulation of stress genes | DREB1, NAC1, CAT, APX, GST | [26] |
| Bacillus amyloliquefaciens FZB42 | Improved growth and drought tolerance in Arabidopsis | Multiple mechanisms, including ethylene and jasmonate pathways | RD29A, RD17, ERD1, LEA14, and more | [27] |
| Microbe | Plant Host | Major Findings | Cytosine Methylation Impact | References |
|---|---|---|---|---|
| Burkholderia phytofirmans strain PsJN | Potato varieties: Red Pontiac and Superior | PsJN inoculation caused minimal DNA methylation changes in Red Pontiac, while Superior exhibited increased overall cytosine methylation. Genes displayed variety-specific responses to PsJN. | Enhanced DNA loci methylation observed in Superior, suppressing PsJN-induced growth stimulation. | [11] |
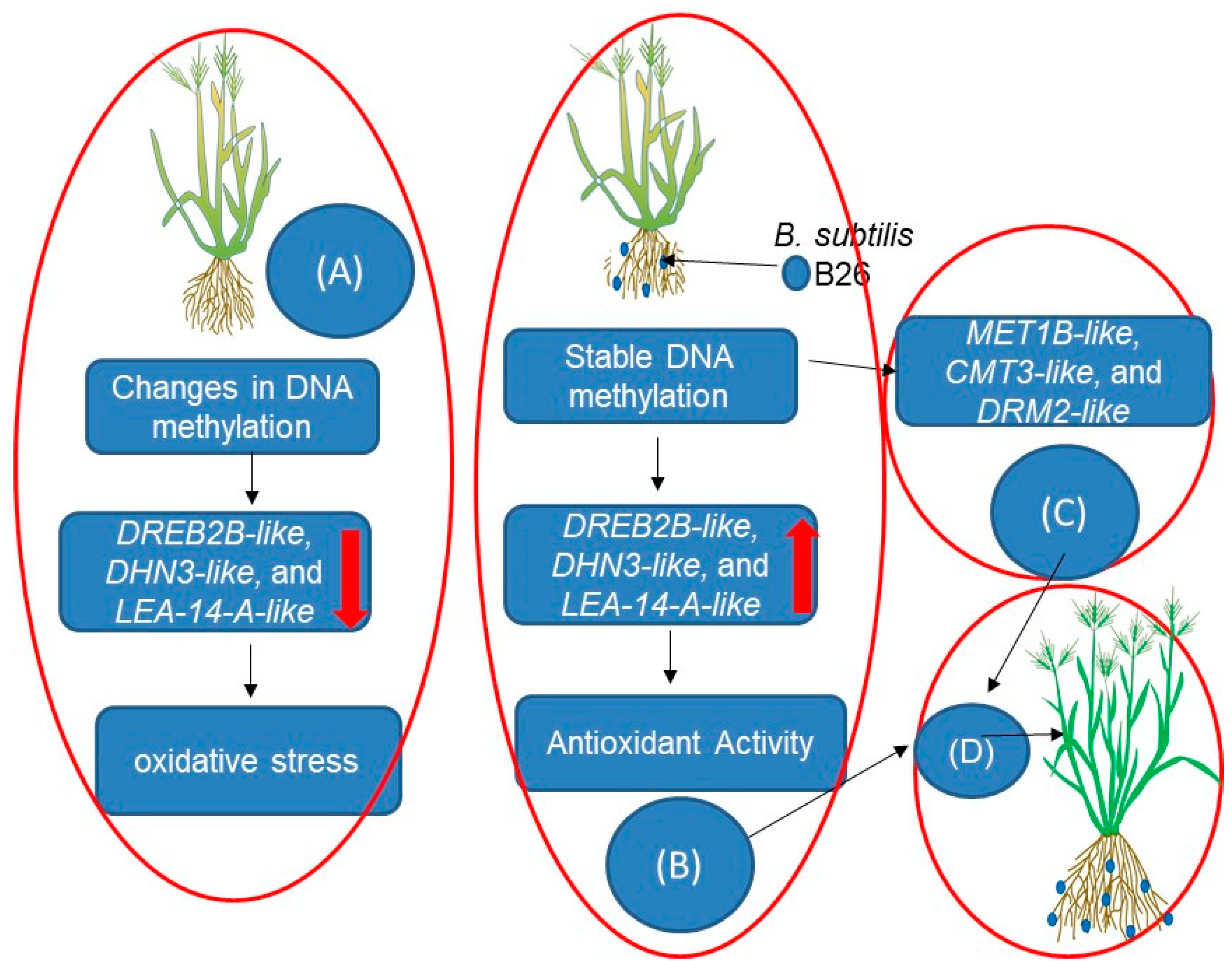
| Bacillus subtilis B26 | Brachypodium distachyon Bd21 | B. subtilis B26 increased plant growth, seed yield, and drought tolerance. Upregulated drought-responsive genes and modulation of DNA methylation genes observed. | DNA methylation changes associated with enhanced drought tolerance, involving specific genes (MET1B-like, CMT3-like, and DRM2-like). | [12] |
| Bacillus group (microbial-based biostimulant) | Maize | Biostimulant increased biomass, oxidative stress regulators, and induced metabolic changes in amino acids, phytohormones, flavonoids, and phenolic acids. | Altered metabolic profiles and gene expression patterns, with potential implications for drought resilience. | [33] |
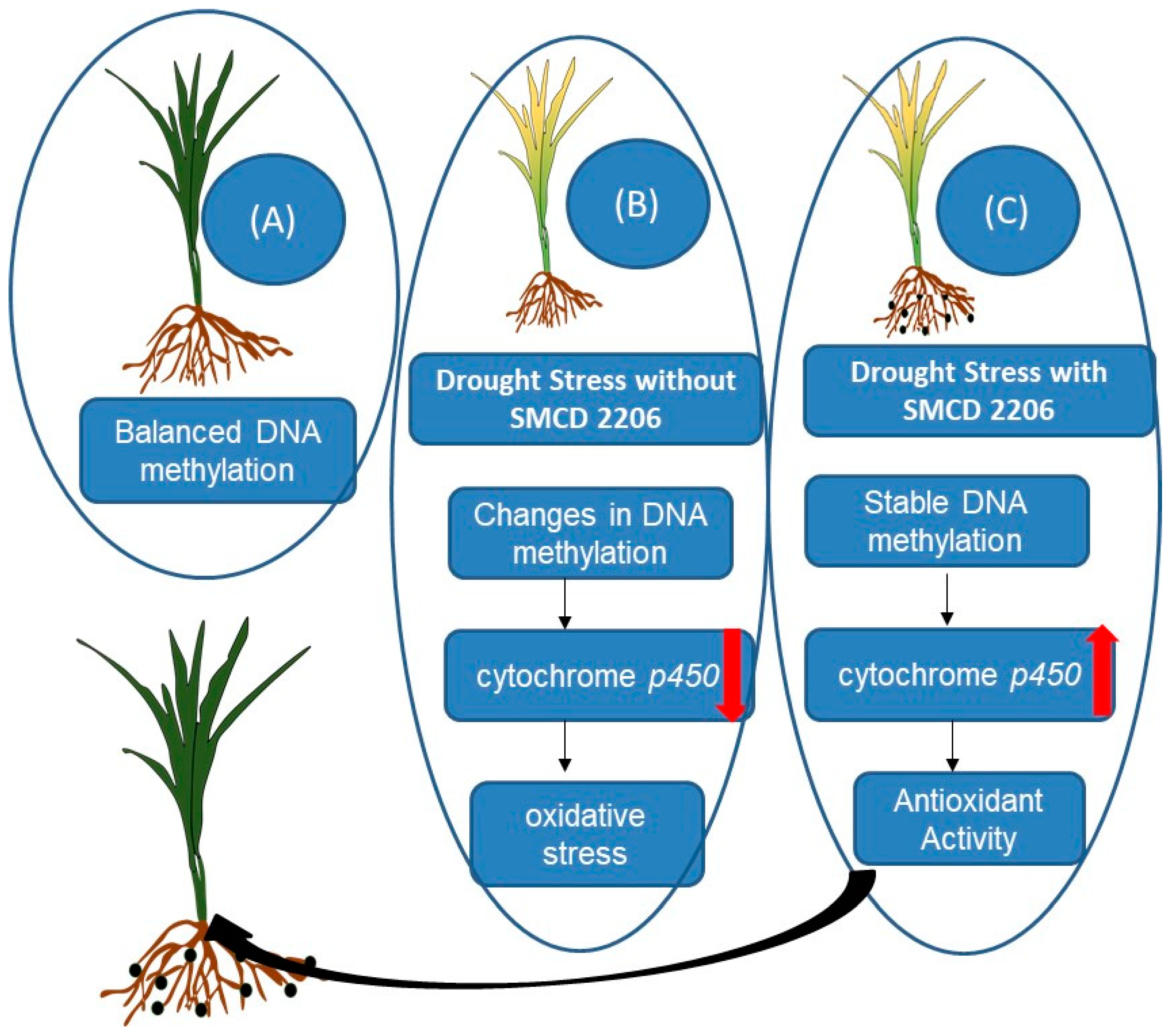
| Endophytic fungus SMCD 2206 | Wheat | SMCD 2206 colonization resulted in similar DNA methylation patterns to unstressed controls in drought-stressed wheat seedlings. Distinct DNA methylation patterns in endophyte-free, drought-stressed plants. | Epigenetic changes associated with SMCD 2206 colonization, with implications for drought resistance. | [36] |
| Microbes | Genes Studied | Hormones Investigated | Drought-Related Findings | References |
|---|---|---|---|---|
| Pseudomonas mandelii #29, Terfezia claveryi, | T. claveryi AQP (TcAQP1), Microtubule-associated | ABA | P. mandelii #29 enhanced fungal colonization and nutrient uptake under drought stress. Upregulated genes included TcAQP1, Microtubule-associated protein, and Predicted 3′−5′ exonuclease. Tripartite interactions improved plant resilience. | [39] |
| Azospirillum brasilense SP-7, Herbaspirillum seropedicae Z-152 | ZmVP14 and, Other genes | ABA, ET | PGPR-inoculated plants showed increased drought tolerance, higher biomass, reduced ABA and ET, and improved osmoregulation. | [40] |
| Gluconacetobacter diazotrophicus PAL5 | Multiple genes involved in hormone pathways | ABA and ET | Inoculated plants exhibited increased drought tolerance, unique gene expression in roots, and ABA-dependent signaling in shoots. | [41] |
| Pseudomonas simiae strain AU | Transcription factors (DREB/EREB), Osmoprotectants | ABA, SA and ET | Upregulation of drought-related genes and hormone pathways, increased proline and sugar levels. | [20] |
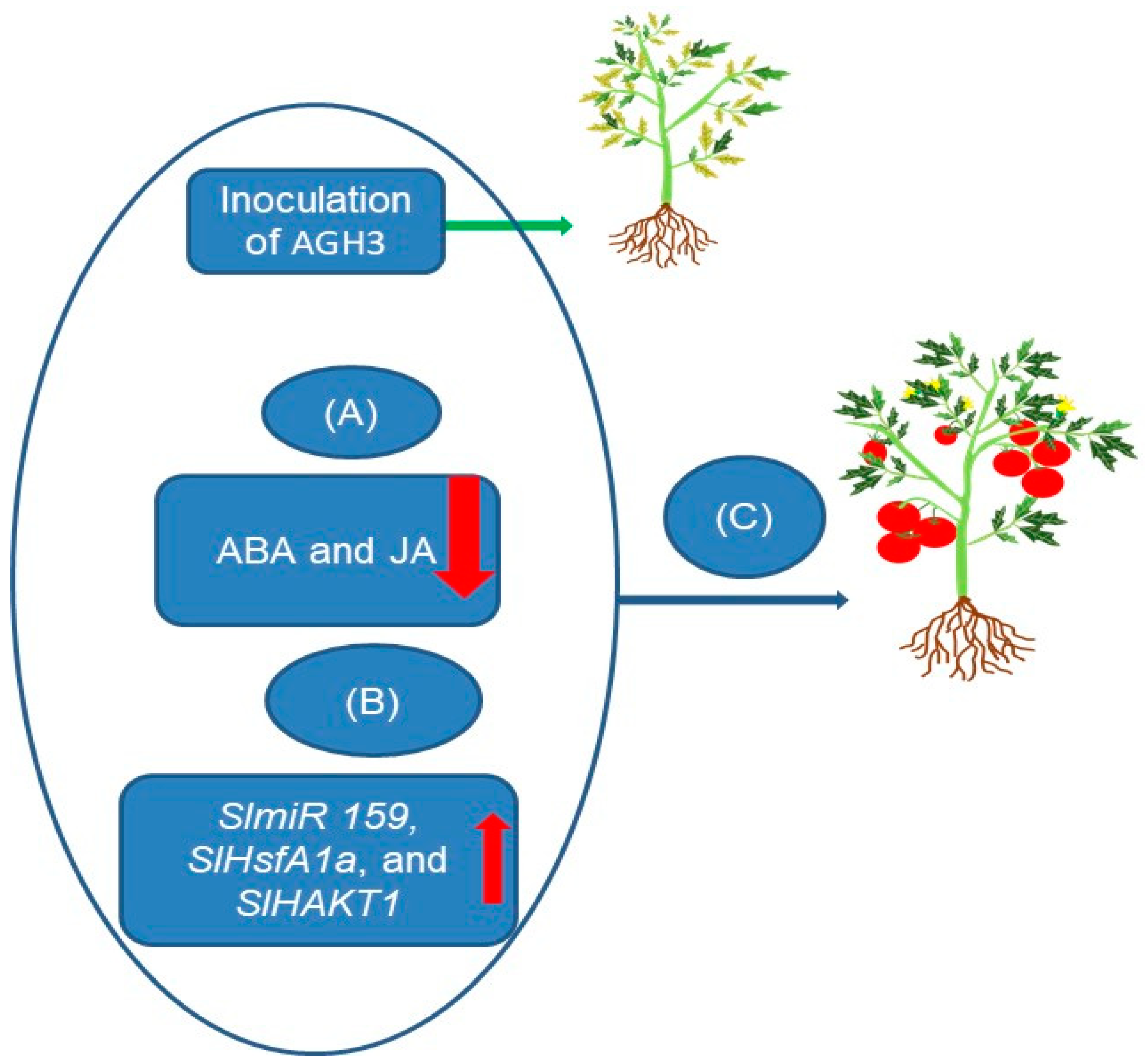
| 55 bacterial strains (AGH3, AGH5, AGH9) | SlmiR 159, SlHsfA1a, SlHAKT1 | ABA, JA | Improved growth, reduced ABA and JA production, and increased gene expression under drought stress. | [6] |
| Rhizophagus irregularis (AM), Bacillus megaterium (Bm) | ZmPIP1;3, ZmTIP1.1, ZmPIP2;2, GintAQPF1 | ABA, JA, SA, IAA | Dual inoculation mitigated drought and high-temperature stress, improving photosynthesis, root hydraulic conductivity, and regulating aquaporin genes and plant sap hormones. | [5] |
| Shewanella putrefaciens MCL-1, Cronobacter dublinensis MKS-1 | SbNCED, SbGA20oX, SbYUC, SbAP2, SbSNAC1, PgDREB2A | ABA, IAA, GA | Endophyte-inoculated plants exhibited improved growth, higher hormone levels, and upregulated genes associated with phytohormone biosynthesis and drought-responsive transcription factors. | [4] |
| Paecilomyces formosus LHL10, Penicillium funiculosum LHLO6 | Drought-related genes (GmDREB2, GmDREB1B, GmERD1, GmRD20) | Endogenous ABA and JA | Co-inoculation improved soybean growth, photosynthetic activity, antioxidant enzyme activities, nutrient uptake, and reduced oxidative damage under drought stress. | [42] |
| Various plant-associated microbiomes | ACC deaminase gene | ET | Bacteria with ACC deaminase enzymes play a role in drought tolerance by degrading ethylene and influencing stress-related gene expression in plants. | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, C.; Uğurlar, F.; Adamakis, I.-D.S. Epigenetic and Hormonal Modulation in Plant–Plant Growth-Promoting Microorganism Symbiosis for Drought-Resilient Agriculture. Int. J. Mol. Sci. 2023, 24, 16064. https://doi.org/10.3390/ijms242216064
Kaya C, Uğurlar F, Adamakis I-DS. Epigenetic and Hormonal Modulation in Plant–Plant Growth-Promoting Microorganism Symbiosis for Drought-Resilient Agriculture. International Journal of Molecular Sciences. 2023; 24(22):16064. https://doi.org/10.3390/ijms242216064
Chicago/Turabian StyleKaya, Cengiz, Ferhat Uğurlar, and Ioannis-Dimosthenis S. Adamakis. 2023. "Epigenetic and Hormonal Modulation in Plant–Plant Growth-Promoting Microorganism Symbiosis for Drought-Resilient Agriculture" International Journal of Molecular Sciences 24, no. 22: 16064. https://doi.org/10.3390/ijms242216064
APA StyleKaya, C., Uğurlar, F., & Adamakis, I.-D. S. (2023). Epigenetic and Hormonal Modulation in Plant–Plant Growth-Promoting Microorganism Symbiosis for Drought-Resilient Agriculture. International Journal of Molecular Sciences, 24(22), 16064. https://doi.org/10.3390/ijms242216064







