Sperm Capacitation and Kinematics in Phodopus Hamsters
Abstract
1. Introduction
2. Results
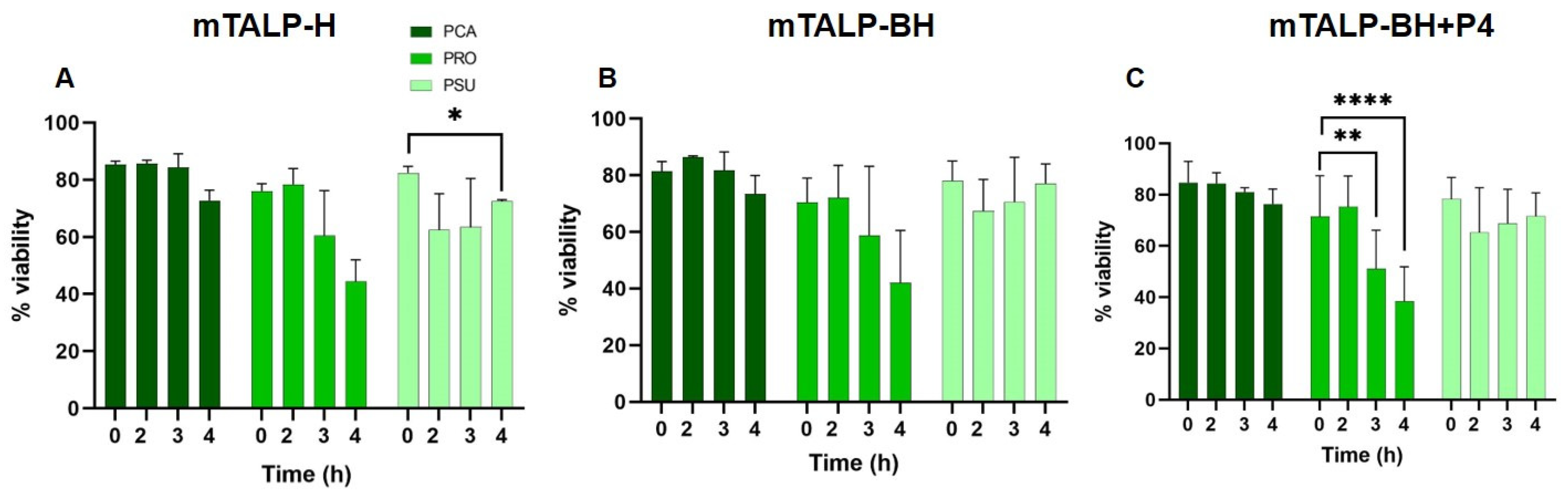
2.1. Sperm Parameters
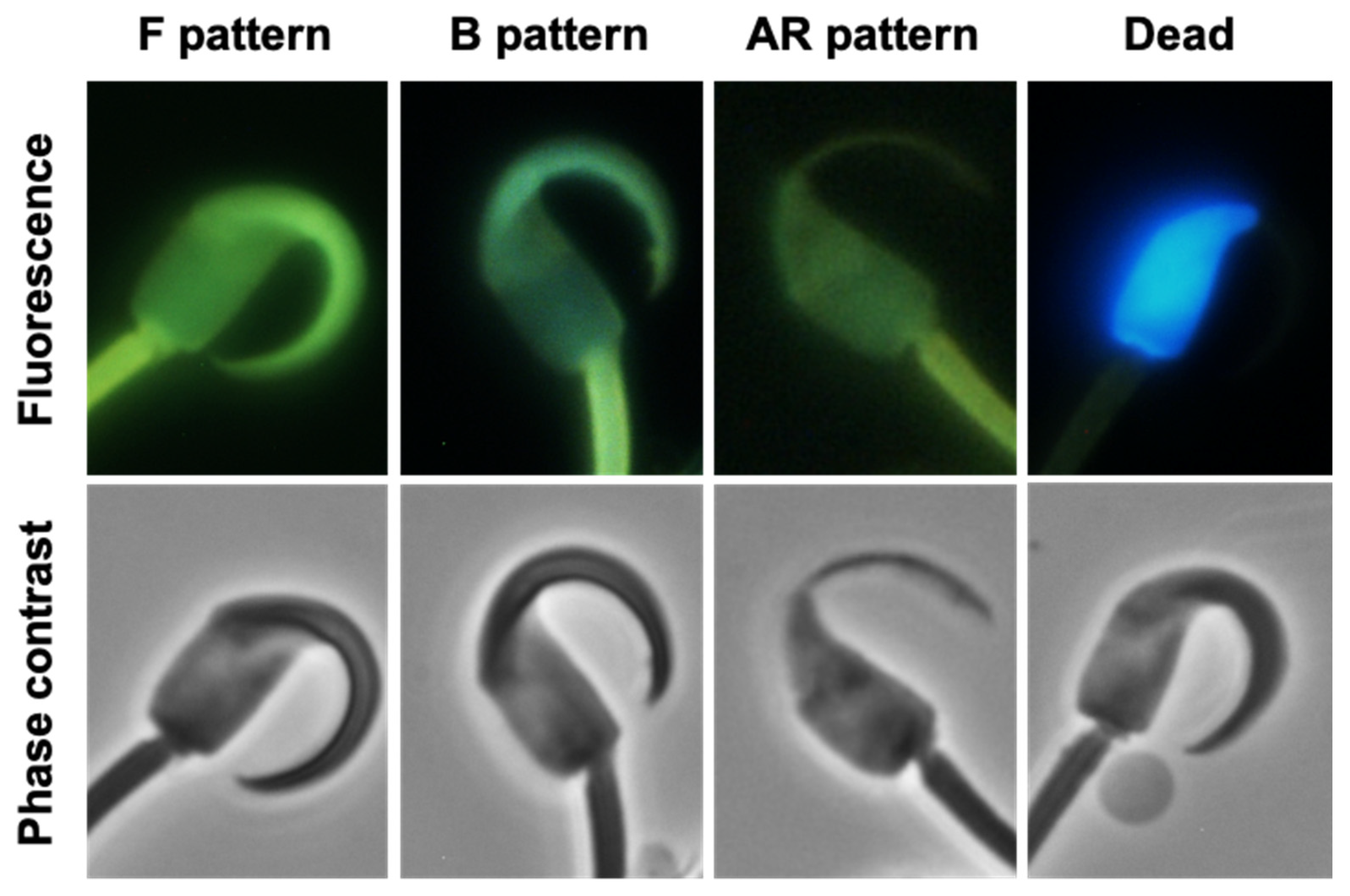
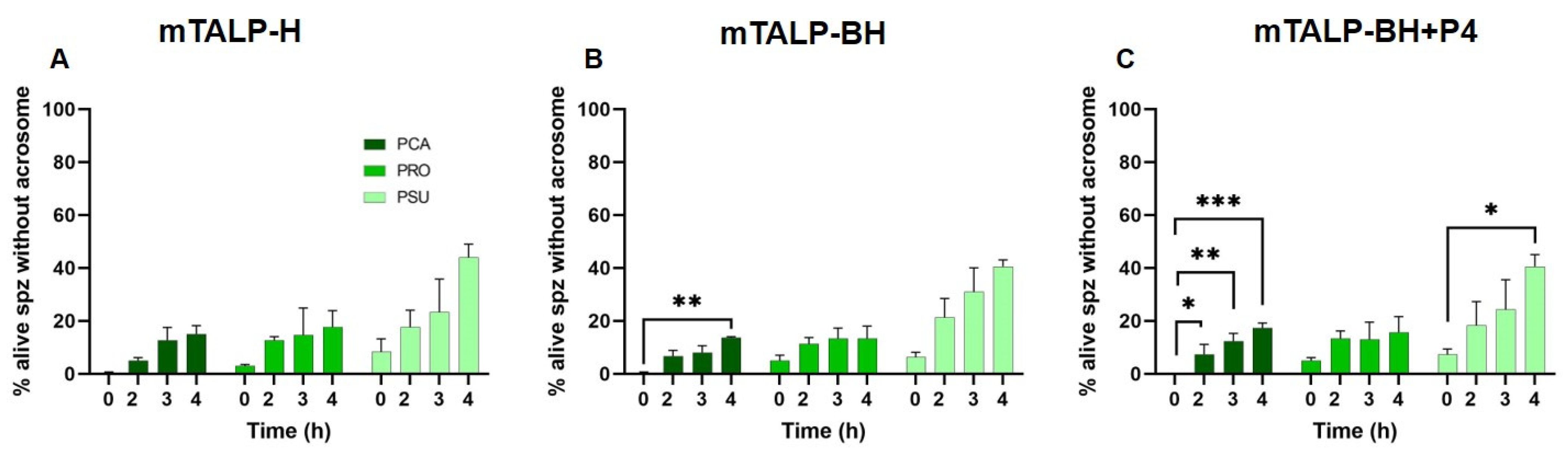
2.2. Changes in CTC Staining Patterns
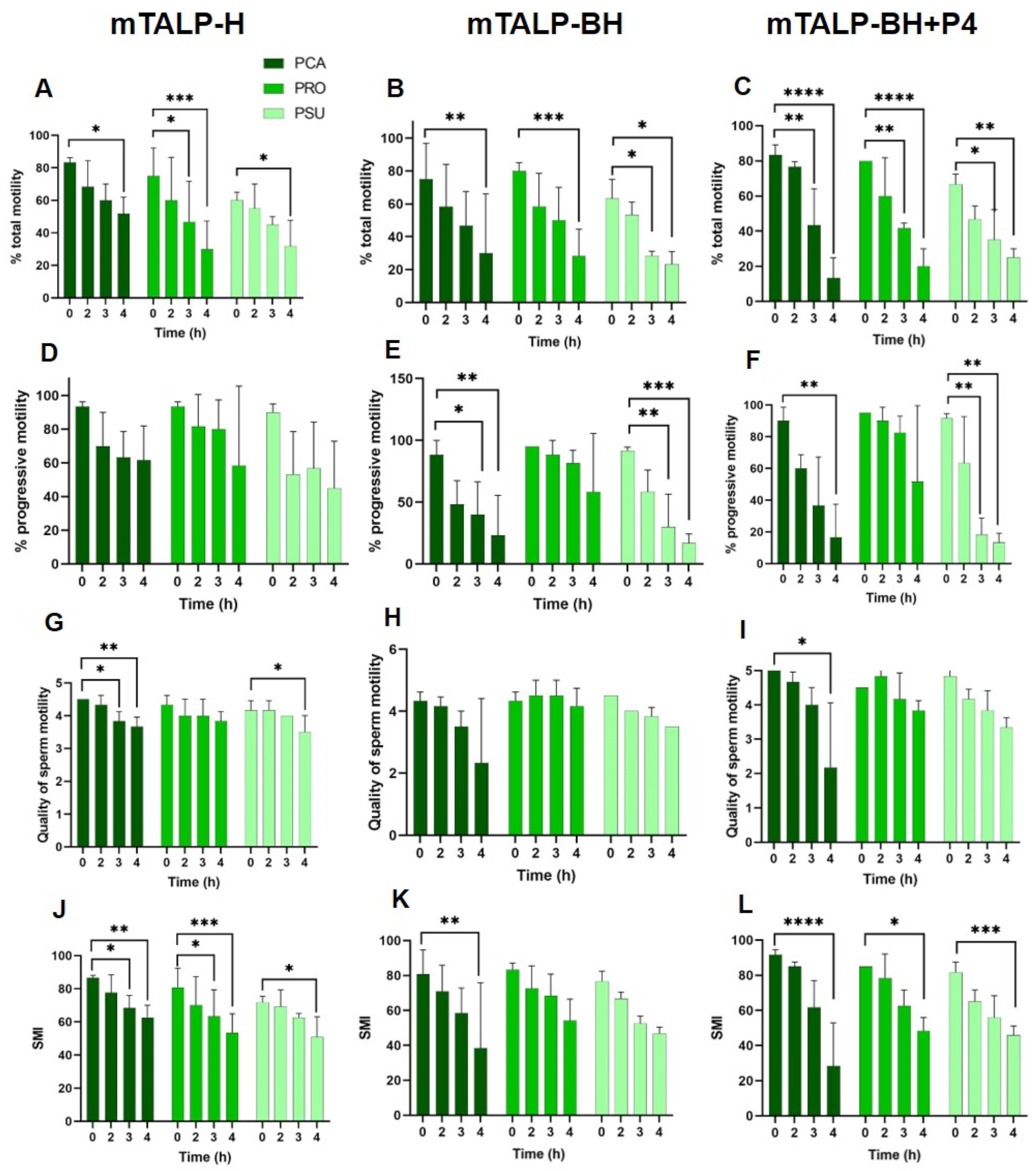
2.3. Sperm Kinematics
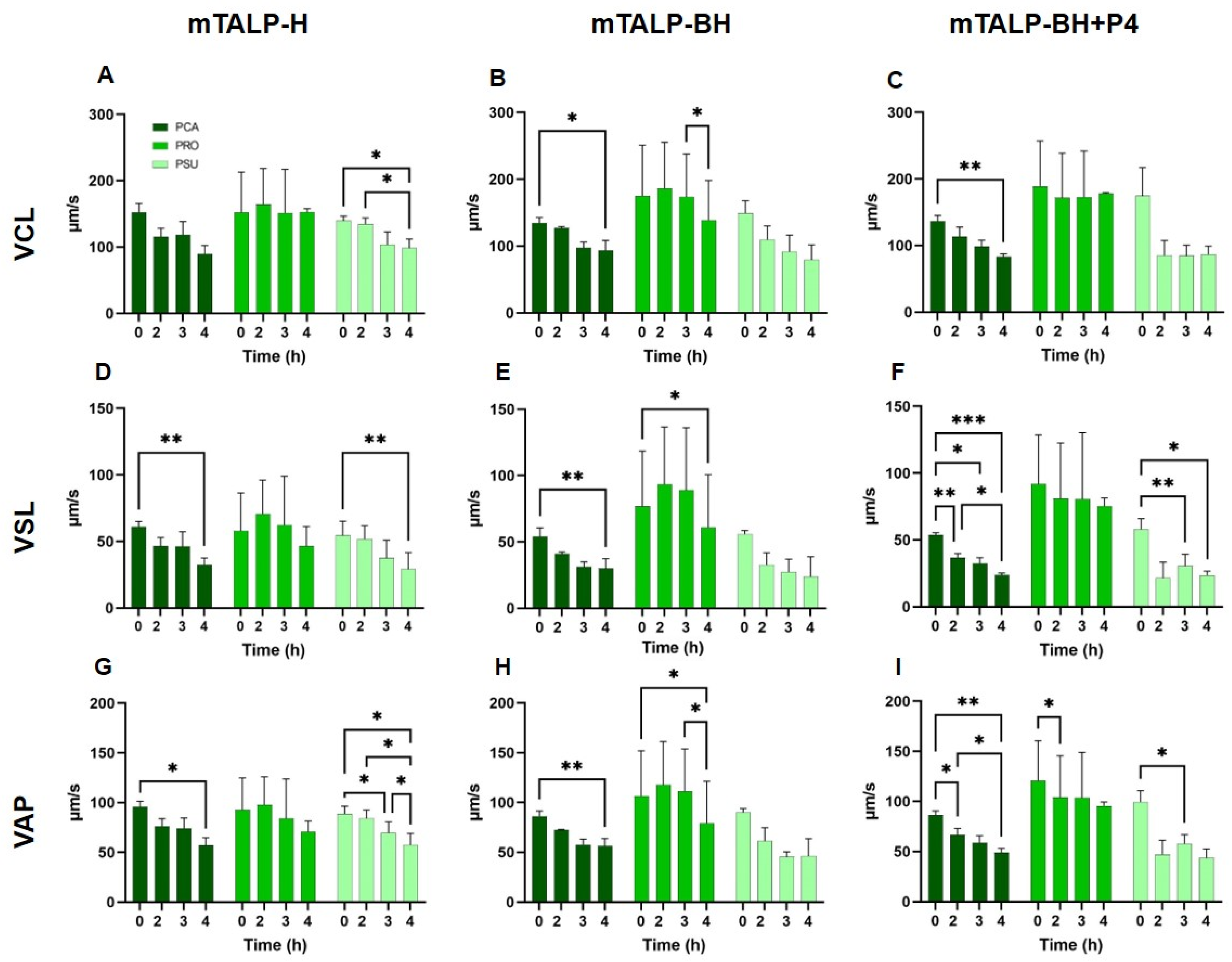
2.3.1. Velocity Parameters
2.3.2. Trajectory Parameters
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Animal Phenotype, Sperm Collection, and Incubation
4.3. Sperm Motility and Viability Assessment
4.4. Capacitation Analysis
4.5. Sperm Kinematics
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Austin, C.R. The capacitation of the mammalian sperm. Nature 1952, 170, 326. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.R. Observations on the penetration of the sperm into the mammalian egg. Aust. J. Biol. Sci. 1951, 4, 581–596. [Google Scholar] [CrossRef]
- Chang, M.C. Fertilizing capacity of spermatozoa deposited into the Fallopian tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.M. Sperm capacitation and fertilization in mammals. Biol. Reprod. 1970, 2, 128–158. [Google Scholar] [CrossRef]
- Bavister, B.D. Capacitation of golden hamster spermatozoa during incubation in culture medium. J. Reprod. Fert. 1973, 35, 161–163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Austin, C.R. Membrane fusion events in fertilization. J. Reprod. Fert. 1975, 44, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R. Mechanisms of fertilization in mammals. In Fertilization and Embryonic Development In Vitro; Mastroianni, L., Biggers, J.D., Eds.; Springer: Boston, MA, USA, 1981; pp. 81–182. [Google Scholar] [CrossRef]
- Buffone, M.G.; Rodriguez-Miranda, E.; Storey, B.T.; Gerton, G.L. Acrosomal exocytosis of mouse sperm progresses in a consistent direction in response to zona pellucida. J. Cell. Physiol. 2009, 220, 611–620. [Google Scholar] [CrossRef]
- Chang, M.C. The meaning of sperm capacitation. A historical perspective. J. Androl. 1984, 5, 45–50. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Chang’s meaning of capacitation: A molecular perspective. Mol. Reprod. Dev. 2016, 83, 860–874. [Google Scholar] [CrossRef]
- Yanagimachi, R. The movement of golden hamster spermatozoa before and after capacitation. J. Reprod. Fertil. 1970, 23, 193–196. [Google Scholar] [CrossRef]
- Puga Molina, L.C.; Luque, G.M.; Balestrini, P.A.; Marín-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular basis of human sperm capacitation. Front. Cell Dev. Biol. 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Fujinoki, M. Regulation and disruption of hamster sperm hyperactivation by progesterone, 17ß-estradiol and diethylstilbestrol. Reprod. Med. Biol. 2014, 13, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.R.; Herod, J.E. Expression of capacitation-dependent changes in chlortetracycline fluorescence patterns in mouse spermatozoa requires a suitable glycolysable substrate. J. Reprod. Fert. 1990, 88, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Roldan, E.R.S. Sperm competition and the evolution of sperm form and function in mammals. Reprod. Dom. Anim. 2019, 54 (Suppl. S4), 14–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Babcock, D.F.; Lardy, H.A. Increased calcium-ion influx is a component of capacitation of spermatozoa. Biochem. J. 1978, 172, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Ikawa, M.; Yamada, S.; Toshimori, K.; Okabe, M. Alkalinization of acrosome measured by GFP as a pH indicator and its relation to sperm capacitation. Dev. Biol. 2001, 237, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Bavister, B.D.; Chen, A.F.; Fu, P.C. Catecholamine requirement for hamster sperm motility in vitro. J. Reprod. Fertil. 1979, 56, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Meizel, S.; Working, P.K. Further evidence suggesting the hormonal stimulation of hamster sperm acrosome reactions by catecholamines in vitro. Biol. Reprod. 1980, 22, 211–216. [Google Scholar] [CrossRef]
- Cornett, L.E.; Meizel, S. Stimulation of in vitro activation and the acrosome reaction of hamster spermatozoa by catecholamines. Proc. Natl. Acad. Sci. USA 1978, 75, 4954–4958. [Google Scholar] [CrossRef]
- Meizel, S.; Lui, C.W.; Working, P.K.; Mrsny, R.J. Taurine and hypotaurine: Their effects on motility, capacitation and the acrosome reaction of hamster sperm in vitro and their presence in sperm and reproductive tract fluids of several mammals. Dev. Growth Differ. 1980, 22, 483–494. [Google Scholar] [CrossRef]
- Brogan, P.T.; Beitsma, M.; Henning, H.; Gadella, B.M.; Stout, T.A. Liquid storage of equine semen: Assessing the effect of D-penicillamine on longevity of ejaculated and epididymal stallion sperm. Anim. Reprod. Sci. 2015, 159, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Leahy, T.; Rickard, J.P.; Aitken, R.J.; de Graaf, S.P. Penicillamine prevents ram sperm agglutination in media that support capacitation. Reproduction 2016, 151, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.; Merino, J.; Bravo, A.; Zambrano, F.; Schulz, M.; Villegas, J.V.; Sanchez, R. Antioxidant effects of penicillamine against in vitro-induced oxidative stress in human spermatozoa. Andrologia 2020, 52, e13553. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Diaz, S.; Oseguera-Lopez, I.; De La Cuesta-Diaz, D.; Garcia-Lopez, B.; Serres, C.; Sanchez-Calabuig, M.J.; Gutierrez-Adan, A.; Perez-Cerezales, S. The presence of D-penicillamine during the in vitro capacitation of stallion spermatozoa prolongs hyperactive-like motility and allows for sperm selection by thermotaxis. Animals 2020, 10, 1467. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Koyama, K.; Huang, W.; Yang, Y.; Yanagawa, Y.; Takahashi, Y.; Nagano, M. Addition of D-penicillamine, hypotaurine, and epinephrine (PHE) mixture to IVF medium maintains motility and longevity of bovine sperm and enhances stable production of blastocysts in vitro. J. Reprod. Dev. 2015, 61, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Tourmente, M.; Sanchez-Rodriguez, A.; Roldan, E.R.S. Effect of motility factors D-penicillamine, hypotaurine and epinephrine on the performance of spermatozoa from five hamster species. Biology 2022, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Fujinoki, M. Melatonin-enhanced hyperactivation of hamster sperm. Reproduction 2008, 136, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Fujinoki, M. Serotonin-enhanced hyperactivation of hamster sperm. Reproduction 2011, 142, 255–266. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Fujinoki, M.; Shibahara, H. Effects of 5-hydroxytryptamine on spermatozoal hyperactivation and in vitro fertilization in mice. J. Reprod. Dev. 2019, 65, 541–550. [Google Scholar] [CrossRef]
- Baldi, E.; Casano, R.; Falsetti, C.; Krausz, C.; Maggi, M.; Forti, G. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J. Androl. 1991, 12, 323–330. [Google Scholar] [CrossRef]
- Libersky, E.A.; Boatman, D.E. Effects of progesterone on in vitro sperm capacitation and egg penetration in the golden hamster. Biol. Reprod. 1995, 53, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Fujinoki, M.; Kitazawa, M.; Inaba, N. Regulation of hyperactivation of hamster spermatozoa by progesterone. Reprod. Med. Biol. 2008, 7, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Roldan, E.R.S.; Murase, T.; Shi, Q.X. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science 1994, 266, 1578–1581. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.X.; Roldan, E.R.S. Bicarbonate/CO2 is not required for zona pellucida- or progesterone-induced acrosomal exocytosis of mouse spermatozoa but is essential for capacitation. Biol. Reprod. 1995, 52, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Llanos, M.N.; Anaabalon, M.C. Studies related to progesterone-induced hamster sperm acrosome reaction. Mol. Reprod. Dev. 1996, 45, 313–319. [Google Scholar] [CrossRef]
- Uhler, M.L.; Leung, A.; Chan, S.Y.; Wang, C. Direct effects of progesterone and antiprogesterone on human sperm hyperactivated motility and acrosome reaction. Fertil. Steril. 1992, 58, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Sueldo, C.E.; Oehninger, S.; Subias, E.; Mahony, M.; Alexander, N.J.; Burkman, L.J.; Acosta, A.A. Effect of progesterone on human zona pellucida sperm binding and oocyte penetrating capacity. Fertil. Steril. 1993, 60, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Baldi, E.; Luconi, M.; Bonaccorsi, L.; Forti, G. Nongenomic effects of progesterone on spermatozoa: Mechanisms of signal transduction and clinical implications. Front Biosci 1998, 3, D1051–D1059. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.V.; Barrat, C.L.R.; Publicover, S.J.; Kirkman-Brown, J.C. Kinetics of the progesterone-induced acrosome reaction and its relation to intracellular calcium responses in individual human spermatozoa. Biol. Reprod. 2006, 75, 933–939. [Google Scholar] [CrossRef]
- Murase, T.; Roldan, E.R.S. Progesterone and the zone pellucida activate different transducing pathways in the sequence of events leading to diacylglycerol generation during mouse sperm. Biochem. J. 1996, 320, 1017–1023. [Google Scholar] [CrossRef]
- Mahé, C.; Zlotkowska, A.M.; Reynaud, K.; Tsikis, G.; Mermillod, P.; Druart, X.; Schoen, J.; Saint-Dizier, M. Sperm migration, selection, survival, and fertilizing ability in the mammalian oviduct. Biol. Reprod. 2021, 105, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Tourmente, M.; Villar-Moya, P.; Varea-Sánchez, M.; Luque-Larena, J.J.; Rial, E.; Roldan, E.R.S. Performance of rodent spermatozoa over time is enhanced by increased ATP concentrations: The role of sperm competition. Biol. Reprod. 2015, 93, 64. [Google Scholar] [CrossRef] [PubMed]
- Sansegundo, E.; Tourmente, M.; Roldan, E.R.S. Energy metabolism and hyperactivation of spermatozoa from three mouse species under capacitating conditions. Cells 2022, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Ogura, A. The golden (Syrian) hamster as a model for the study of reproductive biology: Past, present, and future. Reprod. Med. Biol. 2019, 18, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R.; Chang, M.C. Fertilization of hamster eggs in vitro. Nature 1963, 200, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R.; Chang, M.C. In vitro fertilization of golden hamster ova. J. Exp. Zool. 1964, 156, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Fujinoki, M.; Kawamura, T.; Toda, T.; Ohtake, H.; Ishimoda-Takagi, T.; Shimizu, N.; Yamaoka, S.; Okuno, M. Identification of 36-kDa flagellar phosphoproteins associated with hamster sperm motility. Biomed. Res. 2001, 133, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Fujinoki, M.; Ishimoda-Takagi, T.; Ohtake, H. Serine/threonine phosphorylation associated with hamster sperm hyperactivation. Reprod. Med. Biol. 2004, 3, 223–230. [Google Scholar] [CrossRef]
- Fujinoki, M.; Suzuki, T.; Takayama, T.; Shibahara, H.; Ohtake, H. Profiling of proteins phosphorylated or dephosphorylated during hyperactivation via activation on hamster spermatozoa. Reprod. Med. Biol. 2006, 5, 123–135. [Google Scholar] [CrossRef]
- Tateno, H.; Tamura-Nakano, M.; Kusakabe, H.; Hirohashi, N.; Kawano, N.; Yanagimachi, R. Sperm acrosome status before and during fertilization in the Chinese hamster (Cricetulus griseus), and observation of oviductal vesicles and globules. Mol. Reprod. Dev. 2021, 88, 793–804. [Google Scholar] [CrossRef]
- Yanagimachi, R.; Kamiguchi, Y.; Sugawara, S.; Mikamo, K. Gametes and fertilization in the Chinese hamster. Gamete Res. 1983, 8, 97–117. [Google Scholar] [CrossRef]
- Roldan, E.R.S.; Yanagimachi, R. Cross-fertilization between Syrian and Chinese hamsters. J. Exp. Zool. 1989, 250, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K. The influence of photoperiod and melatonin on testis size, body weight, and pelage colour in the Djungarian hamster (Phodopus sungorus). J. Comp. Physiol. 1973, 85, 267–282. [Google Scholar] [CrossRef]
- Anand, S.; Losee-Olson, S.; Turek, F.W.; Horton, T.H. Differential regulation of luteinizing hormone and follicle-stimulating hormone in male Siberian hamsters by exposure to females and photoperiod. Endocrinology 2002, 143, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Parkening, T.A. In vitro fertilization of Siberian hamster oocytes. J. Exp. Zool. 1990, 254, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Niklowitz, P.; Lerchl, A.; Nieschlag, E. In vitro fertilizing capacity of sperm from FSH-treated photoinhibited Djungarian hamsters (Phodopus sungorus). J. Endocrinol. 1997, 154, 475–481. [Google Scholar] [CrossRef]
- Bavister, B.D. A consistently successful procedure for in vitro fertilization of golden hamster eggs. Gamete Res. 1989, 23, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Libersky, E.A.; Boatman, D.E. Progesterone concentration in serum, follicular fluid, and oviductal fluid of the golden hamster during the periovulatory period. Biol. Reprod. 1995, 53, 477–482. [Google Scholar] [CrossRef]
- Yanagimachi, R. Requirement of extracellular calcium ions for various stages of fertilization and fertilization-related phenomena in the hamster. Gamete Res. 1982, 9, 323–344. [Google Scholar] [CrossRef]
- Gomez Montoto, L.; Varea-Sánchez, M.; Tourmente, M.; Martín-Coello, J.; Luque-Larena, J.J.; Gomendio, M.; Roldan, E.R.S. Sperm competition differentially affects swimming velocity and size of spermatozoa from closely related muroid rodents: Head first. Reproduction 2011, 142, 819–830. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Rodriguez, A.; Idrovo, I.I.D.; Rielo, J.A.; Roldan, E.R.S. Sperm Capacitation and Kinematics in Phodopus Hamsters. Int. J. Mol. Sci. 2023, 24, 16093. https://doi.org/10.3390/ijms242216093
Sanchez-Rodriguez A, Idrovo IID, Rielo JA, Roldan ERS. Sperm Capacitation and Kinematics in Phodopus Hamsters. International Journal of Molecular Sciences. 2023; 24(22):16093. https://doi.org/10.3390/ijms242216093
Chicago/Turabian StyleSanchez-Rodriguez, Ana, Ingrid I. D. Idrovo, Juan Antonio Rielo, and Eduardo R. S. Roldan. 2023. "Sperm Capacitation and Kinematics in Phodopus Hamsters" International Journal of Molecular Sciences 24, no. 22: 16093. https://doi.org/10.3390/ijms242216093
APA StyleSanchez-Rodriguez, A., Idrovo, I. I. D., Rielo, J. A., & Roldan, E. R. S. (2023). Sperm Capacitation and Kinematics in Phodopus Hamsters. International Journal of Molecular Sciences, 24(22), 16093. https://doi.org/10.3390/ijms242216093






