Promotion of Ca2+ Accumulation in Roots by Exogenous Brassinosteroids as a Key Mechanism for Their Enhancement of Plant Salt Tolerance: A Meta-Analysis and Systematic Review
Abstract
:1. Introduction
- What is the best application strategy for BRs to improve seed germination rate?
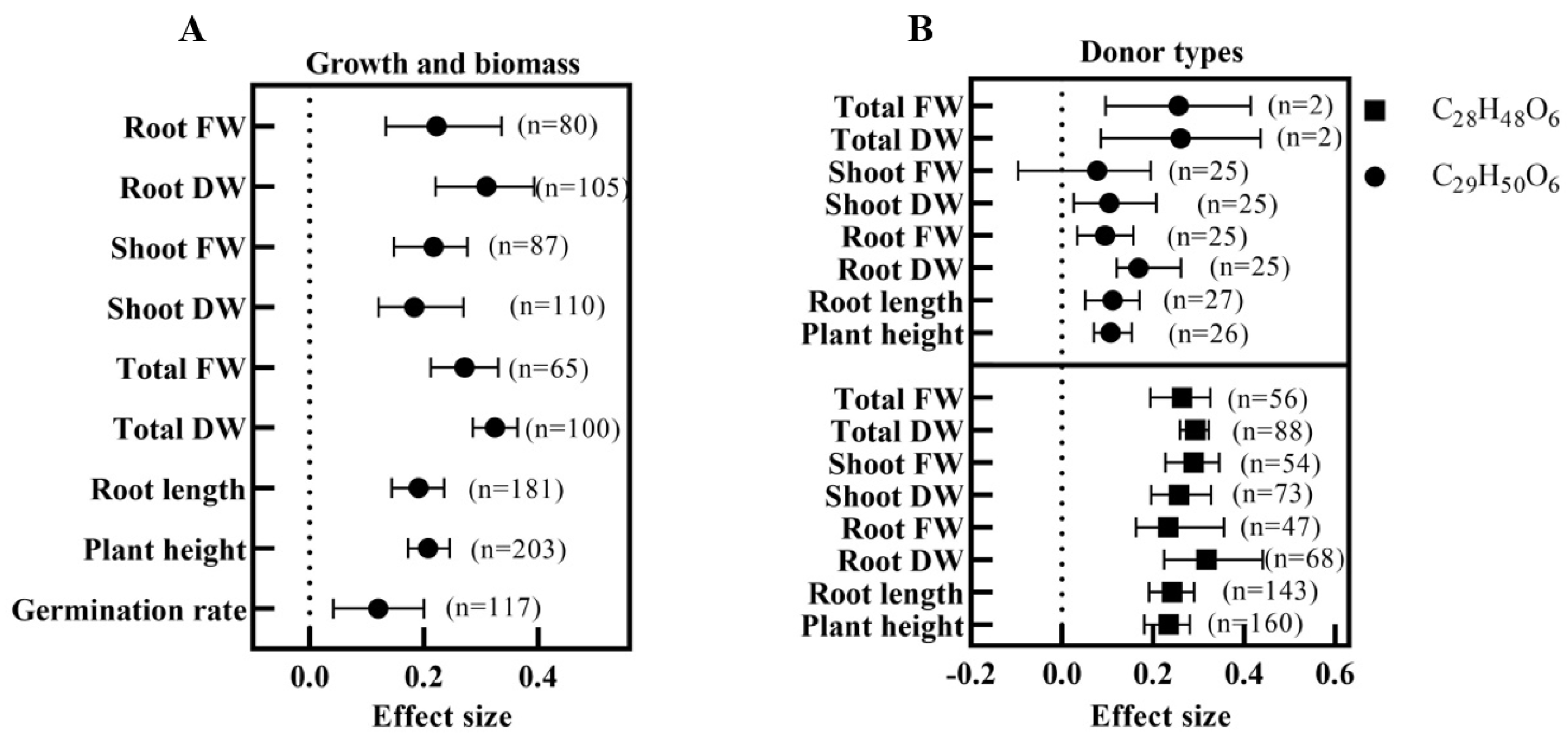
- What are the best donor compounds (C29H50O6 or C28H48O6), concentrations, and application methods for exogenous BRs in the seedling stage?
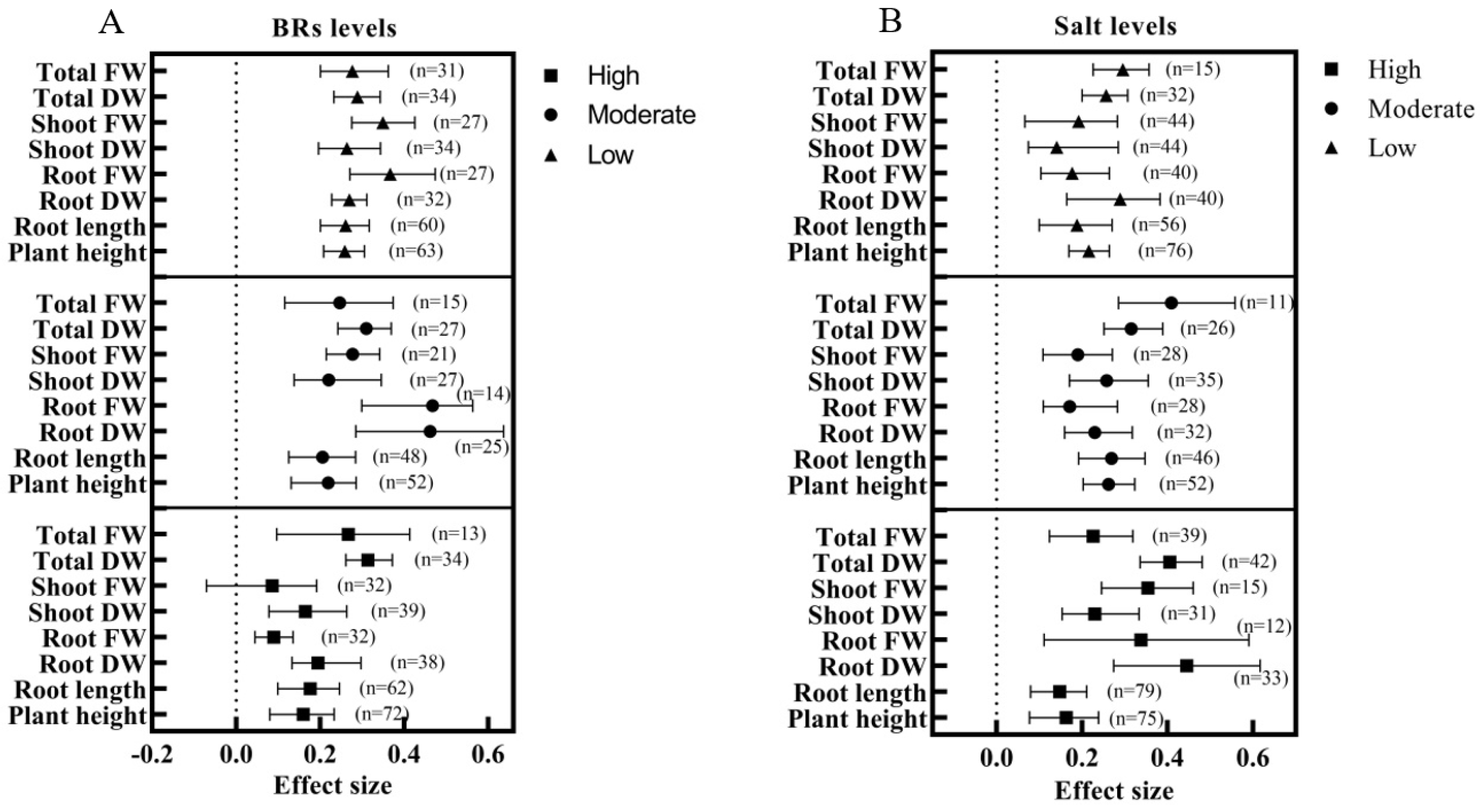
- What are the differences between exogenous BRs in alleviating different levels of salt stress?
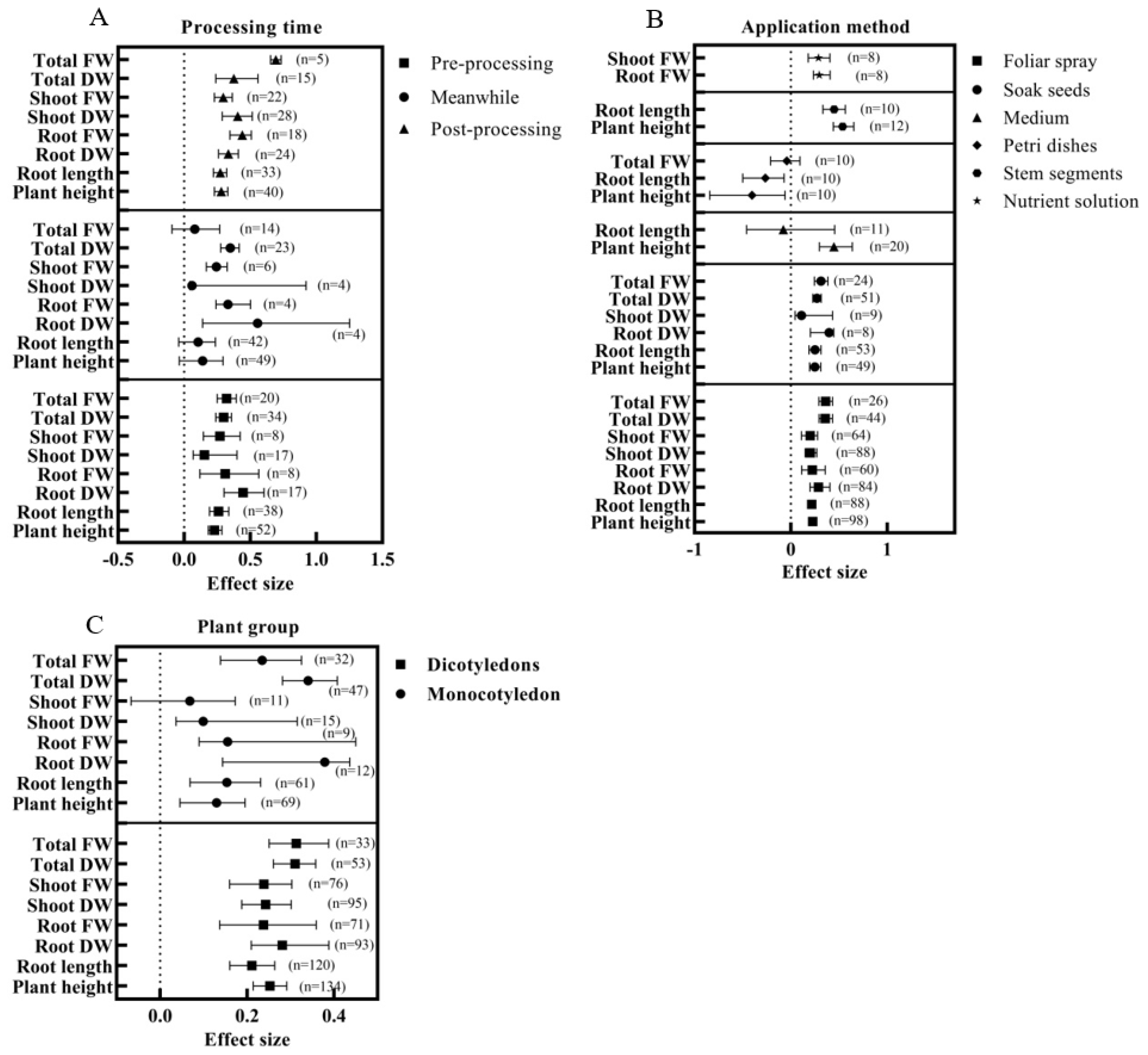
- Does the effect of exogenous BRs on plant salt tolerance vary with plant taxonomy (monocotyledon vs. dicotyledon; herbaceous vs. woody)?
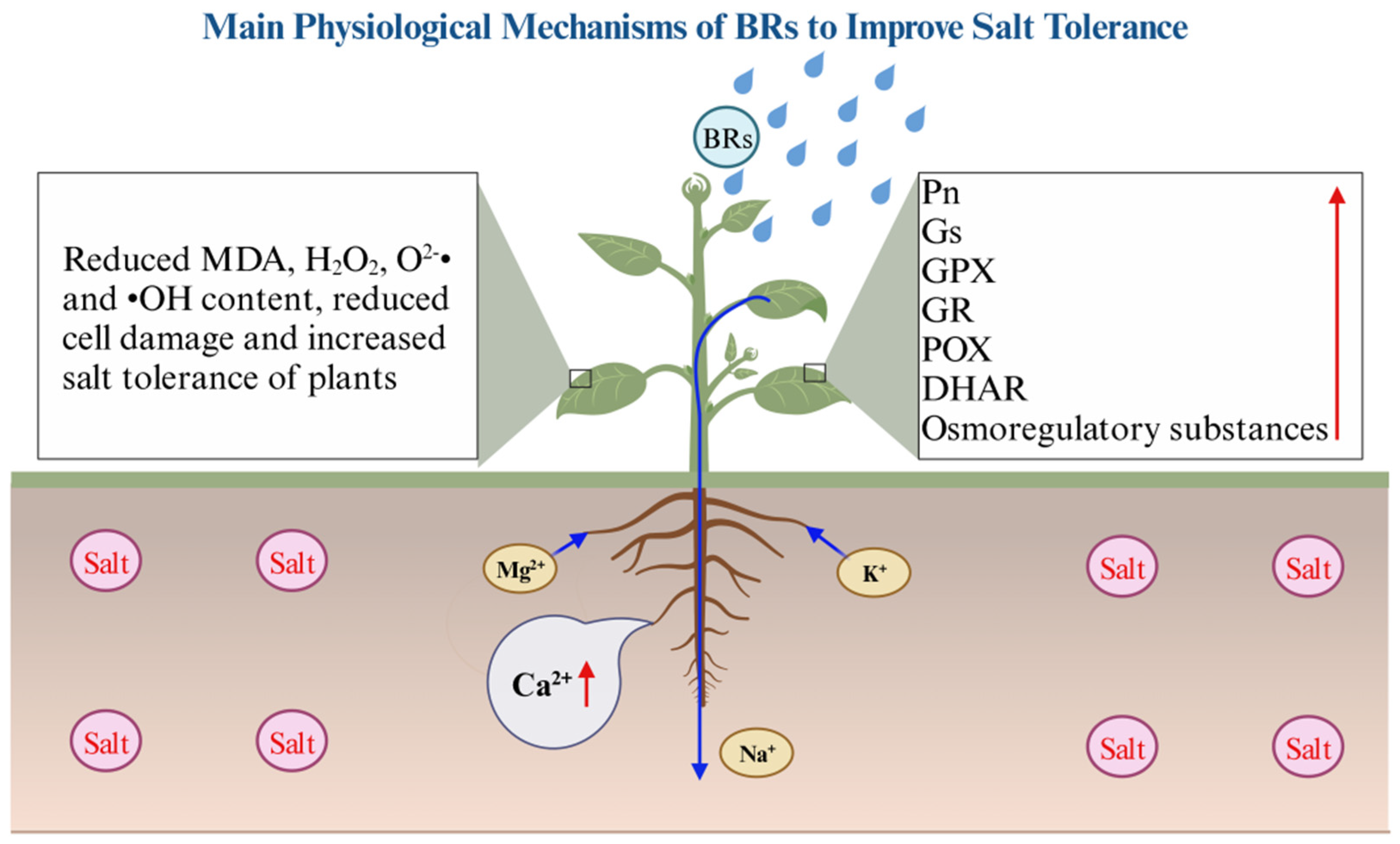
- How do exogenous BRs affect plant growth (plant height, root length, and biomass), antioxidant substances (ascorbic acid (ASA), reduced glutathione (GSH), dehydroascorbate reductase (DHAR), superoxide dismutase (SOD), guaiacol peroxidase (POX), POD, glutathione reductase (GR), glutathione peroxidase (GPX), catalase (CAT), and APX), photosynthesis (chlorophyll content, net photosynthetic rate (Pn), E, stomatal conductance (Gs), and Ci), ion changes (Ca2+, Na+, K+, and Mg2+), and other standard physiological parameters? Which physiological processes are most important in BRs increasing the plant salt tolerance levels?
2. Materials and Methods
2.1. Literature Screening
2.2. Data Extraction and Classification
2.3. Meta-Analysis
3. Results
3.1. Data Overview
3.2. Effect of Exogenous BRs on Germination Rate under Salt Stress and Subgroup Analysis
3.3. Effect of Exogenous BRs on Plant Growth and Biomass under Salt Stress
3.4. Optimal Application Program for BRs Based on Growth and Biomass Analysis
3.5. Effect of Exogenous BRs on Photosynthesis of Plants under Salt Stress
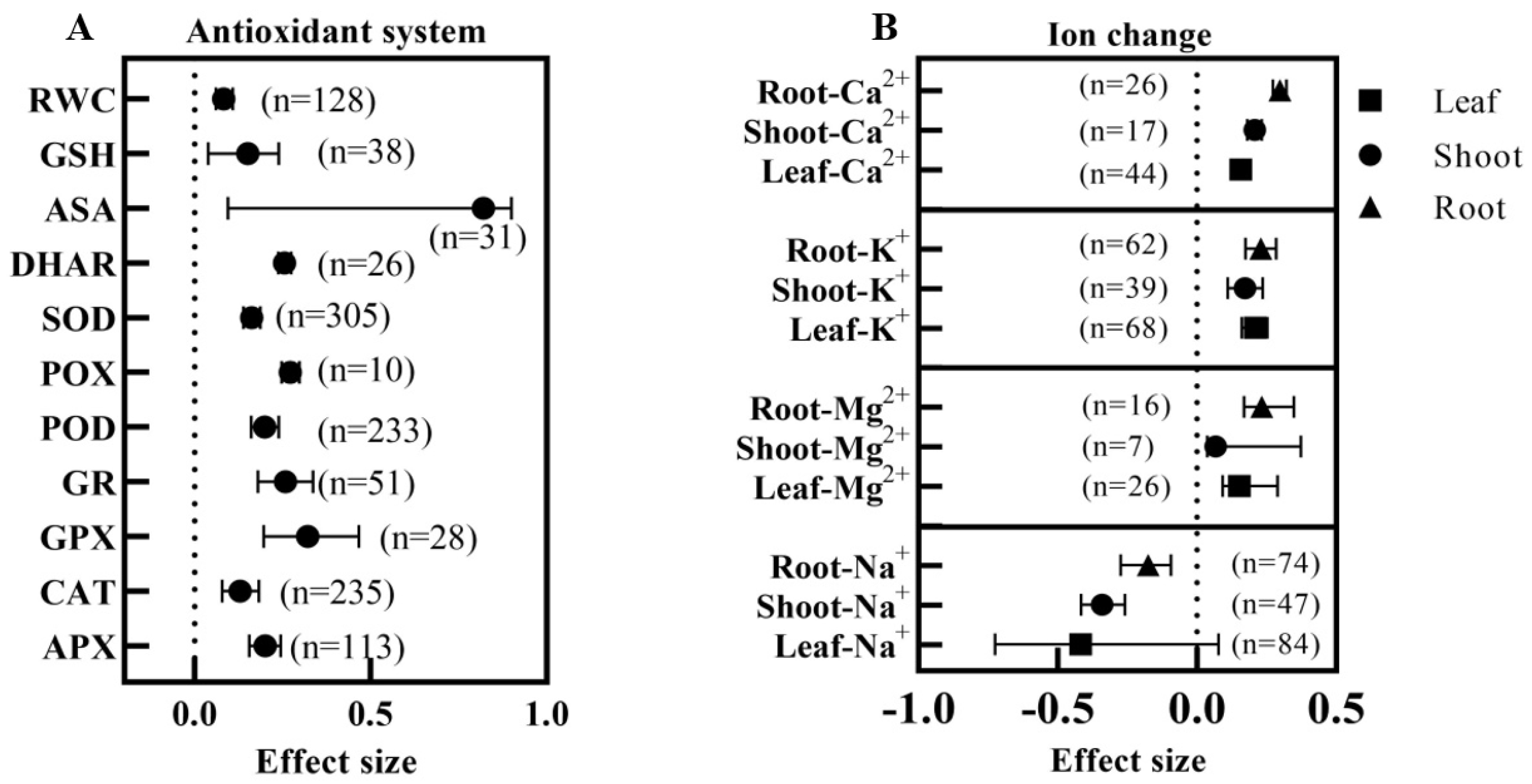
3.6. Effect of Exogenous BRs on Oxidative Damage System and Osmoregulatory Capacity of Plants under Salt Stress
3.7. Effect of Exogenous BRs on the Cation Content of Plants under Salt Stress
4. Discussion
4.1. Effect of Exogenous BRs on the Germination Rate of Different Plants and the Optimal Method of the Germination Period
4.2. Effects of Exogenous BRs on Growth and Biomass of Seedling Plants under Salt Stress and Their Optimal Usage
4.3. Effect of Exogenous BRs on the Physiological and Biochemical Levels of Plants under Salt Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability Statement
Acknowledgments
Conflicts of Interest
References
- Matishov, G.G.; Grigorenko, K.S. Causes of salinization of the Gulf of Taganrog. Dokl. Earth Sci. 2017, 477, 1311–1315. [Google Scholar] [CrossRef]
- Litalien, A.; Zeeb, B. Curing the earth: A review of anthropogenic soil salinization and plant-based strategies for sustainable mitigation. Sci. Total Environ. 2019, 698, 134235. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2017, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, L.; Zhao, F.; Li, J.; Zhang, X.; Kong, X.; Wu, H.; Zhang, Z. Plant Salinity Stress Response and Nano-Enabled Plant Salt Tolerance. Front. Plant Sci. 2022, 13, 843994. [Google Scholar] [CrossRef]
- Zhao, K.; Song, J.; Fan, H.; Zhou, S.; Zhao, M. Growth response to ionic and osmotic stress of NaCl in salt-tolerant and salt-sensitive maize. J. Integr. Plant Biol. 2010, 52, 468–475. [Google Scholar] [CrossRef]
- Kim, T.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.; Sun, Y.; Burlingame, A.L.; Wang, Z. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009, 11, 1254–1260. [Google Scholar] [CrossRef]
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K.; Mandava, N.; Worley, J.F.; Warthen, J.D.; Steffens, G.L.; Flippen-Anderson, J.L.; Cook, J.C. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Shiang, S.; Xiangfeng, Y.; Xiang, L.; Zhihua, Q.; Yu, L.; Xiangdong, L.; Xingyin, J. Brassinolide can improve drought tolerance of maize seedlings under drought stress: By inducing the photosynthetic performance, antioxidant capacity and ZmMYB gene expression of maize seedlings. J. Soil Sci. Plant Nutr. 2022, 22, 2092–2104. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.; Yan, Y.; Gao, L.; He, C.; Wang, J.; Yuan, Q.; Miao, L.; Li, S.; Di, Q.; et al. Proteome and phosphoproteome analysis of 2,4-epibrassinolide-mediated cold stress response in cucumber seedlings. Front. Plant Sci. 2023, 14, 1104036. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Rehman, S.U.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotox. Environ. Safe 2017, 147, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Abd Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Wirth, S.; Egamberdieva, D. Regulatory roles of 24-epibrassinolide in tolerance of Acacia gerrardii Benth to salt stress. Bioengineered 2017, 9, 61–71. [Google Scholar] [CrossRef]
- Ahmad, H.; Hayat, S.; Ali, M.; Liu, H.; Chen, X.; Li, J.; Cheng, Z. The Protective Role of 28-Homobrassinolide and Glomus versiforme in Cucumber to Withstand Saline Stress. Plants 2019, 9, 42. [Google Scholar] [CrossRef]
- He, X.; Wan, Z.; Jin, N.; Jin, L.; Zhang, G.; Lyu, J.; Liu, Z.; Luo, S.; Yu, J. Enhancement of cucumber resistance under salt stress by 2, 4-epibrassinolide lactones. Front. Plant Sci. 2022, 13, 1023178. [Google Scholar] [CrossRef]
- Steinhorst, L.; He, G.; Moore, L.K.; Schültke, S.; Schmitz-Thom, I.; Cao, Y.; Hashimoto, K.; Andrés, Z.; Piepenburg, K.; Ragel, P.; et al. A Ca2+-sensor switch for tolerance to elevated salt stress in Arabidopsis. Dev. Cell 2022, 57, 2081–2094.e7. [Google Scholar] [CrossRef]
- Han, F.; Sun, M.; He, W.; Cui, X.; Pan, H.; Wang, H.; Song, F.; Lou, Y.; Zhuge, Y. Ameliorating effects of exogenous Ca2+ on foxtail millet seedlings under salt stress. Funct. Plant Biol. 2019, 46, 407–416. [Google Scholar] [CrossRef]
- Chen, Y.; Ge, J.; Liu, Y.; Li, R.; Zhang, R.; Li, K.; Huo, Z.; Xu, K.; Wei, H.; Dai, Q. 24-Epibrassnolide Alleviates the Adverse Effect of Salinity on Rice Grain Yield through Enhanced Antioxidant Enzyme and Improved K+/Na+ Homeostasis. Agronomy 2022, 12, 2499. [Google Scholar] [CrossRef]
- Sharma, I.; Ching, E.; Saini, S.; Bhardwaj, R.; Pati, P.K. Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol. Biochem. 2013, 69, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. the meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1298–1310. [Google Scholar] [CrossRef]
- Lehmann, A.; Rillig, M.C. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops—A meta-analysis. Soil Biol. Biochem. 2014, 81, 147–158. [Google Scholar] [CrossRef]
- Yuzhe, W.; Siyu, G.; Yaoyu, L.; Laiye, Q. Meta-Analysis of Effects of Melatonin Treatment on Plant Drought Stress Alleviation. Agriculture 2022, 12, 1335. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Wei, X.; Jahan, I.; Hasanuzzaman, M.; Sabuj, Z.H.; Zulfiqar, F.; Chen, J.; Iqbal, R.; Dastogeer, K.M.G.; Sohag, A.A.M.; et al. Exogenous nitric oxide promotes salinity tolerance in plants: A meta-analysis. Front. Plant Sci. 2022, 13, 957735. [Google Scholar] [CrossRef] [PubMed]
- Gurevitch, J.; Hedges, L.V. statistical issues in ecological meta-analyses. Ecology 1999, 80, 1298–1310. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Coulter, J.A.; Xie, J.; Luo, Z.; Zhang, R.; Deng, X.; Li, L. Winter wheat yield and water use efficiency response to organic fertilization in northern China: A meta-analysis. Agric. Water Manag. 2020, 229, 105934. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2018, 651, 2354–2364. [Google Scholar] [CrossRef]
- Wang, L.; Coulter, J.A.; Palta, J.A.; Xie, J.; Luo, Z.; Li, L.; Carberry, P.; Li, Q.; Deng, X. Mulching-Induced Changes in Tuber Yield and Nitrogen Use Efficiency in Potato in China: A Meta-Analysis. Agronomy 2019, 9, 793. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A.; Arteca, R.N.; Foolad, M.R. The Physiological, Biochemical and Molecular Roles of Brassinosteroids and Salicylic Acid in Plant Processes and Salt Tolerance. Crit. Rev. Plant Sci. 2010, 29, 162–190. [Google Scholar] [CrossRef]
- Alam, P.; Albalawi, T.H.; Altalayan, F.H.; Bakht, M.A.; Ahanger, M.A.; Raja, V.; Ashraf, M.; Ahmad, P. 24-Epibrassinolide (EBR) Confers Tolerance against NaCl Stress in Soybean Plants by Up-Regulating Antioxidant System, Ascorbate-Glutathione Cycle, and Glyoxalase System. Biomolecules 2019, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Efimova, M.V.; Khripach, V.A.; Boyko, E.V.; Malofii, M.K.; Kolomeichuk, L.V.; Murgan, O.K.; Vidershpun, A.N.; Mukhamatdinova, E.A.; Kuznetsov, V.V. The Priming of Potato Plants Induced by Brassinosteroids Reduces Oxidative Stress and Increases Salt Tolerance. Dokl. Biol. Sci. Proc. Acad. Sci. USSR Biol. Sci. Sect. 2018, 478, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, Y.; Zhu, L.; Li, Z.; Deng, B. Omethoate treatment mitigates high salt stress inhibited maize seed germination. Pest. Biochem. Physiol. 2018, 144, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.A.; Balal, R.M.; Pervez, M.A.; Garcia-Sanchez, F.; Gimeno, V.; Abbas, T.; Mattson, N.S.; Riaz, A. Treatment with 24-epibrassinolide mitigates NaCl-induced toxicity by enhancing carbohydrate metabolism, osmolyte accumulation, and antioxidant activity in Pisum sativum. Turk. J. Bot. 2014, 38, 511–525. [Google Scholar] [CrossRef]
- Liu, J.; Yang, R.; Jian, N.; Wei, L.; Ye, L.; Wang, R.; Gao, H.; Zheng, Q. Putrescine metabolism modulates the biphasic effects of brassinosteroids on canola and Arabidopsis salt tolerance. Plant Cell Environ. 2020, 43, 1348–1359. [Google Scholar] [CrossRef]
- Galal, A. 24-epibrassinolide application enhances growth and biochemical aspects of squash under salt stress conditions. Acta Biol. Hung. 2018, 69, 182–196. [Google Scholar] [CrossRef]
- Azhar, N.; Su, N.; Shabala, L.; Shabala, S. Exogenously Applied 24-Epibrassinolide (EBL) Ameliorates Detrimental Effects of Salinity by Reducing K+ Efflux via Depolarization-Activated K+ Channels. Plant Cell Physiol. 2017, 58, 802–810. [Google Scholar] [CrossRef]
- Cai, Z.; Gao, Q. Comparative physiological and biochemical mechanisms of salt tolerance in five contrasting highland quinoa cultivars. BMC Plant Biol. 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Liu, Y.; Su, M.; Han, Z. Effects of NaCl Stress on the Growth, Physiological Characteristics and Anatomical Structures of Populus talassica × Populus euphratica Seedlings. Plants 2022, 11, 3025. [Google Scholar] [CrossRef]
- Tang, H.; Bai, J.; Chen, F.; Liu, Y.; Lou, Y. Effects of salinity and temperature on tuber sprouting and growth of Schoenoplectus nipponicus. Ecosphere 2021, 12, e03448. [Google Scholar] [CrossRef]
- Agami, R.A. Alleviating the adverse effects of NaCl stress in maize seedlings by pretreating seeds with salicylic acid and 24-epibrassinolide. S. Afr. J. Bot. 2013, 88, 171–177. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.S.; Hayat, S.; Ahmad, A.; Tahir, I. Efficacy of brassinosteroid analogues in the mitigation of toxic effects of salt stress in Brassica juncea plants. J. Environ. Biol. 2017, 38, 27–36. [Google Scholar] [CrossRef]
- Ahmad, H.; Hayat, S.; Ali, M.; Ghani, M.I.; Cheng, Z. Regulation of growth and physiological traits of cucumber (ITCucumis sativus&IT L.) through various levels of 28-homobrassinolide under salt stress conditions. Can. J. Plant Sci. 2018, 98, 132–140. [Google Scholar] [CrossRef]
- Ahmad, H.; Hayat, S.; Ali, M.; Liu, T.; Cheng, Z. The combination of arbuscular mycorrhizal fungi inoculation (Glomus versiforme) and 28-homobrassinolide spraying intervals improves growth by enhancing photosynthesis, nutrient absorption, and antioxidant system in cucumber (Cucumis sativus L.) under salinity. Ecol. Evol. 2018, 8, 5724–5740. [Google Scholar] [CrossRef]
- Ahmad, P.; Abd Allah, E.F.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Siddique, K.H.M. Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate-glutathione cycle and secondary metabolites. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Chen, Y.; Xiang, Y.; Hu, Z.; Gao, Y.; Zhang, Y.; Chen, M.; Khaldun, A.B.M.; Yan, X.; Fan, J. Transcriptomic profiling revealed the role of 24-epibrassinolide in alleviating salt stress damage in tall fescue (Festuca arundinacea). Front. Plant Sci. 2022, 13, 976341. [Google Scholar] [CrossRef]
- de Oliveira, V.P.; Roque Lima, M.D.; Serrao Da Silva, B.R.; Batista, B.L.; Da Silva Lobato, A.K. Brassinosteroids Confer Tolerance to Salt Stress in Eucalyptus urophylla Plants Enhancing Homeostasis, Antioxidant Metabolism and Leaf Anatomy. J. Plant Growth Regul. 2019, 38, 557–573. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Yang, Q.; Li, T. Short-term Salt Stress Strongly Affects Dynamic Photosynthesis, but not Steady-State Photosynthesis, in Tomato (Solanum lycopersicum). Environ. Exp. Bot. 2018, 149, 109–119. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotox. Environ. Safe. 2016, 137, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Hayat, N.; Afroz, N.; Rehman, S.; Bukhari, S.H.; Iqbal, K.; Khatoon, A.; Taimur, N.; Sakhi, S.; Ahmad, N.; Ullah, R.; et al. Plant--Derived Smoke Ameliorates Salt Stress in Wheat by Enhancing Expressions of Stress--Responsive Genes and Antioxidant Enzymatic Activity. Agronomy 2021, 12, 28. [Google Scholar] [CrossRef]
- Shen, Z.; Pu, X.; Wang, S.; Dong, X.; Cheng, X.; Cheng, M. Silicon improves ion homeostasis and growth of liquorice under salt stress by reducing plant Na+ uptake. Sci. Rep. 2022, 12, 5089. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Li, Y.; Luo, X.; Song, S.; Chen, Y.; Wang, X.; Mao, D.; Chen, L.; Luan, S. Rice Na+-Permeable Transporter OsHAK12 Mediates Shoots Na+ Exclusion in Response to Salt Stress. Front. Plant Sci. 2021, 12, 771746. [Google Scholar] [CrossRef]
- Jiang, X.; Gao, Y.; Zhou, H.; Chen, J.; Wu, J.; Zhang, S. Apoplastic calmodulin promotes self-incompatibility pollen tube growth by enhancing calcium influx and reactive oxygen species concentration in Pyrus pyrifolia. Plant Cell Rep. 2013, 33, 255–263. [Google Scholar] [CrossRef]
- Dong, Q.; Wallrad, L.; Almutairi, B.O.; Kudla, J. Ca2+ signaling in plant responses to abiotic stresses. J. Integr. Plant Biol. 2022, 64, 287–300. [Google Scholar] [CrossRef]
- Manishankar, P.; Wang, N.; Köster, P.; Alatar, A.A.; Kudla, J. Calcium signaling during salt stress and in the regulation of ion homeostasis. J. Exp. Bot. 2018, 69, 4215–4226. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Jin, L.; Peng, R. Crosstalk between Ca2+ and Other Regulators Assists Plants in Responding to Abiotic Stress. Plants 2022, 11, 1351. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Z.; Wu, M.; Wang, W.; Wang, Y.; Nie, S. Enhanced brassinosteroid signaling via the overexpression of SlBRI1 positively regulates the chilling stress tolerance of tomato. Plant Sci. 2022, 320, 111281. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, J.; Liu, D.; Wei, Y.; Wang, Y.; Li, X. Characterization of bHLH/HLH genes that are involved in brassinosteroid (BR) signaling in fiber development of cotton (Gossypium hirsutum). BMC Plant Biol. 2018, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Hayat, S.; Fariduddin, Q.; Ahmad, A. 24-Epibrassinolide protects against the stress generated by salinity and nickel in Brassica juncea. Chemosphere 2008, 72, 1387–1392. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Hayat, S.; Wijaya, L.; Anaji, A. Foliar application of 28-homobrassinolide mitigates salinity stress by increasing the efficiency of photosynthesis in Brassica juncea. Acta Bot. Bras. 2013, 27, 502–505. [Google Scholar] [CrossRef]
- Amraee, L.; Rahmani, F.; Abdollahi Mandoulakani, B. Exogenous application of 24-epibrassinosteroid mitigates NaCl toxicity in flax by modifying free amino acids profile and antioxidant defence system. Funct. Plant Biol. 2020, 47, 565–575. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Sheng, W.; Yu, Y.; Zhang, L.; Ceng, H.; Chen, G. Effects of Exogenous 2,4-Epibrassinolide on Physiological Characteristics of Rice Seedlings Under Salt Stress. Mol. Plant Breed. 2021, 19, 2740–2746. [Google Scholar] [CrossRef]
- Arora, N.; Bhardwaj, R.; Sharma, P.; Arora, H.K. 28-Homobrassinolide alleviates oxidative stress in salttreated maize (Zea mays L.) plants. Braz. J. Plant Physiol. 2008, 20, 153–157. [Google Scholar] [CrossRef]
- Chang, D.; Yang, Y.; Wang, Y.; Zhang, X.; Zhang, F.; Li, F. Effects of 24-Epi Brassinolide on Seed Germination under Stresses of Salt and PEG in Cotton. Acta Agric. Boreali-Occident. Sin. 2015, 24, 96–101. [Google Scholar] [CrossRef]
- Ding, H.; Zhu, X.; Zhu, Z.; Yang, S.; Zha, D.; Wu, X. Amelioration of salt-induced oxidative stress in eggplant by application of 24-epibrassinolide. Biol. Plant. 2012, 56, 767–770. [Google Scholar] [CrossRef]
- Dong, Y.R.; Zhang, Y.B.; Zhao, D.X.; Geng, B.; Lou, Q.N.; Li, Y.Z.; Wang, Z.H.; Guo, G. Mitigating effect of exogenous 24-epibrassinolide on mulberry seedlings under NaCl stress. J. Nucl. Agric. Sci. 2021, 35, 1466–1475. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.; Hu, G.; Chen, W.; Zhuge, Y.; Wang, Z.; He, M.R. Role of exogenous 24-epibrassinolide in enhancing the salt tolerance of wheat seedlings. J. Soil Sci. Plant Nutr. 2017, 17, 554–569. [Google Scholar] [CrossRef]
- Efimova, M.V.; Savchuk, A.L.; Hasan, J.A.K.; Litvinovskaya, R.P.; Khripach, V.A.; Kholodova, V.P.; Kuznetsov, V.V. Physiological mechanisms of enhancing salt tolerance of oilseed rape plants with brassinosteroids. Russ. J. Plant Physiol. 2014, 61, 733–743. [Google Scholar] [CrossRef]
- Ekinci, M.; Yildirim, E.; Dursun, A.; Turan, M. Mitigation of Salt Stress in Lettuce (Lactuca sativa L. var. Crispa) by Seed and Foliar 24-epibrassinolide Treatments. Hortscience 2012, 47, 631–636. [Google Scholar] [CrossRef]
- El-Mashad, A.A.A.; Mohamed, H.I. Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma 2012, 249, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wu, X.; Guan, X.; Zheng, C.; Zhao, H.; Gu, Z.; Liu, W.; Chen, J.; Zheng, Q. Concentration effects and its physiological mechanism of soaking seeds with brassinolide on tomato seed germination under salt stress. Acta Ecol. Sin. 2021, 41, 1857–1867. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Khalil, R.R.A.E.; Mir, B.A.; Yusuf, M.; Ahmad, A. 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ. Monit. Assess. 2013, 185, 7845–7856. [Google Scholar] [CrossRef] [PubMed]
- Fariduddin, Q.; Mir, B.A.; Yusuf, M.; Ahmad, A. 24-epibrassinolide and/or putrescine trigger physiological and biochemical responses for the salt stress mitigation in Cucumis sativus L. Photosynthetica 2014, 52, 464–474. [Google Scholar] [CrossRef]
- Fedina, E.O. Effect of 24-epibrassinolide on pea protein tyrosine phosphorylation after salinity action. Russ. J. Plant Physiol. 2013, 60, 351–358. [Google Scholar] [CrossRef]
- Furio, R.N.; Salazar, S.M.; Mariotti-Martinez, J.A.; Martinez-Zamora, G.M.; Coll, Y.; Diaz-Ricci, J.C. Brassinosteroid Applications Enhance the Tolerance to Abiotic Stresses, Production and Quality of Strawberry Fruits. Horticulturae. 2022, 8, 572. [Google Scholar] [CrossRef]
- Gong, Z.Y.; Hu, Z.H.; Wang, Y.J. Effects of Exogenous EBR on Photosynthetic Physiology of Cowpea Under Waterlogging, Drought and Salt Stress. N. Hortic. 2022, 9–20. [Google Scholar] [CrossRef]
- Groszyk, J.; Szechynska-Hebda, M. Effects of 24-Epibrassinolide, Bikinin, and Brassinazole on Barley Growth under Salinity Stress Are Genotype- and Dose-Dependent. Agronomy 2021, 11, 259. [Google Scholar] [CrossRef]
- Gupta, P.; Seth, C.S. Interactive role of exogenous 24 Epibrassinolide and endogenous NO in Brassica juncea L. under salinity stress: Evidence for NR-dependent NO biosynthesis. Nitric Oxide-Biol. Chem. 2020, 97, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Seth, C.S. 24-Epibrassinolide Regulates Functional Components of Nitric Oxide Signalling and Antioxidant Defense Pathways to Alleviate Salinity Stress in Brassica juncea L. cv. Varuna. J. Plant Growth Regul. 2023, 42, 4207–4222. [Google Scholar] [CrossRef]
- Gupta, P.; Srivastava, S.; Seth, C.S. 24-Epibrassinolide and Sodium Nitroprusside alleviate the salinity stress in Brassica juncea L. cv. Varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil 2017, 411, 483–498. [Google Scholar] [CrossRef]
- Hayat, S.; Khalique, G.; Wani, A.S.; Alyemeni, M.N.; Ahmad, A. Protection of growth in response to 28-homobrassinolide under the stress of cadmium and salinity in wheat. Int. J. Biol. Macromol. 2014, 64, 130–136. [Google Scholar] [CrossRef]
- Hayat, S.; Maheshwari, P.; Wani, A.S.; Irfan, M.; Alyemeni, M.N.; Ahmad, A. Comparative effect of 28 homobrassinolide and salicylic of NaCl stress in Brassica juncea L. Plant Physiol. Biochem. 2012, 53, 61–68. [Google Scholar] [CrossRef]
- Hegazi, A.M.; El-Shraiy, A.M.; Ghoname, A.A. Mitigation of Salt Stress Negative Effects on Sweet Pepper Using Arbuscular Mycorrhizal Fungi (AMF), Bacillus Megaterium and Brassinosteroids (BRs). Gesunde Pflanz. 2017, 69, 91–102. [Google Scholar] [CrossRef]
- Hou, H. Effects of Brassinolide on Seed Germination of Rice Under Salt Stress. Chin. J. Trop. Agric. 2020, 40, 1–6. [Google Scholar]
- Hu, Y.; Xia, S.; Su, Y.; Wang, H.; Luo, W.; Su, S.; Xiao, L. Brassinolide Increases Potato Root Growth In Vitro in a Dose-Dependent Way and Alleviates Salinity Stress. BioMed Res. Int. 2016, 2016, 8231873. [Google Scholar] [CrossRef]
- Hua, Z.; Li, X. Effects of Brassinolide on Physiological and Biochemical Characteristics of Scutellaria baicalensis Seedlings under Salt Stress. Acta Agric. Jiangxi 2021, 33, 21–26. [Google Scholar] [CrossRef]
- Jin-huan, L.; Anjum, S.A.; Mei-ru, L.; Jian-hang, N.; Ran, W.; Ji-xuan, S.; Jun, L.; Xue-feng, Z.; Ashraf, U.; San-gen, W. Modulation of morpho-physiological traits of leymus chinensis (trin.) through exogenous application of brassinolide under salt stress. J. Anim. Plant Sci. 2015, 25, 1055–1062. [Google Scholar]
- Jinlong, L.; Huiling, G.; Lizhou, H.; Changhai, W.; Gengmao, Z.; Xueying, W.; Qingsong, Z. Role of Plant Pigments in the 24-epibrassinolide Ameliorating Salt Stress in Canola. Acta Bot. Boreali-Occident. Sin. 2013, 33, 90–100. [Google Scholar] [CrossRef]
- Kagale, S.; Divi, U.K.; Krochko, J.E.; Keller, W.A.; Krishna, P. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 2007, 225, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Karlidag, H.; Yildirim, E.; Turan, M. Role of 24-epibrassinolide in mitigating the adverse effects of salt stress on stomatal conductance, membrane permeability, and leaf water content, ionic composition in salt stressed strawberry (Fragaria×ananassa). Sci. Hortic. 2011, 130, 133–140. [Google Scholar] [CrossRef]
- Kaya, C.; Aydemir, S.; Akram, N.A.; Ashraf, M. Epibrassinolide Application Regulates Some Key Physio-biochemical Attributes As Well As Oxidative Defense System in Maize Plants Grown Under Saline Stress. J. Plant Growth Regul. 2018, 37, 1244–1257. [Google Scholar] [CrossRef]
- Khalid, A.; Aftab, F. Effect of exogenous application of 24-epibrassinolide on growth, protein contents, and antioxidant enzyme activities of in vitro-grown Solanum tuberosum L. under salt stress. In Vitro Cell. Dev. Biol.-Plant 2016, 52, 81–91. [Google Scholar] [CrossRef]
- Kolomeichuk, L.V.; Danilova, E.D.; Khripach, V.A.; Zhabinskyi, V.N.; Kuznetsov, V.V.; Efimova, M.V. Ability of Lactone- and Ketone-Containing Brassinosteroids to Induce Priming in Rapeseed Plants to Salt Stress. Russ. J. Plant Physiol. 2021, 68, 499–509. [Google Scholar] [CrossRef]
- Kolomeichuk, L.V.; Khripach, V.A.; Kuznetsov, V.V.; Efimova, M.V. Comparison of Protective Reactions of Rape Seeds to Chloride Salination at Exposure to Epibrassinolide before or during Salt Stress. Dokl. Biochem. Biophys. 2022, 502, 25–29. [Google Scholar] [CrossRef]
- Kolomeichuk, L.V.; Efimova, M.V.; Zlobin, I.E.; Kreslavski, V.D.; Murgan, O.K.; Kovtun, I.S.; Khripach, V.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. 24-Epibrassinolide alleviates the toxic effects of NaCl on photosynthetic processes in potato plants. Photosynth. Res. 2020, 146, 151–163. [Google Scholar] [CrossRef]
- Kou, J. Effects of 2,4-Epibrassinolide on Germination and Physiological Characteristics of Avena sativa L. Seeds under NaCl Stress. Acta Agrestia Sin. 2019, 27, 1562–1568. [Google Scholar] [CrossRef]
- Kou, J. Effects of Exogenous 2,4-Epibrassinolide on Photosynthetic Characteristics of Oat Seedlings under NaCl Stress. Acta Agric. Boreali-Sin. 2020, 35, 79–87. [Google Scholar] [CrossRef]
- Kou, J. Effects of exogenous 2,4-epibrassinolide on the absorption, transportation and allocation of inorganic ions in Avena sativa L.seedlings under NaCl stress. Chin. J. Ecol. 2020, 39, 855–864. [Google Scholar] [CrossRef]
- Kou, J. Mitigating effect of exogenous 2, 4-epibrassinolide on the inhibition of oat seed germination under salt stress. Grassl. Sci. 2020, 37, 916–925. [Google Scholar]
- Kou, J. Physiological Response of Salt Tolerance of Medicago sativa Seedlings Induced by Exogenous 2, 4-Epibrassinolide. Acta Agric. Boreali-Sin. 2020, 35, 133–140. [Google Scholar] [CrossRef]
- Kou, J. Physiological responses of Medicago sativa seed germination induced by exogenous 2,4-epibrassinolide under salt stress. Grassl. Turf 2020, 40, 8–14. [Google Scholar] [CrossRef]
- Kou, J.; Kang, W.; Miao, Y.; Shi, S. Effect of exogenous 2,4-epibrassinolide on the uptake, transport, and disputation of ions, and photosynthetic characteristics of Medicago sativa seedlings under NaCl stress. Acta Prata. Sin. 2016, 25, 91–103. [Google Scholar] [CrossRef]
- Kou, J.; Kang, W.; Miao, Y.; Shi, S. Effect of exogenous 2,4-epibrassinolide on trace element absorption and chlorophyll fluorescence of Medicago sativa L. seedlings under NaCl stress. Chin. J. Eco-Agric. 2016, 345–355. [Google Scholar] [CrossRef]
- Kou, J.; Shi, S. 2,4-Epibrassinolide Germination of Alfa Seeds under Salt Stress and the Impact of Seedling Growth; Grassland: Wageningen, The Netherlands, 2005. [Google Scholar]
- Kou, J.; Shi, S. 2,4-epibrassinolide protection aginest root growth inhibition and oxidative damage of Medicago sativa L. seedling under NaCl stress. Chin. J. Eco-Agric. 2015, 23, 1010–1019. [Google Scholar] [CrossRef]
- Lei, X.; Wan, C.; Tao, J.; Leng, J.; Wu, Y.; Wang, J.; Wang, P.; Yang, Q.; Feng, B.; Gao, J. Effects of soaking seeds with MT and EBR on germination and seedling growth in buckwheat under salt stress. Acta Agron. Sin. 2022, 48, 1210–1221. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Yao, Q.; Bai, L.; Hou, L.; Shi, Y. Effects of brassinolide on seedling growth and osmotic regulation characteristics of tomato under iso-osmotic salt stress. J. Northwest AF Univ. (Nat. Sci. Ed.) 2020, 48, 130–136. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Yao, Q.; Zhang, Y.; Hou, L.; Shi, Y. Effects of Exogenous BR on Growth and Physiological Resistance of Tomato Seedlings under Different Salt Stresses. Shandong Agric. Sci. 2019, 51, 50–54. [Google Scholar] [CrossRef]
- Li, T.; Gao, Y.; Ma, X.; Chen, Y.; Wang, Y.; Ma, J. Effects of Exogenous Brassinosteroid on Photosynthesis of Three Spesies of Populus under Drought, Salt and Copper Stress. Genom. Appl. Biol. 2016, 35, 218–226. [Google Scholar] [CrossRef]
- Li, W.; Sun, J.; Zhang, X.; Ahmad, N.; Hou, L.; Zhao, C.; Pan, J.; Tian, R.; Wang, X.; Zhao, S. The Mechanisms Underlying Salt Resistance Mediated by Exogenous Application of 24-Epibrassinolide in Peanut. Int. J. Mol. Sci. 2022, 23, 6376. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, S.; Xu, J.; Song, J.; Zhu, L. The alternative oxidase pathway is involved in the BR-induced salt resistance in mustard. Acta Physiol. Plant. 2018, 40, 171. [Google Scholar] [CrossRef]
- Liaqat, S.; Umar, S.; Saffeullah, P.; Iqbal, N.; Siddiqi, T.O.; Khan, M.I.R. Protective Effect of 24-Epibrassinolide on Barley Plants Growing Under Combined Stress of Salinity and Potassium Deficiency. J. Plant Growth Regul. 2020, 39, 1543–1558. [Google Scholar] [CrossRef]
- Litvinovskaya, R.P.; Shkliarevskyi, M.A.; Kolupaev, Y.E.; Kokorev, A.I.; Khripach, V.A.; Dmitriev, A.P. Effect of 24-Epicastasterone and Its Monosalicylate on Salt Resistance of Arabidopsis thaliana Wild Type and the Salicylate-Deficit NahG Transformants. Russ. J. Plant Physiol. 2022, 69, 35. [Google Scholar] [CrossRef]
- Liu, J.; Gao, H.; Wang, X.; Zheng, Q.; Wang, C.; Wang, X.; Wang, Q. Effects of 24-epibrassinolide on plant growth, osmotic regulation and ion homeostasis of salt-stressed canola. Plant Biol. 2014, 16, 440–450. [Google Scholar] [CrossRef]
- Ma, M.; Liu, R.; Zheng, C.; Liu, W.; Yin, X.; Liu, J.; Wang, Z.; Zheng, Q. Regulation of exogenous brassinosteroid on growth of salt-stressed canola seedlings and its physiological mechanism. Acta Ecol. Sin. 2015, 35, 1837–1844. [Google Scholar] [CrossRef]
- Ma, Q.; Gu, W. Regulation of sodium nitroprusside and brassinolide on osmotic adjustment of Cichorium intybus L. roots under salt stress. Jiangsu Agric. Sci. 2018, 46, 99–101. [Google Scholar] [CrossRef]
- Mehmood, S.; Siddiqi, E.H.; Nawaz, I.; Nasir, N. 24-epibrassinolide modulates biomass production, gas exchange characteristics and inorganic nutrients in canola (Brassica napus L.) under salt stress. Pak. J. Bot. 2022, 54, 1199–1209. [Google Scholar] [CrossRef]
- Mokari-Firuzsalari, S.; Khomari, S.; Seyed-Sharifi, R.; Goli-Kalanpa, E.; Azizpour, K. The Combined Influence of Zinc and Epibrassinolide Increase Tolerance to Salt Stress in Brassica napus L. Russ. J. Plant Physiol. 2019, 66, 240–249. [Google Scholar] [CrossRef]
- Mu, D.; Feng, N.; Zheng, D.; Zhou, H.; Liu, L.; Chen, G. Studies on the Physiological Mechanism of Brassinolide to Improve the Resistance of Rice Seedlings to NaCl Stress. Water Air Soil Pollut. 2022, 233, 238. [Google Scholar] [CrossRef]
- Mu, D.; Feng, N.; Zheng, D.; Zhou, H.; Liu, L.; Chen, G.; Mu, B. Physiological mechanism of exogenous brassinolide alleviating salt stress injury in rice seedlings. Sci. Rep. 2022, 12, 24039. [Google Scholar] [CrossRef] [PubMed]
- Nejad-Alimoradi, F.; Nasibi, F.; Kalantari, K.M. 24-epibrassinolide pre-treatment alleviates the salt-induced deleterious effects in medicinal pumpkin (Cucurbita pepo) by enhancement of GABA content and enzymatic antioxidants. S. Afr. J. Bot. 2019, 124, 111–117. [Google Scholar] [CrossRef]
- Otie, V.; Udo, I.; Shao, Y.; Itam, M.O.; Okamoto, H.; An, P.; Eneji, E.A. Salinity Effects on Morpho-Physiological and Yield Traits of Soybean (Glycine max L.) as Mediated by Foliar Spray with Brassinolide. Plants 2021, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Chen, C.; Wang, H.; Zheng, T.; Feng, L.; Jiang, Y. Effect of exogenous substances on seed germination of wild gypsophila under salt stress. Xiandai Nongcun Keji 2020, 77–79. [Google Scholar]
- Plazek, A.; Tatrzanska, M.; Maciejewski, M.; Dziurka, M.; Dubert, F. Effects of zearalenone and 24-epibrassinolide on the salt tolerance of selected monocotyledonous crop plants. J. Appl. Bot. Food Qual. 2017, 90, 280–287. [Google Scholar] [CrossRef]
- Raju, A.D.; Parihar, P.; Singh, R.; Kumar, J.; Prasad, S.M. Synergistic action of indole acetic acid with homobrassinolide in easing the NaCl-induced toxicity in Solanum melongena L. seedlings. Acta Physiol. Plant. 2020, 42, 68. [Google Scholar] [CrossRef]
- Sadeghi, F.; Shekafandeh, A. Effect of 24-epibrassinolide on salinity-induced changes in loquat (Eriobotrya japonica Lindl). J. Appl. Bot. Food Qual. 2014, 87, 182–189. [Google Scholar] [CrossRef]
- Semida, W.M.; Rady, M.M. Pre-soaking in 24-epibrassinolide or salicylic acid improves seed germination, seedling growth, and anti-oxidant capacity in Phaseolus vulgaris L. grown under NaCl stress. J. Horticult. Sci. Biotechnol. 2014, 89, 338–344. [Google Scholar] [CrossRef]
- Shahid, M.A.; Pervez, M.A.; Balal, R.M.; Mattson, N.S.; Rashid, A.; Ahmad, R.; Ayyub, C.M.; Abbas, T. Brassinosteroid (24-epibrassinolide) enhances growth and alleviates the deleterious effects induced by salt stress in pea (Pisum sativum L.). Aust. J. Crop Sci. 2011, 5, 500–510. [Google Scholar]
- Shahzad, R.; Harlina, P.W.; Ewas, M.; Zhenyuan, P.; Nie, X.; Gallego, P.P.; Khan, S.U.; Nishawy, E.; Khan, A.H.; Jia, H. Foliar applied 24-epibrassinolide alleviates salt stress in rice (Oryza sativa L.) by suppression of ABA levels and upregulation of secondary metabolites. J. Plant Interact. 2021, 16, 533–549. [Google Scholar] [CrossRef]
- Shang, Q.; Song, S.; Zhang, Z.; Guo, S. Exogenous Brassinosteroid Induced the Salt Resistance of Cucumber (Cucumis sativus L.) Seedlings. Sci. Agric. Sin. 2006, 39, 1872–1877. [Google Scholar]
- Shu, H.M.; Guo, S.Q.; Gong, Y.Y.; Jiang, L.; Zhu, J.W.; Ni, W.C. RNA-seq analysis reveals a key role of brassinolide-regulated pathways in NaCl-stressed cotton. Biol. Plant. 2017, 61, 667–674. [Google Scholar] [CrossRef]
- Shu, H.; Guo, S.; Gong, Y.; Ni, W. Effects of brassinolide on leaf physiological characteristics and differential gene expression profiles of NaCl-stressed cotton. Chin. J. Appl. Ecol. 2016, 27, 150–156. [Google Scholar] [CrossRef]
- Shu, H.; Guo, S.; Gong, Y.; Mamat, P.; Ni, W. Effects of Brassinosteroid on Salinity Tolerance of Cotton. Agric. Sci. Technol. 2014, 15, 1433–1437. [Google Scholar] [CrossRef]
- Shu, H.; Guo, S.; Shen, X.; Ni, W. Cotton physiology affected by brassinosteroid under NaCl stress. Jiangsu J. Agric. Sci. 2011, 27, 1198–1202. [Google Scholar]
- Siddiqui, H.; Yusuf, M.; Faraz, A.; Faizan, M.; Sami, F.; Hayat, S. 24-Epibrassinolide supplemented with silicon enhances the photosynthetic efficiency of Brassica juncea under salt stress. S. Afr. J. Bot. 2018, 118, 120–128. [Google Scholar] [CrossRef]
- Singh, S.; Jakhar, S.; Rao, S. Improvement in salt tolerance of Vigna mungo (L.) Hepper by exogenously applied 24-epibrassinolide. Legume Res. 2020, 43, 647–652. [Google Scholar] [CrossRef]
- Sivakumar, R.; Priya, S.J. PGRs and nutrient consortium effect on water relations, photosynthesis, catalase enzyme and yield of blackgram under salinity stress. Legume Res. 2021, 44, 413–418. [Google Scholar] [CrossRef]
- Soliman, M.; Elkelish, A.; Souad, T.; Alhaithloul, H.; Farooq, M. Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiol. Mol. Biol. Plants 2020, 26, 501–511. [Google Scholar] [CrossRef]
- Sousa, D.J.P.; Nogueira, G.A.S.; Teixeira, K.B.S.; Monteiro, G.G.T.N.; Brito, A.E.A.; Nascimento, V.R.; Albuquerque, G.D.P.; Oliveira, T.J.M.; Souza, L.C.; Freitas, J.M.N.; et al. Mitigation of the effects of salt stress in cowpea bean through the exogenous aplication of brassinosteroid. Braz. J. Biol. 2022, 82, e260818. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.Q.; Serra Messias, W.F.; Pereira, Y.C.; Serrao Da Silva, B.R.; Silva Guedes Lobato, E.M.; Alyemeni, M.N.; Ahmad, P.; Da Silva Lobato, A.K. Pretreatment with 24-Epibrassinolide Synergistically Protects Root Structures and Chloroplastic Pigments and Upregulates Antioxidant Enzymes and Biomass in Na+-Stressed Tomato Plants. J. Plant Growth Regul. 2022, 41, 2869–2885. [Google Scholar] [CrossRef]
- Soylemez, S.; Kaya, C.; Dikilitas, S.K. Promotive effects of epibrassinolide on plant growth, fruit yield, antioxidant, and mineral nutrition of saline stressed tomato plants. Pak. J. Bot. 2017, 49, 1655–1661. [Google Scholar]
- Su, Q.; Zheng, X.; Tian, Y.; Wang, C. Exogenous Brassinolide Alleviates Salt Stress in Malus hupehensis Rehd. by Regulating the Transcription of NHX-Type Na+(K+)/H+ Antiporters. Front. Plant Sci. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; An, M.; Han, L.; Xu, L. Effects of Exogenously Applied 2,4-Epibrassinolide on the Seedlings of Perennial Ryegrass under NaCl Stress. Acta Agrestia Sin. 2014, 22, 1045–1050. [Google Scholar] [CrossRef]
- Sun, S.; An, M.; Han, L.; Yin, S. Foliar Application of 24-Epibrassinolide Improved Salt Stress Tolerance of Perennial Ryegrass. Hortscience 2015, 50, 1518–1523. [Google Scholar] [CrossRef]
- Tofighi, C.; Khavari-Nejad, R.A.; Najafi, F.; Razavi, K.; Rejali, F. Physiological and molecular responses of wheat plants to mycorrhizal and epibrassinolide interactions under salinity. Plant Biosyst. 2021, 155, 1075–1080. [Google Scholar] [CrossRef]
- Tofighi, C.; Khavari-Nejad, R.A.; Najafi, F.; Razavi, K.; Rejali, F. Responses of wheat plants to interactions of 24-epibrassinolide and Glomus mosseae in saline condition. Physiol. Mol. Biol. Plants 2017, 23, 557–564. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y. Effect of brassinolide on the stress resistance in Pinus elliotth engelm seedlings. J. Nanjing For. Univ. (Nat. Sci.) 1993, 17, 27–31. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Zhou, Y.; Li, B.; Nie, S. Physiological regulation of brassinosteroids on seed germination and seedling growth in Lolium perenne in response to salt stress. Pratacultural Sci. 2021, 38, 1110–1118. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Yue, J.; You, Y.; Zhang, L. BRs, photosynthetic pigments, and chlorophyll fluorescence parameters in Cinnamomum camphora seedlings with NaCl stress. J. Zhejiang A F Univ. 2017, 34, 20–27. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Zhang, J.; Yue, J. Effects of exogenous 2,4-epibrassinolide on antioxidant enzyme activities of camphor seedlings under salt stress. J. Zhejiang Univ. 2017, 43, 476–482. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Zhang, L. Effects of exogenous 24-epibrassinolide on chlorophyll content and chlorophyll fluorescence characteristics of camphor seedlings under salt stress. J. Zhejiang Univ. (Agric. Life Sci.) 2017, 43, 45–53. [Google Scholar]
- Wang, W.; Ma, D.; Zhao, L.; Ma, Q. Effects of 2,4-table Brassinolide on Enzyme Activity and Root ion Distribution and Absorption in Alfalfa Seedlings. Acta Agrestia Sin. 2021, 29, 1363–1368. [Google Scholar]
- Wang, X.; Ji, X.; Liu, L.; Ji, B.; Tian, Y. Effects of epibrassinolide on ion absorption and distribution in Medicago species under NaCl stress. Acta Prataculturae Sin. 2018, 27, 110–119. [Google Scholar] [CrossRef]
- Wei, L.J.; Deng, X.G.; Zhu, T.; Zheng, T.; Li, P.X.; Wu, J.Q.; Zhang, D.W.; Lin, H.H. Ethylene is Involved in Brassinosteroids Induced Alternative Respiratory Pathway in Cucumber (Cucumis sativus L.) Seedlings Response to Abiotic Stress. Front. Plant Sci. 2015, 6, 982. [Google Scholar] [CrossRef]
- Wei, S.; Ji, B.; Li, Z.; Gu, W. Effect of brassinolide on physiological characteristics of maize seedlings under salt stress. J. Northeast Agric. Univ. 2018, 49, 9–16. [Google Scholar] [CrossRef]
- Wu, X.; Cha, D.; Zhu, Z.; Li, X. Effects of Exogenous 24-Epibrassinolide on Seed Germination, Physiological Characteristics of Eggplant Seedlings under NaCl Stress. Plant Physiol. J. 2011, 47, 607–612. [Google Scholar] [CrossRef]
- Yan, H.; Peng, Y.; Zhao, X.; Lu, Y. Effect of Exogenous 24-epibrassinolide on Seed Germination and Seedling Growth of Maize under Different Stress. J. Nucl. Agric. Sci. 2016, 30, 988–996. [Google Scholar] [CrossRef]
- Yang, W.W.; Liu, Y.; Nie, S.M. Effect of Exogenous Brassinosteroids on Germination of Tomato Seeds under Salt Stress. Hortic. Seed 2022, 42, 43–46. [Google Scholar] [CrossRef]
- Yuan, H.; Guo, W.; Zhao, L.; Yu, Y.; Cheng, L.; Chen, S.; Kang, Q.; Song, X.; Chen, J.; Wu, G. Effect of exogenous brassinolide treatment on seed germination and seedling physiological characteristics of flax under NaCl stress. J. Northeast Agric. Univ. 2019, 50, 11–16. [Google Scholar] [CrossRef]
- Yue, J.; Fu, Z.; Zhang, L.; Zhang, Z.; Zhang, J. The Positive Effect of Different 24-epiBL Pretreatments on Salinity Tolerance in Robinia pseudoacacia L. Seedlings. Forests 2019, 10, 4. [Google Scholar] [CrossRef]
- Yue, J.; You, Y.; Zhang, L.; Fu, Z.; Wang, J.; Zhang, J.; Guy, R.D. Exogenous 24-Epibrassinolide Alleviates Effects of Salt Stress on Chloroplasts and Photosynthesis in Robinia pseudoacacia L. Seedlings. J. Plant Growth Regul. 2019, 38, 669–682. [Google Scholar] [CrossRef]
- Yue, J.; Zhang, J.; You, Y.; Wang, J.; Zhang, L.; Fu, Z.; Wang, S.; Yi, X. Effects of Brassinostreoids on photosynthesis and ultrastructure of chloroplasts in Robinia pseudoacacia seedlings under salt stress. J. Northwest A F Univ. (Nat. Sci. Ed.) 2017, 45, 56–66. [Google Scholar] [CrossRef]
- Yusuf, M.; Fariduddin, Q.; Khan, T.A.; Hayat, S. Epibrassinolide reverses the stress generated by combination of excess aluminum and salt in two wheat cultivars through altered proline metabolism and antioxidants. S. Afr. J. Bot. 2017, 112, 391–398. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, S.; Zhang, W.; Li, J.; Zhang, G. Effects of exogenous 2, 4- epibrassinolide on growth and photosynthetic physiological characteristics of cucumber seedlings under cadmium stress. Acta Bot. Boreali-Occident. Sin. 2022, 42, 272–279. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, S.; Li, Q.; Ye, P. Effects of EBR Immersion on Seed Germination of Lycium ruthenicum under Salt Stress. For. Sci. Technol. 2021, 59–61. [Google Scholar] [CrossRef]
- Zheng, C.; Fan, C.; Zheng, Q.; Liu, W.; Chen, J.; Ding, W.; Li, P. Growth of tomato seedlings under salt stress with external application of 2,4-epibrassinolide and the influence of physiological characteristics. J. Zhejiang Agric. Sci. 2022, 63, 991–995. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, J.; Liu, R.; Wu, H.; Jiang, C.; Wang, C.; Guan, Y. Temporal and spatial distributions of sodium and polyamines regulated by brassinosteroids in enhancing tomato salt resistance. Plant Soil 2016, 400, 147–164. [Google Scholar] [CrossRef]
- Zhou, N. Effect of 24-Epibrassinolide on Germination of Cucumber Seeds under Salt Stress. J. Hainan Trop. Ocean Univ. 2016, 23, 66–68. [Google Scholar] [CrossRef]
- Zhou, Y.; Luan, X.; Wang, L.; Zhang, Z.; Hui, Z. Effects of EBR Pretreatment on Antioxidant Substances and Enzyme Activities of Grapevine Seedling Leaves under Salt Stress. Acta Bot. Boreali-Occident. Sin. 2018, 38, 291–297. [Google Scholar] [CrossRef]
- Zhu, T.; Deng, X.; Tan, W.; Zhou, X.; Luo, S.; Han, X.; Zhang, D.; Lin, H. Nitric oxide is involved in brassinosteroid-induced alternative respiratory pathway in Nicotiana benthamiana seedlings’ response to salt stress. Physiol. Plant 2016, 156, 150–163. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chai, J.; Liu, W.; Zhu, X.; Liu, H.; Wei, X. Promotion of Ca2+ Accumulation in Roots by Exogenous Brassinosteroids as a Key Mechanism for Their Enhancement of Plant Salt Tolerance: A Meta-Analysis and Systematic Review. Int. J. Mol. Sci. 2023, 24, 16123. https://doi.org/10.3390/ijms242216123
Wang X, Chai J, Liu W, Zhu X, Liu H, Wei X. Promotion of Ca2+ Accumulation in Roots by Exogenous Brassinosteroids as a Key Mechanism for Their Enhancement of Plant Salt Tolerance: A Meta-Analysis and Systematic Review. International Journal of Molecular Sciences. 2023; 24(22):16123. https://doi.org/10.3390/ijms242216123
Chicago/Turabian StyleWang, Xian, Jiali Chai, Wenyu Liu, Xiaolin Zhu, Haixun Liu, and Xiaohong Wei. 2023. "Promotion of Ca2+ Accumulation in Roots by Exogenous Brassinosteroids as a Key Mechanism for Their Enhancement of Plant Salt Tolerance: A Meta-Analysis and Systematic Review" International Journal of Molecular Sciences 24, no. 22: 16123. https://doi.org/10.3390/ijms242216123
APA StyleWang, X., Chai, J., Liu, W., Zhu, X., Liu, H., & Wei, X. (2023). Promotion of Ca2+ Accumulation in Roots by Exogenous Brassinosteroids as a Key Mechanism for Their Enhancement of Plant Salt Tolerance: A Meta-Analysis and Systematic Review. International Journal of Molecular Sciences, 24(22), 16123. https://doi.org/10.3390/ijms242216123




