Facile Fabrication of Energetic Nanocomposite Materials by Polydopamine
Abstract
:1. Introduction
2. Results and Discussion
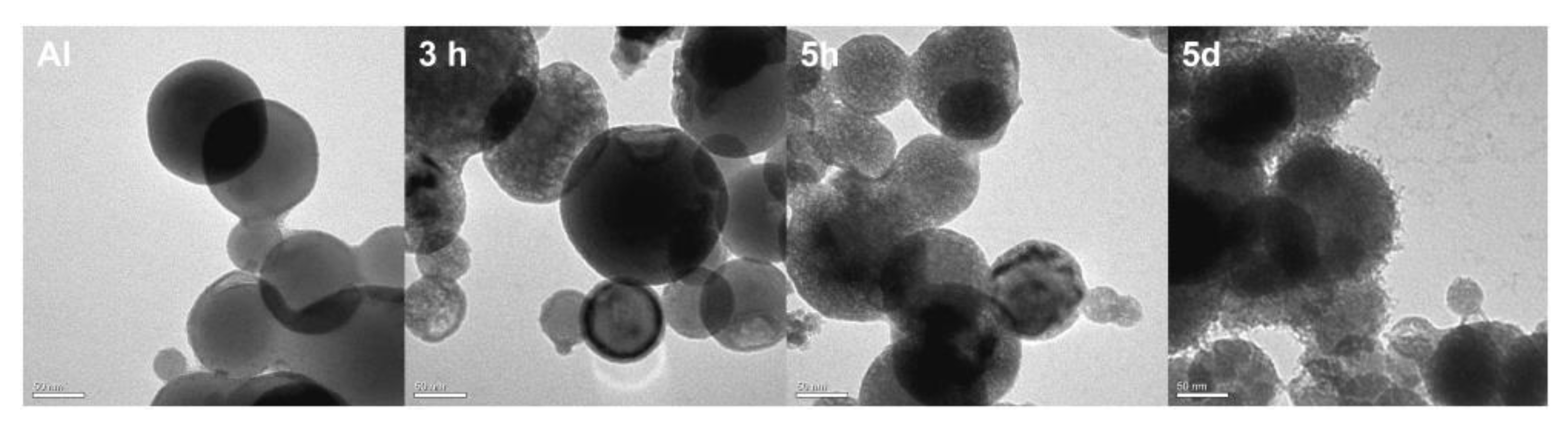
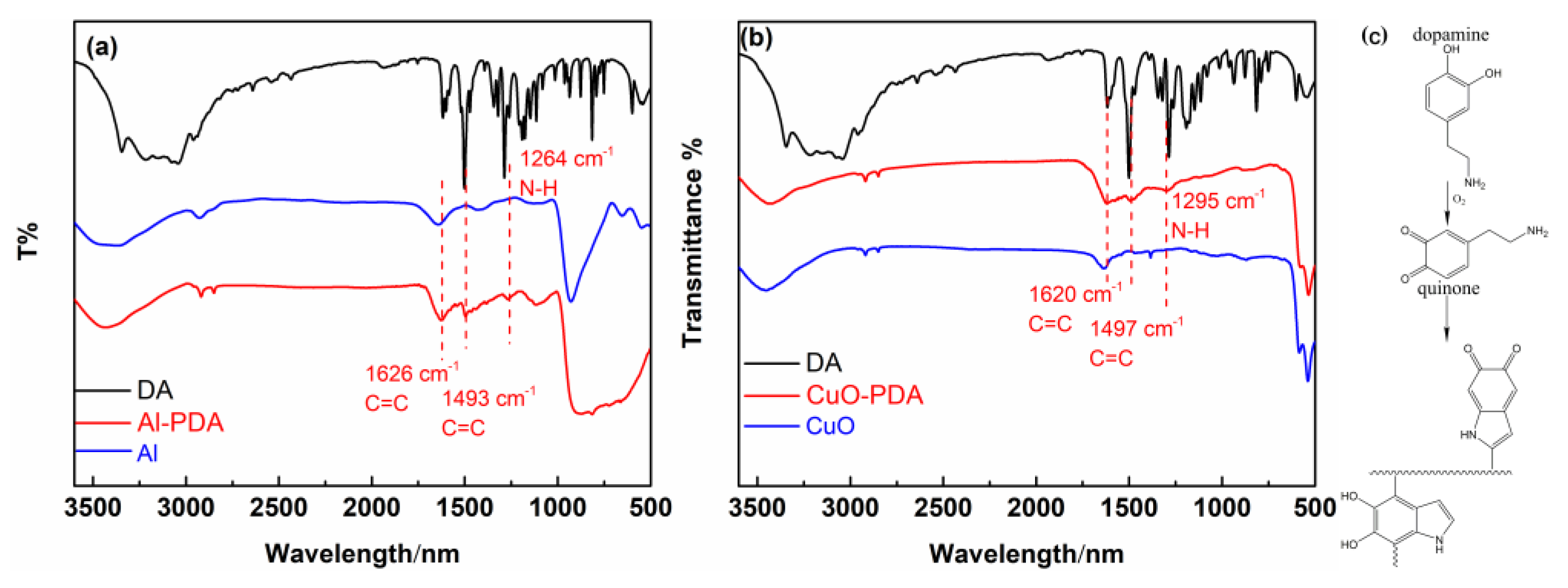
2.1. Polydopamine Coated Nanoparticles
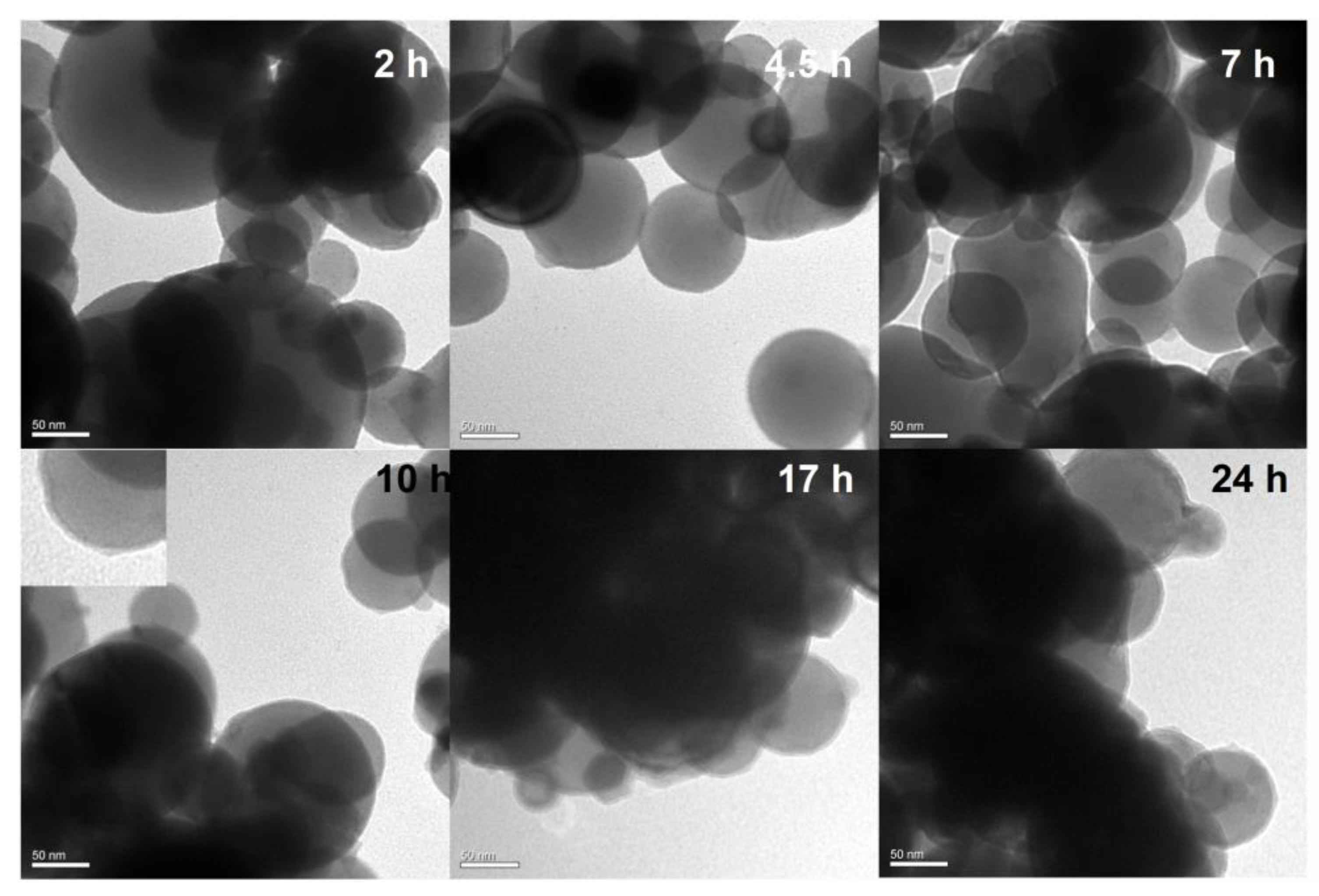
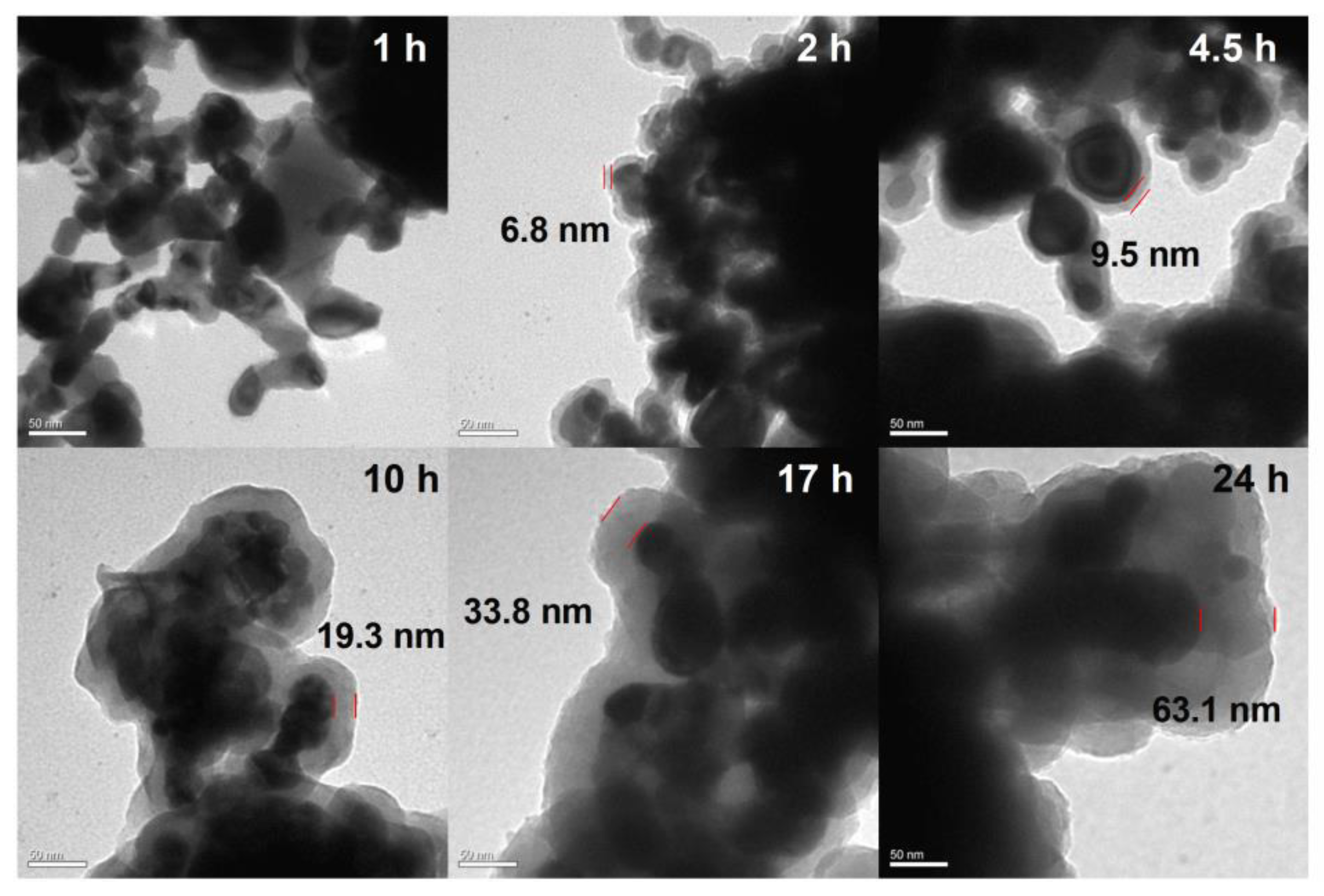
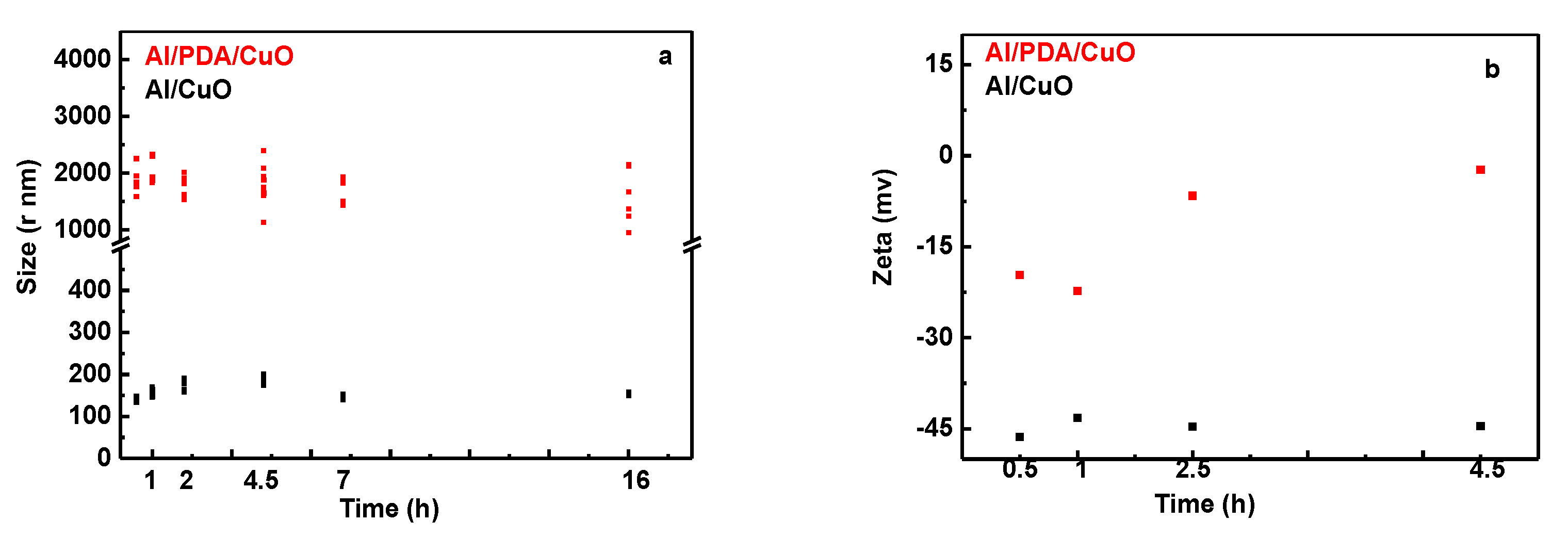
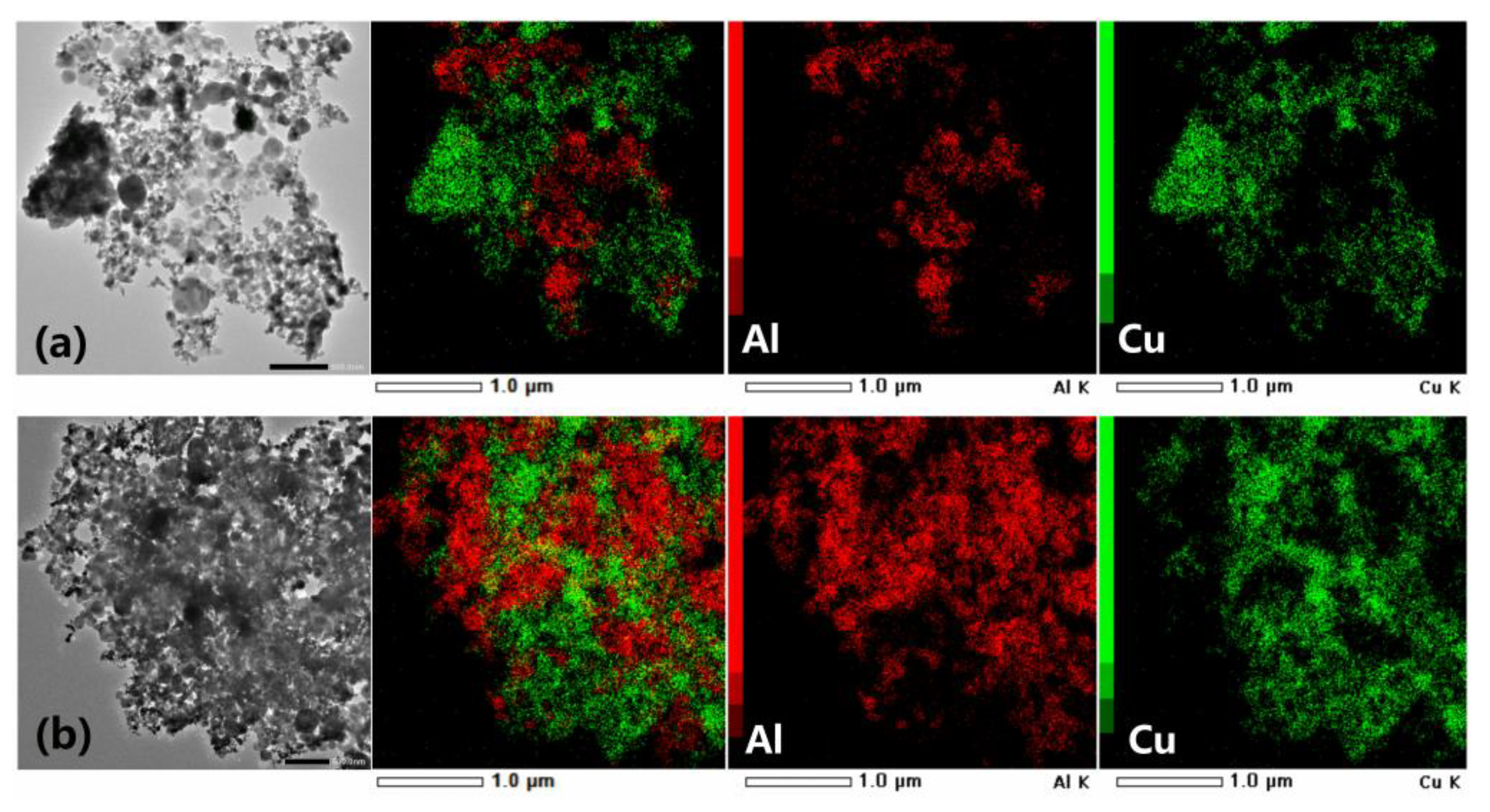
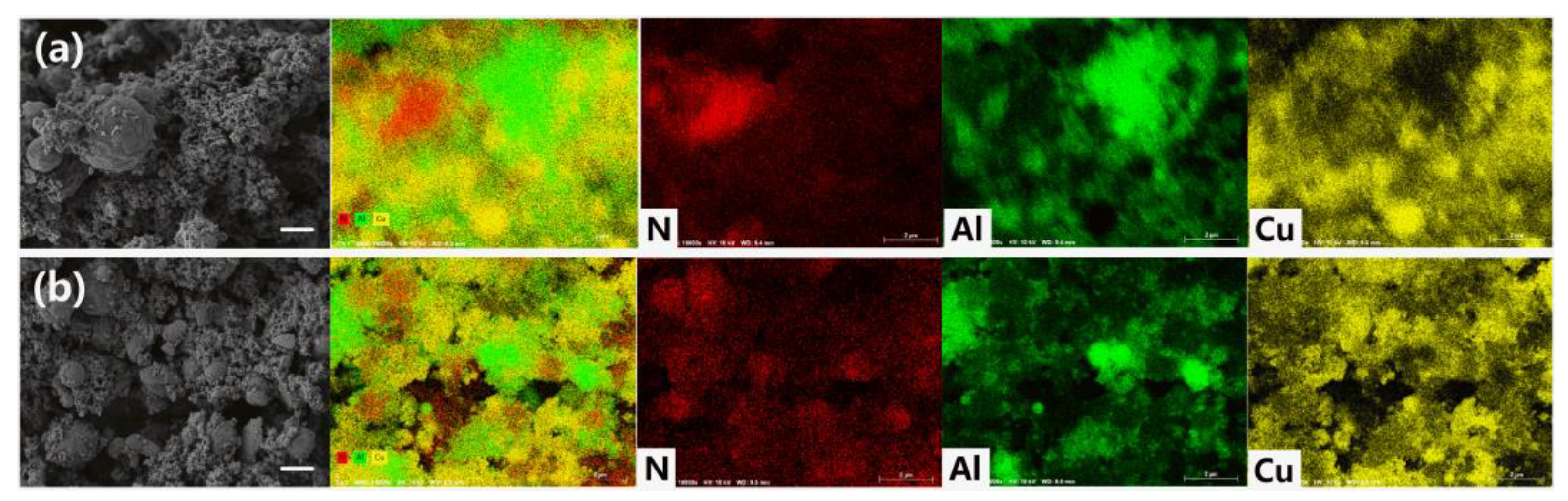
2.2. Preparation of Al/PDA/CuO Composite
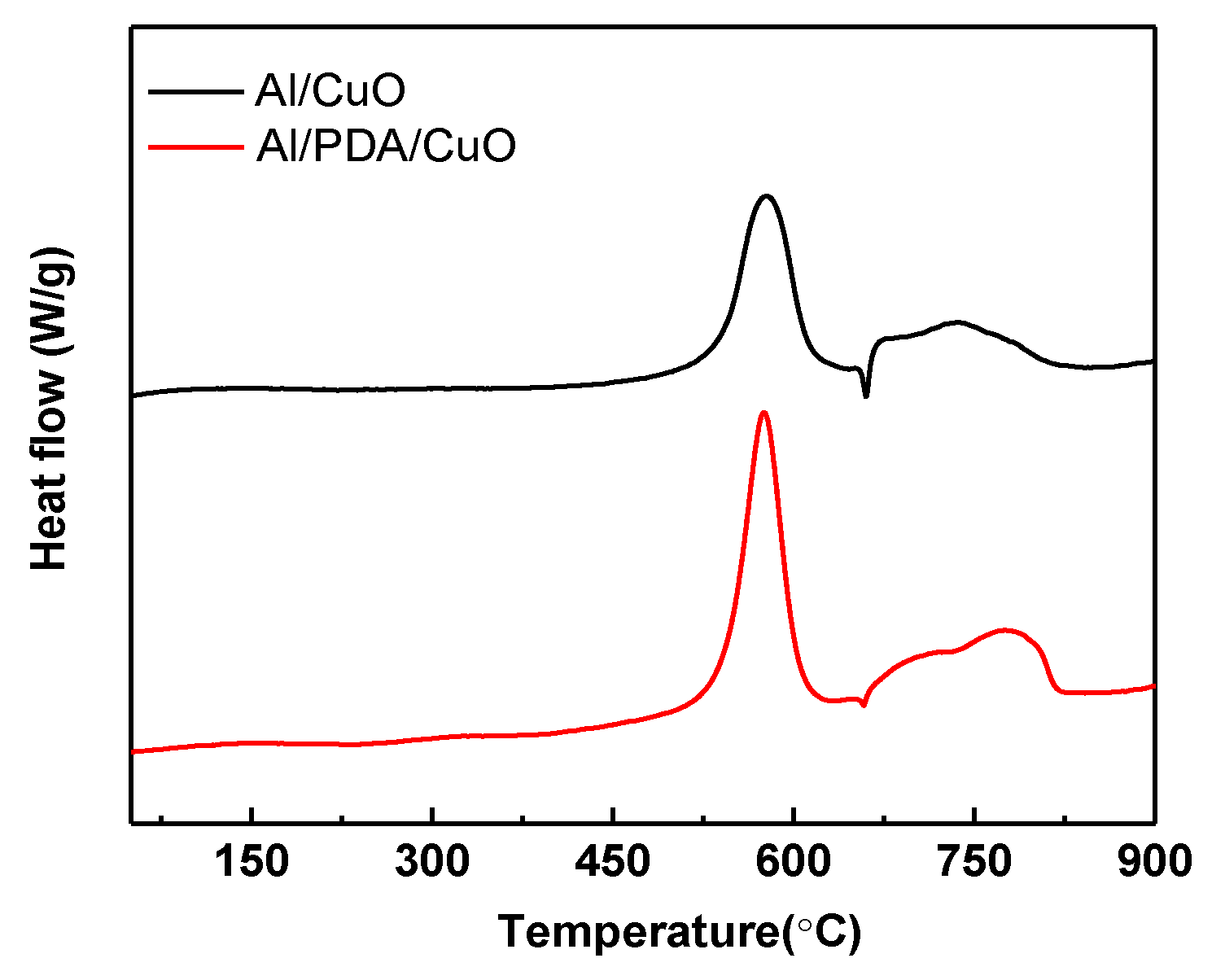
2.3. Thermal Decomposition Performance of Al/PDA/CuO Energetic Nanocomposite
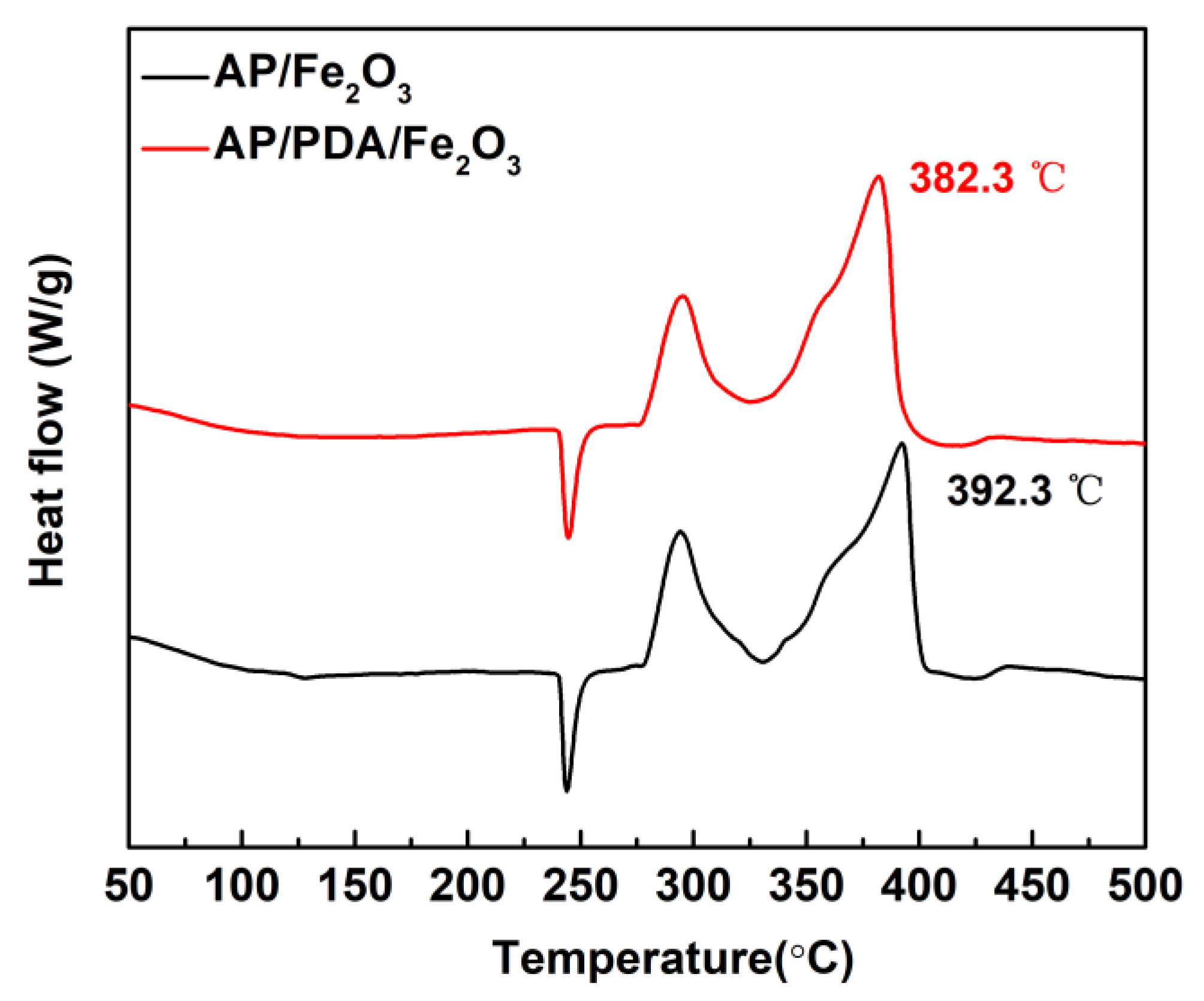
2.4. PDA-Assembled Inorganic Oxidant/Nanometal Catalyst AP/Fe2O3
2.5. PDA-Assembled Organic Energetic Material: HMX/Al/CuO Nanothermite
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Sui, H.; Li, Y.; Wen, J.Z. Energetic characteristics of the Al/CuO core-shell composite micro-particles fabricated as spherical colloids. Thermochim. Acta 2020, 689, 178656. [Google Scholar] [CrossRef]
- Shin, M.S.; Kim, J.K.; Kim, J.W.; Moraes, C.A.M.; Kim, H.S.; Koo, K.K. Reaction characteristics of Al/Fe2O3 nanocomposites. J. Ind. Eng. Chem. 2012, 18, 1768–1773. [Google Scholar] [CrossRef]
- Dai, J.; Xu, J.; Wang, F.; Tai, Y.; Shen, Y.; Shen, R.; Ye, Y. Facile formation of nitrocellulose-coated Al/Bi2O3 nanothermites with excellent energy output and improved electrostatic discharge safety. Mater. Des. 2018, 143, 93–103. [Google Scholar] [CrossRef]
- Sheikhpour, A.; Hosseini, S.G.; Tavangar, S.; Keshavarz, M.H. The influence of magnesium powder on the thermal behavior of Al–CuO thermite mixture. J. Therm. Anal. Calorim. 2017, 129, 1847–1854. [Google Scholar] [CrossRef]
- Parimi, V.S.; Huang, S.; Zheng, X. Enhancing ignition and combustion of micron-sized aluminum by adding porous silicon. Proc. Combust. Inst. 2017, 36, 2317–2324. [Google Scholar] [CrossRef]
- Kim, S.B.; Kim, K.J.; Cho, M.H.; Kim, J.H.; Kim, K.T.; Kim, S.H. Micro- and Nanoscale Energetic Materials as Effective Heat Energy Sources for Enhanced Gas Generators. ACS Appl. Mater. Interfaces 2016, 8, 9405–9412. [Google Scholar] [CrossRef]
- Lyu, J.Y.; Yang, S.L.; Wu, S.; Tang, G.; Yang, W.; Yan, Q.L. Burning rate modulation for composite propellants by interfacial control of Al@AP with precise catalysis of CuO. Combust. Flame 2022, 240, 112029. [Google Scholar]
- Zhang, T.; Wang, Z.; Li, G.; Luo, Y. Tuning the reactivity of Al/Fe2O3 nanoenergetic materials via an approach combining soft template self-assembly with sol–gel process process. J. Solid State Chem. 2015, 230, 1–7. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, Z.; Li, G.; Wang, Z.; Zhao, B.; Luo, Y. Electrostatic interactions for directed assembly of high performance nanostructured energetic materials of Al/Fe2O3/multi-walled carbon nanotube (MWCNT). J. Solid State Chem. 2016, 237, 394–403. [Google Scholar] [CrossRef]
- Tillotson, T.M.; Gash, A.E.; Simpson, R.L.; Hrubesh, L.W.; Satcher, J.H.; Poco, J.F. Nanostructured energetic materials using sol–gel methodologies. J. Non-Cryst. Solids 2001, 285, 338–345. [Google Scholar] [CrossRef]
- Séverac, F.; Alphonse, P.; Estève, A.; Bancaud, A.; Rossi, C. High-Energy Al/CuO Nanocomposites Obtained by DNA-Directed Assembly. Adv. Funct. Mater. 2012, 22, 323–329. [Google Scholar] [CrossRef]
- Xu, D.; Yang, Y.; Cheng, H.; Li, Y.Y.; Zhang, K. Integration of nano-Al with Co3O4 nanorods to realize high-exothermic core–shell nanoenergetic materials on a silicon substrate. Combust. Flame 2012, 159, 2202–2209. [Google Scholar] [CrossRef]
- Mursalat, M.; Schoenitz, M.; Dreizin, E.L. Effect of premilling Al and CuO in acetonitrile on properties of Al·CuO thermites prepared by arrested reactive milling. Combust. Flame 2020, 214, 57–64. [Google Scholar] [CrossRef]
- Shi, K.; Guo, X.; Chen, L.; Huang, S.; Zhao, L.; Ji, J.; Zhou, X. Alcohol-thermal synthesis of approximately core-shell structured Al@CuO nanothermite with improved heat-release and combustion characteristics. Combust. Flame 2021, 228, 331–339. [Google Scholar] [CrossRef]
- Chien, H.W.; Kuo, W.H.; Wang, M.J.; Tsai, S.W.; Tsai, W.B. Tunable micropatterned substrates based on poly(dopamine) deposition via microcontact printing. Langmuir 2012, 28, 5775–5782. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Liu, W.; Wang, Z.; Singamaneni, S.; Wang, R. Multifunctional Surface Modification of Nanodiamonds Based on Dopamine Polymerization. Langmuir 2018, 34, 4036–4042. [Google Scholar] [CrossRef]
- Mondin, G.; Wisser, F.M.; Leifert, A.; Mohamed-Noriega, N.; Grothe, J.; Dorfler, S.; Kaskel, S. Metal deposition by electroless plating on polydopamine functionalized micro- and nanoparticles. J. Colloid Interface Sci. 2013, 411, 187–193. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Wang, P.; He, W.; Duan, H. Robust Nanoparticle-DNA Conjugates Based on Mussel-Inspired Polydopamine Coating for Cell Imaging and Tailored Self-Assembly. Bioconjugate Chem. 2016, 27, 815–823. [Google Scholar] [CrossRef]
- Yang, S.H.; Kang, S.M.; Lee, K.B.; Chung, T.D.; Lee, H.; Choi, I.S. Mussel-inspired encapsulation and functionalization of individual yeast cells. J. Am. Chem. Soc. 2011, 133, 2795–2797. [Google Scholar] [CrossRef]
- Lin, C.; Gong, F.; Yang, Z.; Zhao, X.; Li, Y.; Zeng, C.; Li, J.; Guo, S. Core-Shell Structured HMX@Polydopamine Energetic Microspheres: Synergistically Enhanced Mechanical, Thermal, and Safety Performances. Polymers 2019, 11, 568. [Google Scholar] [CrossRef]
- Zhu, Q.; Xiao, C.; Xie, X.; Zheng, B.H.; Li, S.B.; Luo, G. Thermal Decomposition Enhancement of HMX by Bonding with TiO2 Nanoparticles. Propellants Explos. Pyrotech. 2019, 44, 438–446. [Google Scholar] [CrossRef]
- Zhu, Q.; Xiao, C.; Li, S.; Luo, G. Bioinspired Fabrication of Insensitive HMX Particles with Polydopamine Coating. Propellants Explos. Pyrotech. 2016, 41, 1092–1097. [Google Scholar] [CrossRef]
- Liang, L.; Guo, X.; Liao, X.; Chang, Z. Improve the interfacial adhesion, corrosion resistance and combustion properties of aluminum powder by modification of nickel and dopamine. Appl. Surf. Sci. 2020, 508, 144790. [Google Scholar] [CrossRef]
- Lin, C.; Zeng, C.; Wen, Y.; Gong, F.; He, G.; Li, Y.; Yang, Z.; Ding, L.; Li, J.; Guo, S. Litchi-like Core-Shell HMX@HPW@PDA Microparticles for Polymer-Bonded Energetic Composites with Low Sensitivity and High Mechanical Properties. ACS Appl. Mater. Interfaces 2020, 12, 4002–4013. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, P.J.; Gong, F.; Tao, B.; Gu, J.; Yang, Z.; Yan, Q.L. Tuning the Reactivity of Metastable Intermixed Composite n-Al/PTFE by Polydopamine Interfacial Control. ACS Appl. Mater. Interfaces 2018, 10, 32849–32858. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.Y.; Yu, J.H.; Tang, D.Y.; He, W.; Tao, B.W.; Guo, X.; Yan, Q.L. Unexpected burning rate independence of composite propellants on the pressure by fine interfacial control of fuel/oxidizer. J. Chem. Eng. 2020, 388, 124320. [Google Scholar] [CrossRef]
- He, W.; Tao, B.; Yang, Z.; Yang, G.; Guo, X.; Liu, P.J.; Yan, Q.L. Mussel-inspired polydopamine-directed crystal growth of core-shell n-Al@PDA@CuO metastable intermixed composites. J. Chem. Eng. 2019, 369, 1093–1101. [Google Scholar] [CrossRef]
- Sipos, P. The structure of Al (III) in strongly alkaline aluminate solutions—A review. J. Mol. Liq. 2009, 146, 1–14. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, X.L.; Niu, H.Y.; Ma, Y.R.; Li, W.H.; Cai, Y.Q. In Situ growth of gold nanoparticles onto polydopamine-encapsulated magnetic microspheres for catalytic reduction of nitrobenzene. Appl. Catal. B Environ. 2013, 134–135, 26–33. [Google Scholar] [CrossRef]
- Yu, F.; Chen, S.; Chen, Y.; Li, H.; Yang, L.; Chen, Y.; Yin, Y. Experimental and theoretical analysis of polymerization reaction process on the polydopamine membranes and its corrosion protection properties for 304 Stainless Steel. J. Mol. Struct. 2010, 982, 152–161. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Q.; Wu, S.; Gou, Z.; Wu, X.; Xu, D. Starch/chitosan films reinforced with polydopamine modified MMT: Effects of dopamine concentration. Food Hydrocoll. 2016, 61, 678–684. [Google Scholar] [CrossRef]
- Jin, M.; Song, Z.; Liu, W.; Wang, G.; Xian, M. Biofunctionalization of HMX with Peptides via Polydopamine Crosslinking for Assembling an HMX@Al@CuO Nanoenergetic Composite. Nanomaterials 2023, 13, 1837. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Liu, W.; Xian, M.; Jin, M. Facile Fabrication of Energetic Nanocomposite Materials by Polydopamine. Int. J. Mol. Sci. 2023, 24, 16199. https://doi.org/10.3390/ijms242216199
Song Z, Liu W, Xian M, Jin M. Facile Fabrication of Energetic Nanocomposite Materials by Polydopamine. International Journal of Molecular Sciences. 2023; 24(22):16199. https://doi.org/10.3390/ijms242216199
Chicago/Turabian StyleSong, Zhanxin, Wei Liu, Mo Xian, and Miaomiao Jin. 2023. "Facile Fabrication of Energetic Nanocomposite Materials by Polydopamine" International Journal of Molecular Sciences 24, no. 22: 16199. https://doi.org/10.3390/ijms242216199



