An Emerging Strategy for Neuroinflammation Treatment: Combined Cannabidiol and Angiotensin Receptor Blockers Treatments Effectively Inhibit Glial Nitric Oxide Release
Abstract
1. Introduction
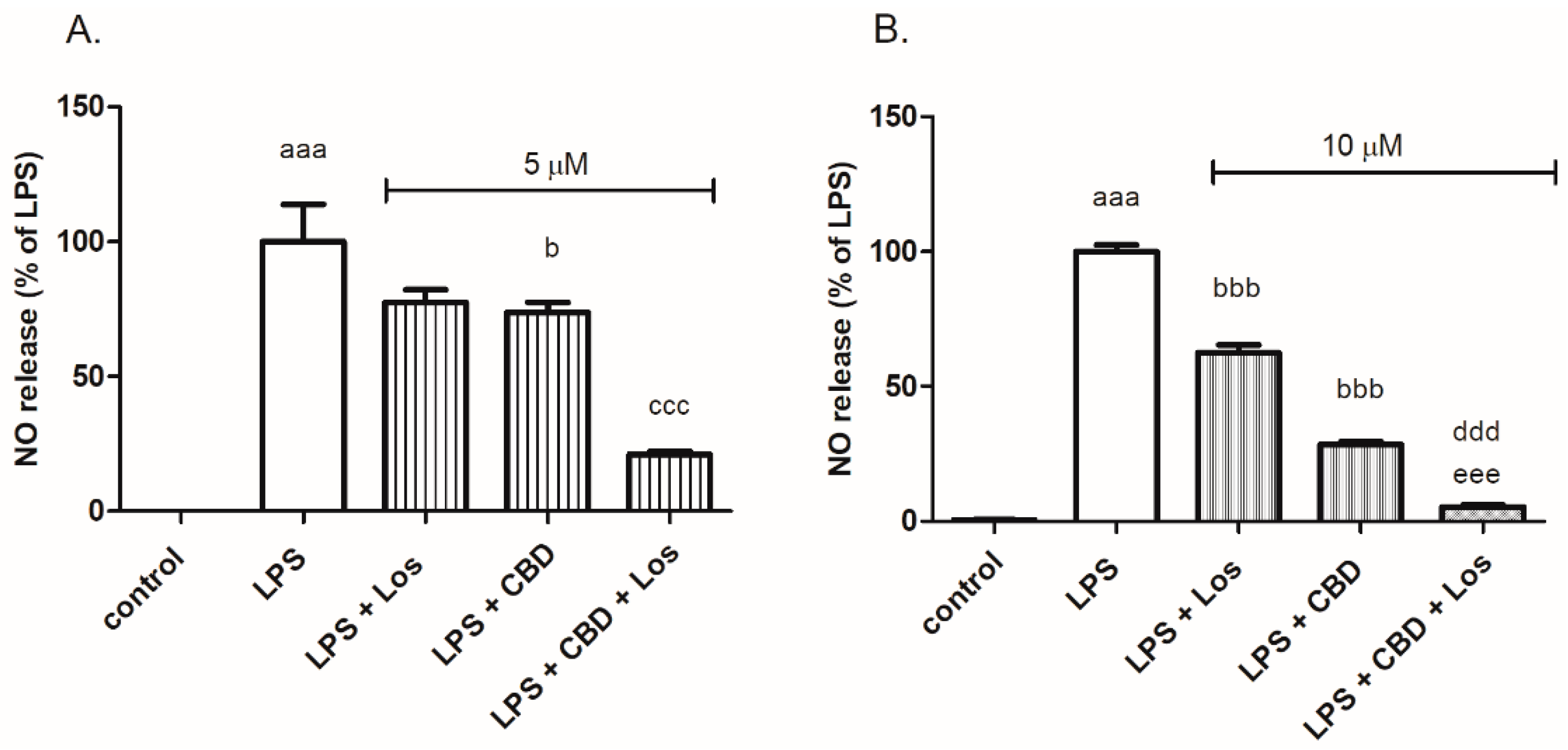
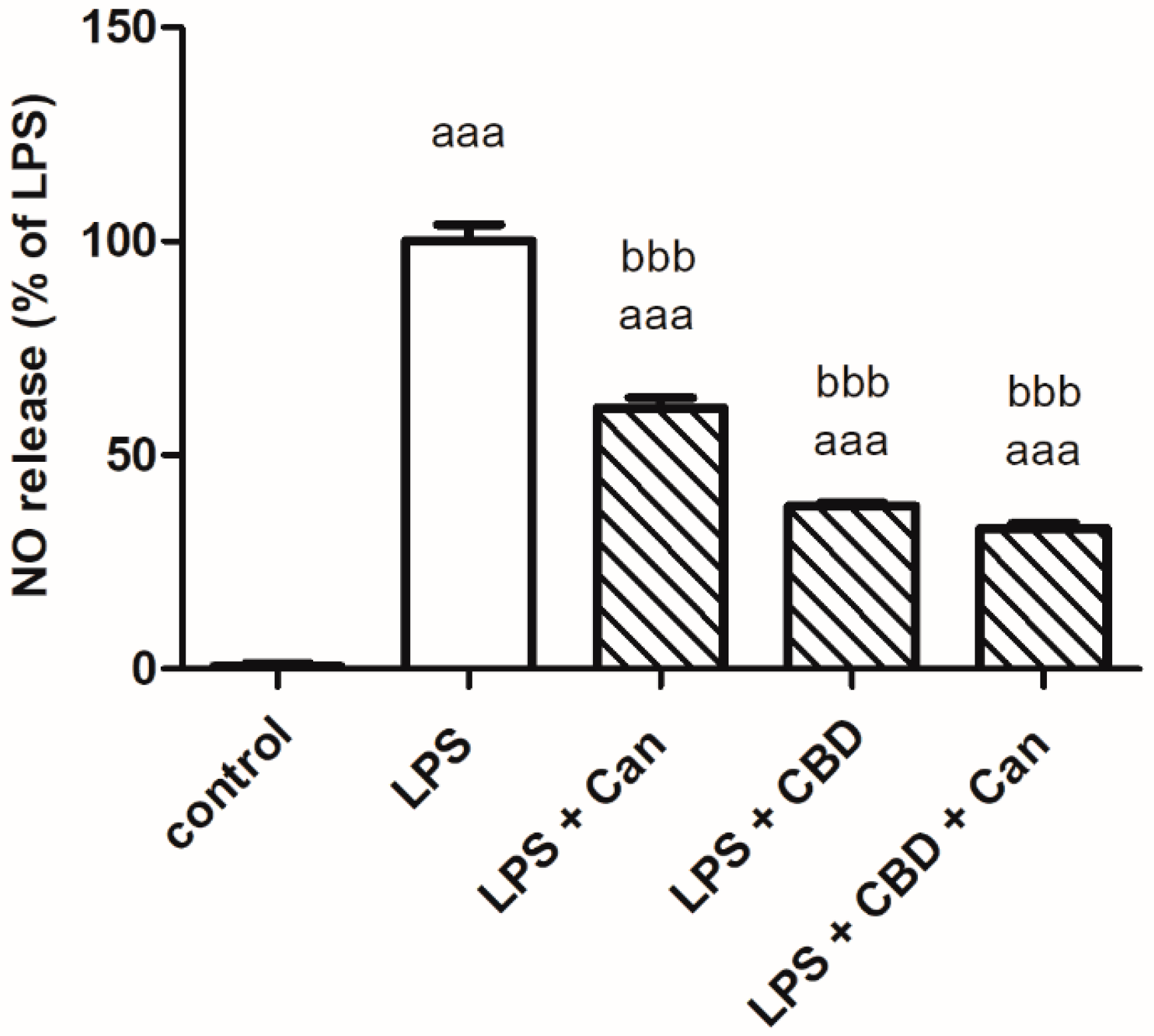
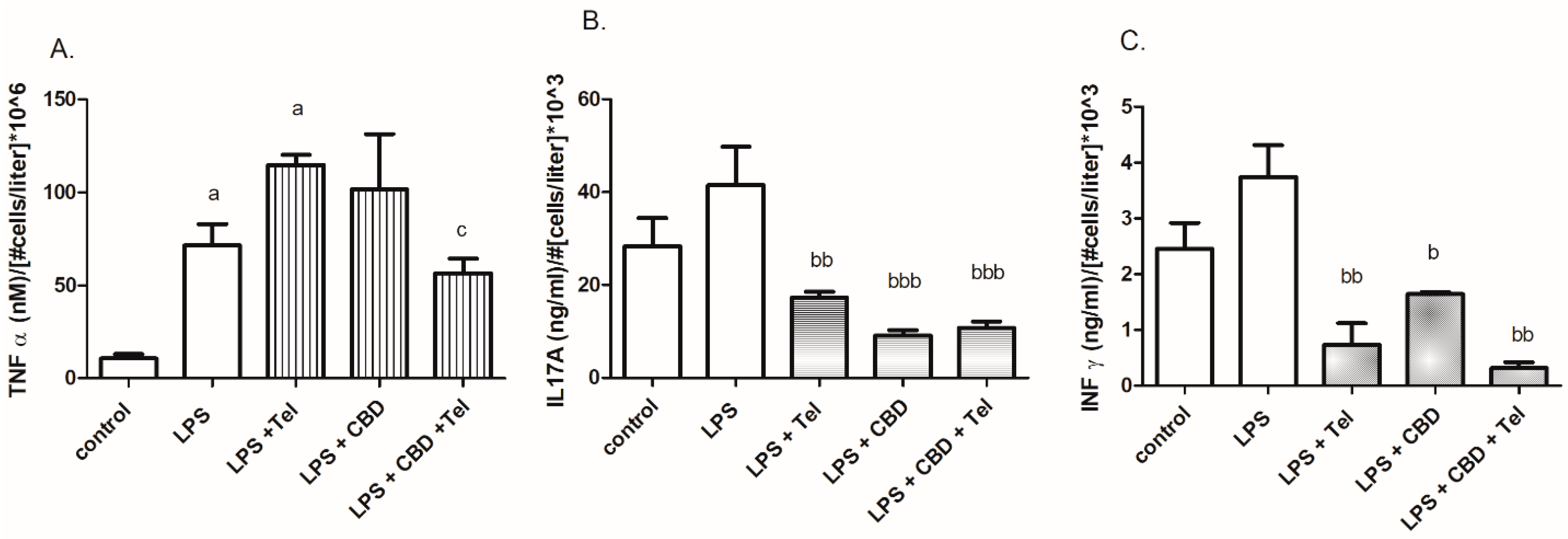
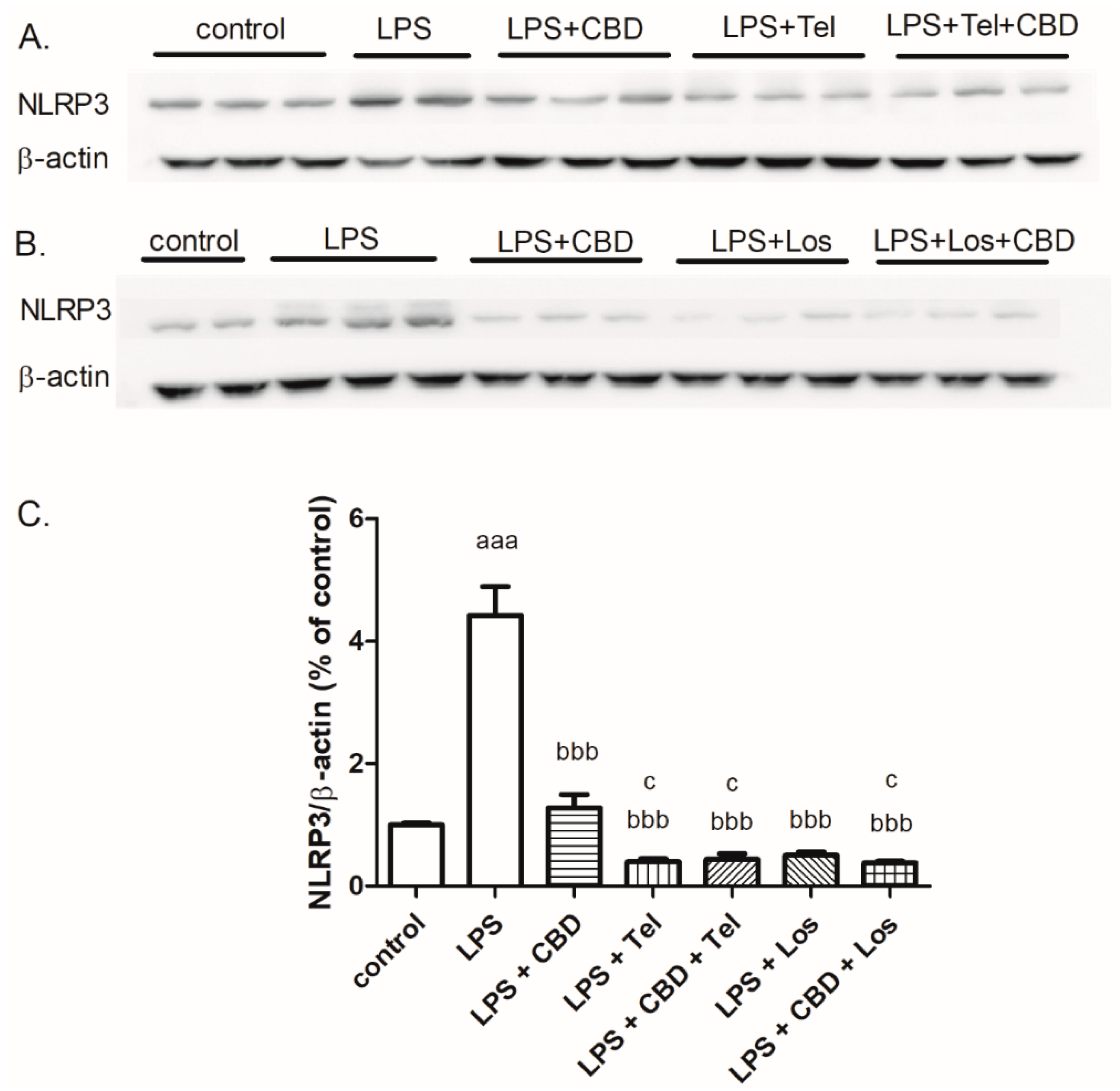
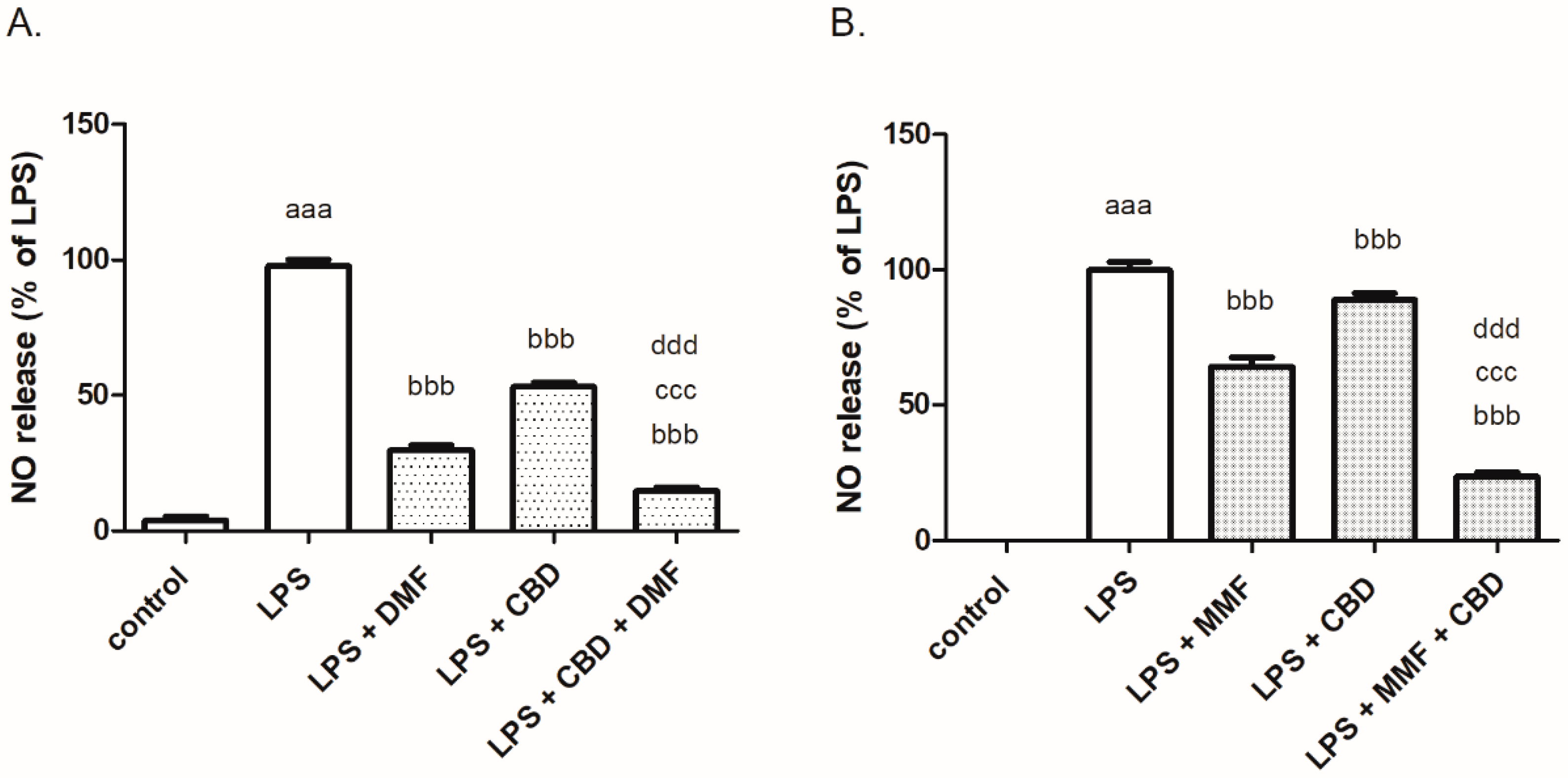
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Determination of NO Levels (Griess Reaction)
4.3. SDS-PAGE and Western Blot Analysis
4.4. Determination of Cytokine Levels
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuppo, E.E.; Arias, H.R. The role of inflammation in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2005, 37, 289–305. [Google Scholar] [CrossRef]
- Griffin, W.S. Inflammation and neurodegenerative diseases. Am. J. Clin. Nutr. 2006, 83, 470S–474S. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef]
- Jonnala, R.R.; Buccafusco, J.J. Inhibition of nerve growth factor signaling by peroxynitrite. J. Neurosci. Res. 2001, 63, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Mendieta, L.; Zenteno, E.; Guevara, J.; Limon, I.D. The role of NOS in the impairment of spatial memory and damaged neurons in rats injected with amyloid beta 25–35 into the temporal cortex. Pharmacol. Biochem. Behav. 2011, 98, 67–75. [Google Scholar] [CrossRef]
- Maezawa, I.; Zimin, P.I.; Wulff, H.; Jin, L.W. Amyloid-β protein oligomer at low nanomolar concentrations activates microglia and induces microglial neurotoxicity. J. Biol. Chem. 2011, 286, 3693–3706. [Google Scholar] [CrossRef]
- Amato, S.; Arnold, A. Modeling Microglia Activation and Inflammation-Based Neuroprotectant Strategies during Ischemic Stroke. Bull. Math. Biol. 2021, 83, 72. [Google Scholar] [CrossRef]
- Zuroff, L.; Daley, D.; Black, K.L.; Koronyo-Hamaoui, M. Clearance of cerebral Aβ in Alzheimer’s disease: Reassessing the role of microglia and monocytes. Cell Mol. Life Sci. 2017, 74, 2167–2201. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef] [PubMed]
- Meissner, H.; Cascella, M. Cannabidiol (CBD); StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Pertwee, R.G. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005, 76, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Δ9-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and interferon-β/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 2010, 285, 1616–1626. [Google Scholar] [CrossRef]
- Torika, N.; Asraf, K.; Danon, A.; Apte, R.N.; Fleisher-Berkovich, S. Telmisartan Modulates Glial Activation: In Vitro and In Vivo Studies. PLoS ONE 2016, 11, e0155823. [Google Scholar] [CrossRef] [PubMed]
- Karnik, S.S.; Unal, H.; Kemp, J.R.; Tirupula, K.C.; Eguchi, S.; Vanderheyden, P.M.; Thomas, W.G. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin Receptors: Interpreters of Pathophysiological Angiotensinergic Stimuli. Pharmacol. Rev. 2015, 67, 754–819, Erratum in Pharmacol. Rev. 2015, 67, 820. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Garrido-Gil, P.; Rodriguez-Perez, A.I.; Fernandez-Rodriguez, P.; Lanciego, J.L.; Labandeira-Garcia, J.L. Expression of angiotensinogen and receptors for angiotensin and prorenin in the rat and monkey striatal neurons and glial cells. Brain Struct. Funct. 2017, 222, 2559–2571. [Google Scholar] [CrossRef]
- Wiesmann, M.; Roelofs, M.; van der Lugt, R.; Heerschap, A.; Kiliaan, A.J.; Claassen, J.A. Angiotensin II, hypertension and angiotensin II receptor antagonism: Roles in the behavioural and brain pathology of a mouse model of Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 2017, 37, 2396–2413. [Google Scholar] [CrossRef]
- Li, J.; Brasier, A.R. Angiotensinogen gene activation by angiotensin II is mediated by the rel A (nuclear factor-kappaB p65) transcription factor: One mechanism for the renin angiotensin system positive feedback loop in hepatocytes. Mol. Endocrinol. 1996, 10, 252–264. [Google Scholar] [CrossRef][Green Version]
- Saavedra, J.M. Angiotensin II AT1 receptor blockers as treatments for inflammatory brain disorders. Clin. Sci. 2012, 123, 567–590. [Google Scholar] [CrossRef]
- Saavedra, J.M. Evidence to Consider Angiotensin II Receptor Blockers for the Treatment of Early Alzheimer’s Disease. Cell Mol. Neurobiol. 2016, 36, 259–279. [Google Scholar] [CrossRef]
- Blume, A.; Herdegen, T.; Unger, T. Angiotensin peptides and inducible transcription factors. J. Mol. Med. 1999, 77, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Collotta, D.; Collino, M. NLRP3 Inflammasome Signaling Platform as New Pharmacological Target for Metaflammation. Diabetes Res.-Open J. 2017, 3, e1–e3. [Google Scholar] [CrossRef]
- Gaborik, Z.; Jagadeesh, G.; Zhang, M.; Spat, A.; Catt, K.J.; Hunyady, L. The role of a conserved region of the second intracellular loop in AT1 angiotensin receptor activation and signaling. Endocrinology 2003, 144, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- So, H.S.; Oh, J.; Chung, Y.T.; Moon, Y.J.; Kim, D.H.; Moon, B.S.; Lee, H.S.; Baek, S.W.; Park, C.; Lim, Y.S.; et al. The water extract of Samultang protects the lipopolysaccharide (LPS)/phorbol 12-myristate 13-acetate (PMA)-induced damage and nitric oxide production of C6 glial cells via down-regulation of NF-κB. Gen. Pharmacol. 2000, 34, 303–310. [Google Scholar] [CrossRef]
- Zhou, X.; MY, A.Z.; Li, D.; Wang, J. Telmisartan ameliorates LPS-induced pneumonia in rats through regulation of the PPARγ/NF-κB pathway. Microbiol. Immunol. 2022, 66, 371–378. [Google Scholar] [CrossRef]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Gao, F.; Coppola, G.; Geschwind, D.; Vogel, Z. Microarray and pathway analysis reveal distinct mechanisms underlying cannabinoid-mediated modulation of LPS-induced activation of BV-2 microglial cells. PLoS ONE 2013, 8, e61462. [Google Scholar] [CrossRef]
- Hlozek, T.; Uttl, L.; Kaderabek, L.; Balikova, M.; Lhotkova, E.; Horsley, R.R.; Novakova, P.; Sichova, K.; Stefkova, K.; Tyls, F.; et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol. 2017, 27, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghezi, Z.Z.; Busbee, P.B.; Alghetaa, H.; Nagarkatti, P.S.; Nagarkatti, M. Combination of cannabinoids, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), mitigates experimental autoimmune encephalomyelitis (EAE) by altering the gut microbiome. Brain Behav. Immun. 2019, 82, 25–35. [Google Scholar] [CrossRef]
- King, K.M.; Myers, A.M.; Soroka-Monzo, A.J.; Tuma, R.F.; Tallarida, R.J.; Walker, E.A.; Ward, S.J. Single and combined effects of Δ9-tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br. J. Pharmacol. 2017, 174, 2832–2841. [Google Scholar] [CrossRef]
- Aso, E.; Sanchez-Pla, A.; Vegas-Lozano, E.; Maldonado, R.; Ferrer, I. Cannabis-based medicine reduces multiple pathological processes in AβPP/PS1 mice. J. Alzheimer’s Dis. 2015, 43, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Sagredo, O.; Pazos, M.R.; Satta, V.; Ramos, J.A.; Pertwee, R.G.; Fernandez-Ruiz, J. Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington’s disease. J. Neurosci. Res. 2011, 89, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Sonsalla, P.K.; Richardson, J.R. Coordinated role of voltage-gated sodium channels and the Na+/H+ exchanger in sustaining microglial activation during inflammation. Toxicol. Appl. Pharmacol. 2013, 273, 355–364. [Google Scholar] [CrossRef]
- Jacques, M.T.; Saso, L.; Farina, M. LPS-Activated Microglial Cell-Derived Conditioned Medium Protects HT22 Neuronal Cells against Glutamate-Induced Ferroptosis. Int. J. Mol. Sci. 2023, 24, 2910. [Google Scholar] [CrossRef] [PubMed]
- Torika, N.; Asraf, K.; Cohen, H.; Fleisher-Berkovich, S. Intranasal telmisartan ameliorates brain pathology in five familial Alzheimer’s disease mice. Brain Behav. Immun. 2017, 64, 80–90. [Google Scholar] [CrossRef] [PubMed]
- von Bernhardi, R.; Eugenin-von Bernhardi, L.; Eugenin, J. Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, M.; Miyano, K.; Moriyama, N.; Taniguchi, M.; Watanabe, T. Angiotensin type 1 receptor antagonist inhibits lipopolysaccharide-induced stimulation of rat microglial cells by suppressing nuclear factor κB and activator protein-1 activation. Eur. J. Neurosci. 2008, 27, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.; Eldahshan, W.; Fagan, S.C.; Ergul, A. Within the Brain: The Renin Angiotensin System. Int. J. Mol. Sci. 2018, 19, 876. [Google Scholar] [CrossRef]
- Kim, J.M.; Heo, H.S.; Choi, Y.J.; Ye, B.H.; Ha, Y.M.; Seo, A.Y.; Yu, B.P.; Leeuwenburgh, C.; Chung, H.Y.; Carter, C.S. Inhibition of NF-κB-induced inflammatory responses by angiotensin II antagonists in aged rat kidney. Exp. Gerontol. 2011, 46, 542–548. [Google Scholar] [CrossRef]
- Lin, X.H.; Yuece, B.; Li, Y.Y.; Feng, Y.J.; Feng, J.Y.; Yu, L.Y.; Li, K.; Li, Y.N.; Storr, M. A novel CB receptor GPR55 and its ligands are involved in regulation of gut movement in rodents. Neurogastroenterol. Motil. 2011, 23, 862-e342. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.L.; Nagarkatti, P.S.; Nagarkatti, M. Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS ONE 2011, 6, e18281. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An overview of some pharmacological aspects. J. Clin. Pharmacol. 2002, 42, 11S–19S. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J. In the coming year we should abandon interferons and glatiramer acetate as first-line therapy for MS: Yes. Mult. Scler. 2013, 19, 24–25. [Google Scholar] [CrossRef]
- Fox, R.J.; Miller, D.H.; Phillips, J.T.; Hutchinson, M.; Havrdova, E.; Kita, M.; Yang, M.; Raghupathi, K.; Novas, M.; Sweetser, M.T.; et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N. Engl. J. Med. 2012, 367, 1087–1097, Correction in N. Engl. J. Med. 2012, 367, 1673. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.S.; Matos, M.F.; Richter, K.E.; Li, B.; Scannevin, R.H. The NRF2 transcriptional target, OSGIN1, contributes to monomethyl fumarate-mediated cytoprotection in human astrocytes. Sci. Rep. 2017, 7, 42054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, J.; Zhou, Y.; Xiao, Q.; Chen, Q.; Wang, H.; Lan, J.; Wu, L.; Peng, Y. Short-term exposure to dimethyl fumarate (DMF) inhibits LPS-induced IκBζ expression in macrophages. Front. Pharmacol. 2023, 14, 1114897. [Google Scholar] [CrossRef]
- de Almeida, D.L.; Devi, L.A. Diversity of molecular targets and signaling pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleisher-Berkovich, S.; Battaglia, V.; Baratta, F.; Brusa, P.; Ventura, Y.; Sharon, N.; Dahan, A.; Collino, M.; Ben-Shabat, S. An Emerging Strategy for Neuroinflammation Treatment: Combined Cannabidiol and Angiotensin Receptor Blockers Treatments Effectively Inhibit Glial Nitric Oxide Release. Int. J. Mol. Sci. 2023, 24, 16254. https://doi.org/10.3390/ijms242216254
Fleisher-Berkovich S, Battaglia V, Baratta F, Brusa P, Ventura Y, Sharon N, Dahan A, Collino M, Ben-Shabat S. An Emerging Strategy for Neuroinflammation Treatment: Combined Cannabidiol and Angiotensin Receptor Blockers Treatments Effectively Inhibit Glial Nitric Oxide Release. International Journal of Molecular Sciences. 2023; 24(22):16254. https://doi.org/10.3390/ijms242216254
Chicago/Turabian StyleFleisher-Berkovich, Sigal, Veronica Battaglia, Francesca Baratta, Paola Brusa, Yvonne Ventura, Nitzan Sharon, Arik Dahan, Massimo Collino, and Shimon Ben-Shabat. 2023. "An Emerging Strategy for Neuroinflammation Treatment: Combined Cannabidiol and Angiotensin Receptor Blockers Treatments Effectively Inhibit Glial Nitric Oxide Release" International Journal of Molecular Sciences 24, no. 22: 16254. https://doi.org/10.3390/ijms242216254
APA StyleFleisher-Berkovich, S., Battaglia, V., Baratta, F., Brusa, P., Ventura, Y., Sharon, N., Dahan, A., Collino, M., & Ben-Shabat, S. (2023). An Emerging Strategy for Neuroinflammation Treatment: Combined Cannabidiol and Angiotensin Receptor Blockers Treatments Effectively Inhibit Glial Nitric Oxide Release. International Journal of Molecular Sciences, 24(22), 16254. https://doi.org/10.3390/ijms242216254






