YKL-40 and the Cellular Metabolic Profile in Parkinson’s Disease
Abstract
1. Introduction
2. Results
2.1. Patients
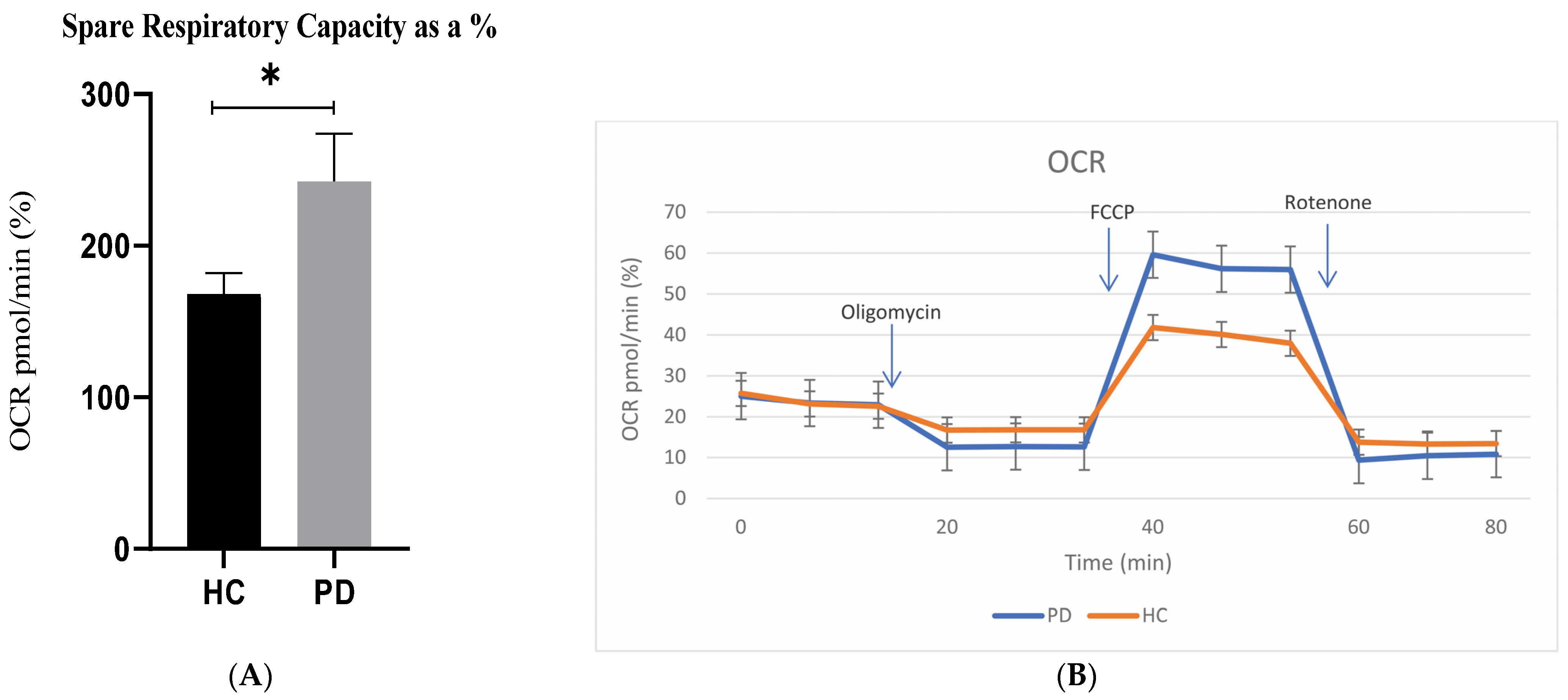
2.2. Mitochondrial Activity
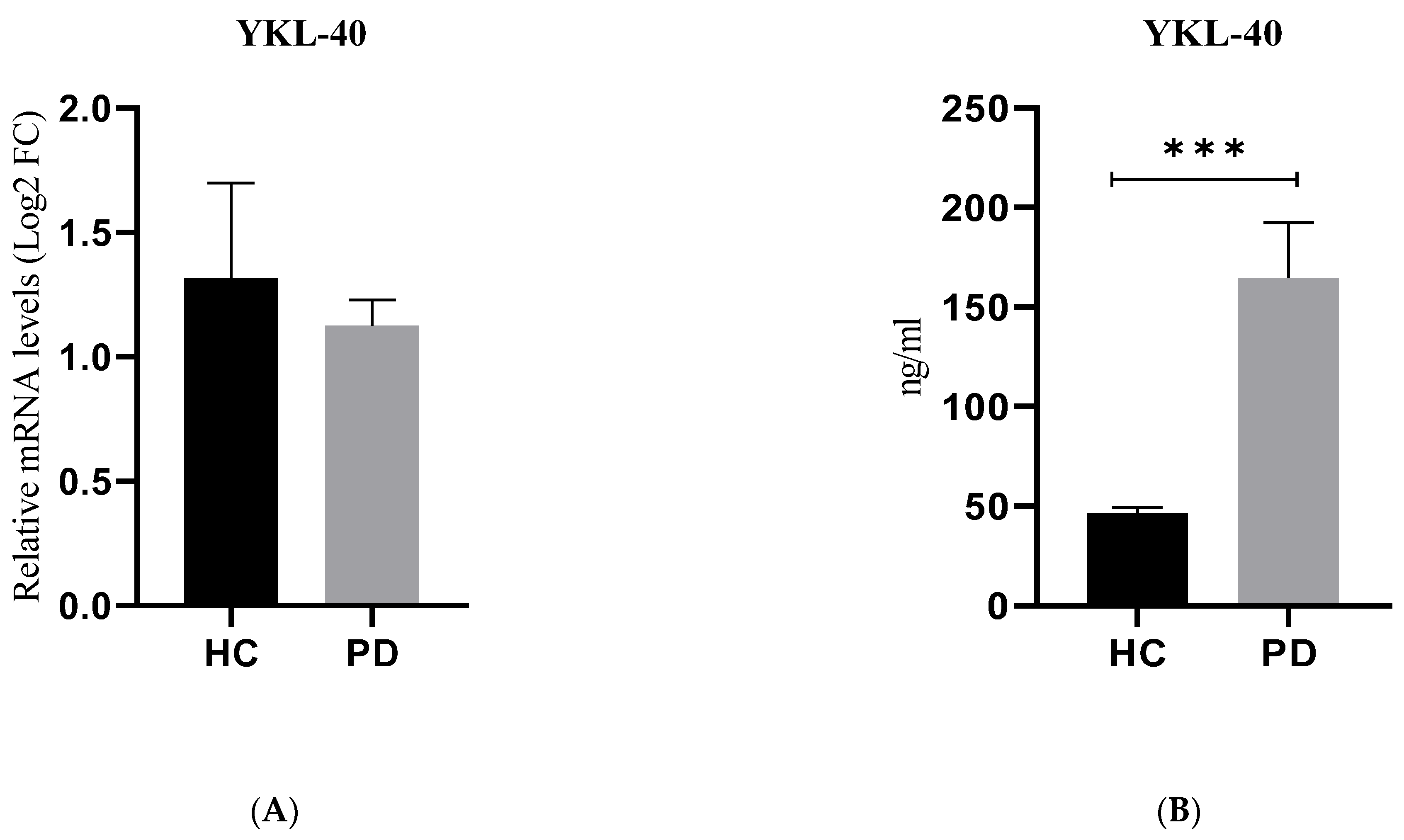
2.3. Gene and Protein Expression of YKL-40
2.4. Correlation Analysis
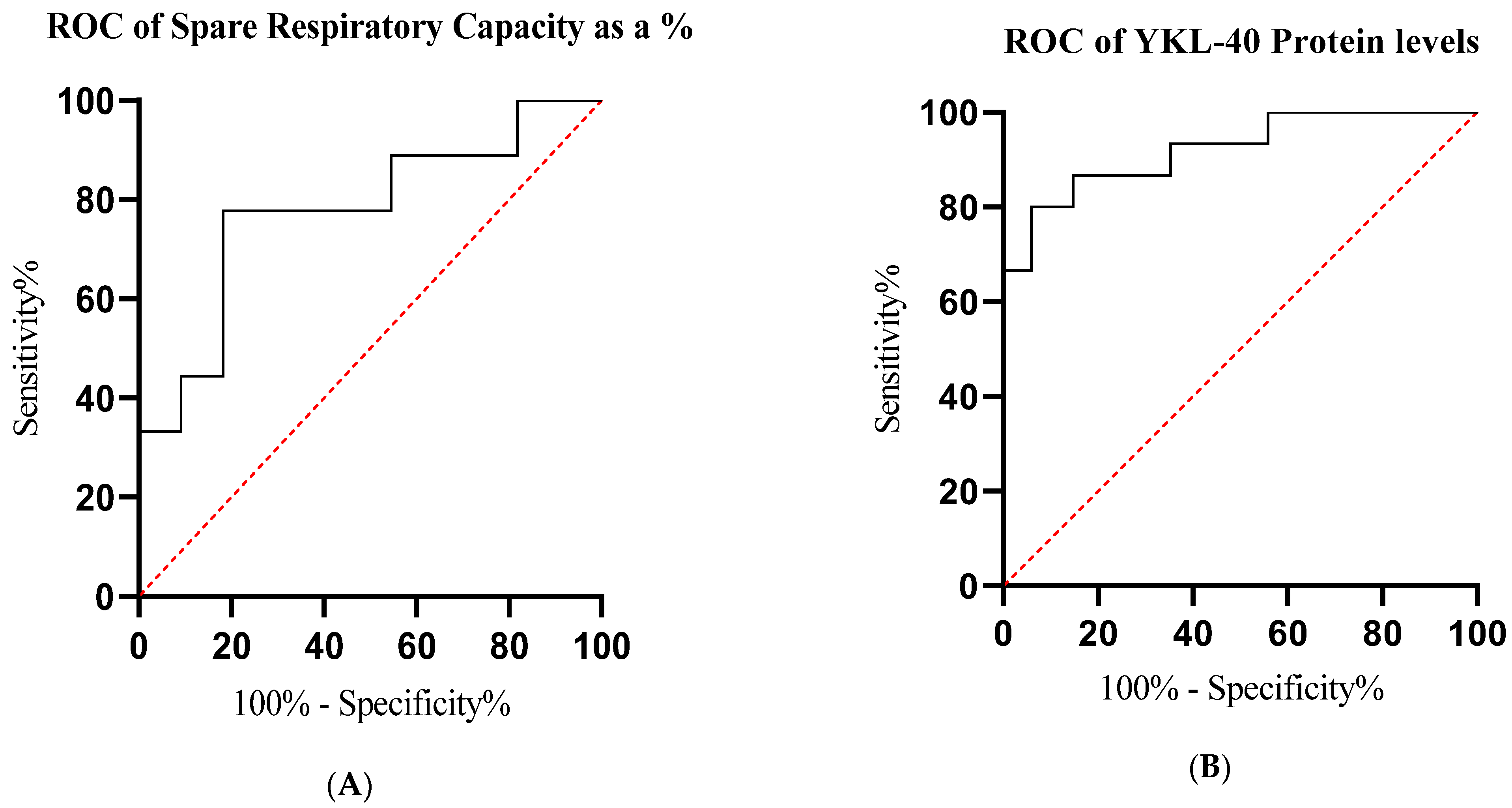
2.5. Receiver Operating Characteristic (ROC) Analysis
3. Discussion
4. Materials and Methods
4.1. Patients and Controls
4.2. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
4.3. Metabolic Analysis in Real Time
4.4. Mito Stress Test
4.5. YKL-40 Gene Expression by qPCR
4.6. Detection of YKL-40 in Plasma
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalia, V.; Lang, E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Váradi, C. Clinical features of Parkinson’s disease: The evolution of critical symptoms. Biology. 2020, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T. The prevalence of Parkinson’s disease: A systematic review and metaanalysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef]
- Collier, J.; Kanaan, M.; Kordower, H. Ageing as a primary risk factor for Parkinson’s disease: Evidence from studies of non-human primates. Nat. Rev. Neurosci. 2011, 12, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Davis, L.; Sue, M. Mitochondrial dysfunction in Parkinson’s disease: New mechanistic insights and therapeutic perspectives. Curr. Neurol. Neurosci. 2018, 18, 18–21. [Google Scholar] [CrossRef]
- Rango, M.; Bresolin, N. Brain mitochondria, aging, and Parkinson’s disease. Genes 2018, 9, 250. [Google Scholar] [CrossRef]
- Yadava, N.; Nicholls, G. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J. Neurosci. 2007, 27, 7310–7317. [Google Scholar] [CrossRef]
- Renkema, G. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur. J. Biochem. 1998, 251, 504–509. [Google Scholar] [CrossRef]
- Jensen, B.V.; Johansen, J.S.; Price, P.A. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin. Cancer Res. 2003, 9, 4423–4434. [Google Scholar]
- Hakala, B.E.; White, C.; Recklies, A.D. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of the chitinase protein family. J. Biol. Chem. 1993, 268, 25803–25810. [Google Scholar] [CrossRef]
- Brasso, K. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate 2006, 66, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Shao, R. YKL-40 acts as an angiogenic factor to promote tumor angiogenesis. Front. Physiol. 2013, 4, 122. [Google Scholar] [CrossRef] [PubMed]
- Prakash, M.; Bodas, M.; Prakash, D.; Nawani, N.; Khetmalas, M.; Mandal, A. Diverse pathological implications of YKL-40: Answers may lie in ‘outside-in’ signaling. Cell Signal. 2013, 25, 1567–1573. [Google Scholar] [CrossRef]
- Riabov, V.; Gudima, A.; Wang, N.; Mickley, A.; Orekhov, A.; Kzhyshkowska, J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front. Physiol. 2014, 5, 75. [Google Scholar] [CrossRef]
- González-Rodríguez, P.; Zampese, E.; Stout, K.; Guzman, N.; Ilijic, E.; Yang, B.; Tkatch, T.; Stavarache, A.; Wokosin, L.; Gao, L.; et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature 2021, 599, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Depp, C.; Ryan, J.; Johnston, I.; Alegre-Abarrategui, J.; Evetts, S.; Rolinski, M.; Baig, F.; Ruffmann, C.; Simon, K.; et al. Mitochondrial dysfunction and increased glycolysis in prodromal and early Parkinson’s blood cells. Mov. Disord. 2018, 33, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, T.; Gu, Y.; Sun, X. Mitochondrial dysfunction in Parkinson’s disease: From mechanistic insights to therapy. Front. Aging Neurosci. 2022, 14, 885500. [Google Scholar] [CrossRef] [PubMed]
- Malpartida, A.; Williamson, M.; Narendra, D.; Wade-Martins, R.; Ryan, B. Mitochondrial dysfunction and mitophagy in Parkinson’s disease: From mechanism to therapy. Trends Biochem. Sci. 2021, 46, 329–343. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.; Greenamyre, J. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef]
- Tanner, C.; Kamel, F.; Ross, G. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Salvatori, I.; Zenuni, H.; Grillo, P.; Valle, C.; Martella, G.; Mercuri, N.; Ferri, A. Pattern of Mitochondrial Respiration in Peripheral Blood Cells of Patients with Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 10863. [Google Scholar] [CrossRef] [PubMed]
- Minchev, D.; Kazakova, M.; Sarafian, V. Neuroinflammation and Autophagy in Parkinson’s Disease—Novel Perspectives. Int. J. Mol. Sci. 2022, 23, 14997. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Bolognin, S.; Antony, P.; Nickels, S.; Poovathingal, S.; Salamanca, L.; Magni, S.; Perfeito, R.; Hoel, F.; Qing, X.; et al. Neural stem cells of Parkinson’s disease patients exhibit aberrant mitochondrial morphology and functionality. Stem Cell Rep. 2019, 12, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Valdinocci, D.; Simões, R.; Kovarova, J.; Cunha-Oliveira, T.; Neuzil, J.; Pountney, D. Intracellular and intercellular mitochondrial dynamics in Parkinson’s disease. Front. Neurosci. 2019, 13, 930. [Google Scholar] [CrossRef] [PubMed]
- Lauro, C.; Limatola, C. Metabolic reprograming of microglia in the regulation of the innate inflammatory response. Front. Immunol. 2020, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Booth, H.D.; Hirst, W.D.; Wade-Martins, R. The Role of Astrocyte Dysfunction in Parkinson’s Disease Pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef]
- Flynn, M.; Choi, S.; Day, N.; Gerencser, A.; Hubbard, A.; Melov, S. Impaired spare respiratory capacity in cortical synaptosomes from Sod2 null mice. Free. Radic. Biol. Med. 2011, 50, 866–873. [Google Scholar] [CrossRef][Green Version]
- Schapira, H.; Tolosa, E. Molecular and clinical prodrome of Parkinson disease: Implications for treatment. Nat. Rev. Neurol. 2010, 6, 309–317. [Google Scholar] [CrossRef]
- Bell, S.; De Marco, M.; Barnes, K.; Shaw, P.; Ferraiuolo, L.; Blackburn, D.; Mortiboys, H.; Venneri, A. Deficits in mitochondrial spare respiratory capacity contribute to the neuropsychological changes of Alzheimer’s disease. J. Pers. Med. 2020, 10, 32. [Google Scholar] [CrossRef]
- Gandhi, P.; Chen, S.; Wilson-Delfosse, A. Leucine-rich repeat kinase 2 (LRRK2): A key player in the pathogenesis of Parkinson’s disease. J. Neurosci. Res. 2009, 87, 1283–1295. [Google Scholar] [CrossRef]
- Ludtmann, M.; Angelova, P.; Ninkina, N.; Gandhi, S.; Buchman, V.; Abramov, A. Monomeric alpha-synuclein exerts a physiological role on brain ATP synthase. J. Neurosci. 2016, 36, 10510–10521. [Google Scholar] [CrossRef] [PubMed]
- Teves, Y.; Bhargava, V. Parkinson’s disease skin fibroblasts display signature alterations in growth, redox homeostasis, mitochondrial function, and autophagy. Front. Neurosci. 2018, 11, 737. [Google Scholar] [CrossRef] [PubMed]
- Angajala, A.; Lim, S. Diverse roles of mitochondria in immune responses: Novel insights into immuno-metabolism. Front. Immunol. 2018, 9, 1605. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.; Pearce, J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef]
- Carr, L.; Kelman, A.; Wu, G.; Gopaul, R.; Senkevitch, E.; Aghvanyan, A. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 2010, 185, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Lane, N.; Hamaker, M.; Bose, S.; Gouw, A.; Barbi, J. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012, 15, 110–121. [Google Scholar] [CrossRef]
- Hall, S.; Janelidze, S.; Surova, Y.; Hansson, O. Cerebrospinal fluid concentrations of inflammatory markers in Parkinson’s disease and atypical parkinsonian disorders. Sci. Rep. 2018, 8, 13276. [Google Scholar] [CrossRef]
- Magdalinou, N.; Paterson, W.; Schott, J.; Fox, N.; Mummery, C.; Blennow, K.; Bhatia, K.; Morris, H.; Giunti, P.; Warner, T. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1240–1247. [Google Scholar] [CrossRef]
- Dichev, V.; Mehterov, N.; Kazakova, M.; Karalilova, R.; Batalov, A.; Sarafian, V. The lncRNAs/miR-30e/CHI3L1 axis is dysregulated in systemic sclerosis. Biomedicines 2022, 10, 496. [Google Scholar] [CrossRef]
- Villar-Piqué, A.; Schmitz, M.; Hermann, P.; Goebel, S.; Bunck, T.; Varges, D.; Ferrer, I.; Riggert, J.; Llorens, F.; Zerr, I. Plasma YKL-40 in the spectrum of neurodegenerative dementia. J. Neuroinflamm. 2019, 16, 1–5. [Google Scholar] [CrossRef]
- Sanfilippo, C.; Castrogiovanni, P.; Imbesi, R.; Musumeci, G.; Vecchio, M.; Volti, G.L.; Tibullo, D.; Broggi, G.; Caltabiano, R.; Ulivieri, M.; et al. Sex-dependent neuro-deconvolution analysis of Alzheimer’s disease brain transcriptomes according to CHI3L1 expression levels. J. Neuroimmunol. 2022, 373, 577977. [Google Scholar] [CrossRef] [PubMed]
- Wilczyńska, K.; Maciejczyk, M.; Zalewska, A.; Waszkiewicz, N. Serum amyloid biomarkers, tau protein and YKL-40 utility in detection, differential diagnosing, and monitoring of dementia. Front. Psychiatry 2021, 12, 725511. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Hertze, J.; Lautner, R.; Zetterberg, H.; Nägga, K.; Höglund, K.; Basun, H.; Annas, P.; Lannfelt, L.; Andreasen, N.; et al. Microglial markers are elevated in the prodromal phase of Alzheimer’s disease and vascular dementia. J. Alzheimer’s Dis. 2013, 33, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Bojesen, E.; Johansen, S.; Nordestgaard, G. Plasma YKL-40 levels in healthy subjects from the general population. Clin. Chim. Acta 2011, 412, 709–712. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gevezova, M.; Kazakova, M.; Trenova, A.; Sarafian, V. YKL-40 and the Cellular Metabolic Profile in Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 16297. https://doi.org/10.3390/ijms242216297
Gevezova M, Kazakova M, Trenova A, Sarafian V. YKL-40 and the Cellular Metabolic Profile in Parkinson’s Disease. International Journal of Molecular Sciences. 2023; 24(22):16297. https://doi.org/10.3390/ijms242216297
Chicago/Turabian StyleGevezova, Maria, Maria Kazakova, Anastasia Trenova, and Victoria Sarafian. 2023. "YKL-40 and the Cellular Metabolic Profile in Parkinson’s Disease" International Journal of Molecular Sciences 24, no. 22: 16297. https://doi.org/10.3390/ijms242216297
APA StyleGevezova, M., Kazakova, M., Trenova, A., & Sarafian, V. (2023). YKL-40 and the Cellular Metabolic Profile in Parkinson’s Disease. International Journal of Molecular Sciences, 24(22), 16297. https://doi.org/10.3390/ijms242216297




