Association between Decreased SGK1 and Increased Intestinal α-Synuclein in an MPTP Mouse Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
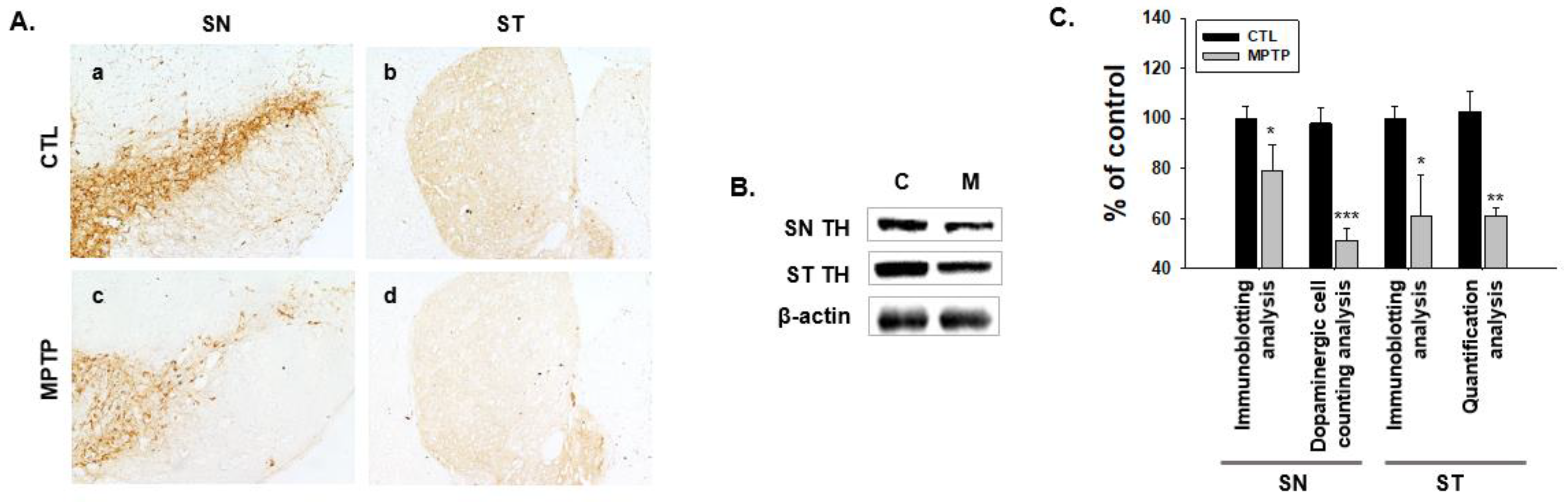
2.1. MPTP-Induced PD Model
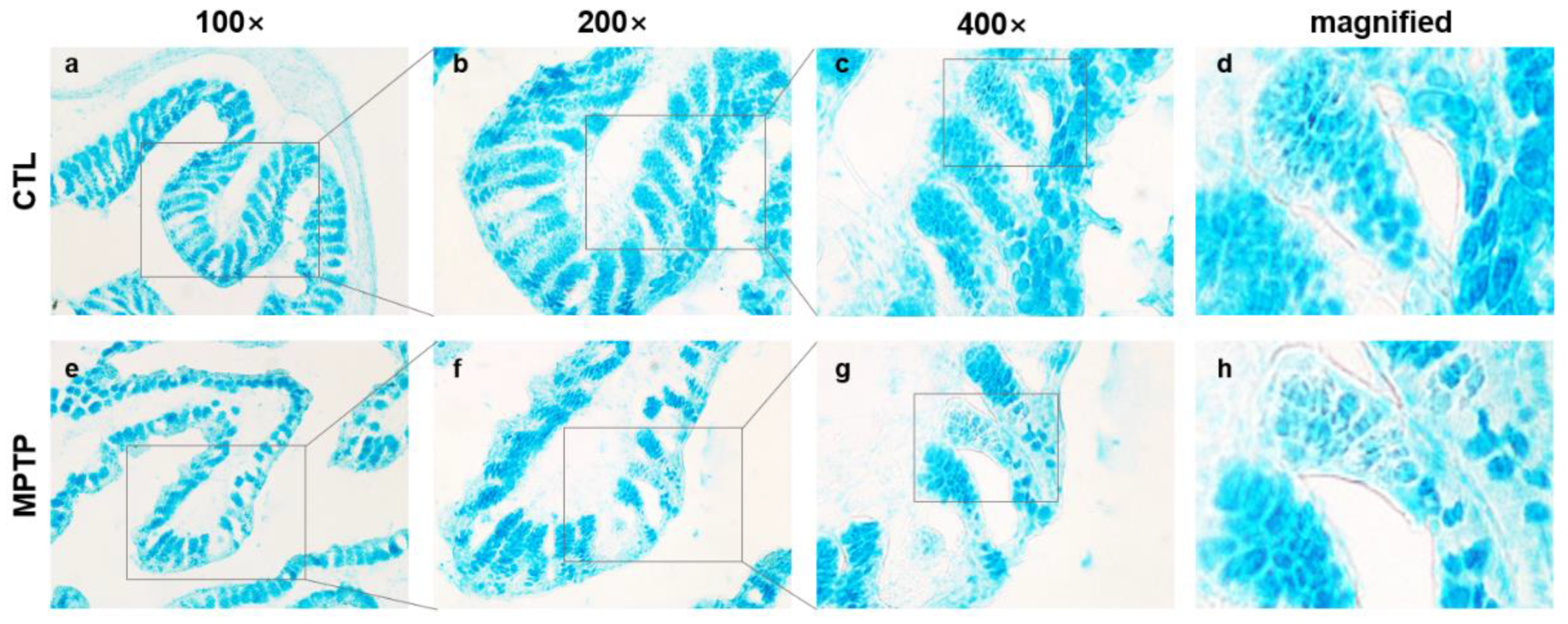
2.2. Morphological Changes in the Colon in the MPTP-Induced PD Model
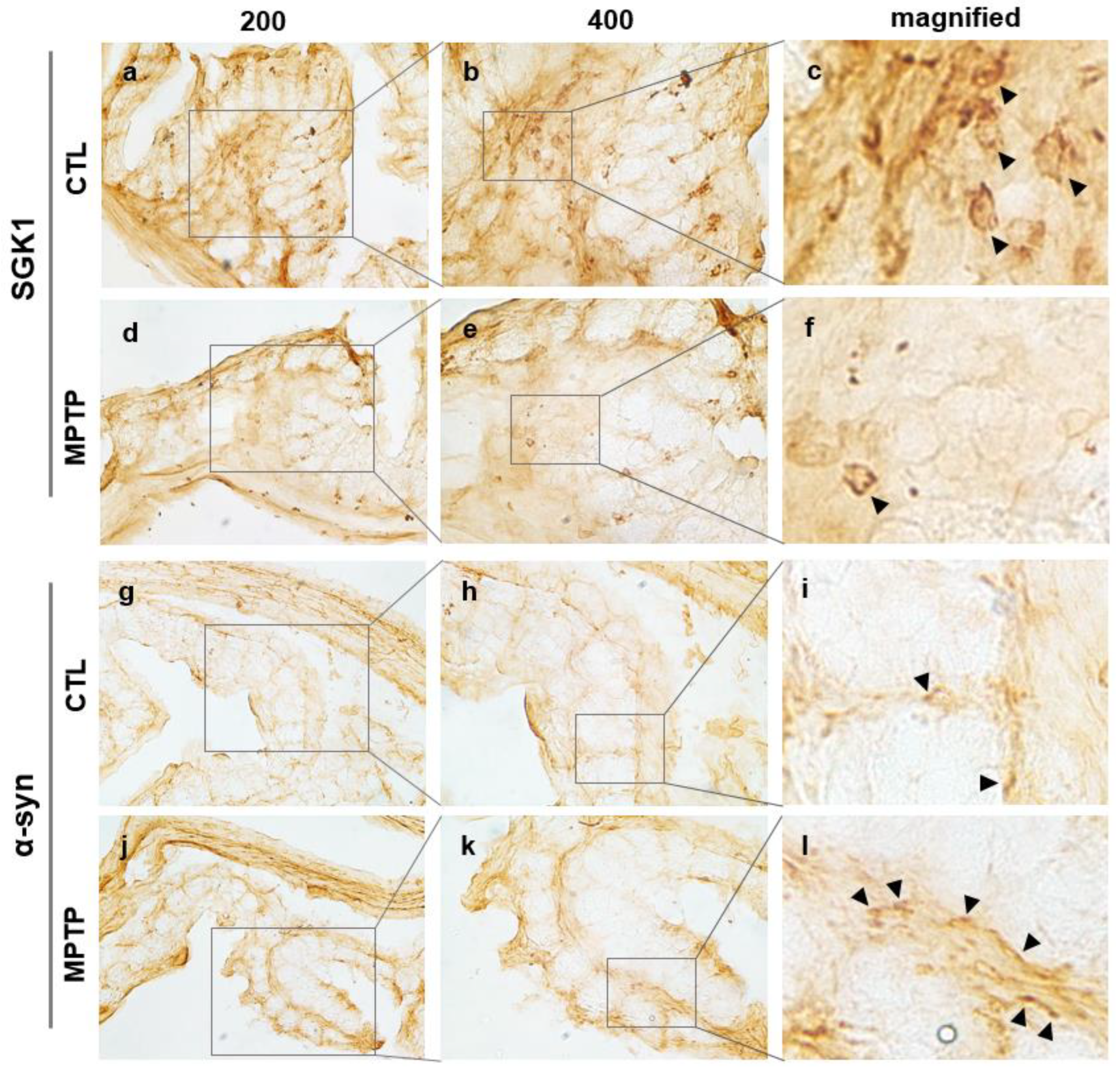
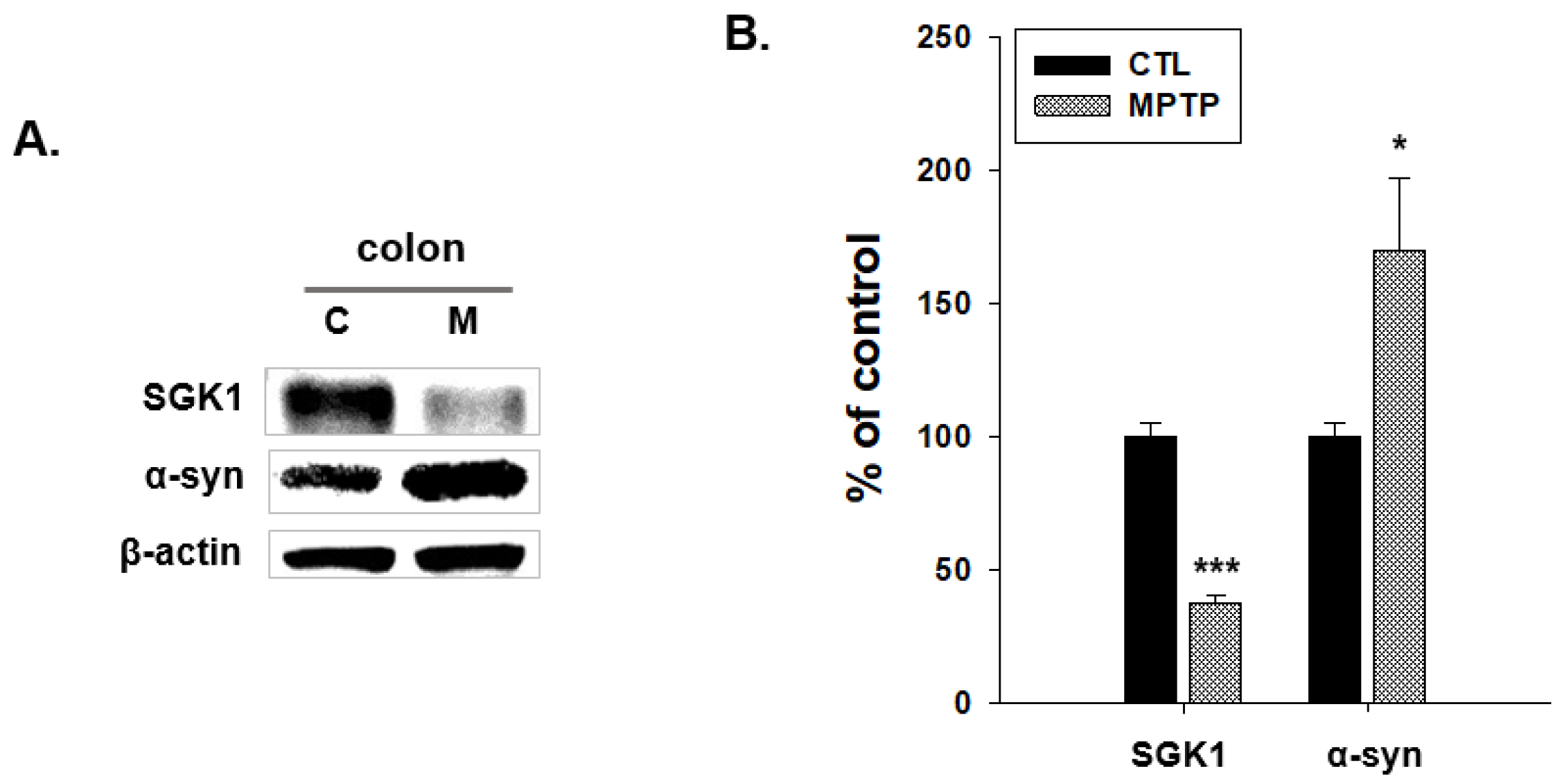
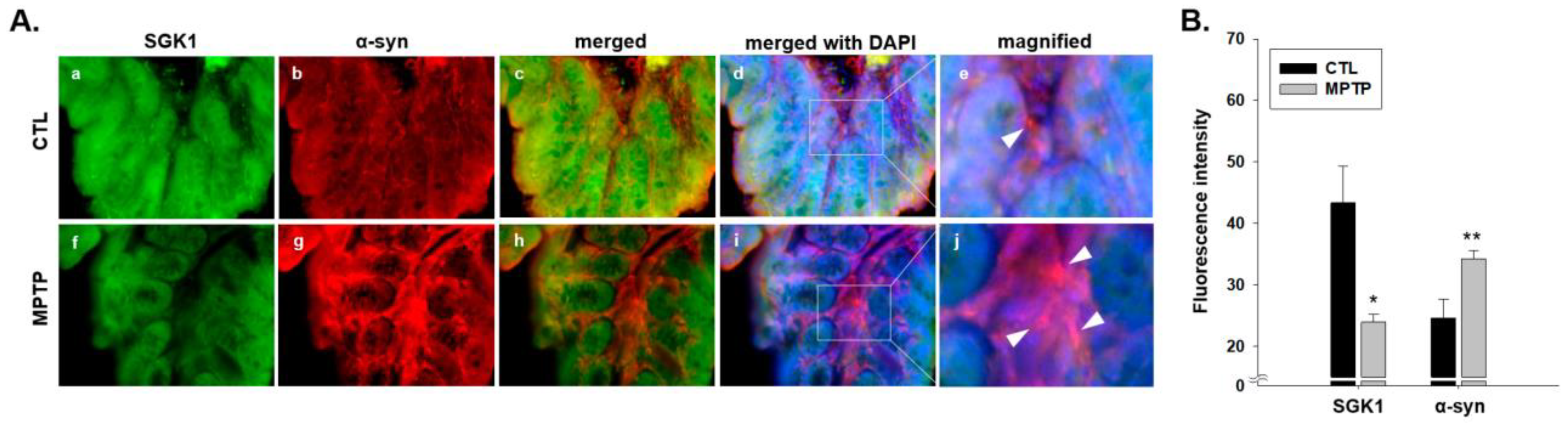
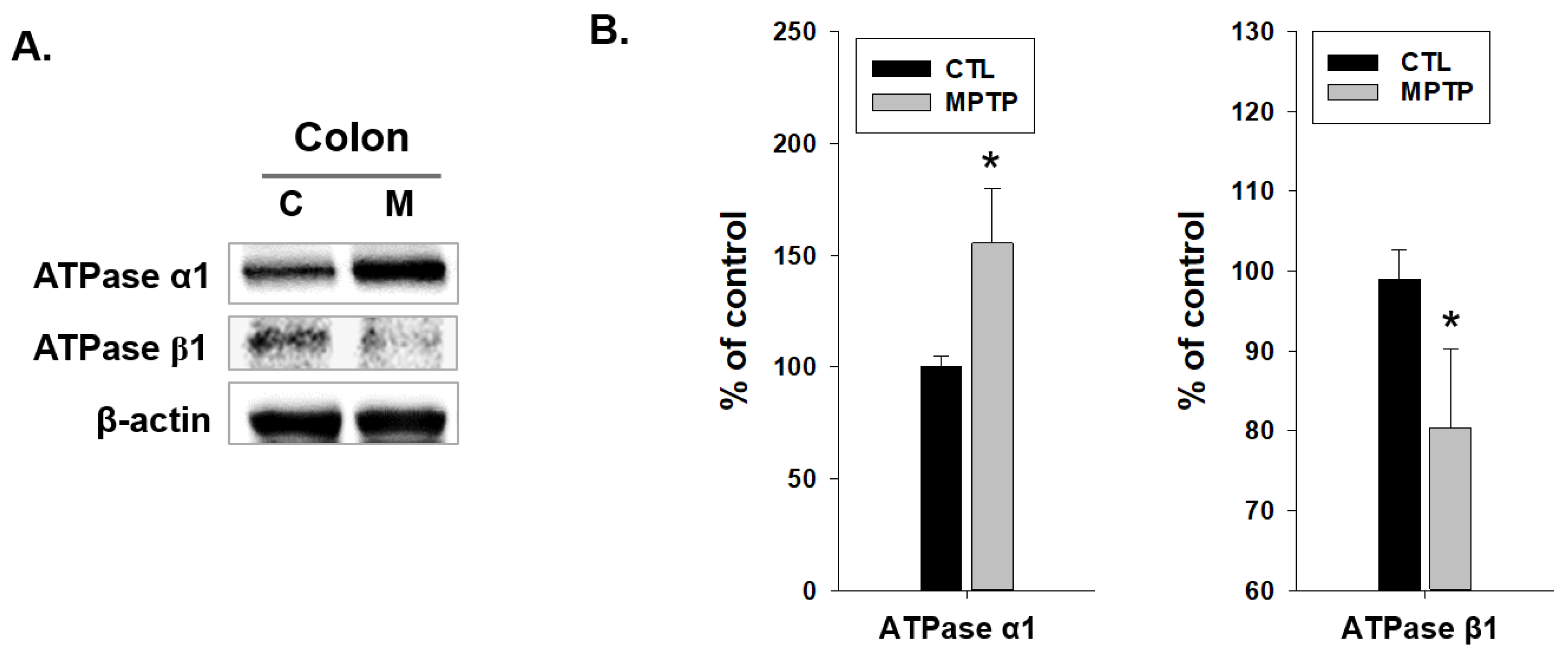
2.3. SGK1 and α-Syn Expression in the Colon of MPTP-Induced PD Model
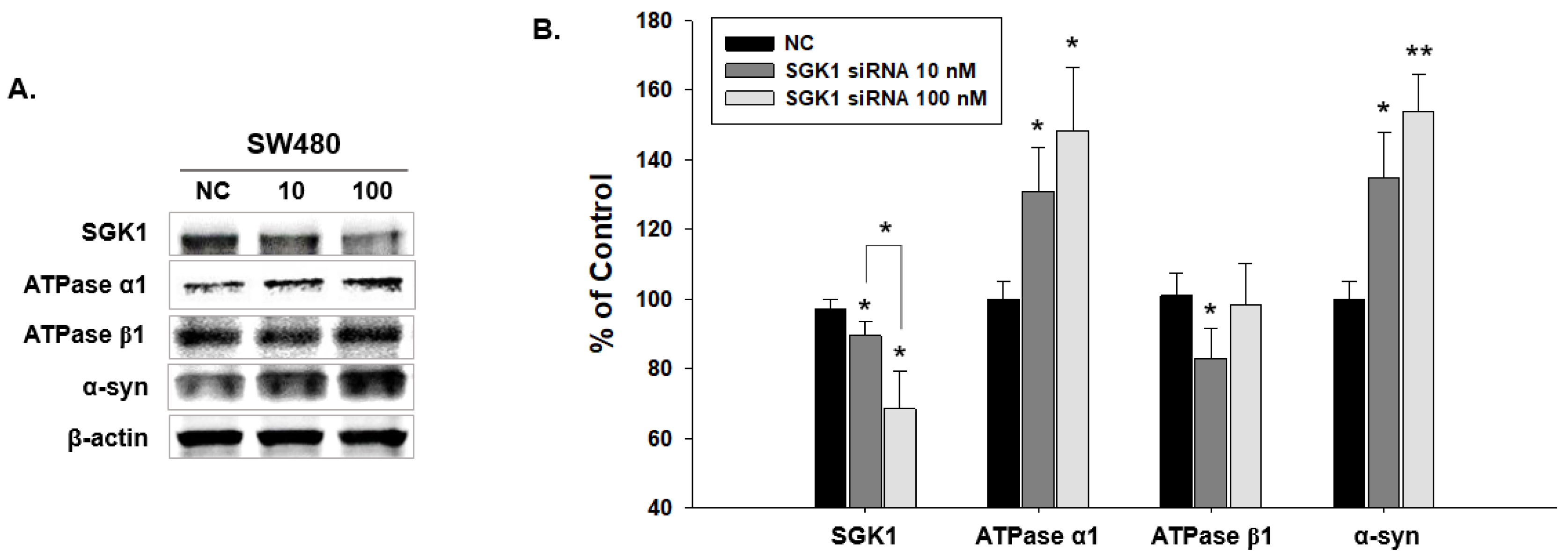
2.4. Expressional Changes in Factors Related to SGK1 Following SW480 Knockdown by SGK1 siRNA
3. Discussion
4. Materials and Methods
4.1. MPTP Model of PD
4.2. Immunohistochemistry
4.3. Alcian Blue Staining
4.4. Western Blotting
4.5. Immunofluorescence Microscopy
4.6. Cell Lines and Cultures
4.7. siRNA Transfection
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tolosa, E.; Wenning, G.; Poewe, W. The diagnosis of Parkinson’s disease. Lancet Neurol. 2006, 5, 75–86. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Jost, W.H.; Schimrigk, K. Constipation in Parkinson’s disease. Klin. Wochenschr. 1991, 69, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H. Gastrointestinal motility problems in patients with Parkinson’s disease. Effects of antiparkinsonian treatment and guidelines for management. Drugs Aging 1997, 10, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003, 2, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Noorian, A.R.; Taylor, G.; Anitha, M.; Bernhard, D.; Srinivasan, S.; Greene, J.G. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Exp. Neurol. 2007, 207, 4–12. [Google Scholar] [CrossRef]
- Sakakibara, R.; Odaka, T.; Uchiyama, T.; Asahina, M.; Yamaguchi, K.; Yamaguchi, T.; Yamanishi, T.; Hattori, T. Colonic transit time and rectoanal videomanometry in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 268–272. [Google Scholar] [CrossRef]
- Devos, D.; Lebouvier, T.; Lardeux, B.; Biraud, M.; Rouaud, T.; Pouclet, H.; Coron, E.; Bruley des Varannes, S.; Naveilhan, P.; Nguyen, J.M.; et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 2013, 50, 42–48. [Google Scholar] [CrossRef]
- Fasano, A.; Visanji, N.P.; Liu, L.W.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef]
- Shannon, K.M.; Keshavarzian, A.; Dodiya, H.B.; Jakate, S.; Kordower, J.H. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov. Disord. 2012, 27, 716–719. [Google Scholar] [CrossRef]
- Pouclet, H.; Lebouvier, T.; Coron, E.; des Varannes, S.B.; Rouaud, T.; Roy, M.; Neunlist, M.; Derkinderen, P. A comparison between rectal and colonic biopsies to detect Lewy pathology in Parkinson’s disease. Neurobiol. Dis. 2012, 45, 305–309. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rub, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Visanji, N.P.; Marras, C.; Hazrati, L.N.; Liu, L.W.; Lang, A.E. Alimentary, my dear Watson? The challenges of enteric alpha-synuclein as a Parkinson’s disease biomarker. Mov. Disord. 2014, 29, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.G.; Corbille, A.G.; Letournel, F.; Kordower, J.H.; Kremer, T.; Munoz, D.G.; Intorcia, A.; Hentz, J.; Adler, C.H.; Sue, L.I.; et al. Multicenter Assessment of Immunohistochemical Methods for Pathological Alpha-Synuclein in Sigmoid Colon of Autopsied Parkinson’s Disease and Control Subjects. J. Parkinson’s Dis. 2016, 6, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.; Turkalp, Z.T.; Munoz, D.G. Enteric alpha-synuclein expression is increased in Parkinson’s disease but not Alzheimer’s disease. Mov. Disord. 2013, 28, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zuo, X.; Li, Y.; Zhang, C.; Zhou, M.; Zhang, Y.A.; Ueda, K.; Chan, P. Inhibition of tyrosine hydroxylase expression in alpha-synuclein-transfected dopaminergic neuronal cells. Neurosci. Lett. 2004, 367, 34–39. [Google Scholar] [CrossRef]
- Beekes, M. The Neural Gut-Brain Axis of Pathological Protein Aggregation in Parkinson’s Disease and Its Counterpart in Peroral Prion Infections. Viruses 2021, 13, 1394. [Google Scholar] [CrossRef]
- Cockfield, J.A.; Schafer, Z.T. SGK1-regulated metabolism: Key for the survival of cells detached from the extracellular matrix. Mol. Cell Oncol. 2021, 8, 1976583. [Google Scholar] [CrossRef]
- Mercado, E.; Paniagua, N.; Sanchez-Robles, E.M.; Giron, R.; Alvarez de la Rosa, D.; Giraldez, T.; Goicoechea, C. SGK1.1 isoform is involved in nociceptive modulation, offering a protective effect against noxious cold stimulus in a sexually dimorphic manner. Pharmacol. Biochem. Behav. 2022, 212, 173302. [Google Scholar] [CrossRef]
- Park, J.C.; Jeon, Y.J.; Jang, Y.S.; Cho, J.; Choi, D.H.; Han, J.S. SGK1 knockdown in the medial prefrontal cortex reduces resistance to stress-induced memory impairment. Eur. Neuropsychopharmacol. 2021, 45, 29–34. [Google Scholar] [CrossRef]
- Lang, F.; Strutz-Seebohm, N.; Seebohm, G.; Lang, U.E. Significance of SGK1 in the regulation of neuronal function. J. Physiol. 2010, 588, 3349–3354. [Google Scholar] [CrossRef] [PubMed]
- Schoenebeck, B.; Bader, V.; Zhu, X.R.; Schmitz, B.; Lubbert, H.; Stichel, C.C. Sgk1, a cell survival response in neurodegenerative diseases. Mol. Cell Neurosci. 2005, 30, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Hollister, R.D.; Page, K.J.; Hyman, B.T. Distribution of the messenger RNA for the extracellularly regulated kinases 1, 2 and 3 in rat brain: Effects of excitotoxic hippocampal lesions. Neuroscience 1997, 79, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Cohen, P. Regulation and physiological roles of serum- and glucocorticoid-induced protein kinase isoforms. Sci. STKE 2001, 2001, re17. [Google Scholar] [CrossRef]
- Rangone, H.; Poizat, G.; Troncoso, J.; Ross, C.A.; MacDonald, M.E.; Saudou, F.; Humbert, S. The serum- and glucocorticoid-induced kinase SGK inhibits mutant huntingtin-induced toxicity by phosphorylating serine 421 of huntingtin. Eur. J. Neurosci. 2004, 19, 273–279. [Google Scholar] [CrossRef]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimer’s Dis. 2014, 42 (Suppl. S3), S125–S152. [Google Scholar] [CrossRef]
- Heo, E.J.; Lee, Y.; Hyung Seo, M.; Yeo, S. Association between SGK1 and alpha-synuclein in skeletal muscle in an MPTP-induced Parkinson’s disease model. Neurosci. Lett. 2023, 814, 137464. [Google Scholar] [CrossRef]
- Yeo, S.; Sung, B.; Hong, Y.M.; van den Noort, M.; Bosch, P.; Lee, S.H.; Song, J.; Park, S.K.; Lim, S. Decreased expression of serum- and glucocorticoid-inducible kinase 1 (SGK1) promotes alpha-synuclein increase related with down-regulation of dopaminergic cell in the Substantia Nigra of chronic MPTP-induced Parkinsonism mice and in SH-SY5Y cells. Gene 2018, 661, 189–195. [Google Scholar] [CrossRef]
- Alvarez de la Rosa, D.; Gimenez, I.; Forbush, B.; Canessa, C.M. SGK1 activates Na+-K+-ATPase in amphibian renal epithelial cells. Am. J. Physiol. Cell Physiol. 2006, 290, C492–C498. [Google Scholar] [CrossRef]
- Henke, G.; Setiawan, I.; Bohmer, C.; Lang, F. Activation of Na+/K+-ATPase by the serum and glucocorticoid-dependent kinase isoforms. Kidney Blood Press. Res. 2002, 25, 370–374. [Google Scholar] [CrossRef]
- Koumangoye, R.; Omer, S.; Kabeer, M.H.; Delpire, E. Novel Human NKCC1 Mutations Cause Defects in Goblet Cell Mucus Secretion and Chronic Inflammation. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Zecevic, M.; Heitzmann, D.; Camargo, S.M.; Verrey, F. SGK1 increases Na,K-ATP cell-surface expression and function in Xenopus laevis oocytes. Pflug. Arch. 2004, 448, 29–35. [Google Scholar] [CrossRef]
- Markossian, S.; Kreydiyyeh, S.I. TNF-alpha down-regulates the Na+-K+ ATPase and the Na+-K+-2Cl-cotransporter in the rat colon via PGE2. Cytokine 2005, 30, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Allgayer, H.; Brown, L.; Kruis, W.; Erdmann, E.; Paumgartner, G. Inhibition of human colonic (Na+ + K+)-ATPase by arachidonic and linoleic acid. Naunyn Schmiedeberg’s Arch. Pharmacol. 1986, 332, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wan, Q. Research advances on the role of Na(+)-K(+)-ATPase regulation in pulmonary edema clearance of acute respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2021, 33, 1011–1016. [Google Scholar] [PubMed]
- Geering, K.; Beggah, A.; Good, P.; Girardet, S.; Roy, S.; Schaer, D.; Jaunin, P. Oligomerization and maturation of Na,K-ATPase: Functional interaction of the cytoplasmic NH2 terminus of the beta subunit with the alpha subunit. J. Cell Biol. 1996, 133, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. The functional role of beta subunits in oligomeric P-type ATPases. J. Bioenerg. Biomembr. 2001, 33, 425–438. [Google Scholar] [CrossRef]
- Ashmore, L.J.; Hrizo, S.L.; Paul, S.M.; Van Voorhies, W.A.; Beitel, G.J.; Palladino, M.J. Novel mutations affecting the Na, K ATPase alpha model complex neurological diseases and implicate the sodium pump in increased longevity. Hum. Genet. 2009, 126, 431–447. [Google Scholar] [CrossRef]
- Holm, T.H.; Lykke-Hartmann, K. Insights into the Pathology of the alpha3 Na(+)/K(+)-ATPase Ion Pump in Neurological Disorders; Lessons from Animal Models. Front. Physiol. 2016, 7, 209. [Google Scholar] [CrossRef]
- Lang, F.; Stournaras, C.; Alesutan, I. Regulation of transport across cell membranes by the serum- and glucocorticoid-inducible kinase SGK1. Mol. Membr. Biol. 2014, 31, 29–36. [Google Scholar] [CrossRef]
- Setiawan, I.; Henke, G.; Feng, Y.; Bohmer, C.; Vasilets, L.A.; Schwarz, W.; Lang, F. Stimulation of Xenopus oocyte Na(+),K(+)ATPase by the serum and glucocorticoid-dependent kinase sgk1. Pflug. Arch. 2002, 444, 426–431. [Google Scholar] [CrossRef]
- Kwon, O.C.; Song, J.J.; Yang, Y.; Kim, S.H.; Kim, J.Y.; Seok, M.J.; Hwang, I.; Yu, J.W.; Karmacharya, J.; Maeng, H.J.; et al. SGK1 inhibition in glia ameliorates pathologies and symptoms in Parkinson disease animal models. EMBO Mol. Med. 2021, 13, e13076. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Shumilina, E. Regulation of ion channels by the serum- and glucocorticoid-inducible kinase SGK1. FASEB J. 2013, 27, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Howard, S.; LoGrasso, P.V. Serum- and Glucocorticoid-Inducible Kinase 1 Confers Protection in Cell-Based and in In Vivo Neurotoxin Models via the c-Jun N-Terminal Kinase Signaling Pathway. Mol. Cell Biol. 2015, 35, 1992–2006. [Google Scholar] [CrossRef] [PubMed]
- Stichel, C.C.; Schoenebeck, B.; Foguet, M.; Siebertz, B.; Bader, V.; Zhu, X.R.; Lubbert, H. sgk1, a member of an RNA cluster associated with cell death in a model of Parkinson’s disease. Eur. J. Neurosci. 2005, 21, 301–316. [Google Scholar] [CrossRef]
- Chen, X.; Tagliaferro, P.; Kareva, T.; Yarygina, O.; Kholodilov, N.; Burke, R.E. Neurotrophic effects of serum- and glucocorticoid-inducible kinase on adult murine mesencephalic dopamine neurons. J. Neurosci. 2012, 32, 11299–11308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.H.; Kwon, D.; Kim, S.-H.; Yeo, S. Association between Decreased SGK1 and Increased Intestinal α-Synuclein in an MPTP Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 16408. https://doi.org/10.3390/ijms242216408
Seo MH, Kwon D, Kim S-H, Yeo S. Association between Decreased SGK1 and Increased Intestinal α-Synuclein in an MPTP Mouse Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2023; 24(22):16408. https://doi.org/10.3390/ijms242216408
Chicago/Turabian StyleSeo, Min Hyung, Dasom Kwon, Soo-Hwan Kim, and Sujung Yeo. 2023. "Association between Decreased SGK1 and Increased Intestinal α-Synuclein in an MPTP Mouse Model of Parkinson’s Disease" International Journal of Molecular Sciences 24, no. 22: 16408. https://doi.org/10.3390/ijms242216408
APA StyleSeo, M. H., Kwon, D., Kim, S.-H., & Yeo, S. (2023). Association between Decreased SGK1 and Increased Intestinal α-Synuclein in an MPTP Mouse Model of Parkinson’s Disease. International Journal of Molecular Sciences, 24(22), 16408. https://doi.org/10.3390/ijms242216408





