The Relationships between Intestinal Permeability and Target Antibodies for a Spectrum of Autoimmune Diseases
Abstract
1. Introduction
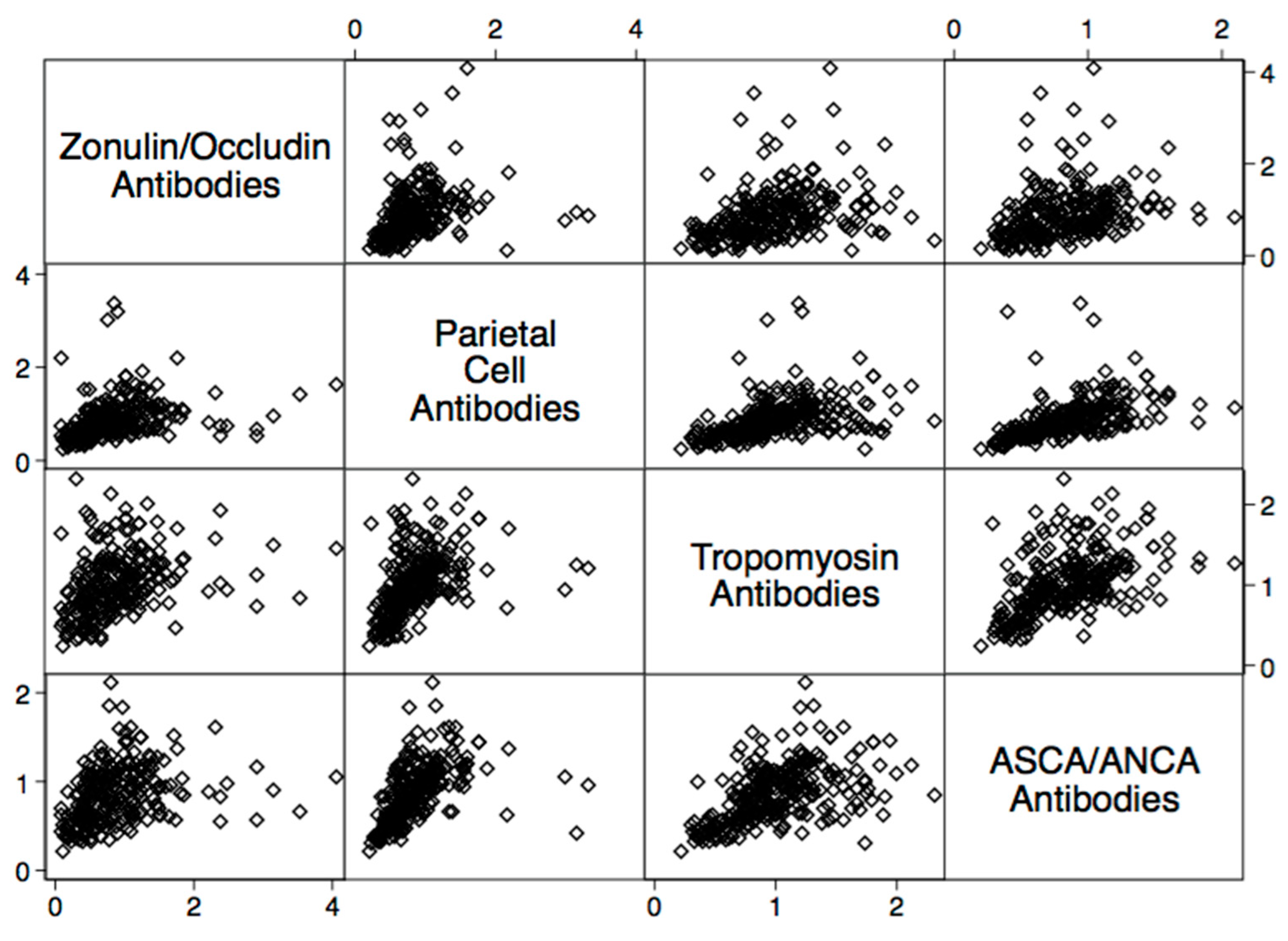
2. Results
3. Discussion
4. Methods and Materials
4.1. Data Set
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lerner, A.; Jeremias, P.; Matthias, T. The world incidence and prevalence of autoimmune disease is increasing. Int. J. Celiac Dis. 2015, 3, 151–155. [Google Scholar] [CrossRef]
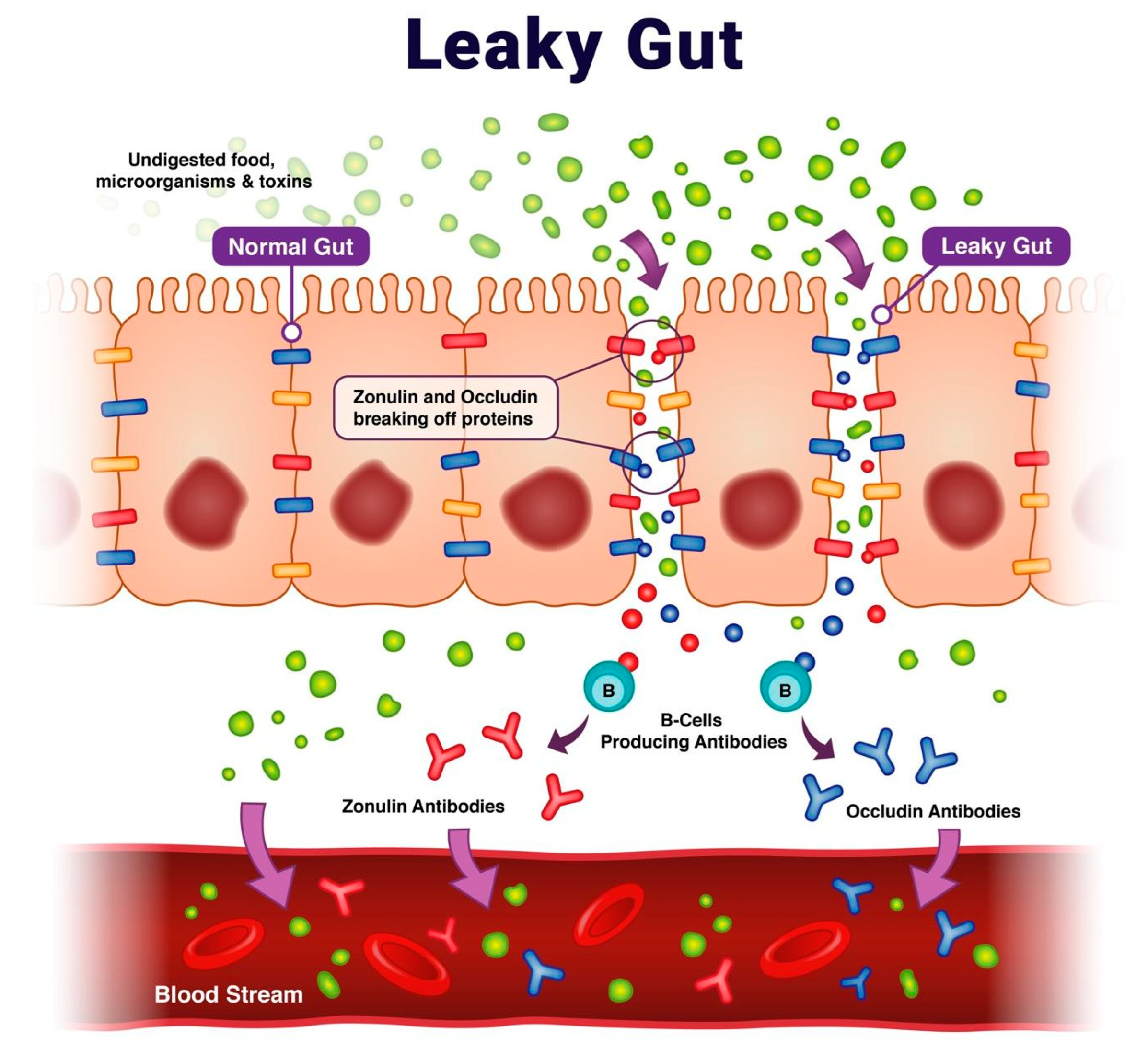
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky gut as a danger signal for autoimmune diseases. Front. Immunol. 2017, 23, 598. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, E.; Shimokawa, C.; Steimle, A.; Desai, M.S.; Ohno, H. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat. Rev. Immunol. 2023, 23, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Shea-Donohue, T. Mechanisms of disease: The role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 416–422. [Google Scholar] [CrossRef]
- Charoensappakit, A.; Sae-Khow, K.; Leelahavanichkul, A. gut barrier damage and gut translocation of pathogen molecules in lupus, an impact of innate immunity (macrophages and neutrophils) in autoimmune disease. Int. J. Mol. Sci. 2022, 23, 8223. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Leaky gut and autoimmune diseases. Clin. Rev. Allergy Immunol. 2012, 42, 71–78. [Google Scholar] [CrossRef]
- Paray, B.A.; Albeshr, M.F.; Jan, A.T.; Rather, I.A. Leaky gut and autoimmunity: An intricate balance in individuals health and the diseased state. Int. J. Mol. Sci. 2020, 21, 9770. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef]
- Simeonova, D.; Ivanovska, M.; Murdjeva, M.; Carvalho, A.F.; Maes, M. Recognizing the leaky gut as a trans-diagnostic target for neuroimmune disorders using clinical chemistry and molecular immunology assays. Curr. Top. Med. Chem. 2018, 18, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Vojdani, E.; Kharrazian, D. Fluctuation of zonulin levels in blood vs stability of antibodies. World J. Gastroenterol. 2017, 23, 5669–5679. [Google Scholar] [CrossRef]
- Skardelly, M.; Armbruster, F.P.; Meixensberger, J.; Hilbig, H. Expression of Zonulin, c-kit, and glial fibrillary acidic protein in human gliomas. Transl. Oncol. 2009, 2, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Coránguez, M.; Segovia, J.; López-Ornelas, A.; Puerta-Guardo, H.; Ludert, J.; Chávez, B.; Meraz-Cruz, N.; González-Mariscal, L. Transmigration of neural stem cells across the blood brain barrier induced by glioma cells. PLoS ONE 2013, 8, e60655. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef] [PubMed]
- Buscarinu, M.C.; Romano, S.; Mechelli, R.; Pizzolato Umeton, R.; Ferraldeschi, M.; Fornasiero, A.; Reniè, R.; Cerasoli, B.; Morena, E.; Romano, C.; et al. Intestinal permeability in relapsing-remitting multiple sclerosis. Neurotherapeutics 2018, 15, 68–74. [Google Scholar] [CrossRef]
- Nouri, M.; Bredberg, A.; Weström, B.; Lavasani, S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS ONE 2014, 9, e106335. [Google Scholar] [CrossRef]
- Vaahtovuo, J.; Munukka, E.; Korkeamäki, M.; Luukkainen, R.; Toivanen, P. Fecal microbiota in early rheumatoid arthritis. J. Rheumatol. 2008, 35, 1500–1505. [Google Scholar]
- Azzouz, D.F.; Silverman, G.J. Is gut microbial LPS a potential trigger of juvenile idiopathic arthritis? J. Rheumatol. 2017, 44, 1569–1571. [Google Scholar] [CrossRef]
- Taneja, V. Arthritis susceptibility and the gut microbiome. FEBS Lett. 2014, 588, 4244–4249. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Rosado, M.M.; D’Amelio, R. Infectious agents and inflammation: The role of microbiota in autoimmune arthritis. Front. Microbiol. 2018, 8, 2696. [Google Scholar] [CrossRef]
- Pisetsky, D.S. How the gut inflames the joints. Ann. Rheum. Dis. 2018, 77, 634–635. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Guss, J.D.; Luna, M.; Goldring, S.R. Links between the microbiome and bone. J. Bone Miner. Res. 2016, 31, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.R.; Ward, W.E.; Comelli, E.M. Gut microbiota-bone axis. Crit. Rev. Food Sci. Nutr. 2017, 57, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Carratù, R.; Secondulfo, M.; de Magistris, L.; Iafusco, D.; Urio, A.; Carbone, M.G.; Pontoni, G.; Cartenì, M.; Prisco, F. Altered intestinal permeability to mannitol in diabetes mellitus type I. J. Pediatr. Gastroenterol. Nutr. 1999, 28, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Kuitunen, M.; Saukkonen, T.; Ilonen, J.; Akerblom, H.K.; Savilahti, E. Intestinal permeability to mannitol and lactulose in children with type 1 diabetes with the HLA-DQB1*02 allele. Autoimmunity 2002, 35, 365–368. [Google Scholar] [CrossRef]
- Sapone, A.; de Magistris, L.; Pietzak, M.; Clemente, M.G.; Tripathi, A.; Cucca, F.; Lampis, R.; Kryszak, D.; Cartenì, M.; Generoso, M.; et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006, 55, 1443–1449. [Google Scholar] [CrossRef]
- Secondulfo, M.; Iafusco, D.; Carratù, R.; deMagistris, L.; Sapone, A.; Generoso, M.; Mezzogiomo, A.; Sasso, F.C.; Cartenì, M.; De Rosa, R.; et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig. Liver Dis. 2004, 36, 35–45. [Google Scholar] [CrossRef]
- Terjung, B.; Spengler, U. Atypical p-ANCA in PSC and AIH: A hint toward a “leaky gut”? Clin. Rev. Allergy Immunol. 2009, 36, 40–51. [Google Scholar] [CrossRef]
- Lin, R.; Zhou, L.; Zhang, J.; Wang, B. Abnormal intestinal permeability and microbiota in patients with autoimmune hepatitis. Int. J. Clin. Exp. Pathol. 2015, 8, 5153–5160. [Google Scholar]
- Kobayashi, T.; Iwaki, M.; Nakajima, A.; Nogami, A.; Yoneda, M. Current research on the pathogenesis of NAFLD/NASH and the gut-liver axis: Gut microbiota, dysbiosis, and leaky-gut syndrome. Int. J. Mol. Sci. 2022, 23, 11689. [Google Scholar] [CrossRef]
- Fugger, L.; Jensen, L.T.; Rossjohn, J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell 2020, 181, 63–80. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in leaky gut syndrome: Intestinal dysbiosis and autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Chang, S.H.; Ko, Y.F.; Hwang, T.L.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 2022, 33, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Vella, A. What to do about the leaky gut. Gut 2022, 71, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Christovich, A.; Luo, X.M. Gut microbiota, leaky gut, and autoimmune diseases. Front. Immunol. 2022, 13, 946248. [Google Scholar] [CrossRef]
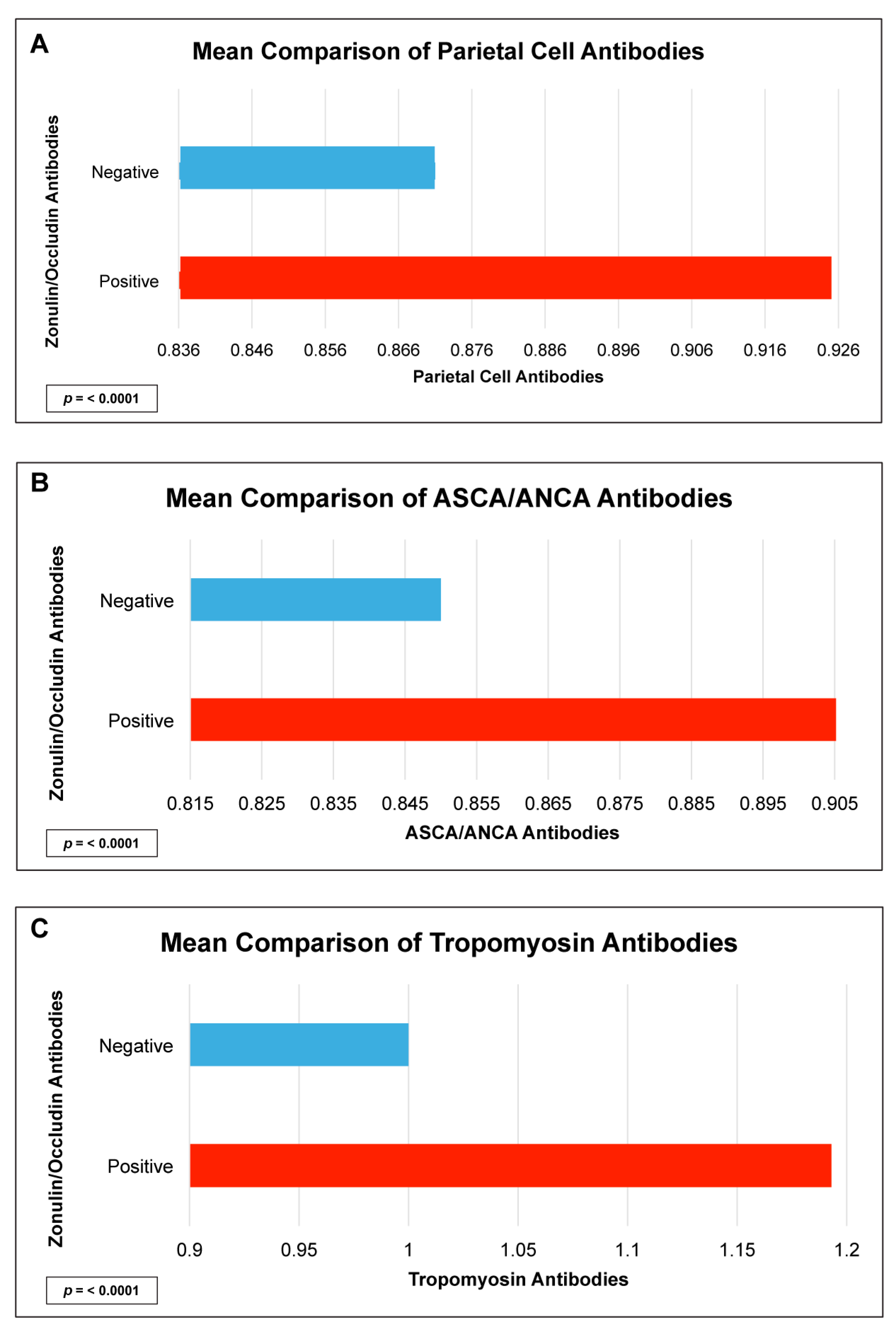

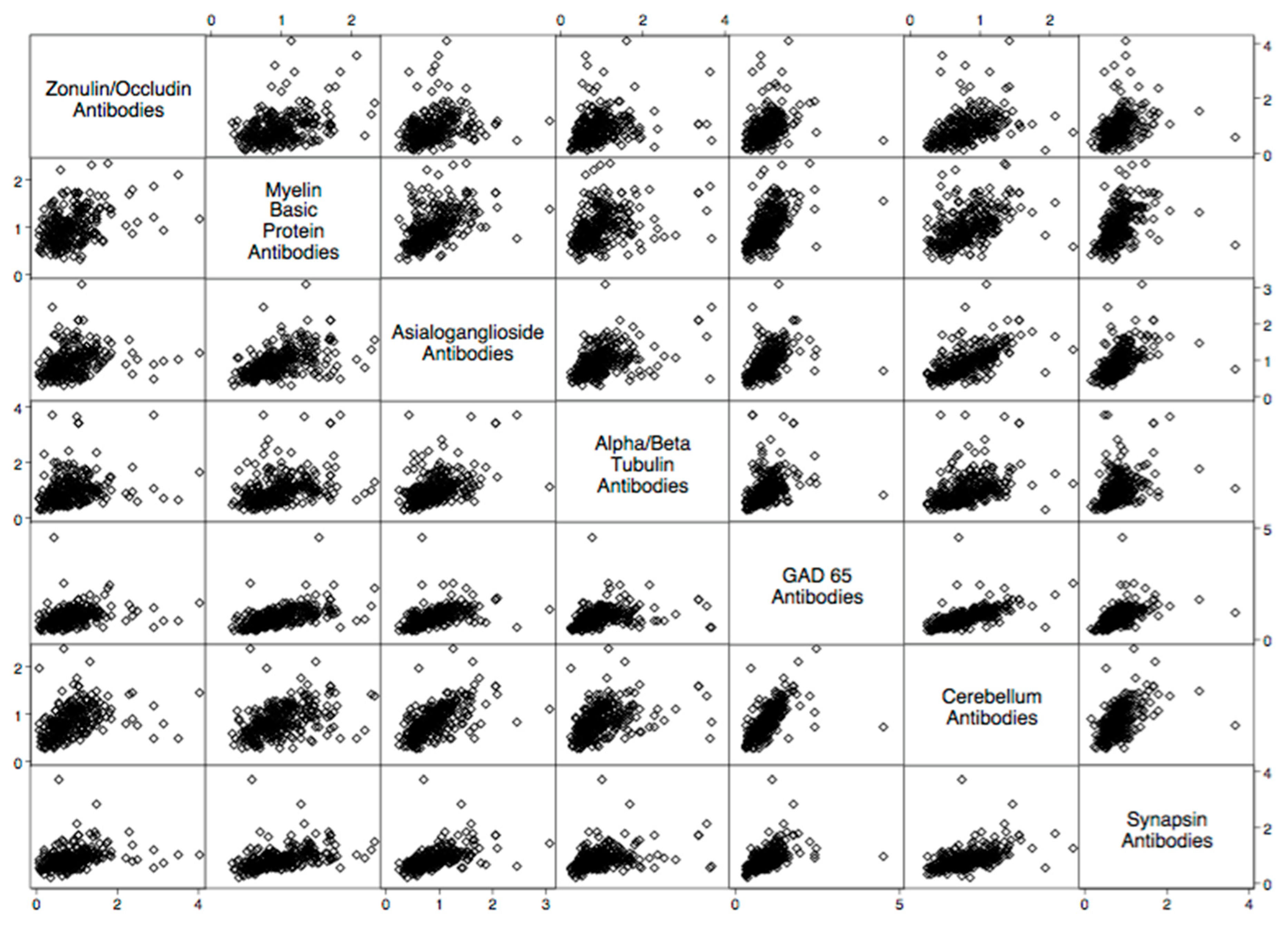
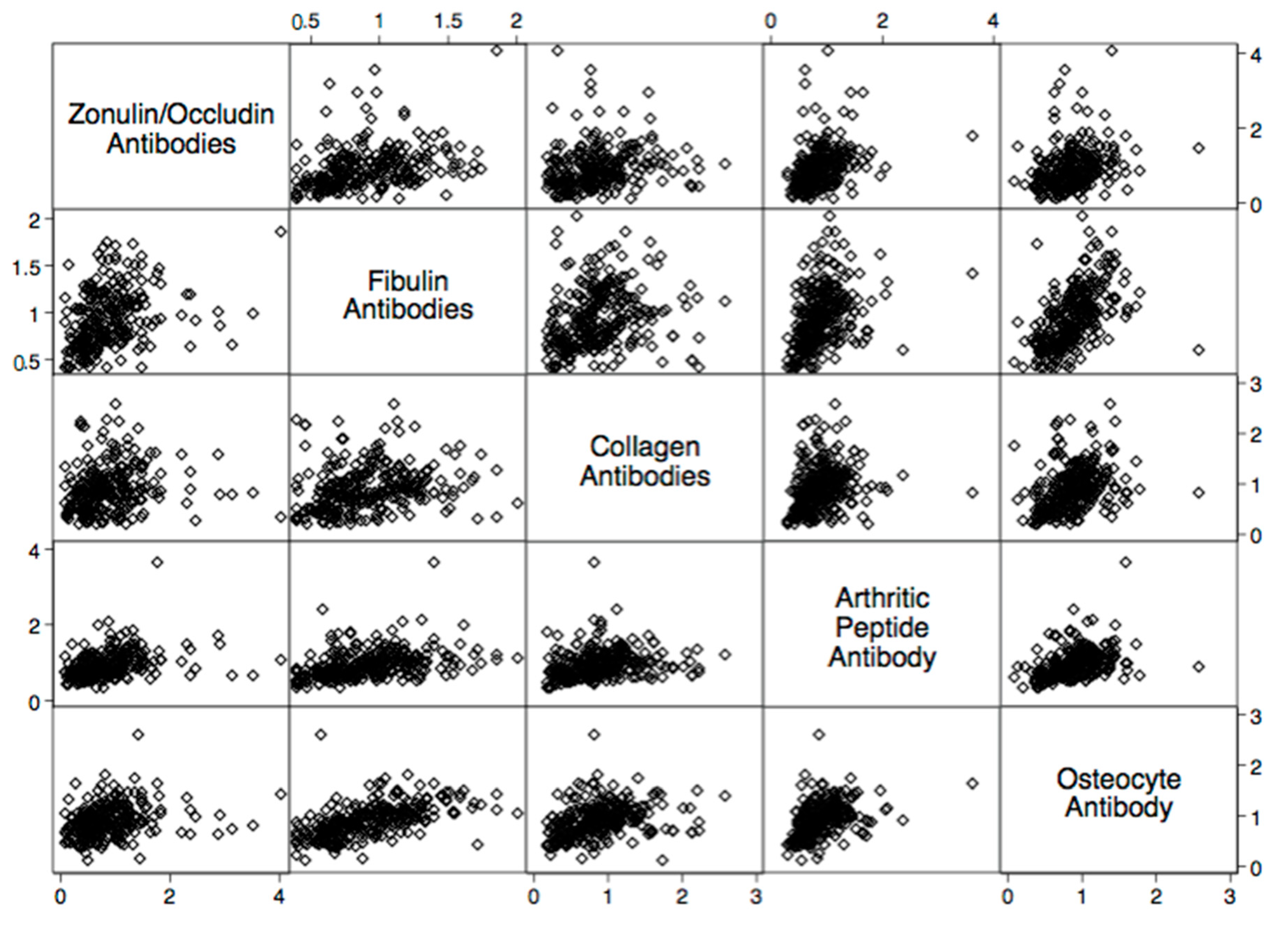
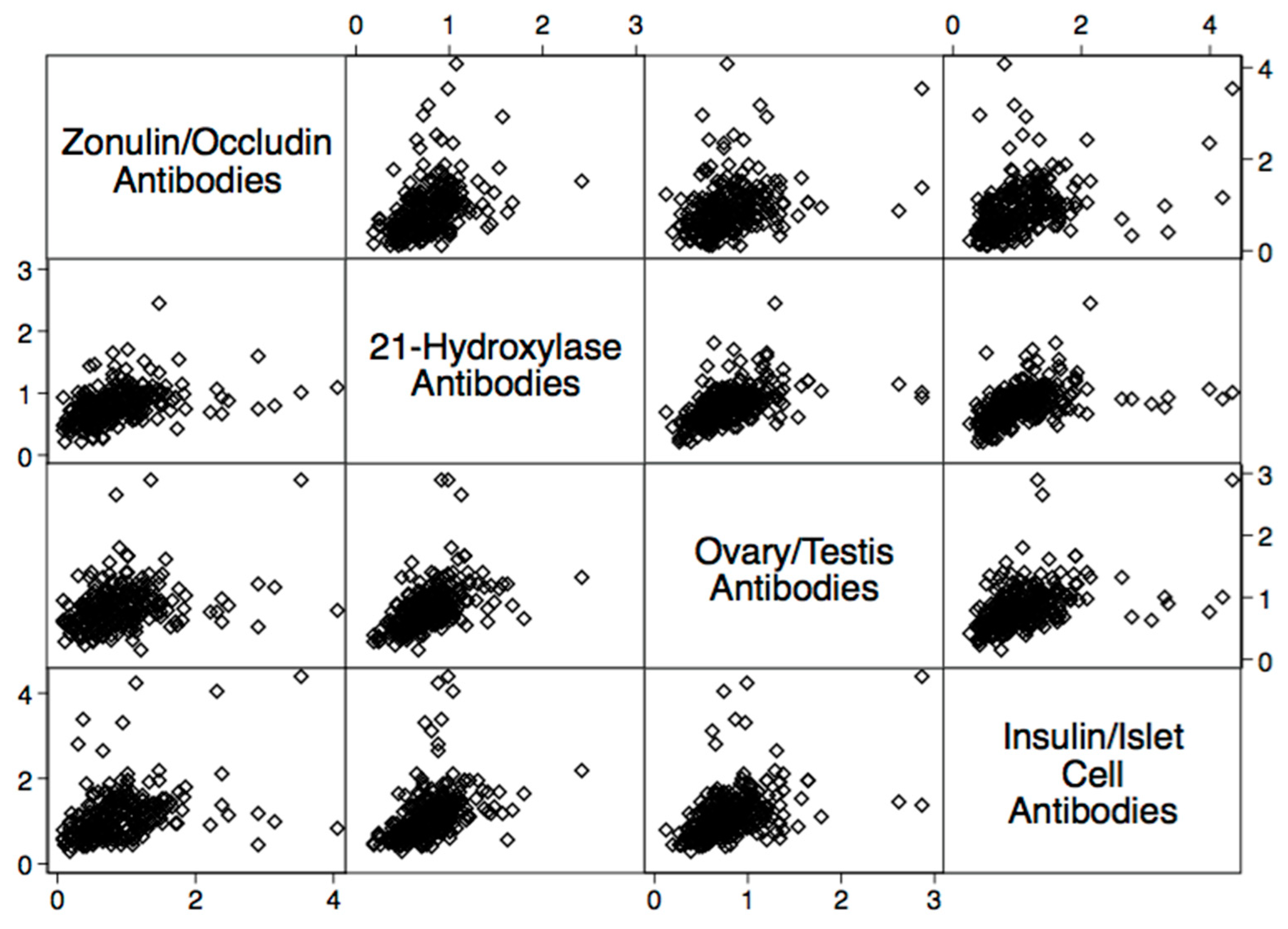

| Autoantibody | Odd Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Parietal Cell + ATPase IgG + IgA | 5.8 | 2.6–12.8 | <0.0001 |
| Intrinsic Factor IgG + IgA | 1.7 | 1.1–2.7 | 0.025 |
| ASCA + ANCA IgG + IgA | 8.4 | 3.5–20.2 | <0.0001 |
| Tropomyosin IgG + IgA | 7.0 | 3.3–15.0 | <0.0001 |
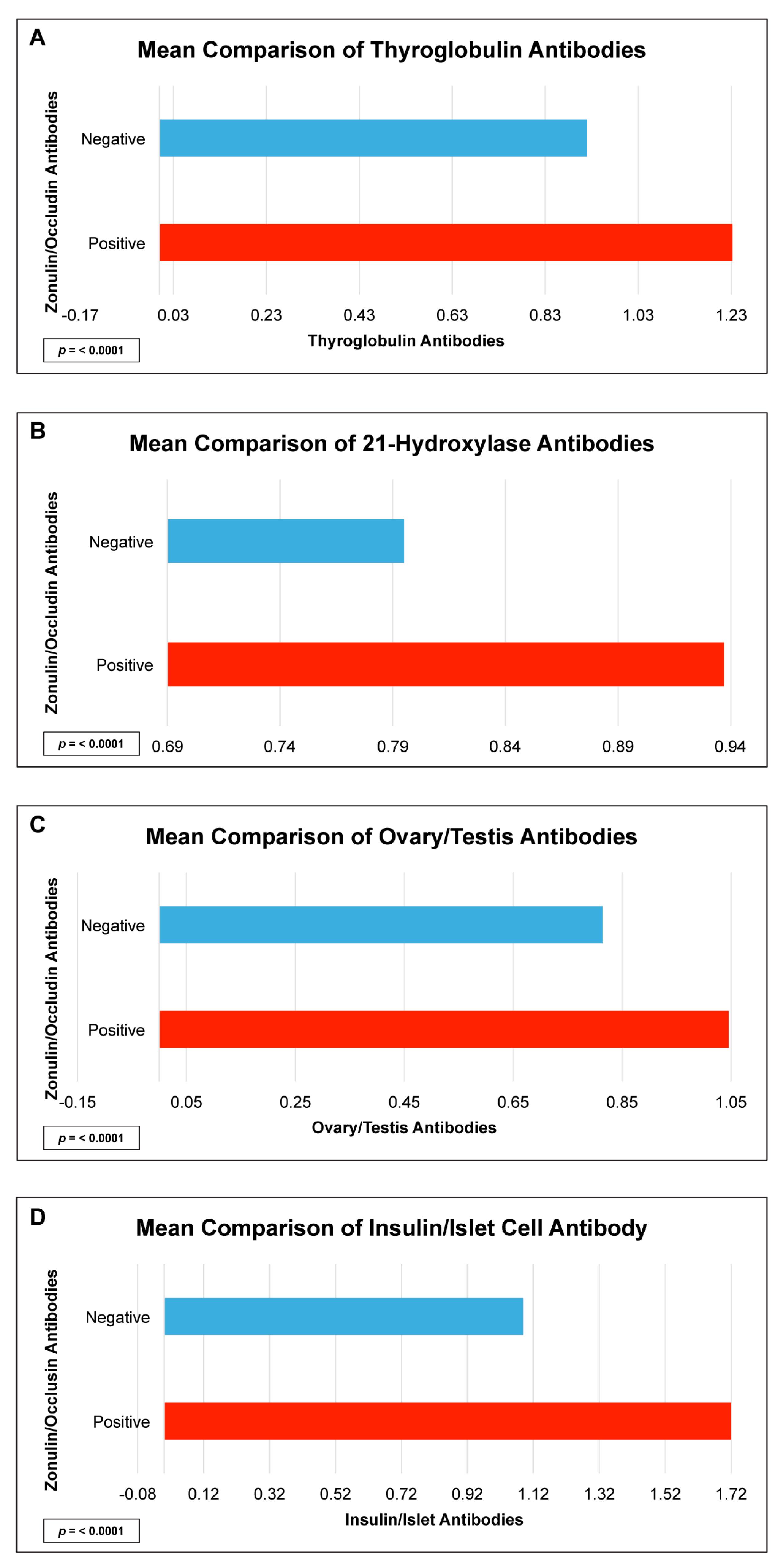
| Thyroglobulin IgG + IgA | 1.6 | 0.3–1.0 | <0.034 |
| Thyroid Peroxidase IgG + IgA | 2.4 | 1.4–4.2 | 0.002 |
| 21 Hydroxylase IgG + IgA | 28.0 | 8.7–89.0 | <0.0001 |
| Myocardial Peptide IgG + IgA | 9.0 | 4.1–19.2 | <0.0001 |
| Alpha-Myosin IgG + IgA | 10.4 | 4.5–23.1 | <0.0001 |
| Phospholipid IgG + IgA | 2.5 | 1.3–5.0 | 0.009 |
| Platelet Glycoprotein IgG + IgA | 5.4 | 2.3–13.0 | <0.0001 |
| Ovary/Testis IgG + IgA | 7.1 | 3.0–17.7 | <0.0001 |
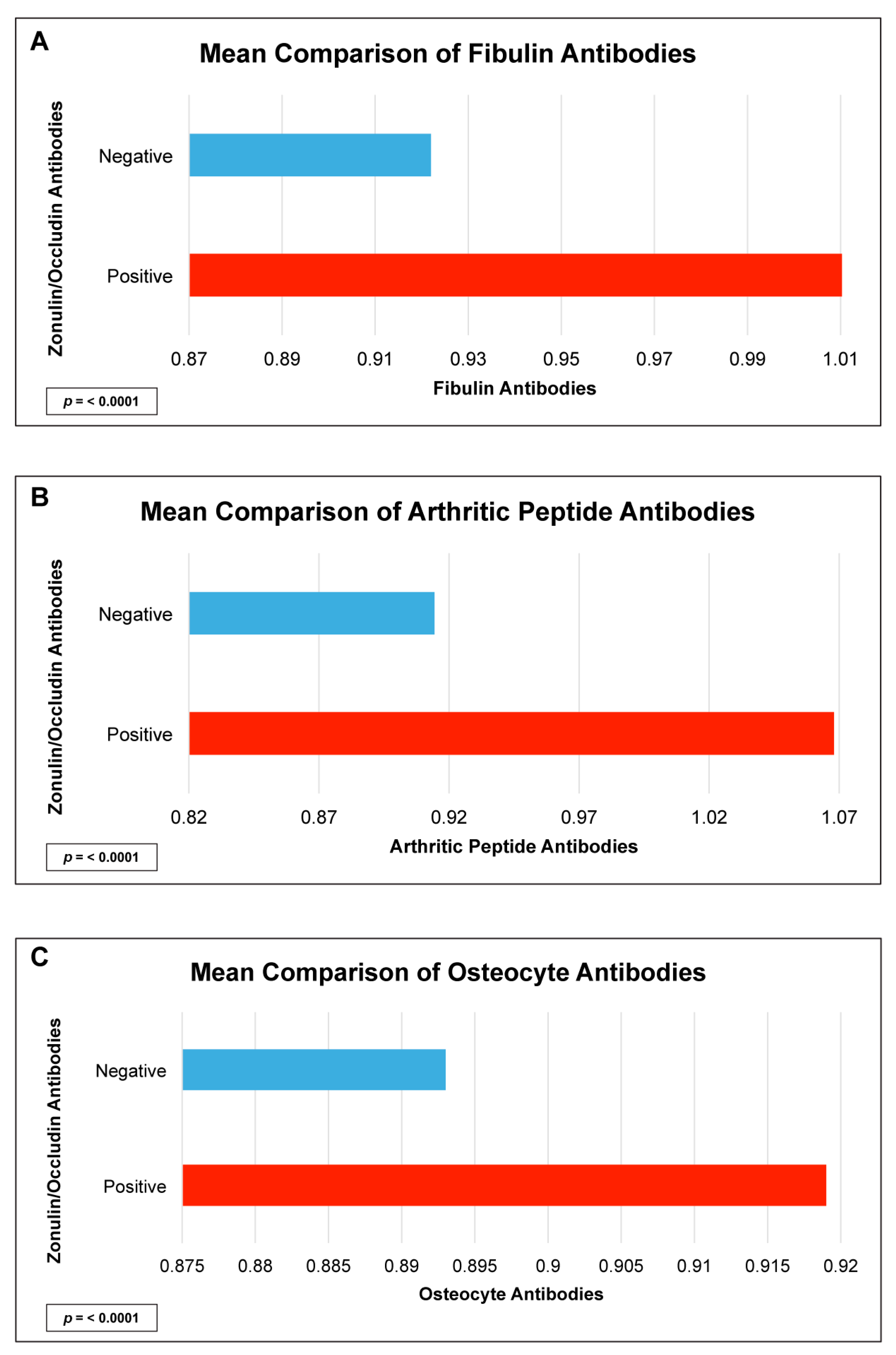
| Fibulin IgG + IgA | 7.2 | 3.0–17.0 | <0.0001 |
| Collagen Complex IgG + IgA | 2.2 | 1.3–4.0 | 0.005 |
| Arthritic Peptide IgG + IgA | 30.0 | 10.0–77.8 | <0.0001 |
| Osteocyte IgG + IgA | 6.6 | 2.8–15.7 | <0.0001 |
| Cytochrome P450 IgG + IgA | 22.3 | 8.0–64.4 | <0.0001 |
| Insulin + Islet Cell Antibody IgG + IgA | 0.9 | 0.6–1.6 | <0.0001 |
| Glutamic Acid Decarboxylase-65 IgG + IgA | 10.0 | 4.2–23.3 | <0.0001 |
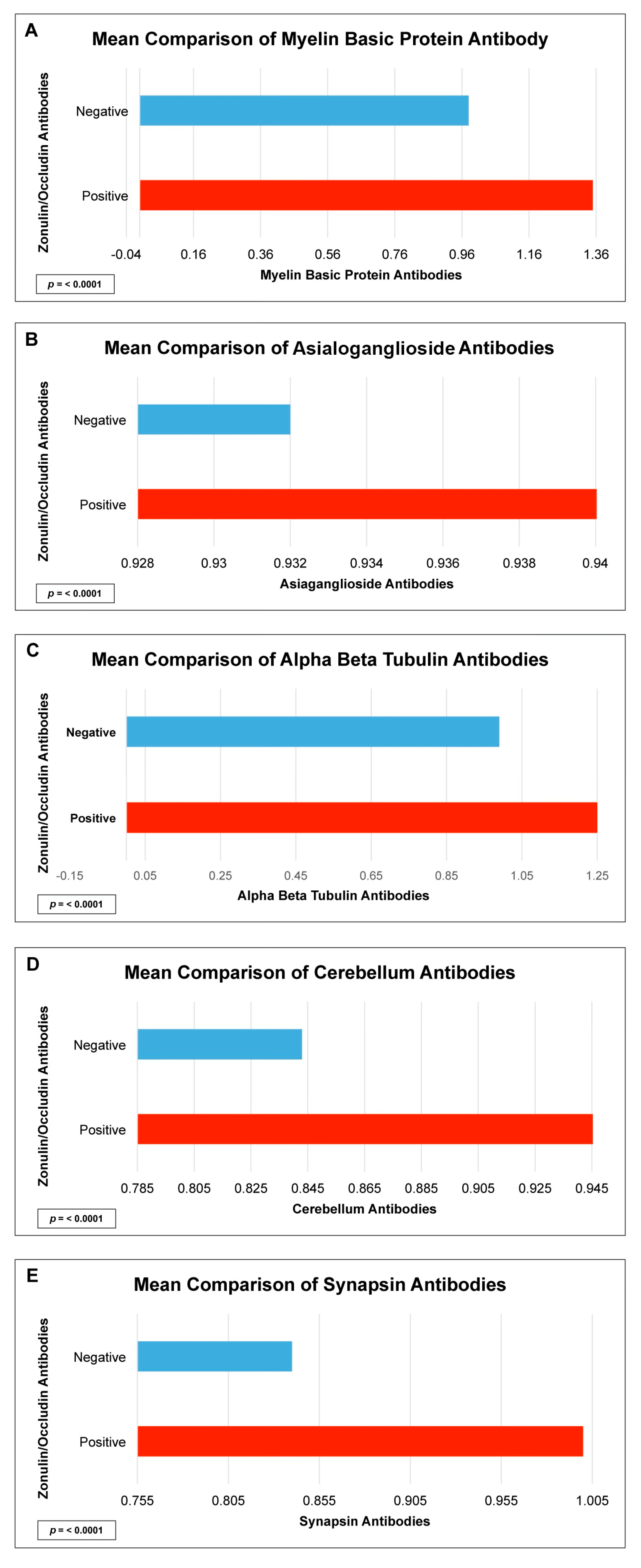
| Myelin Basic Protein IgG + IgA | 6.9 | 3.2–15.0 | <0.0001 |
| Asialoganglioside IgG + IgA | 6.2 | 2.9–13.3 | <0.0001 |
| Alpha + Beta Tubulin IgG + IgA | 2.7 | 1.6–4.6 | <0.0001 |
| Cerebellar IgG + IgA | 22.0 | 8.0–59.9 | <0.0001 |
| Synapsin IgG + IgA | 8.7 | 3.3–22.5 | <0.0001 |
| Autoantibody | Correlation Coefficient | p-Value |
|---|---|---|
| Parietal Cell + ATPase IgG + IgA | 0.4 | <0.0001 |
| Intrinsic Factor IgG + IgA | 0.3 | <0.0001 |
| ASCA + ANCA IgG + IgA | 0.4 | <0.0001 |
| Tropomyosin IgG + IgA | 0.4 | <0.0001 |
| Thyroglobulin IgG + IgA | 0.2 | 0.02 |
| Thyroid Peroxidase IgG + IgA | 0.2 | 0.001 |
| 21 Hydroxylase IgG + IgA | 0.4 | <0.0001 |
| Myocardial Peptide IgG + IgA | 0.3 | <0.0001 |
| Alpha-Myosin IgG + IgA | 0.5 | <0.0001 |
| Phospholipid IgG + IgA | 0.3 | <0.0001 |
| Platelet Glycoprotein IgG + IgA | 0.3 | <0.0001 |
| Ovary/Testis IgG + IgA | 0.4 | <0.0001 |
| Fibulin IgG + IgA | 0.3 | <0.0001 |
| Collagen Complex IgG + IgA | 0.2 | <0.0001 |
| Arthritic Peptide IgG + IgA | 0.2 | 0.0005 |
| Osteocyte IgG + IgA | 0.2 | 0.004 |
| Cytochrome P450 IgG + IgA | 0.3 | <0.0001 |
| Insulin + Islet Cell Antibody IgG + IgA | 0.2 | 0.0004 |
| Glutamic Acid Decarboxylase-65 IgG + IgA | 0.4 | <0.0000 |
| Myelin Basic Protein IgG + IgA | 0.3 | <0.0000 |
| Asialoganglioside IgG + IgA | 0.3 | <0.0001 |
| Alpha + Beta Tubulin IgG + IgA | 0.3 | <0.0001 |
| Cerebellar IgG + IgA | 0.4 | <0.0001 |
| Synapsin IgG + IgA | 0.3 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharrazian, D.; Herbert, M.; Lambert, J. The Relationships between Intestinal Permeability and Target Antibodies for a Spectrum of Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 16352. https://doi.org/10.3390/ijms242216352
Kharrazian D, Herbert M, Lambert J. The Relationships between Intestinal Permeability and Target Antibodies for a Spectrum of Autoimmune Diseases. International Journal of Molecular Sciences. 2023; 24(22):16352. https://doi.org/10.3390/ijms242216352
Chicago/Turabian StyleKharrazian, Datis, Martha Herbert, and Jama Lambert. 2023. "The Relationships between Intestinal Permeability and Target Antibodies for a Spectrum of Autoimmune Diseases" International Journal of Molecular Sciences 24, no. 22: 16352. https://doi.org/10.3390/ijms242216352
APA StyleKharrazian, D., Herbert, M., & Lambert, J. (2023). The Relationships between Intestinal Permeability and Target Antibodies for a Spectrum of Autoimmune Diseases. International Journal of Molecular Sciences, 24(22), 16352. https://doi.org/10.3390/ijms242216352







