Fatal Hymenoptera Venom–Triggered Anaphylaxis in Patients with Unrecognized Clonal Mast Cell Disorder—Is Mastocytosis to Blame?
Abstract
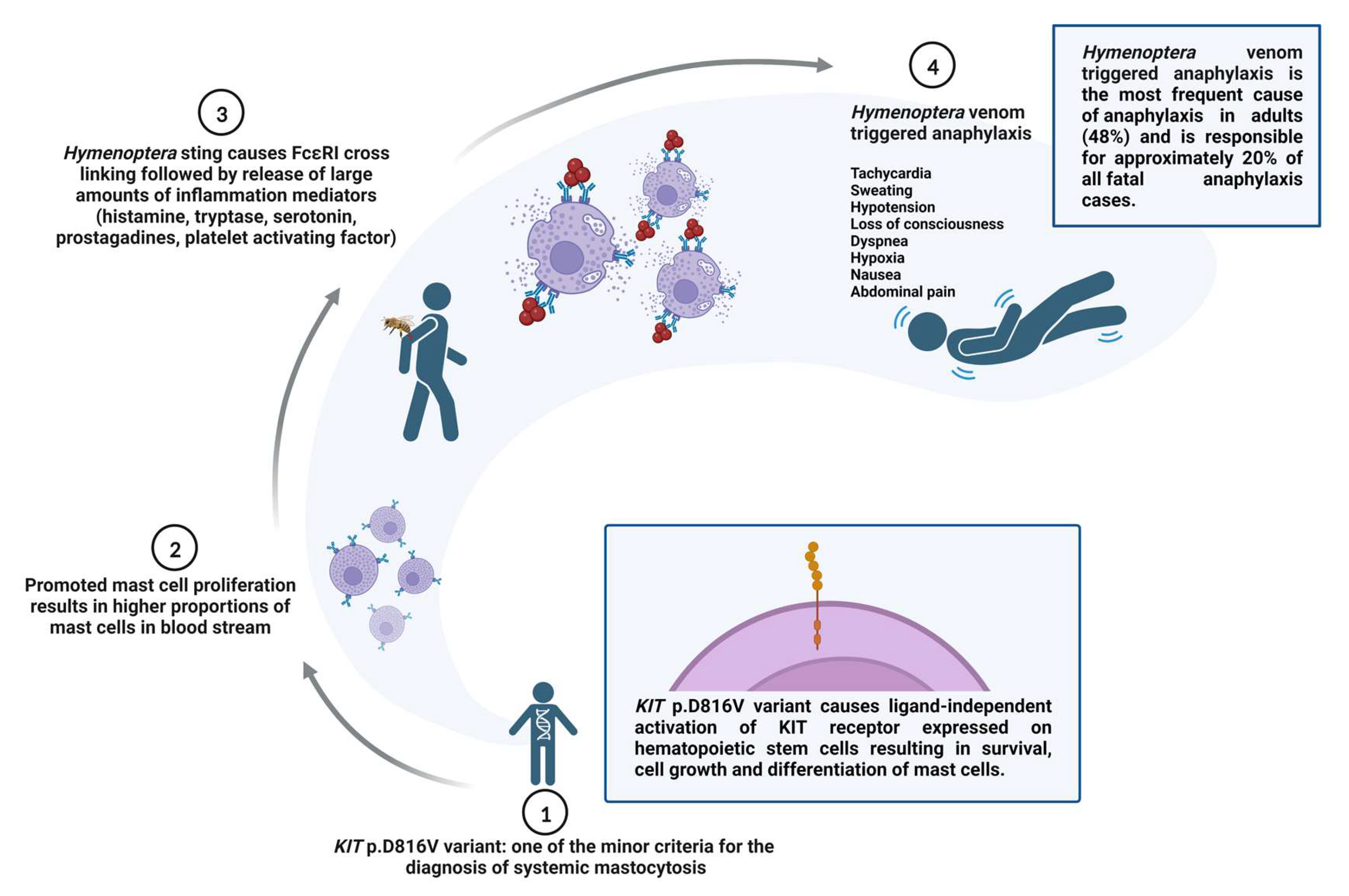
:1. Introduction
2. Results
2.1. Fatal Anaphylaxis in Slovenia
2.2. Fatal HVA in a Beekeeper (Case 1)
2.3. Fatal HVA during VIT in a Patient with Normal BST (Case 2)
2.4. Fatal HVA after VIT Discontinuation (Case 3)
3. Discussion
4. Materials and Methods
4.1. National Register of Causes of Death Database
4.2. Cases
4.3. Specific IgE and Total Serum Tryptase Testing
4.4. KIT p.D816V Missense Variant Assay
4.5. Tryptase Genotyping
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muraro, A.; Worm, M.; Alviani, C.; Cardona, V.; DunnGalvin, A.; Garvey, L.H.; Riggioni, C.; de Silva, D.; Angier, E.; Arasi, S.; et al. EAACI guidelines: Anaphylaxis (2021 update). Allergy 2022, 77, 357–377. [Google Scholar] [CrossRef]
- Reber, L.L.; Hernandez, J.D.; Galli, S.J. The pathophysiology of anaphylaxis. J. Allergy Clin. Immunol. 2017, 140, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Kačar, M.; Rijavec, M.; Šelb, J.; Korošec, P. Clonal mast cell disorders and hereditary α-tryptasemia as risk factors for anaphylaxis. Clin. Exp. Allergy 2023, 53, 392–404. [Google Scholar] [CrossRef]
- Bilò, M.B.; Pravettoni, V.; Bignardi, D.; Bonadonna, P.; Mauro, M.; Novembre, E.; Quercia, O.; Cilia, M.; Cortellini, G.; Costantino, M.; et al. Hymenoptera venom allergy: Management of children and adults in clinical practice. J. Investig. Allergol. Clin. Immunol. 2019, 29, 180–205. [Google Scholar] [CrossRef] [PubMed]
- Bilò, M.B.; Bonifazi, F. The natural history and epidemiology of insect venom allergy: Clinical implications. Clin. Exp. Allergy 2009, 39, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Worm, M.; Moneret-Vautrin, A.; Scherer, K.; Lang, R.; Fernandez-Rivas, M.; Cardona, V.; Kowalski, M.L.; Jutel, M.; Poziomkowska-Gesicka, I.; Papadopoulos, N.G.; et al. First European data from the network of severe allergic reactions (NORA). Allergy 2014, 69, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Jerschow, E.; Umasunthar, T.; Lin, R.; Campbell, D.E.; Boyle, R.J. Fatal Anaphylaxis: Mortality Rate and Risk Factors. J. Allergy Clin. Immunol. Pract. 2017, 5, 1169–1178. [Google Scholar] [CrossRef]
- Bilò, M.B.; Corsi, A.; Martini, M.; Penza, E.; Grippo, F.; Bignardi, D. Fatal anaphylaxis in Italy: Analysis of cause-of-death national data, 2004–2016. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J. Anaphylaxis. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021; pp. 71–72. [Google Scholar]
- Valent, P.; Sotlar, K.; Horny, H.-P.; Arock, M.; Akin, C. World Health Organization Classification and Diagnosis of Mastocytosis Update 2023 and Future Perspectives. Immunol. Allergy Clin. N. Am. 2023, 43, 627–649. [Google Scholar] [CrossRef]
- Lyons, J.J.; Chovanec, J.; O’Connell, M.P.; Liu, Y.; Šelb, J.; Zanotti, R.; Bai, Y.; Kim, J.; Le, Q.T.; DiMaggio, T.; et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J. Allergy Clin. Immunol. 2021, 147, 622–632. [Google Scholar] [CrossRef]
- Šelb, J.; Rijavec, M.; Eržen, R.; Zidarn, M.; Kopač, P.; Škerget, M.; Bajrović, N.; Luzar, A.D.; Park, Y.H.; Liu, Y.; et al. Routine KIT p.D816V screening identifies clonal mast cell disease in patients with Hymenoptera allergy regularly missed using baseline tryptase levels alone. J. Allergy Clin. Immunol. 2021, 148, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Hartmann, K.; Schwaab, J.; Alvarez-Twose, I.; Brockow, K.; Bonadonna, P.; Hermine, O.; Niedoszytko, M.; Carter, M.C.; Hoermann, G.; et al. Personalized Management Strategies in Mast Cell Disorders: ECNM-AIM User’s Guide for Daily Clinical Practice. J. Allergy Clin. Immunol. Pract. 2022, 10, 1999–2012. [Google Scholar] [CrossRef]
- Wagner, N.; Fritze, D.; Przybilla, B.; Hagedorn, M.; Ruëff, F. Fatal anaphylactic sting reaction in a patient with mastocytosis. Int. Arch. Allergy Immunol. 2008, 146, 162–163. [Google Scholar] [CrossRef]
- Elberink, J.N.G.O.; De Monchy, J.G.R.; Kors, J.W.; Van Doormaal, J.J.; Dubois, A.E.J. Fatal anaphylaxis after a yellow jacket sting, despite venom immunotherapy, in two patients with mastocytosis. J. Allergy Clin. Immunol. 1997, 99, 153–154. [Google Scholar] [CrossRef]
- Vos, B.J.P.R.; van Anrooij, B.; van Doormaal, J.J.; Dubois, A.E.J.; Oude Elberink, J.N.G. Fatal Anaphylaxis to Yellow Jacket Stings in Mastocytosis: Options for Identification and Treatment of At-Risk Patients. J. Allergy Clin. Immunol. Pract. 2017, 5, 1264–1271. [Google Scholar] [CrossRef]
- Chatain, C.; Sedillot, N.; Thomas, M.; Pernollet, M.; Bocquet, A.; Boccon-Gibod, I.; Bouillet, L.; Leccia, M. Fatal hymenoptera venom anaphylaxis by undetected clonal mast cell disorder: A better identification of high risk patients is needed. Rev. Med. Interne 2021, 42, 869–874. [Google Scholar] [CrossRef]
- Bonadonna, P.; Zanotti, R.; Pagani, M.; Bonifacio, M.; Scaffidi, L.; Olivieri, E.; Franchini, M.; Reccardini, F.; Costantino, M.T.; Roncallo, C.; et al. Anaphylactic Reactions After Discontinuation of Hymenoptera Venom Immunotherapy: A Clonal Mast Cell Disorder Should Be Suspected. J. Allergy Clin. Immunol. Pract. 2018, 6, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Garland, J.; Ondruschka, B.; Da Broi, U.; Palmiere, C.; Tse, R. Post mortem tryptase: A review of literature on its use, sampling and interpretation in the investigation of fatal anaphylaxis. Forensic Sci. Int. 2020, 314, 110415. [Google Scholar] [CrossRef] [PubMed]
- Tse, R.; Wong, C.X.; Kesha, K.; Garland, J.; Tran, Y.; Anne, S.; Elstub, H.; Cala, A.; Palmiere, C.; Patchett, K. Post mortem tryptase cut-off level for anaphylactic death. Forensic Sci. Int. 2018, 284, 5–8. [Google Scholar] [CrossRef]
- Feás, X.; Vidal, C.; Remesar, S. What We Know about Sting-Related Deaths? Human Fatalities Caused by Hornet, Wasp and Bee Stings in Europe (1994–2016). Biology 2022, 11, 282. [Google Scholar] [CrossRef]
- Blank, S.; Pehlivanli, S.; Methe, H.; Schmidt-Weber, C.B.; Biedermann, T.; Horny, H.P.; Kristensen, T.; Amar, Y.; Köberle, M.; Brockow, K.; et al. Fatal anaphylaxis following a hornet sting in a yellow jacket venom-sensitized patient with undetected monoclonal mast cell activation syndrome and without previous history of a systemic sting reaction. J. Allergy Clin. Immunol. Pract. 2020, 8, 401–403. [Google Scholar] [CrossRef]
- Heldring, N.; Kahn, L.; Zilg, B. Fatal anaphylactic shock: A review of postmortem biomarkers and diagnostics. Forensic Sci. Int. 2021, 323, 110814. [Google Scholar] [CrossRef]
- Pouessel, G.; Alonzo, S.; Divaret-Chauveau, A.; Dumond, P.; Bradatan, E.; Liabeuf, V.; Beaumont, P.; Tscheiller, S.; Diesnis, R.; Renaudin, J.-M.; et al. Fatal and near-fatal anaphylaxis: The Allergy-Vigilance® Network data (2002–2020). Allergy Eur. J. Allergy Clin. Immunol. 2023, 78, 1628–1638. [Google Scholar] [CrossRef]
- Šelb, J.; Rijavec, M.; Kopač, P.; Lyons, J.J.; Korošec, P. HαT is associated with increased risk for severe Hymenoptera venom-triggered anaphylaxis. J. Allergy Clin. Immunol. 2022, 278, 4–5. [Google Scholar] [CrossRef]
- De Puysseleyr, L.P.; Ebo, D.G.; Elst, J.; Faber, M.A.; van der Poorten, M.L.; Van Gasse, A.L.; Bridts, C.H.; Mertens, C.; Van Houdt, M.; Hagendorens, M.M.; et al. Diagnosis of Primary Mast Cell Disorders in Anaphylaxis: Value of KIT D816V in Peripheral Blood. J. Allergy Clin. Immunol. Pract. 2021, 9, 3176–3187. [Google Scholar] [CrossRef]
- Onnes, M.C.; Alheraky, A.; Nawijn, M.C.; Sluijter, T.E.; Mulder, A.B.; Arends, S.; Elberink, H.N.G.O. Detection of clonal mast cell disease in wasp venom allergic patients with normal tryptase. Clin. Transl. Allergy 2022, 12, e12174. [Google Scholar] [CrossRef]
- Dölle-Bierke, S.; Siebenhaar, F.; Burmeister, T.; Worm, M. Detection of KIT D816V mutation in patients with severe anaphylaxis and normal basal tryptase—First data from the Anaphylaxis Registry (NORA). J. Allergy Clin. Immunol. 2019, 144, 1448–1450. [Google Scholar] [CrossRef]
- Navarro-Navarro, P.; Álvarez-Twose, I.; Pérez-Pons, A.; Henriques, A.; Mayado, A.; García-Montero, A.C.; Sánchez-Muñoz, L.; González-López, O.; Matito, A.; Caldas, C.; et al. KITD816V mutation in blood for the diagnostic screening of systemic mastocytosis and mast cell activation syndromes. Allergy Eur. J. Allergy Clin. Immunol. 2023, 78, 1347–1359. [Google Scholar] [CrossRef]
- Gülsen, A.; Ruëff, F.; Jappe, U. Omalizumab ensures compatibility to bee venom immunotherapy (VIT) after VIT-induced anaphylaxis in a patient with systemic mastocytosis. Allergol. Sel. 2021, 5, 128. [Google Scholar] [CrossRef]
- Broesby-Olsen, S.; Vestergaard, H.; Mortz, C.G.; Jensen, B.; Havelund, T.; Hermann, A.P.; Siebenhaar, F.; Møller, M.B.; Kristensen, T.K.; Bindslev-Jensen, C.; et al. Omalizumab prevents anaphylaxis and improves symptoms in systemic mastocytosis: Efficacy and safety observations. Allergy 2018, 73, 230–238. [Google Scholar] [CrossRef]
- Pumphrey, R.S.H. Lessons for management of anaphylaxis from a study of fatal reactions. Clin. Exp. Allergy 2000, 30, 1144–1150. [Google Scholar] [CrossRef]
- International Classification of Diseases (ICD) [Internet]. Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 9 August 2023).
- Bonadonna, P.; Perbellini, O.; Passalacqua, G.; Caruso, B.; Colarossi, S.; Dal Fior, D.; Castellani, L.; Bonetto, C.; Frattini, F.; Dama, A.; et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J. Allergy Clin. Immunol. 2009, 123, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.B. Diagnostic Value of Tryptase in Anaphylaxis and Mastocytosis. Immunol. Allergy Clin. N. Am. 2006, 26, 451–463. [Google Scholar] [CrossRef]
- Kristensen, T.; Vestergaard, H.; Bindslev-Jensen, C.; Møller, M.B.; Broesby-Olsen, S. Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am. J. Hematol. 2014, 89, 493–498. [Google Scholar] [CrossRef]
- Lyons, J.J.; Yu, X.; Hughes, J.D.; Le, Q.T.; Jamil, A.; Bai, Y.; Ho, N.; Zhao, M.; Liu, Y.; O’Connell, M.P.; et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat. Genet. 2016, 48, 1564–1569. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rijavec, M.; Inkret, J.; Bidovec-Stojković, U.; Carli, T.; Frelih, N.; Kukec, A.; Korošec, P.; Košnik, M. Fatal Hymenoptera Venom–Triggered Anaphylaxis in Patients with Unrecognized Clonal Mast Cell Disorder—Is Mastocytosis to Blame? Int. J. Mol. Sci. 2023, 24, 16368. https://doi.org/10.3390/ijms242216368
Rijavec M, Inkret J, Bidovec-Stojković U, Carli T, Frelih N, Kukec A, Korošec P, Košnik M. Fatal Hymenoptera Venom–Triggered Anaphylaxis in Patients with Unrecognized Clonal Mast Cell Disorder—Is Mastocytosis to Blame? International Journal of Molecular Sciences. 2023; 24(22):16368. https://doi.org/10.3390/ijms242216368
Chicago/Turabian StyleRijavec, Matija, Jezerka Inkret, Urška Bidovec-Stojković, Tanja Carli, Nina Frelih, Andreja Kukec, Peter Korošec, and Mitja Košnik. 2023. "Fatal Hymenoptera Venom–Triggered Anaphylaxis in Patients with Unrecognized Clonal Mast Cell Disorder—Is Mastocytosis to Blame?" International Journal of Molecular Sciences 24, no. 22: 16368. https://doi.org/10.3390/ijms242216368
APA StyleRijavec, M., Inkret, J., Bidovec-Stojković, U., Carli, T., Frelih, N., Kukec, A., Korošec, P., & Košnik, M. (2023). Fatal Hymenoptera Venom–Triggered Anaphylaxis in Patients with Unrecognized Clonal Mast Cell Disorder—Is Mastocytosis to Blame? International Journal of Molecular Sciences, 24(22), 16368. https://doi.org/10.3390/ijms242216368







