Genetic Heterogeneity Underlying Phenotypes with Early-Onset Cerebellar Atrophy
Abstract
:1. Introduction
2. Results
3. Discussion
4. Patients and Methods
4.1. Patients and Clinical Features
4.2. Genetic and Genomic Studies
4.3. Structural Modelling of SETX p.I1942T, CACNA1G p.A961T and TBCD p.N1033K
4.4. Transcript Analysis of CPLANE1, c.7588+7A>G and c.2747-1981_6172-78del, and PI4KA, c.3845C>T
4.5. In Silico Analyses and Protein Expression Levels of CLK2 p.Y372H
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ataullah, A.H.M.; Naqvi, I.A. Cerebellar Dysfunction; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Schmahmann, J.D. The cerebellum and cognition. Neurosci. Lett. 2018, 688, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Tavano, A.; Grasso, R.; Gagliardi, C.; Triulzi, F.; Bresolin, N.; Fabbro, F.; Borgatti, R. Disorders of cognitive and affective development in cerebellar malformations. Brain 2007, 130, 2646–2660. [Google Scholar] [CrossRef] [PubMed]
- Al-Maawali, A.; Blaser, S.; Yoon, G. Diagnostic approach to childhood-onset cerebellar atrophy: A 10-year retrospective study of 300 patients. J. Child Neurol. 2012, 27, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Chemin, J.; Siquier-Pernet, K.; Nicouleau, M.; Barcia, G.; Ahmad, A.; Medina-Cano, D.; Hanein, S.; Altin, N.; Hubert, L.; Bole-Feysot, C.; et al. De novo mutation screening in childhood-onset cerebellar atrophy identifies gain-of-function mutations in the CACNA1G calcium channel gene. Brain 2018, 141, 1998–2013. [Google Scholar] [CrossRef]
- Poretti, A.; Wolf, N.I.; Boltshauser, E. Differential diagnosis of cerebellar atrophy in childhood: An update. Neuropediatrics 2015, 46, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Sancho, P.; Andrés-Bordería, A.; Gorría-Redondo, N.; Llano, K.; Martínez-Rubio, D.; Yoldi-Petri, M.E.; Blumkin, L.; de la Fuente, P.R.; Gil-Ortiz, F.; Fernández-Murga, L.; et al. Expanding the β-III spectrin-associated phenotypes toward non-progressive congenital ataxias with neurodegeneration. Int. J. Mol. Sci. 2021, 22, 2505. [Google Scholar] [CrossRef]
- Martínez-Rubio, D.; Rodríguez-Prieto, Á.; Sancho, P.; Navarro-González, C.; Gorría-Redondo, N.; Miquel-Leal, J.; Marco-Marín, C.; Jenkins, A.; Soriano-Navarro, M.; Hernández, A.; et al. Protein misfolding and clearance in the pathogenesis of a new infantile onset ataxia caused by mutations in PRDX3. Hum. Mol. Genet. 2022, 31, 3897–3913. [Google Scholar] [CrossRef]
- Martínez-Rubio, D.; Hinarejos, I.; Sancho, P.; Gorría-Redondo, N.; Bernadó-Fonz, R.; Tello, C.; Marco-Marín, C.; Martí-Carrera, I.; Martínez-González, M.J.; García-Ribes, A.; et al. Mutations, genes, and phenotypes related to movement disorders and ataxias. Int. J. Mol. Sci. 2022, 23, 11847. [Google Scholar] [CrossRef]
- Darling, A.; Aguilera-Albesa, S.; Tello, C.A.; Serrano, M.; Tomás, M.; Camino-León, R.; Fernández-Ramos, J.; Jiménez-Escrig, A.; Poó, P.; O’Callaghan, M.; et al. PLA2G6-associated neurodegeneration: New insights into brain abnormalities and disease progression. Park. Relat. Disord. 2018, 61, 179–186. [Google Scholar] [CrossRef]
- Tello, C.; Darling, A.; Lupo, V.; Pérez-Dueñas, B.; Espinós, C. On the complexity of clinical and molecular bases of neurodegeneration with brain iron accumulation. Clin. Genet. 2017, 93, 731–740. [Google Scholar] [CrossRef]
- Coarelli, G.; Coutelier, M.; Durr, A. Autosomal dominant cerebellar ataxias: New genes and progress towards treatments. Lancet Neurol. 2023, 22, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Perez-Lloret, S.; Doldan, L.; Cerquetti, D.; Balej, J.; Millar Vernetti, P.; Hawkes, H.; Cammarota, A.; Merello, M. Autosomal dominant cerebellar ataxias: A systematic review of clinical features. Eur. J. Neurol. 2014, 21, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Synofzik, M.; Puccio, H.; Mochel, F.; Schöls, L. Autosomal recessive cerebellar ataxias: Paving the way toward targeted molecular therapies. Neuron 2019, 101, 560–583. [Google Scholar] [CrossRef]
- Rossi, M.; Anheim, M.; Durr, A.; Klein, C.; Koenig, M.; Synofzik, M.; Marras, C.; van de Warrenburg, B.P.; International Parkinson and Movement Disorder Society Task Force on Classification and Nomenclature of Genetic Movement Disorders. The genetic nomenclature of recessive cerebellar ataxias. Mov. Disord. 2018, 33, 1056–1076. [Google Scholar] [CrossRef] [PubMed]
- Sancho, P.; Sánchez-Monteagudo, A.; Collado, A.; Marco-Marín, C.; Domínguez-González, C.; Camacho, A.; Knecht, E.; Espinós, C.; Lupo, V. A newly distal hereditary motor neuropathy caused by a rare AIFM1 mutation. Neurogenetics 2017, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Sancho, P.; Bartesaghi, L.; Miossec, O.; García-García, F.; Ramírez-Jiménez, L.; Siddell, A.; Åkesson, E.; Hedlund, E.; Laššuthová, P.; I Pascual-Pascual, S.; et al. Characterization of molecular mechanisms underlying the axonal Charcot–Marie–Tooth neuropathy caused by MORC2 mutations. Hum. Mol. Genet. 2019, 28, 1629–1644. [Google Scholar] [CrossRef]
- Abbott, J.A.; Meyer-Schuman, R.; Lupo, V.; Feely, S.; Mademan, I.; Oprescu, S.N.; Griffin, L.B.; Alberti, M.A.; Casasnovas, C.; Aharoni, S.; et al. Substrate interaction defects in histidyl-tRNA synthetase linked to dominant axonal peripheral neuropathy. Hum. Mutat. 2018, 39, 415–432. [Google Scholar] [CrossRef]
- Leonaitė, B.; Han, Z.; Basquin, J.; Bonneau, F.; Libri, D.; Porrua, O.; Conti, E. Sen1 has unique structural features grafted on the architecture of the Upf1-like helicase family. EMBO J. 2017, 36, 1590–1604. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Anesthesia Analg. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, G.; Wu, Q.; Wu, K.; Li, R.; Lei, J.; Pan, X.; Yan, N. Cryo-EM structures of apo and antagonist-bound human Ca(v)3.1. Nature 2019, 576, 492–497. [Google Scholar] [CrossRef]
- Wu, J.; Yan, Z.; Li, Z.; Qian, X.; Lu, S.; Dong, M.; Zhou, Q.; Yan, N. Structure of the voltage-gated calcium channel Cav1.1 at 3.6 Å resolution. Nature 2016, 537, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Flex, E.; Niceta, M.; Cecchetti, S.; Thiffault, I.; Au, M.G.; Capuano, A.; Piermarini, E.; Ivanova, A.A.; Francis, J.W.; Chillemi, G.; et al. Biallelic mutations in TBCD, encoding the tubulin folding cofactor D, perturb microtubule dynamics and cause early-onset encephalopathy. Am. J. Hum. Genet. 2016, 99, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Fanarraga, M.L.; Bellido, J.; Jaén, C.; Villegas, J.C.; Zabala, J.C. TBCD links centriologenesis, spindle microtubule dynamics, and midbody abscission in human cells. PLoS ONE 2010, 5, e8846. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Haas, W.; Gygi, S.P.; Puigserver, P. Cdc2-like kinase 2 is an insulin-regulated suppressor of hepatic gluconeogenesis. Cell Metab. 2010, 11, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Nayler, O.; Schnorrer, F.; Stamm, S.; Ullrich, A. The cellular localization of the murine serine/arginine-rich protein kinase CLK2 is regulated by serine 141 autophosphorylation. J. Biol. Chem. 1998, 273, 34341–34348. [Google Scholar] [CrossRef]
- Poretti, A.; Wolf, N.I.; Boltshauser, E. Differential diagnosis of cerebellar atrophy in childhood. Eur. J. Paediatr. Neurol. 2008, 12, 155–167. [Google Scholar] [CrossRef]
- Renaud, M.; Tranchant, C.; Koenig, M.; Anheim, M. Autosomal recessive cerebellar ataxias with elevated alpha-fetoprotein: Uncommon diseases, common biomarker. Mov. Disord. 2020, 35, 2139–2149. [Google Scholar] [CrossRef]
- Anheim, M.; Monga, B.; Fleury, M.; Charles, P.; Barbot, C.; Salih, M.; Delaunoy, J.P.; Fritsch, M.; Arning, L.; Synofzik, M.; et al. Ataxia with oculomotor apraxia type 2: Clinical, biological and genotype/phenotype correlation study of a cohort of 90 patients. Brain 2009, 132, 2688–2698. [Google Scholar] [CrossRef]
- Moreira, M.-C.; Klur, S.; Watanabe, M.; Németh, A.H.; Le Ber, I.; Moniz, J.-C.; Tranchant, C.; Aubourg, P.; Tazir, M.; Schöls, L.; et al. Senataxin, the ortholog of a yeast RNA helicase, is mutant in ataxia-ocular apraxia 2. Nat. Genet. 2004, 36, 225–227. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Bennett, C.L.; Huynh, H.M.; Blair, I.P.; Puls, I.; Irobi, J.; Dierick, I.; Abel, A.; Kennerson, M.L.; Rabin, B.A.; et al. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am. J. Hum. Genet. 2004, 74, 1128–1135. [Google Scholar] [CrossRef]
- Coutelier, M.; Coarelli, G.; Monin, M.-L.; Konop, J.; Davoine, C.-S.; Tesson, C.; Valter, R.; Anheim, M.; Behin, A.; Castelnovo, G.; et al. A panel study on patients with dominant cerebellar ataxia highlights the frequency of channelopathies. Brain 2017, 140, 1579–1594. [Google Scholar] [CrossRef] [PubMed]
- Gauquelin, L.; Hartley, T.; Tarnopolsky, M.; Dyment, D.A.; Brais, B.; Geraghty, M.T.; Tétreault, M.; Ahmed, S.; Rojas, S.; Choquet, K.; et al. Channelopathies are a frequent cause of genetic ataxias associated with cerebellar atrophy. Mov. Disord. Clin. Pr. 2020, 7, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Coutelier, M.; Blesneac, I.; Monteil, A.; Monin, M.-L.; Ando, K.; Mundwiller, E.; Brusco, A.; Le Ber, I.; Anheim, M.; Castrioto, A.; et al. A recurrent mutation in CACNA1G alters Cav3.1 T-type calcium-channel conduction and causes autosomal-dominant cerebellar ataxia. Am. J. Hum. Genet. 2015, 97, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Lange, L.M.; Gonzalez-Latapi, P.; Rajalingam, R.; Tijssen, M.A.; Ebrahimi-Fakhari, D.; Gabbert, C.; Ganos, C.; Ghosh, R.; Kumar, K.R.; Lang, A.E.; et al. Nomenclature of genetic movement disorders: Recommendations of the International Parkinson and Movement Disorder Society Task Force—An update. Mov. Disord. 2022, 37, 905–935. [Google Scholar] [CrossRef] [PubMed]
- Renbaum, P.; Kellerman, E.; Jaron, R.; Geiger, D.; Segel, R.; Lee, M.; King, M.C.; Levy-Lahad, E. Spinal muscular atrophy with pontocerebellar hypoplasia is caused by a mutation in the VRK1 gene. Am. J. Hum. Genet. 2009, 85, 281–289. [Google Scholar] [CrossRef]
- Indelicato, E.; Boesch, S. From genotype to phenotype: Expanding the clinical spectrum of CACNA1A variants in the era of next generation sequencing. Front. Neurol. 2021, 12, 639994. [Google Scholar] [CrossRef]
- Kordasiewicz, H.B.; Thompson, R.M.; Clark, H.B.; Gomez, C.M. C-termini of P/Q-type Ca 2+ channel α1A subunits translocate to nuclei and promote polyglutamine-mediated toxicity. Hum. Mol. Genet. 2006, 15, 1587–1599. [Google Scholar] [CrossRef]
- Gao, H.; Boustany, R.-M.N.; Espinola, J.A.; Cotman, S.L.; Srinidhi, L.; Antonellis, K.A.; Gillis, T.; Qin, X.; Liu, S.; Donahue, L.R.; et al. Mutations in a novel CLN6-encoded transmembrane protein cause variant neuronal ceroid lipofuscinosis in man and mouse. Am. J. Hum. Genet. 2002, 70, 324–335. [Google Scholar] [CrossRef]
- Tuermer, A.; Mausbach, S.; Kaade, E.; Damme, M.; Sylvester, M.; Gieselmann, V.; Thelen, M. CLN6 deficiency causes selective changes in the lysosomal protein composition. Proteomics 2021, 21, 2100043. [Google Scholar] [CrossRef]
- Mole, S.E.; Williams, R.E.; Goebel, H.H. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics 2005, 6, 107–126. [Google Scholar] [CrossRef]
- Faruq, M.; Narang, A.; Kumari, R.; Pandey, R.; Garg, A.; Behari, M.; Dash, D.; Srivastava, A.; Mukerji, M. Novel mutations in typical and atypical genetic loci through exome sequencing in autosomal recessive cerebellar ataxia families. Clin. Genet. 2013, 86, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Rus, C.-M.; Weissensteiner, T.; Pereira, C.; Susnea, I.; Danquah, B.D.; Torres, G.M.; Rocha, M.E.; Cozma, C.; Saravanakumar, D.; Mannepalli, S.; et al. Clinical and genetic characterization of a cohort of 97 CLN6 patients tested at a single center. Orphanet J. Rare Dis. 2022, 17, 179. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Vignoli, B.; Ramesh, N.; Polanco, M.J.; Coutelier, M.; Stephen, C.D.; Canossa, M.; Monin, M.-L.; Aeschlimann, P.; Turberville, S.; et al. Mutations in TGM6 induce the unfolded protein response in SCA35. Hum. Mol. Genet. 2017, 26, 3749–3762. [Google Scholar] [CrossRef]
- McMichael, G.; Bainbridge, M.N.; Haan, E.; Corbett, M.; Gardner, A.; Thompson, S.; van Bon, B.W.M.; van Eyk, C.L.; Broadbent, J.; Reynolds, C.; et al. Whole-exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol. Psychiatry 2015, 20, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, R.; Leca, I.; van Dijk, T.; Weiss, J.; van Bon, B.W.; Sergaki, M.C.; Gstrein, T.; Breuss, M.; Tian, G.; Bahi-Buisson, N.; et al. Mutations in MAST1 cause mega-corpus-callosum syndrome with cerebellar hypoplasia and cortical malformations. Neuron 2018, 100, 1354–1368. [Google Scholar] [CrossRef]
- Ben-Mahmoud, A.; Al-Shamsi, A.M.; Ali, B.R.; Al-Gazali, L. Evaluating the role of MAST1 as an intellectual disability disease gene: Identification of a novel de novo variant in a patient with developmental disabilities. J. Mol. Neurosci. 2019, 70, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, M.E.; Cotrina-Vinagre, F.J.; Gómez-Cano, M.d.L.; de Aragón, A.M.; Martín-Hernández, E.; Martínez-Azorín, F. MAST1 variant causes mega-corpus-callosum syndrome with cortical malformations but without cerebellar hypoplasia. Am. J. Med. Genet. Part A 2020, 182, 1483–1490. [Google Scholar] [CrossRef]
- Hecher, L.; Johannsen, J.; Bierhals, T.; Buhk, J.-H.; Hempel, M.; Denecke, J. The clinical picture of a bilateral perisylvian syndrome as the initial symptom of mega-corpus-callosum syndrome due to a MAST1-gene mutation. Neuropediatrics 2020, 51, 435–439. [Google Scholar] [CrossRef]
- Sloboda, N.; Renard, E.; Lambert, L.; Bonnet, C.; Leheup, B.; Todosi, C.; Schmitt, E.; Feillet, F.; Feigerlova, E.; Piton, A.; et al. MAST1-related mega-corpus-callosum syndrome with central hypogonadism. Eur. J. Med Genet. 2023, 66, 104853. [Google Scholar] [CrossRef]
- Gana, S.; Serpieri, V.; Valente, E.M. Genotype–phenotype correlates in Joubert syndrome: A review. Am. J. Med. Genet. Part C Semin. Med Genet. 2022, 190, 72–88. [Google Scholar] [CrossRef]
- Hong, H.; Joo, K.; Park, S.M.; Seo, J.; Kim, M.H.; Shin, E.; Cheong, H.I.; Lee, J.H.; Kim, J. Extraciliary roles of the ciliopathy protein JBTS17 in mitosis and neurogenesis. Ann. Neurol. 2019, 86, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Chaki, M.; Airik, R.; Ghosh, A.K.; Giles, R.H.; Chen, R.; Slaats, G.G.; Wang, H.; Hurd, T.W.; Zhou, W.; Cluckey, A.; et al. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell 2012, 150, 533–548. [Google Scholar] [CrossRef]
- Choi, H.J.C.; Lin, J.-R.; Vannier, J.-B.; Slaats, G.G.; Kile, A.C.; Paulsen, R.D.; Manning, D.K.; Beier, D.R.; Giles, R.H.; Boulton, S.J.; et al. NEK8 links the ATR-regulated replication stress response and S phase CDK activity to renal ciliopathies. Mol. Cell 2013, 51, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Miyake, N.; Fukai, R.; Ohba, C.; Chihara, T.; Miura, M.; Shimizu, H.; Kakita, A.; Imagawa, E.; Shiina, M.; Ogata, K.; et al. Biallelic TBCD mutations cause early-onset neurodegenerative encephalopathy. Am. J. Hum. Genet. 2016, 99, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-Chih, C.; Dennis, H.; Lall, N.; Pham, N.; Liang, B.; Verma, S.; Fresneda, J.N. PEBAT, an intriguing neurodegenerative tubulinopathy caused by a novel homozygous variant in TBCD: A case series and literature Review. Pediatr. Neurol. 2022, 139, 59–64. [Google Scholar] [CrossRef]
- Grønborg, S.; Risom, L.; Ek, J.; Larsen, K.B.; Scheie, D.; Petkov, Y.; Larsen, V.A.; Dunø, M.; Joensen, F.; Østergaard, E. A Faroese founder variant in TBCD causes early onset, progressive encephalopathy with a homogenous clinical course. Eur. J. Hum. Genet. 2018, 26, 1512–1520. [Google Scholar] [CrossRef]
- Verdura, E.; Rodríguez-Palmero, A.; Vélez-Santamaria, V.; Planas-Serra, L.; de la Calle, I.; Raspall-Chaure, M.; Roubertie, A.; Benkirane, M.; Saettini, F.; Pavinato, L.; et al. Biallelic PI4KA variants cause a novel neurodevelopmental syndrome with hypomyelinating leukodystrophy. Brain 2021, 144, 2659–2669. [Google Scholar] [CrossRef]
- Salter, C.G.; Cai, Y.; Lo, B.; Helman, G.; Taylor, H.; McCartney, A.; Leslie, J.S.; Accogli, A.; Zara, F.; Traverso, M.; et al. Biallelic PI4KA variants cause neurological, intestinal and immunological disease. Brain 2021, 144, 3597–3610. [Google Scholar] [CrossRef]
- Nothwang, H.G.; Kim, H.; Aoki, J.; Geisterfer, M.; Kübart, S.; Wegner, R.D.; van Moers, A.; Ashworth, L.K.; Haaf, T.; Bell, J.; et al. Functional hemizygosity of PAFAH1B3 due to a PAFAH1B3-CLK2 fusion gene in a female with mental retardation, ataxia and atrophy of the brain. Hum. Mol. Genet. 2001, 10, 797–806. [Google Scholar] [CrossRef]
- Johnson, K.W.; Smith, K.A. Molecular cloning of a novel human cdc2/CDC28-like protein kinase. J. Biol. Chem. 1991, 266, 3402–3407. [Google Scholar] [CrossRef]
- Kuczynska, Z.; Metin, E.; Liput, M.; Buzanska, L. Covering the Role of PGC-1α in the Nervous System. Cells 2021, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.N.; Zhang, M.Q.; Yu, F.L.; Han, B.; Bao, M.Y.; Yan, H.; Li, X.; Zhang, Y. Peroxisom proliferator-activated receptor-gamma coactivator-1alpha in neurodegenerative disorders: A promising therapeutic target. Biochem. Pharmacol. 2023, 215, 115717. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; Technical and Interpretive Manual WISC-IV US. Psychorp: London, UK, 2014. [Google Scholar]
- Sánchez-Monteagudo, A.; Álvarez-Sauco, M.; Sastre, I.; Martínez-Torres, I.; Lupo, V.; Berenguer, M.; Espinós, C. Author response for “Genetics of Wilson disease and Wilson-like phenotype in a clinical series from eastern Spain”. Clin. Genet. 2020, 97, 758–763. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Schymkowitz, J.; Borg, J.; Stricher, F.; Nys, R.; Rousseau, F.; Serrano, L. The FoldX web server: An online force field. Nucleic Acids Res. 2005, 33, W382–W388. [Google Scholar] [CrossRef]
- Venselaar, H.; Beek, T.A.T.; Kuipers, R.K.; Hekkelman, M.L.; Vriend, G. Protein structure analysis of mutations causing inheritable diseases. An e-Science approach with life scientist friendly interfaces. BMC Bioinform. 2010, 11, 548. [Google Scholar] [CrossRef]
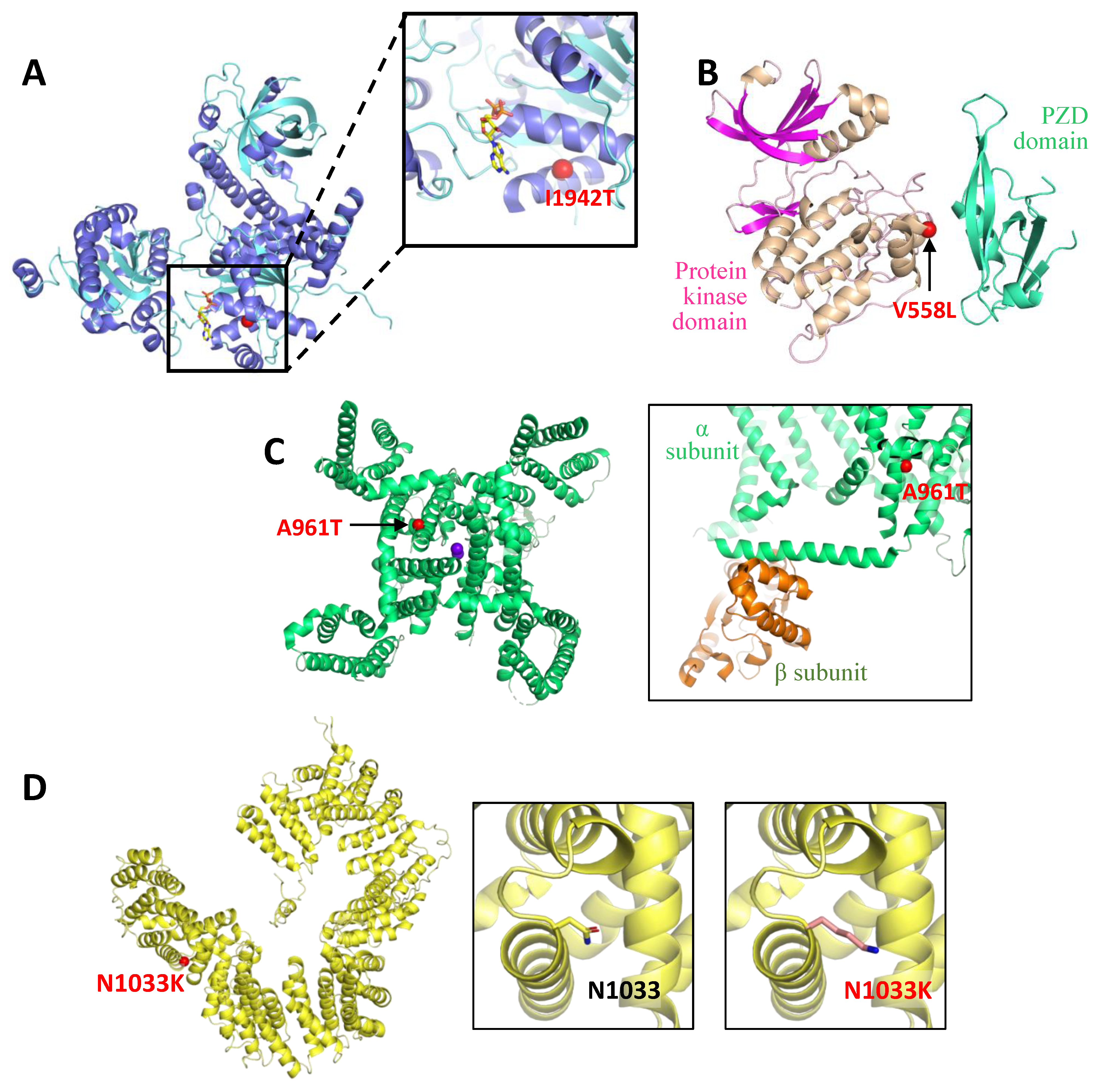

| Patient | Gene | RefSeq | Position † | DNA Change | Protein Change | Prediction ‡ | rs Number (MAF) § | References PMID ‖ | Method |
|---|---|---|---|---|---|---|---|---|---|
| MD-359 | SETX | NM_015046.7 NP_055861.3 | chr9:132297011 | c.5825T>C HOMOZYGOSIS (Consanguinity) | p.I1942T | P | rs773379832 (0.00001195) | 27549087 35872528 33098801 27528516 34426522 | WES-proband |
| MD-353 | CACNA1G | NM_018896.5 NP_061496.2 | chr17:50592063 | c.2881G>A | p.A961T | P | rs886041505 (0) | 29878067 33098379 30842224 32736238 31785789 31836334 | WES-proband |
| MD-309 | CACNA1A | NM_001127221.2 NP_001120693.1 | chr19:13298594 | c.3042C>G | p.Y1014* | P | Novel | Novel | Gene panel |
| MD-556 | CACNA1A | chr19:13505990-13505991 | c.234_235delCT | p.F79Pfs*22 | P | rs762483619 (0.000004023) | NA | WES-trio | |
| MD-471 | CLN6 | NM_017882.3 NP_060352.1 | chr15:68214373 | c.214G>T | p.E72* | P | rs104894483 (0.00006761) | 11791207 35012600 31589614 35505348 25525159 | WES-proband |
| chr15:68208244-68208248 | c.829_836delinsCCT | p.V277Pfs*5 | P | rs1595816474 (NA) | 34440436 35505348 12815591 | ||||
| MD-548 | MAST1 | NM_014975.3 NP_055790.1 | chr19:12865349 | c.1672G>C | p.V558L | P | Novel | Novel | WES-trio |
| MD-392 | CPLANE1 | NM_023073.3 NP_075561.3 | chr5:37164266 | c.7588+7A>G | p.E2512Kfs*18 | P | rs773662834 (0.00002389) | 28431631 | Gene panel |
| chr5:37170413-37215717 | c.2747-1981_6172-78del | p.G916Afs*19 | P | Novel | Novel | ||||
| MD-297 | TBCD | NM_005993.5 NP_005984.3 | chr17:82930629 | c.3099C>G HOMOZYGOSIS (No consanguinity) | p.N1033K | P | rs748615072 (0.000007128) | 29921875 | WES-proband |
| MD-610 | PI4KA | NM_058004.4 NP_477352.3 | chr22:20734450 | c.3845C>T | p.A1282_D1300del | LP | Novel | Novel | WES-trio |
| chr22:20761345 | c.2750T>C | p.F917S | LP | Novel | Novel | ||||
| MD-436 | CLK2 | NM_001294338.2 NP_001281267.1 | chr1:155264494 | c.1120T>C | p.Y374H | LP | Novel | Novel | WES-trio |
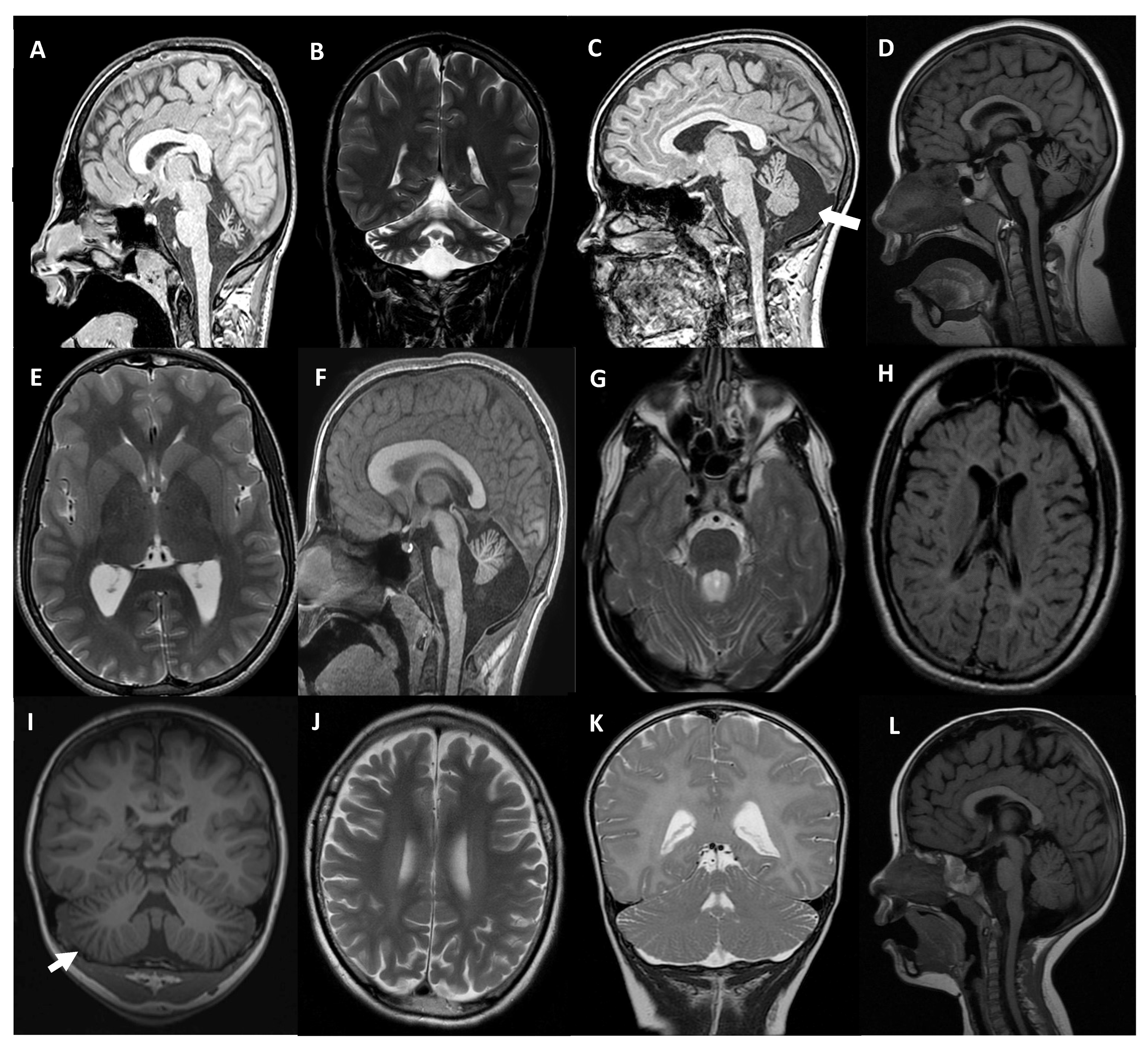
| Patient Sex | Gene | Presentation Inheritance | Disease (OMIM) | Age of Onset Age at Testing | Clinical Presentation | Brain MRI | Cognitive Involvement | Other Neurological Phenotypic Characteristics |
|---|---|---|---|---|---|---|---|---|
| MD-359 female | SETX | Sporadic AR | SCA with axonal neuropathy (606002) | 6 yo 8 yo | Mild truncal ataxia. Bilateral sensorineural deafness. | CA | Average IQ | Learning difficulties |
| MD-353 male | CACNA1G | de novo AD | SCA42 (616795) SCA42 early-onset, severe, with neurodevelopmental deficits (618087) | At birth 17 yo | Congenital arthrogryposis. Ataxia. Gait instability. Dysmetria. | Moderate CA | Moderate Attention deficit | Moderate-severe speech delay. Dystonic head movements. Nystagmus |
| MD-309 male | CACNA1A | de novo AD | Episodic Ataxia type 2 (108500) SCA6 (183086) Developmental and epileptic encephalopathy 42 (617106) Familial hemiplegic migraine type 1 (141500) | 18 mo 12 yo | Gait instability. Basal nystagmus. Dysmetria. Episodes of increased instability that last hours. | Mild vermian atrophy | Mild IQ | Arreflexia |
| MD-556 female | CACNA1A | de novo AD | 6 mo 12 yo | Paroxysmal, episodic, vertigo-like disorder triggered by emotions or fever. Gait instability. | Mild vermian atrophy | Borderline IQ | Strabismus. Mild gait ataxia. EEG abnormalities | |
| MD-471 male | CLN6 | Familial AR | Neuronal Ceroid Lipofuscinosis Type 6A (601780) Type 6B (204300) | 3 yo 5 yo | Dysarthria. Gait instability. ADHD symptoms. | CA White matter T2/FLAIR hyperintensity | Progressive decline | Drop attacks. Visual deficit and gait loss from 6-yo. |
| MD-472 † female | 3 yo 3 yo | Mild stuttering. | Enlarged cerebellar folia, white matter T2/FLAIR hyperintensity | Mild attention deficit | Gait instability, loss of speech and cognitive decline from 4-yo. | |||
| MD-548 male | MAST1 | de novo AD | Mega-corpus-callosum syndrome with cerebellar hypoplasia and cortical malformations (618273) | At birth 13 yo | Ataxia. Lack of speech. | Megacorpus callosum with CA | Severe ID | Absence of language. Mild facial dysmorphic features. Tented upper lip, pointed palate |
| MD-392 male | CPLANE1 | Sporadic AR | Joubert syndrome 17 (614615) | 18 mo 17 yo | Motor delay (independent walk 2 yo). | Molar tooth sign. Cerebellar hypoplasia | Borderline IQ | Ataxia. Oculomotor apraxia. ADHD. |
| MD-297 female | TBCD | Sporadic AR | Early-onset progressive encephalopathy with brain atrophy and thin corpus callosum (PEBAT) (617193) | 15 mo 18 yo | Seizures. Motor impairment. | Progressive CA | Severe ID | Encephalopathy Spastic-dystonic tetraparesis |
| MD-610 male | PI4KA | Sporadic AR | Neurodevelopmental disorder with spasticity, hypomyelinating leukodystrophy, and brain abnormalities (NEDSPLB) (616531) | 20 mo 36 mo | Acute ataxia. | Diffuse hypomyelination Enlarged cerebellar folia | No | Progressive lower limbs spasticity |
| MD-436 female | CLK2 | de novo AD | - | 17 mo 5 yo | Motor delay. Gait instability. Oculomotor apraxia. | Subtle enlargement of vermian folia | Borderline IQ | Global improvement over age. Language delay |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Rubio, D.; Hinarejos, I.; Argente-Escrig, H.; Marco-Marín, C.; Lozano, M.A.; Gorría-Redondo, N.; Lupo, V.; Martí-Carrera, I.; Miranda, C.; Vázquez-López, M.; et al. Genetic Heterogeneity Underlying Phenotypes with Early-Onset Cerebellar Atrophy. Int. J. Mol. Sci. 2023, 24, 16400. https://doi.org/10.3390/ijms242216400
Martínez-Rubio D, Hinarejos I, Argente-Escrig H, Marco-Marín C, Lozano MA, Gorría-Redondo N, Lupo V, Martí-Carrera I, Miranda C, Vázquez-López M, et al. Genetic Heterogeneity Underlying Phenotypes with Early-Onset Cerebellar Atrophy. International Journal of Molecular Sciences. 2023; 24(22):16400. https://doi.org/10.3390/ijms242216400
Chicago/Turabian StyleMartínez-Rubio, Dolores, Isabel Hinarejos, Herminia Argente-Escrig, Clara Marco-Marín, María Ana Lozano, Nerea Gorría-Redondo, Vincenzo Lupo, Itxaso Martí-Carrera, Concepción Miranda, María Vázquez-López, and et al. 2023. "Genetic Heterogeneity Underlying Phenotypes with Early-Onset Cerebellar Atrophy" International Journal of Molecular Sciences 24, no. 22: 16400. https://doi.org/10.3390/ijms242216400
APA StyleMartínez-Rubio, D., Hinarejos, I., Argente-Escrig, H., Marco-Marín, C., Lozano, M. A., Gorría-Redondo, N., Lupo, V., Martí-Carrera, I., Miranda, C., Vázquez-López, M., García-Pérez, A., Marco-Hernández, A. V., Tomás-Vila, M., Aguilera-Albesa, S., & Espinós, C. (2023). Genetic Heterogeneity Underlying Phenotypes with Early-Onset Cerebellar Atrophy. International Journal of Molecular Sciences, 24(22), 16400. https://doi.org/10.3390/ijms242216400






