Identification of Functional Brassinosteroid Receptor Genes in Oaks and Functional Analysis of QmBRI1
Abstract
:1. Introduction
2. Results
2.1. Identification of Functional BR Receptor Genes in Oaks
2.2. Evolution Analysis of Oak Functional BR Receptor Genes
2.3. Transcript Levels of Functional BR Receptors in Q. mongolica
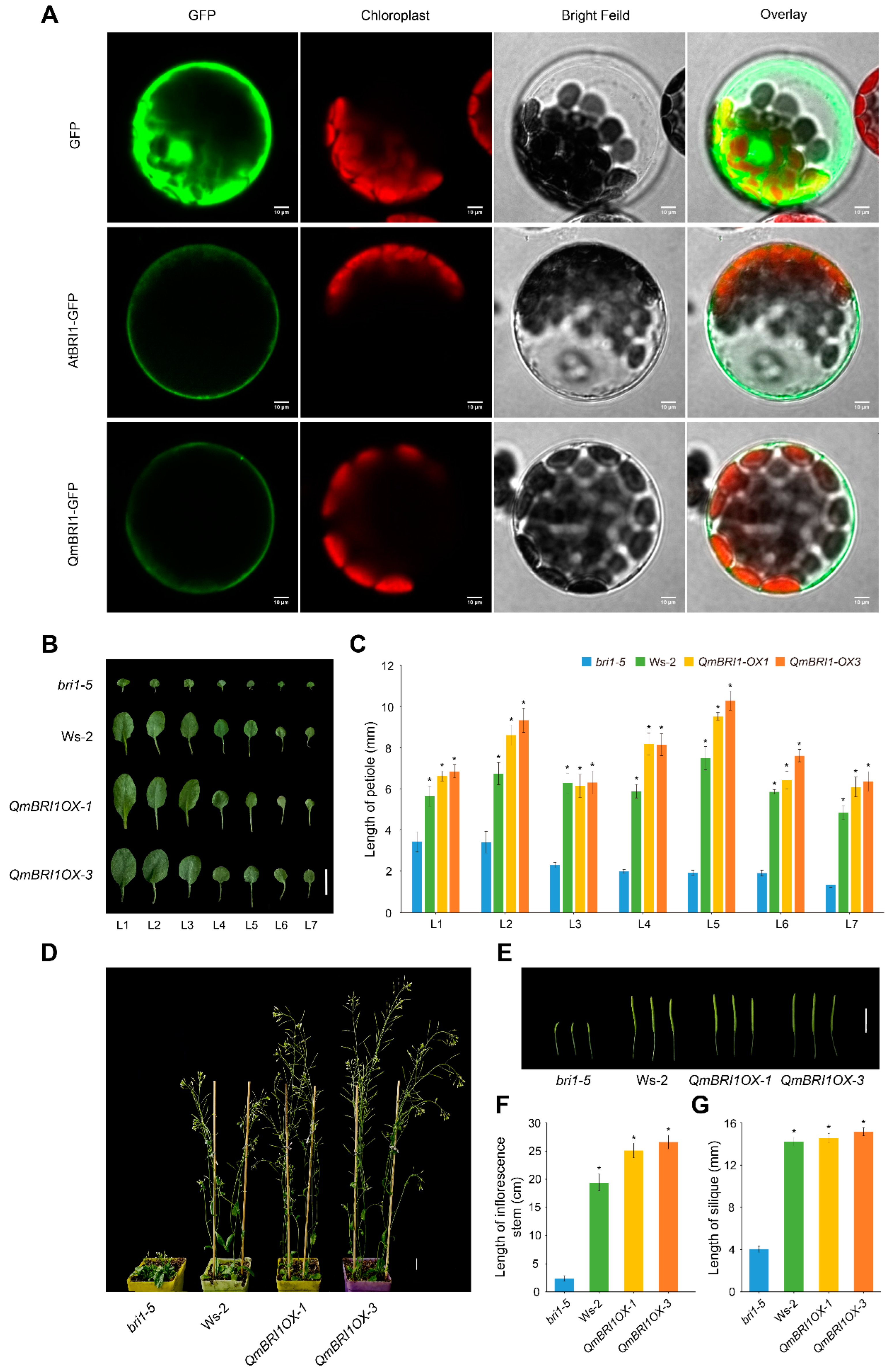
2.4. Subcellular Localization of QmBRI1
2.5. Ectopic Expression of QmBRI1 Restores Growth Retardation in Bri1-5 Mutants
2.6. Overexpression of QmBRI1 in the Bri1-5 Mutant Increases Vascular Bundle Numbers and Xylem Differentiation
3. Discussion
4. Materials and Methods
4.1. Identification and Bioinformatics Analysis of BR Receptor Genes
4.2. Vector Construction, Subcellular Localization, and Plant Transformation
4.3. Plant Growth Conditions, Histological Analysis, and Phenotypic Statistics
4.4. RNA Extraction and qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Z.; Fujioka, S.; Blancaflor, E.B.; Miao, S.; Gou, X.; Li, J. TCP1 modulates brassinosteroid biosynthesis by regulating the expression of the key biosynthetic gene DWARF4 in Arabidopsis thaliana. Plant Cell 2010, 22, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [PubMed]
- De Rybel, B.; Mähönen, A.P.; Helariutta, Y.; Weijers, D. Plant vascular development: From early specification to differentiation. Nat. Rev. Mol. Cell Biol. 2016, 17, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Girard, M.; Morin, H. Lengthening of the duration of xylogenesis engenders disproportionate increases in xylem production. Glob. Chang. Biol. 2014, 20, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Han, S.; Lee, H.; Jeong, B.; Heo, T.; Hyun, T.K.; Kim, K.; Je, B.I.; Lee, H.; Shim, D.; et al. Brassinosteroids facilitate xylem differentiation and wood formation in tomato. Planta 2019, 249, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Cano-Delgado, A.; Seto, H.; Hiranuma, S.; Fujioka, S.; Yoshida, S.; Chory, J. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 2005, 433, 164–167. [Google Scholar] [CrossRef]
- Cano-Delgado, A.; Yin, Y.; Yu, C.; Vafeados, D.; Mora-Garcia, S.; Cheng, J.; Nam, K.H.; Li, J.; Chory, J. BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 2004, 131, 5341–5351. [Google Scholar] [CrossRef]
- Nakamura, A.; Fujioka, S.; Sunohara, H.; Kamiya, N.; Hong, Z.; Inukai, Y.; Miura, K.; Takatsuto, S.; Yoshida, S.; Ueguchi-Tanaka, M.; et al. The role of OsBRI1 and its homologous genes, OsBRL1 and OsBRL3, in rice. Plant Physiol. 2006, 140, 580–590. [Google Scholar] [CrossRef]
- Lozano-Elena, F.; Caño-Delgado, A.I. Emerging roles of vascular brassinosteroid receptors of the BRI1-like family. Curr. Opin. Plant Biol. 2019, 51, 105–113. [Google Scholar] [CrossRef]
- Holton, N.; Caño-Delgado, A.; Harrison, K.; Montoya, T.; Chory, J.; Bishop, G.J. Tomato BRASSINOSTEROID INSENSITIVE1 is required for systemin-induced root elongation in Solanum pimpinellifolium but is not essential for wound signaling. Plant Cell 2007, 19, 1709–1717. [Google Scholar] [CrossRef]
- Peng, S.; Tao, P.; Xu, F.; Wu, A.; Huo, W.; Wang, J. Functional characterization of soybean Glyma04g39610 as a brassinosteroid receptor gene and evolutionary analysis of soybean brassinosteroid receptors. Int. J. Mol. Sci. 2016, 17, 897. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Huang, S.; Wang, S.; Mao, Y.; Liu, J.; Ma, R.; Wang, X. Enhanced brassinosteroid signaling intensity via SlBRI1 overexpression negatively regulates drought resistance in a manner opposite of that via exogenous BR application in tomato. Plant Physiol. Biochem. 2019, 138, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, N.; Geng, Z.; Ji, M.; Wang, S.; Zhuang, Y.; Wang, D.; He, G.; Zhao, S.; Zhou, G.; et al. Integrated transcriptome and proteome analysis reveals brassinosteroid-mediated regulation of cambium initiation and patterning in woody stem. Hortic. Res. 2022, 9, b48. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, D.; Szarejko, I.; Maluszynski, M. New allele of HvBRI1 gene encoding brassinosteroid receptor in barley. J. Appl. Genet. 2011, 52, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Tang, S.; Zhang, Y.; Yue, J.; Xu, J.; Tang, W.; Sun, Y.; Wang, R.; Diao, X.; Zhang, B. Evolutionary analysis and functional characterization of SiBRI1 as a brassinosteroid receptor gene in foxtail millet. BMC Plant Biol. 2021, 21, 291. [Google Scholar] [CrossRef]
- Plomion, C.; Aury, J.M.; Amselem, J.; Leroy, T.; Murat, F.; Duplessis, S.; Faye, S.; Francillonne, N.; Labadie, K.; Le Provost, G.; et al. Oak genome reveals facets of long lifespan. Nat. Plants 2018, 4, 440–452. [Google Scholar] [CrossRef]
- Sork, V.L.; Cokus, S.J.; Fitz-Gibbon, S.T.; Zimin, A.V.; Puiu, D.; Garcia, J.A.; Gugger, P.F.; Henriquez, C.L.; Zhen, Y.; Lohmueller, K.E.; et al. High-quality genome and methylomes illustrate features underlying evolutionary success of oaks. Nat. Commun. 2022, 13, 2047. [Google Scholar] [CrossRef]
- Kudo, K.; Utsumi, Y.; Kuroda, K.; Yamagishi, Y.; Nabeshima, E.; Nakaba, S.; Yasue, K.; Takata, K.; Funada, R. Formation of new networks of earlywood vessels in seedlings of the deciduous ring-porous hardwood Quercus serrata in springtime. Trees 2018, 32, 725–734. [Google Scholar] [CrossRef]
- Ai, W.; Liu, Y.; Mei, M.; Zhang, X.; Tan, E.; Liu, H.; Han, X.; Zhan, H.; Lu, X. A chromosome-scale genome assembly of the Mongolian oak (Quercus mongolica). Mol. Ecol. Resour. 2022, 22, 2396–2410. [Google Scholar] [CrossRef]
- Plomion, C.; Aury, J.; Amselem, J.; Alaeitabar, T.; Barbe, V.; Belser, C.; Bergès, H.; Bodénès, C.; Boudet, N.; Boury, C.; et al. Decoding the oak genome: Public release of sequence data, assembly, annotation and publication strategies. Mol. Ecol. Resour. 2016, 16, 254–265. [Google Scholar] [CrossRef]
- Sork, V.L.; Fitz-Gibbon, S.T.; Puiu, D.; Crepeau, M.; Gugger, P.F.; Sherman, R.; Stevens, K.; Langley, C.H.; Pellegrini, M.; Salzberg, S.L. First draft assembly and annotation of the genome of a California endemic oak Quercus lobata Née (Fagaceae). G3 2016, 6, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.M.; Usié, A.; Barbosa, P.; Barros, P.M.; Capote, T.; Chaves, I.; Simões, F.; Abreu, I.; Carrasquinho, I.; Faro, C.; et al. The draft genome sequence of cork oak. Sci. Data 2018, 5, 180069. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, N.; Jiang, X.; Qin, Z.; Farooq, T.H.; Cao, F.; Li, H. A chromosome-scale genome assembly of Quercus gilva: Insights into the evolution of Quercus section Cyclobalanopsis (Fagaceae). Front. Plant Sci. 2022, 13, 1012277. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wang, L.; Xian, Y.; Xie, X.; Li, W.; Zhao, Y.; Zhang, R.; Qin, X.; Li, D.; Jia, K. A chromosome-level genome assembly of the Chinese cork oak (Quercus variabilis). Front. Plant Sci. 2022, 13, 1001583. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Zhu, Y.; Liu, Y.; Feng, Y.; Lu, R.; Li, Y.; Li, P.; Kremer, A.; Lascoux, M.; Chen, J. Genome-wide analyses of introgression between two sympatric Asian oak species. Nat. Ecol. Evol. 2022, 6, 924–935. [Google Scholar] [CrossRef]
- Byng, J.W.; Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Judd, W.S.; Mabberley, D.J.; Sennikov, A.N.; Soltis, D.E.; Soltis, P.S.; Stevens, P.F. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Noguchi, T.T.; Fujioka, S.S.; Choe, S.S.; Takatsuto, S.S.; Yoshida, S.S.; Yuan, H.H.; Feldmann, K.A.K.A.; Tax, F.E. Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol. 1999, 121, 743–752. [Google Scholar] [CrossRef]
- Wang, M.; Sun, S.; Wu, C.; Han, T.; Wang, Q. Isolation and characterization of the brassinosteroid receptor gene (GmBRI1) from Glycine max. Int. J. Mol. Sci. 2014, 15, 3871–3888. [Google Scholar] [CrossRef]
- Lehmann, F.; Hardtke, C.S. Secondary growth of the Arabidopsis hypocotyl-vascular development in dimensions. Curr. Opin. Plant Biol. 2016, 29, 9–15. [Google Scholar] [CrossRef]
- Baima, S.; Possenti, M.; Matteucci, A.; Wisman, E.; Altamura, M.M.; Ruberti, I.; Morelli, G. The Arabidopsis ATHB-8 HD-Zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol. 2001, 126, 643–655. [Google Scholar] [CrossRef]
- Ibanes, M.; Fabregas, N.; Chory, J.; Cano-Delgado, A.I. Brassinosteroid signaling and auxin transport are required to establish the periodic pattern of Arabidopsis shoot vascular bundles. Proc. Natl. Acad. Sci. USA 2009, 106, 13630–13635. [Google Scholar] [CrossRef]
- Denk, T.; Grimm, G.W.; Manos, P.S.; Deng, M.; Hipp, A.L. An updated infrageneric classification of the oaks: Review of previous taxonomic schemes and synthesis of evolutionary patterns. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Springer International Publishing: Cham, Switzerland, 2017; pp. 13–38. [Google Scholar]
- Wang, H.; Mao, H. On the origin and evolution of plant brassinosteroid receptor kinases. J. Mol. Evol. 2014, 78, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Guerra, M.; Marquès-Bueno, M.; Mora-García, S.; Caño-Delgado, A.I. Delving into the evolutionary origin of steroid sensing in plants. Curr. Opin. Plant Biol. 2020, 57, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, B.; Song, Z.; Zhang, Y.; Yu, C.; Wang, H.; Wang, L.; Zhang, H. PtBRI1.2 promotes shoot growth and wood formation through a brassinosteroid-mediated PtBZR1-PtWNDs module in poplar. J. Exp. Bot. 2021, 72, 6350–6364. [Google Scholar] [CrossRef]
- Zhou, A.; Wang, H.; Walker, J.C.; Li, J. BRL1, a leucine-rich repeat receptor-like protein kinase, is functionally redundant with BRI1 in regulating Arabidopsis brassinosteroid signaling. Plant J. 2004, 40, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Sun, S.; Wang, L.; Wu, Z.; Li, C.; Li, X.; Wang, T.; Leng, L.; Tian, W.; Lu, T.; et al. The RLA1/SMOS1 transcription factor functions with OsBZR1 to regulate brassinosteroid signaling and rice architecture. Plant Cell 2017, 29, 292–309. [Google Scholar] [CrossRef]
- Lamesch, P.; Berardini, T.Z.; Li, D.; Swarbreck, D.; Wilks, C.; Sasidharan, R.; Muller, R.; Dreher, K.; Alexander, D.L.; Garcia-Hernandez, M.; et al. The Arabidopsis Information Resource (TAIR): Improved gene annotation and new tools. Nucleic Acids Res. 2012, 40, D1202–D1210. [Google Scholar] [CrossRef]
- Munz, M.; Tönnies, S.; Balke, W.; Simon, E. Multidimensional gene search with Genehopper. Nucleic Acids Res. 2015, 43, W98–W103. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Chou, K.; Shen, H. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef] [PubMed]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, J.; Tsirigos, K.; Pedersen, M.D.; Jose, J.A.A.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2022. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.A.; Nishiyama, T.; Hasebe, M.; Bowman, J.L.; Gribskov, M.; DePamphilis, C.; Albert, V.A.; Aono, N.; Aoyama, T.; Ambrose, B.A.; et al. The selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science 2011, 6032, 960–963. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Zhu, W.; Hamilton, J.; Lin, H.; Campbell, M.; Childs, K.; Thibaud-Nissen, F.; Malek, R.L.; Lee, Y.; Zheng, L.; et al. The TIGR rice genome annotation resource: Improvements and new features. Nucleic Acids Res. 2007, 35, D883–D887. [Google Scholar] [CrossRef]
- Schnable, P.S.; Ware, D.; Fulton, R.S.; Stein, J.C.; Wei, F.; Pasternak, S.; Liang, C.; Zhang, J.; Fulton, L.; Graves, T.A.; et al. The B73 maize genome: Complexity, diversity, and dynamics. Science 2009, 326, 1112–1115. [Google Scholar] [CrossRef]
- The Tomato Genome Consortium. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- Tuskan, G.A.; DiFazio, S.; Jansson, S.; Bohlmann, J.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; Schein, J.; et al. Genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Ri, H.; An, Z.; Wang, X.; Zhou, J.; Zheng, D.; Wu, H.; Wang, P.; Yang, J.; et al. Deletion and tandem duplications of biosynthetic genes drive the diversity of triterpenoids in Aralia elata. Nat. Commun. 2022, 13, 2224. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Zhao, Y.; Du, W.; Lu, Y.; Gui, L.; Zheng, Z.; Yu, H.; Cui, Y.; Liu, Z.; Cui, T.; et al. Pseudomolecule-level assembly of the Chinese oil tree yellowhorn (Xanthoceras sorbifolium) genome. Gigascience 2019, 8, giz070. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Su, P.; Zhang, Z.; Gao, L.; Wang, J.; Hu, T.; Zhou, J.; Zhang, Y.; Zhao, Y.; Liu, Y.; et al. Genome of tripterygium wilfordii and identification of cytochrome P450 involved in triptolide biosynthesis. Nat. Commun. 2020, 11, 912–971. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, Y.; Zhang, L.; Ji, Y.; Tan, D.; Yuan, H.; Wang, A. The jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell 2017, 29, 1316–1334. [Google Scholar] [CrossRef]
- Yoo, S.; Cho, Y.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Cal. Agric. Exp. Station Circ. 1950, 347, 1–32. [Google Scholar]
- Mei, M.; Ai, W.; Liu, L.; Xu, X.; Lu, X. Genome-wide identification of the auxin response factor (ARF) gene family in Magnolia sieboldii and functional analysis of MsARF5. Front. Plant Sci. 2022, 13, 958816. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Sergeeva, L.; Ligterink, W.; Aloni, R.; Zemach, H.; Doron-Faigenboim, A.; Yang, J.; Zhang, P.; Shabtai, S.; Firon, N. Gibberellin promotes sweetpotato root vascular lignification and reduces storage-root formation. Front. Plant Sci. 2019, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Kawakatsu, T.; Itoh, J.; Miyoshi, K.; Kurata, N.; Alvarez, N.; Veit, B.; Nagato, Y. PLASTOCHRON2 regulates leaf initiation and maturation in rice. Plant Cell 2006, 18, 612–625. [Google Scholar] [CrossRef]
- Zheng, X.; Kang, S.; Jing, Y.; Ren, Z.; Li, L.; Zhou, J.; Berkowitz, G.; Shi, J.; Fu, A.; Lan, W.; et al. Danger-associated peptides close stomata by OST1-independent activation of anion channels in guard cells. Plant Cell 2018, 30, 1132–1146. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



| Name | Gene ID | AA (aa) | SP (aa) | TM (aa) | Mw (kDa) | pI | II | AI | GRAVY |
|---|---|---|---|---|---|---|---|---|---|
| Quercus mongolica | |||||||||
| QmBRI1 | Qm025300 | 1189 | 1–24 | 791–809 | 130.09 | 6.07 | 37.50 | 98.40 | −0.048 |
| QmBRL1 | Qm013727 | 1221 | 1–37 | 835–855 | 132.37 | 5.77 | 35.94 | 97.67 | −0.002 |
| QmBRL2 | Qm012212 | 1133 | 1–29 | 755–775 | 124.57 | 6.01 | 31.92 | 101.21 | −0.062 |
| Quercus lobata | |||||||||
| QlBRI1 | QL06p031490 | 1189 | 1–24 | 791–809 | 129.94 | 5.99 | 36.76 | 98.32 | −0.046 |
| QlBRL1 | QL08p000343 | 1221 | 1–37 | 835–855 | 132.34 | 5.82 | 36.46 | 98.07 | 0.001 |
| QlBRL2 | QL03p039295 | 1133 | 1–29 | 755–775 | 124.50 | 5.94 | 32.33 | 101.21 | −0.058 |
| Quercus suber | |||||||||
| QsBRI1 | XP_023879012.1 | 1189 | 1–24 | 791–809 | 129.82 | 5.91 | 36.44 | 97.34 | −0.051 |
| QsBRL1 | XP_023887804.1 | 1221 | 1–37 | 835–855 | 132.42 | 5.66 | 36.66 | 98.30 | −0.000 |
| QsBRL2 | XP_023912129.1 | 1133 | 1–29 | 755–775 | 124.46 | 5.94 | 31.77 | 100.94 | −0.067 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, W.; Liu, H.; Wang, Y.; Wang, Y.; Wei, J.; Zhang, X.; Lu, X. Identification of Functional Brassinosteroid Receptor Genes in Oaks and Functional Analysis of QmBRI1. Int. J. Mol. Sci. 2023, 24, 16405. https://doi.org/10.3390/ijms242216405
Ai W, Liu H, Wang Y, Wang Y, Wei J, Zhang X, Lu X. Identification of Functional Brassinosteroid Receptor Genes in Oaks and Functional Analysis of QmBRI1. International Journal of Molecular Sciences. 2023; 24(22):16405. https://doi.org/10.3390/ijms242216405
Chicago/Turabian StyleAi, Wanfeng, Hanzhang Liu, Yutao Wang, Yu Wang, Jun Wei, Xiaolin Zhang, and Xiujun Lu. 2023. "Identification of Functional Brassinosteroid Receptor Genes in Oaks and Functional Analysis of QmBRI1" International Journal of Molecular Sciences 24, no. 22: 16405. https://doi.org/10.3390/ijms242216405
APA StyleAi, W., Liu, H., Wang, Y., Wang, Y., Wei, J., Zhang, X., & Lu, X. (2023). Identification of Functional Brassinosteroid Receptor Genes in Oaks and Functional Analysis of QmBRI1. International Journal of Molecular Sciences, 24(22), 16405. https://doi.org/10.3390/ijms242216405






