The Role of Insulin-like Growth Factor I in Mechanisms of Resilience and Vulnerability to Sporadic Alzheimer’s Disease
Abstract
:1. Introduction
2. IGF-I and AD Resilience
Mechanisms of IGF-I-Dependent AD Resilience
3. IGF-I and AD Risk
3.1. Old Age
3.2. Type 2 Diabetes
3.3. Imbalanced Diet
3.4. Sedentary Life
3.5. Low Education
3.6. Stroke
3.7. Post-Traumatic Stress Disorder
3.8. ApoE4
3.9. Traumatic Brain Injury
4. Outlook
5. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Mayeux, R. Epidemiology of neurodegeneration. Annu. Rev. Neurosci. 2003, 26, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Citron, M.; Oltersdorf, T.; Haass, C.; McConlogue, L.; Hung, A.Y.; Seubert, P.; Vigo-Pelfrey, C.; Lieberburg, I.; Selkoe, D.J. Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature 1992, 360, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, R.; Rogaev, E.I.; Liang, Y.; Rogaeva, E.A.; Levesque, G.; Ikeda, M.; Chi, H.; Lin, C.; Li, G.; Holman, K.; et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 1995, 375, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Ryman, D.C.; Acosta-Baena, N.; Aisen, P.S.; Bird, T.; Danek, A.; Fox, N.C.; Goate, A.; Frommelt, P.; Ghetti, B.; Langbaum, J.B.; et al. Symptom onset in autosomal dominant Alzheimer disease: A systematic review and meta-analysis. Neurology 2014, 83, 253–260. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Udeochu, J.C.; Amin, S.; Huang, Y.; Fan, L.; Torres, E.R.S.; Carling, G.K.; Liu, B.; McGurran, H.; Coronas-Samano, G.; Kauwe, G.; et al. Tau activation of microglial cGAS–IFN reduces MEF2C-mediated cognitive resilience. Nat. Neurosci. 2023, 26, 737–750. [Google Scholar] [CrossRef]
- Barker, S.J.; Raju, R.M.; Milman, N.E.P.; Wang, J.; Davila-Velderrain, J.; Gunter-Rahman, F.; Parro, C.C.; Bozzelli, P.L.; Abdurrob, F.; Abdelaal, K.; et al. MEF2 is a key regulator of cognitive potential and confers resilience to neurodegeneration. Sci. Transl. Med. 2021, 13, eabd7695. [Google Scholar] [CrossRef]
- Vogel, J.W.; Vachon-Presseau, E.; Pichet Binette, A.; Tam, A.; Orban, P.; La Joie, R.; Savard, M.; Picard, C.; Poirier, J.; Bellec, P.; et al. Brain properties predict proximity to symptom onset in sporadic Alzheimer’s disease. Brain 2018, 141, 1871–1883. [Google Scholar] [CrossRef]
- Arenaza-Urquijo, E.M.; Vemuri, P. Resistance vs resilience to Alzheimer disease: Clarifying terminology for preclinical studies. Neurology 2018, 90, 695–703. [Google Scholar] [CrossRef]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Kosik, K.S. PErsonalized medicine for effective alzheimer disease treatment. JAMA Neurol. 2015, 72, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.V.; Subramaniam, K.G.; Gregory, J.; Bredesen, A.L.; Coward, C.; Okada, S.; Kelly, L.; Bredesen, D.E. Rationale for a Multi-Factorial Approach for the Reversal of Cognitive Decline in Alzheimer’s Disease and MCI: A Review. Int. J. Mol. Sci. 2023, 24, 1659. [Google Scholar] [CrossRef]
- Aisen, P.S.; Cummings, J.; Jack, C.R., Jr.; Morris, J.C.; Sperling, R.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimer’s Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef]
- Carro, E.; Torres-Aleman, I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur. J. Pharmacol. 2004, 490, 127–133. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Santi, A.; Torres Aleman, I. Insulin peptides as mediators of the impact of life style in Alzheimer’s disease. Brain Plast. 2018, 4, 3–15. [Google Scholar] [CrossRef]
- Almeida, O.P.; Hankey, G.J.; Yeap, B.B.; Paul Chubb, S.A.; Gollege, J.; Flicker, L. Risk of prevalent and incident dementia associated with insulin-like growth factor and insulin-like growth factor-binding protein 3. Mol. Psychiatry 2018, 23, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, R.F.; Janssen, J.A.; Brugts, M.P.; van Duijn, C.M.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A. Insulin-Like Growth Factor-I Receptor Stimulating Activity is Associated with Dementia. J. Alzheimer’s Dis. 2014, 42, 137–142. [Google Scholar] [CrossRef]
- Zegarra-Valdivia, J.A.; Santi, A.; Fernandez de Sevilla, M.E.; Nunez, A.; Torres Aleman, I. Serum Insulin-Like Growth Factor I Deficiency Associates to Alzheimer’s Disease Co-Morbidities. J. Alzheimer’s Dis. 2019, 69, 979–987. [Google Scholar] [CrossRef]
- Amtul, Z.; Hill, D.J.; Arany, E.J.; Cechetto, D.F. Altered Insulin/Insulin-Like Growth Factor Signaling in a Comorbid Rat model of Ischemia and β-Amyloid Toxicity. Sci. Rep. 2018, 8, 5136. [Google Scholar] [CrossRef]
- Galle, S.A.; Geraedts, I.K.; Deijen, J.B.; Milders, M.V.; Drent, M.L. The Interrelationship between Insulin-Like Growth Factor 1, Apolipoprotein E ε4, Lifestyle Factors, and the Aging Body and Brain. J. Prev. Alzheimer’s Dis. 2020, 7, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Aberg, M.A.; Aberg, N.D.; Hedbacker, H.; Oscarsson, J.; Eriksson, P.S. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 2000, 20, 2896–2903. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.M.; de la Vega, A.G.; Torres-Aleman, I. Insulin-like growth factor I restores motor coordination in a rat model of cerebellar ataxia. Proc. Natl. Acad. Sci. USA 1998, 95, 1253–1258. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Jimenez, S.; Mecha, M.; Davila, D.; Guaza, C.; Vitorica, J.; Torres-Aleman, I. Regulation of the phosphatase calcineurin by insulin-like growth factor I unveils a key role of astrocytes in Alzheimer’s pathology. Mol. Psychiatry 2012, 17, 705–718. [Google Scholar] [CrossRef]
- Heck, S.; Lezoualc’h, F.; Engert, S.; Behl, C. Insulin-like growth factor-1-mediated neuroprotection against oxidative stress is associated with activation of nuclear factor kappaB. J. Biol. Chem. 1999, 274, 9828–9835. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.M.; Reinhardt, R.R.; Lee, W.H.; Joncas, G.; Patel, S.C.; Bondy, C.A. Insulin-like growth factor 1 regulates developing brain glucose metabolism. Proc. Natl. Acad. Sci. USA 2000, 97, 10236–10241. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Torres-Aleman, I. The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 2012, 13, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Z.; Shang, C.; Yan, F.; Shi, Y.; Zhang, J.; Qu, B.; Han, H.; Wang, Y.; Li, D.; et al. IGF1-Dependent Synaptic Plasticity of Mitral Cells in Olfactory Memory during Social Learning. Neuron 2017, 95, 106–122.e5. [Google Scholar] [CrossRef] [PubMed]
- Trejo, J.L.; Piriz, J.; Llorens-Martin, M.V.; Fernandez, A.M.; Bolos, M.; LeRoith, D.; Nunez, A.; Torres-Aleman, I. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol. Psychiatry 2007, 12, 1118–1128. [Google Scholar] [CrossRef]
- Santi, A.; Bot, M.; Aleman, A.; Penninx, B.W.J.H.; Aleman, I.T. Circulating insulin-like growth factor I modulates mood and is a biomarker of vulnerability to stress: From mouse to man. Transl. Psychiatry 2018, 8, 142. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Hernandez, E.; Guerrero-Gomez, D.; Miranda-Vizuete, A.; Torres Aleman, I. A network of insulin peptides regulate glucose uptake by astrocytes: Potential new druggable targets for brain hypometabolism. Neuropharmacology 2018, 136 Pt B, 216–222. [Google Scholar] [CrossRef]
- Zegarra-Valdivia, J.A.; Pignatelli, J.; Fernandez de Sevilla, M.E.; Fernandez, A.M.; Munive, V.; Martinez-Rachadell, L.; Nunez, A.; Torres Aleman, I. Insulin-like growth factor I modulates sleep through hypothalamic orexin neurons. FASEB J. 2020, 34, 15975–15990. [Google Scholar] [CrossRef]
- Chaudhari, A.; Gupta, R.; Patel, S.; Velingkaar, N.; Kondratov, R. Cryptochromes regulate IGF-1 production and signaling through control JAK2 dependent STAT5B phosphorylation. Mol. Biol. Cell 2017, 28, 834–842. [Google Scholar] [CrossRef]
- Aberg, M.A.; Aberg, N.D.; Palmer, T.D.; Alborn, A.M.; Carlsson-Skwirut, C.; Bang, P.; Rosengren, L.E.; Olsson, T.; Gage, F.H.; Eriksson, P.S. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol. Cell Neurosci. 2003, 24, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.M.; Fernandez, S.; Carrero, P.; Garcia-Garcia, M.; Torres-Aleman, I. Calcineurin in reactive astrocytes plays a key role in the interplay between proinflammatory and anti-inflammatory signals. J. Neurosci. 2007, 27, 8745–8756. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, A.E.; Zigmond, M.J.; Smith, A.D. IGF-1 protects dopamine neurons against oxidative stress: Association with changes in phosphokinases. Exp. Brain Res. 2016, 234, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Noriega-Prieto, J.A.; Maglio, L.E.; Zegarra-Valdivia, J.A.; Pignatelli, J.; Fernandez, A.M.; Martinez-Rachadell, L.; Fernandes, J.; Nunez, A.; Araque, A.; Torres-Aleman, I.; et al. Astrocytic IGF-IRs Induce Adenosine-Mediated Inhibitory Downregulation and Improve Sensory Discrimination. J. Neurosci. 2021, 41, 4768–4781. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Garzon, E.; Fernandez, A.M.; Perez-Alvarez, A.; Genis, L.; Bascunana, P.; Fernandez de la Rosa, R.; Delgado, M.; Angel Pozo, M.; Moreno, E.; McCormick, P.J.; et al. The insulin-like growth factor I receptor regulates glucose transport by astrocytes. Glia 2016, 64, 1962–1971. [Google Scholar] [CrossRef]
- Gómez-Isla, T.; Frosch, M.P. Lesions without symptoms: Understanding resilience to Alzheimer disease neuropathological changes. Nat. Rev. Neurol. 2022, 18, 323–332. [Google Scholar] [CrossRef]
- Eissman, J.M.; Dumitrescu, L.; Mahoney, E.R.; Smith, A.N.; Mukherjee, S.; Lee, M.L.; Scollard, P.; Choi, S.E.; Bush, W.S.; Engelman, C.D.; et al. Sex differences in the genetic architecture of cognitive resilience to Alzheimer’s disease. Brain 2022, 145, 2541–2554. [Google Scholar] [CrossRef]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef] [PubMed]
- Lopera, F.; Marino, C.; Chandrahas, A.S.; O’Hare, M.; Villalba-Moreno, N.D.; Aguillon, D.; Baena, A.; Sanchez, J.S.; Vila-Castelar, C.; Ramirez Gomez, L.; et al. Resilience to autosomal dominant Alzheimer’s disease in a Reelin-COLBOS heterozygous man. Nat. Med. 2023, 29, 1243–1252. [Google Scholar] [CrossRef]
- Lesuis, S.L.; Hoeijmakers, L.; Korosi, A.; de Rooij, S.R.; Swaab, D.F.; Kessels, H.W.; Lucassen, P.J.; Krugers, H.J. Vulnerability and resilience to Alzheimer’s disease: Early life conditions modulate neuropathology and determine cognitive reserve. Alzheimer’s Res. Ther. 2018, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.S.; Blazey, T.; Metcalf, N.V.; McAvoy, M.P.; Strain, J.F.; Rahmani, M.; Durbin, T.J.; Xiong, C.; Benzinger, T.L.; Morris, J.C.; et al. Brain aerobic glycolysis and resilience in Alzheimer disease. Proc. Natl. Acad. Sci. USA 2023, 120, e2212256120. [Google Scholar] [CrossRef] [PubMed]
- Fracassi, A.; Marcatti, M.; Tumurbaatar, B.; Woltjer, R.; Moreno, S.; Taglialatela, G. TREM2-induced activation of microglia contributes to synaptic integrity in cognitively intact aged individuals with Alzheimer’s neuropathology. Brain Pathol. 2023, 33, e13108. [Google Scholar] [CrossRef]
- Boros, B.D.; Greathouse, K.M.; Gentry, E.G.; Curtis, K.A.; Birchall, E.L.; Gearing, M.; Herskowitz, J.H. Dendritic spines provide cognitive resilience against Alzheimer’s disease. Ann. Neurol. 2017, 82, 602–614. [Google Scholar] [CrossRef]
- Nuñez, A.; Zegarra-Valdivia, J.; Fernandez de Sevilla, D.; Pignatelli, J.; Torres Aleman, I. The neurobiology of insulin-like growth factor I: From neuroprotection to modulation of brain states. Mol. Psychiatry 2023, 28, 3220–3230. [Google Scholar] [CrossRef]
- Neuner, S.M.; Telpoukhovskaia, M.; Menon, V.; O’Connell, K.M.S.; Hohman, T.J.; Kaczorowski, C.C. Translational approaches to understanding resilience to Alzheimer’s disease. Trends Neurosci. 2022, 45, 369–383. [Google Scholar] [CrossRef]
- Perez-Nievas, B.G.; Stein, T.D.; Tai, H.C.; Dols-Icardo, O.; Scotton, T.C.; Barroeta-Espar, I.; Fernandez-Carballo, L.; de Munain, E.L.; Perez, J.; Marquie, M.; et al. Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain 2013, 136 Pt 8, 2510–2526. [Google Scholar] [CrossRef]
- Stefaniak, O.; Dobrzyńska, M.; Drzymała-Czyż, S.; Przysławski, J. Diet in the Prevention of Alzheimer’s Disease: Current Knowledge and Future Research Requirements. Nutrients 2022, 14, 4564. [Google Scholar] [CrossRef]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 2018, 361, eaan8821. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.Q.; Dore, K. Novel approaches to increase synaptic resilience as potential treatments for Alzheimer’s disease. Semin. Cell Dev. Biol. 2023, 139, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Corpas, R.; Griñán-Ferré, C.; Palomera-Ávalos, V.; Porquet, D.; García de Frutos, P.; Franciscato Cozzolino, S.M.; Rodríguez-Farré, E.; Pallàs, M.; Sanfeliu, C.; Cardoso, B.R. Melatonin induces mechanisms of brain resilience against neurodegeneration. J. Pineal Res. 2018, 65, e12515. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, S.T.; Scheff, S.W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann. Neurol. 1990, 27, 457–464. [Google Scholar] [CrossRef]
- O’Kusky, J.R.; Ye, P.; D’Ercole, A.J. Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J. Neurosci. 2000, 20, 8435–8442. [Google Scholar] [CrossRef]
- Nieto-Estevez, V.; Defterali, C.; Vicario-Abejon, C. IGF-I: A Key Growth Factor that Regulates Neurogenesis and Synaptogenesis from Embryonic to Adult Stages of the Brain. Front. Neurosci. 2016, 10, 52. [Google Scholar] [CrossRef]
- Lee, M.; Kim, E.J.; Kim, M.J.; Yum, M.S.; Yeom, J.; Kim, K. Insulin-Like Growth Factor-1 Promotes Synaptogenesis Signaling, a Major Dysregulated Pathway in Malformation of Cortical Development, in a Rat Model. Mol. Neurobiol. 2023, 60, 3299–3310. [Google Scholar] [CrossRef]
- Niblock, M.M.; Brunso-Bechtold, J.K.; Riddle, D.R. Insulin-like growth factor I stimulates dendritic growth in primary somatosensory cortex. J. Neurosci. 2000, 20, 4165–4176. [Google Scholar] [CrossRef]
- Gazit, N.; Vertkin, I.; Shapira, I.; Helm, M.; Slomowitz, E.; Sheiba, M.; Mor, Y.; Rizzoli, S.; Slutsky, I. IGF-1 Receptor Differentially Regulates Spontaneous and Evoked Transmission via Mitochondria at Hippocampal Synapses. Neuron 2016, 89, 583–597. [Google Scholar] [CrossRef]
- Katsenelson, M.; Shapira, I.; Abbas, E.; Jevdokimenko, K.; Styr, B.; Ruggiero, A.; Aïd, S.; Fornasiero, E.F.; Holzenberger, M.; Rizzoli, S.O.; et al. IGF-1 receptor regulates upward firing rate homeostasis via the mitochondrial calcium uniporter. Proc. Natl. Acad. Sci. USA 2022, 119, e2121040119. [Google Scholar] [CrossRef] [PubMed]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, A.; Barres, B.A.; Bennett, M.L. Microglia: Scapegoat, saboteur, or something else? Science 2013, 339, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Parachikova, A.; Agadjanyan, M.G.; Cribbs, D.H.; Blurton-Jones, M.; Perreau, V.; Rogers, J.; Beach, T.G.; Cotman, C.W. Inflammatory changes parallel the early stages of Alzheimer disease. Neurobiol. Aging 2007, 28, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Hinz, R.; Gentleman, S.; Hampshire, A.; Dani, M.; Brooks, D.J.; Edison, P. Neuroinflammation is independently associated with brain network dysfunction in Alzheimer’s disease. Mol. Psychiatry 2023, 28, 1303–1311. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease-a double-edged sword. Neuron 2002, 35, 419. [Google Scholar] [CrossRef]
- Pinto-Benito, D.; Paradela-Leal, C.; Ganchala, D.; de Castro-Molina, P.; Arevalo, M.A. IGF-1 regulates astrocytic phagocytosis and inflammation through the p110α isoform of PI3K in a sex-specific manner. Glia 2022, 70, 1153–1169. [Google Scholar] [CrossRef]
- Herrera, M.L.; Bandín, S.; Champarini, L.G.; Hereñú, C.B.; Bellini, M.J. Intramuscular insulin-like growth factor-1 gene therapy modulates reactive microglia after traumatic brain injury. Brain Res. Bull. 2021, 175, 196–204. [Google Scholar] [CrossRef]
- O’Donnell, S.L.; Frederick, T.J.; Krady, J.K.; Vannucci, S.J.; Wood, T.L. IGF-I and microglia/macrophage proliferation in the ischemic mouse brain. Glia 2002, 39, 85–97. [Google Scholar] [CrossRef]
- Ivan, D.C.; Berve, K.C.; Walthert, S.; Monaco, G.; Borst, K.; Bouillet, E.; Ferreira, F.; Lee, H.; Steudler, J.; Buch, T.; et al. Insulin-like growth factor-1 receptor controls the function of CNS-resident macrophages and their contribution to neuroinflammation. Acta Neuropathol. Commun. 2023, 11, 35. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Costa-Besada, M.A.; Labandeira, C.M.; Villar-Cheda, B.; Rodríguez-Perez, A.I. Insulin-Like Growth Factor-1 and Neuroinflammation. Front. Aging Neurosci. 2017, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Ogundele, O.M.; Lee, C.C.; Francis, J. Age-dependent alterations to paraventricular nucleus insulin-like growth factor 1 receptor as a possible link between sympathoexcitation and inflammation. J. Neurochem. 2016, 139, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef]
- Markesbery, W.R.; Carney, J.M. Oxidative alterations in Alzheimer’s disease. Brain Pathol. 1999, 9, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Fracassi, A.; Marcatti, M.; Zolochevska, O.; Tabor, N.; Woltjer, R.; Moreno, S.; Taglialatela, G. Oxidative Damage and Antioxidant Response in Frontal Cortex of Demented and Nondemented Individuals with Alzheimer’s Neuropathology. J. Neurosci. 2021, 41, 538–554. [Google Scholar] [CrossRef]
- Desbois-Mouthon, C.; Cadoret, A.; Blivet-Van Eggelpoël, M.J.; Bertrand, F.; Cherqui, G.; Perret, C.; Capeau, J. Insulin and IGF-1 stimulate the β-catenin pathway through two signalling cascades involving GSK-3β inhibition and Ras activation. Oncogene 2001, 20, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.M.; Tseng, V.; Wang, J.; Wang, D.; Matyakhina, L.; Bondy, C.A. Tau is hyperphosphorylated in the insulin-like growth factor-I null brain. Endocrinology 2005, 146, 5086–5091. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M., 3rd; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Sorrentino, V.; Romani, M.; Mouchiroud, L.; Beck, J.S.; Zhang, H.; D’Amico, D.; Moullan, N.; Potenza, F.; Schmid, A.W.; Rietsch, S.; et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 2017, 552, 187–193. [Google Scholar] [CrossRef]
- Carro, E.; Trejo, J.L.; Gomez-Isla, T.; LeRoith, D.; Torres-Aleman, I. Serum insulin-like growth factor I regulates brain amyloid-β levels. Nat. Med. 2002, 8, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Adlerz, L.; Holback, S.; Multhaup, G.; Iverfeldt, K. IGF-1-induced processing of the amyloid precursor protein family is mediated by different signaling pathways. J. Biol. Chem. 2007, 282, 10203–10209. [Google Scholar] [CrossRef] [PubMed]
- Selles, M.C.; Fortuna, J.T.S.; Zappa-Villar, M.F.; de Faria, Y.P.R.; Souza, A.S.; Suemoto, C.K.; Leite, R.E.P.; Rodriguez, R.D.; Grinberg, L.T.; Reggiani, P.C.; et al. Adenovirus-Mediated Transduction of Insulin-Like Growth Factor 1 Protects Hippocampal Neurons from the Toxicity of Aβ Oligomers and Prevents Memory Loss in an Alzheimer Mouse Model. Mol. Neurobiol. 2020, 57, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Eichhoff, G.; Adelsberger, H.; Abramowski, D.; Wiederhold, K.H.; Haass, C.; Staufenbiel, M.; Konnerth, A.; Garaschuk, O. Clusters of Hyperactive Neurons Near Amyloid Plaques in a Mouse Model of Alzheimer’s Disease. Science 2008, 321, 1686–1689. [Google Scholar] [CrossRef]
- Koelewijn, L.; Lancaster, T.M.; Linden, D.; Dima, D.C.; Routley, B.C.; Magazzini, L.; Barawi, K.; Brindley, L.; Adams, R.; Tansey, K.E.; et al. Oscillatory hyperactivity and hyperconnectivity in young APOE-ɛ4 carriers and hypoconnectivity in Alzheimer’s disease. eLife 2019, 8, e36011. [Google Scholar] [CrossRef]
- Lee, Y.F.; Russ, A.N.; Zhao, Q.; Perle, S.J.; Maci, M.; Miller, M.R.; Hou, S.S.; Algamal, M.; Zhao, Z.; Li, H.; et al. Optogenetic targeting of astrocytes restores slow brain rhythm function and slows Alzheimer’s disease pathology. Sci. Rep. 2023, 13, 13075. [Google Scholar] [CrossRef]
- Bosson, A.; Paumier, A.; Boisseau, S.; Jacquier-Sarlin, M.; Buisson, A.; Albrieux, M. TRPA1 channels promote astrocytic Ca(2+) hyperactivity and synaptic dysfunction mediated by oligomeric forms of amyloid-β peptide. Mol. Neurodegener. 2017, 12, 53. [Google Scholar] [CrossRef]
- Kuchibhotla, K.V.; Lattarulo, C.R.; Hyman, B.T.; Bacskai, B.J. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 2009, 323, 1211–1215. [Google Scholar] [CrossRef]
- Lines, J.; Baraibar, A.M.; Fang, C.; Martin, E.D.; Aguilar, J.; Lee, M.K.; Araque, A.; Kofuji, P. Astrocyte-neuronal network interplay is disrupted in Alzheimer’s disease mice. Glia 2022, 70, 368–378. [Google Scholar] [CrossRef]
- Calafate, S.; Özturan, G.; Thrupp, N.; Vanderlinden, J.; Santa-Marinha, L.; Morais-Ribeiro, R.; Ruggiero, A.; Bozic, I.; Rusterholz, T.; Lorente-Echeverría, B.; et al. Early alterations in the MCH system link aberrant neuronal activity and sleep disturbances in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2023, 26, 1021–1031. [Google Scholar] [CrossRef]
- Harris, S.S.; Wolf, F.; De Strooper, B.; Busche, M.A. Tipping the Scales: Peptide-Dependent Dysregulation of Neural Circuit Dynamics in Alzheimer’s Disease. Neuron 2020, 107, 417–435. [Google Scholar] [PubMed]
- Huijbers, W.; Schultz, A.P.; Papp, K.V.; LaPoint, M.R.; Hanseeuw, B.; Chhatwal, J.P.; Hedden, T.; Johnson, K.A.; Sperling, R.A. Tau Accumulation in Clinically Normal Older Adults Is Associated with Hippocampal Hyperactivity. J. Neurosci. 2019, 39, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Nuriel, T.; Angulo, S.L.; Khan, U.; Ashok, A.; Chen, Q.; Figueroa, H.Y.; Emrani, S.; Liu, L.; Herman, M.; Barrett, G.; et al. Neuronal hyperactivity due to loss of inhibitory tone in APOE4 mice lacking Alzheimer’s disease-like pathology. Nat. Commun. 2017, 8, 1464. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Sevilla, M.E.; Pignatelli, J.; Zegarra-Valdivia, J.A.; Mendez, P.; Nuñez, A.; Torres Alemán, I. Insulin-like growth factor I mitigates post-traumatic stress by inhibiting AMP-kinase in orexin neurons. Mol. Psychiatry 2022, 27, 2182–2196. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.M.; Hernandez-Garzon, E.; Perez-Domper, P.; Perez-Alvarez, A.; Mederos, S.; Matsui, T.; Santi, A.; Trueba-Saiz, A.; Garcia-Guerra, L.; Pose-Utrilla, J.; et al. Insulin Regulates Astrocytic Glucose Handling Through Cooperation With IGF-I. Diabetes 2017, 66, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Farias Quipildor, G.; Mao, K.; Beltran, P.J.; Barzilai, N.; Huffman, D.M. Modulation of Glucose Production by Central Insulin Requires IGF-1 Receptors in AgRP Neurons. Diabetes 2021, 70, 2237–2249. [Google Scholar] [CrossRef]
- Love, S.; Miners, J.S. Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathol. 2016, 131, 645–658. [Google Scholar] [CrossRef]
- Lopez-Lopez, C.; LeRoith, D.; Torres-Aleman, I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl. Acad. Sci. USA 2004, 101, 9833–9838. [Google Scholar] [CrossRef]
- Lachman, M.E.; Agrigoroaei, S.; Murphy, C.; Tun, P.A. Frequent cognitive activity compensates for education differences in episodic memory. Am. J. Geriatr. Psychiatry 2010, 18, 4–10. [Google Scholar] [CrossRef]
- Rovio, S.; Kåreholt, I.; Helkala, E.L.; Viitanen, M.; Winblad, B.; Tuomilehto, J.; Soininen, H.; Nissinen, A.; Kivipelto, M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005, 4, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, N.; Fischer, C.E.; Schweizer, T.A.; Munoz, D.G. Cognitive resilience and severe Alzheimer’s disease neuropathology. Aging Brain 2023, 3, 100065. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; Cholerton, B.A.; et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch. Neurol. 2010, 67, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ohline, S.M.; Abraham, W.C. Environmental enrichment effects on synaptic and cellular physiology of hippocampal neurons. Neuropharmacology 2019, 145 Pt A, 3–12. [Google Scholar] [CrossRef]
- Nishijima, T.; Piriz, J.; Duflot, S.; Fernandez, A.M.; Gaitan, G.; Gomez-Pinedo, U.; Verdugo, J.M.; Leroy, F.; Soya, H.; Nunez, A.; et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron 2010, 67, 834–846. [Google Scholar] [CrossRef]
- Carro, E.; Nunez, A.; Busiguina, S.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 2000, 20, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Torres-Aleman, I. Circulating insulin-like growth factor I and cognitive function: Neuromodulation throughout the lifespan. Prog. Neurobiol. 2009, 89, 256–265. [Google Scholar] [CrossRef]
- Pharaoh, G.; Owen, D.; Yeganeh, A.; Premkumar, P.; Farley, J.; Bhaskaran, S.; Ashpole, N.; Kinter, M.; Van Remmen, H.; Logan, S. Disparate Central and Peripheral Effects of Circulating IGF-1 Deficiency on Tissue Mitochondrial Function. Mol. Neurobiol. 2019, 57, 1317–1331. [Google Scholar] [CrossRef]
- Zegarra-Valdivia, J.A.; Chaves-Coira, I.; Fernandez de Sevilla, M.E.; Martinez-Rachadell, L.; Esparza, J.; Torres-Aleman, I.; Nunez, A. Reduced Insulin-Like Growth Factor-I Effects in the Basal Forebrain of Aging Mouse. Front. Aging Neurosci. 2021, 13, 682388. [Google Scholar] [CrossRef]
- Muller, A.P.; Fernandez, A.M.; Haas, C.; Zimmer, E.; Portela, L.V.; Torres-Aleman, I. Reduced brain insulin-like growth factor I function during aging. Mol. Cell Neurosci. 2012, 49, 9–12. [Google Scholar] [CrossRef]
- Gong, Z.; Kennedy, O.; Sun, H.; Wu, Y.; Williams, G.A.; Klein, L.; Cardoso, L.; Matheny, R.W.; Hubbard, G.B.; Ikeno, Y.; et al. Reductions in serum IGF-1 during aging impair health span. Aging Cell 2014, 13, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Bickel, M.A.; Csik, B.; Gulej, R.; Ungvari, A.; Nyul-Toth, A.; Conley, S.M. Cell non-autonomous regulation of cerebrovascular aging processes by the somatotropic axis. Front. Endocrinol. 2023, 14, 1087053. [Google Scholar] [CrossRef] [PubMed]
- Zyzak, D.R.; Otto, T.; Eichenbaum, H.; Gallagher, M. Cognitive decline associated with normal aging in rats: A neuropsychological approach. Learn. Mem. 1995, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Frater, J.; Lie, D.; Bartlett, P.; McGrath, J.J. Insulin-like Growth Factor 1 (IGF-1) as a marker of cognitive decline in normal ageing: A review. Ageing Res. Rev. 2018, 42, 14–27. [Google Scholar] [CrossRef]
- Munive, V.; Zegarra-Valdivia, J.A.; Herrero-Labrador, R.; Fernandez, A.M.; Aleman, I.T. Loss of the interaction between estradiol and insulin-like growth factor I in brain endothelial cells associates to changes in mood homeostasis during peri-menopause in mice. Aging 2019, 11, 174–184. [Google Scholar] [CrossRef]
- Celaya, A.M.; Rodríguez-de la Rosa, L.; Bermúdez-Muñoz, J.M.; Zubeldia, J.M.; Romá-Mateo, C.; Avendaño, C.; Pallardó, F.V.; Varela-Nieto, I. IGF-1 Haploinsufficiency Causes Age-Related Chronic Cochlear Inflammation and Increases Noise-Induced Hearing Loss. Cells 2021, 10, 1686. [Google Scholar] [CrossRef]
- García-Magro, N.; Zegarra-Valdivia, J.A.; Troyas-Martinez, S.; Torres-Aleman, I.; Nuñez, A. Response Facilitation Induced by Insulin-like Growth Factor-I in the Primary Somatosensory Cortex of Mice Was Reduced in Aging. Cells 2022, 11, 717. [Google Scholar] [CrossRef]
- Acosta-Rodriguez, V.A.; Rijo-Ferreira, F.; Green, C.B.; Takahashi, J.S. Importance of circadian timing for aging and longevity. Nat. Commun. 2021, 12, 2862. [Google Scholar] [CrossRef]
- Olsson, M.; Arlig, J.; Hedner, J.; Blennow, K.; Zetterberg, H. Sleep deprivation and cerebrospinal fluid biomarkers for Alzheimer’s disease. Sleep 2018, 41, zsy025. [Google Scholar] [CrossRef]
- Kang, J.E.; Lim, M.M.; Bateman, R.J.; Lee, J.J.; Smyth, L.P.; Cirrito, J.R.; Fujiki, N.; Nishino, S.; Holtzman, D.M. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science 2009, 326, 1005–1007. [Google Scholar] [CrossRef]
- Crosby, P.; Hamnett, R.; Putker, M.; Hoyle, N.P.; Reed, M.; Karam, C.J.; Maywood, E.S.; Stangherlin, A.; Chesham, J.E.; Hayter, E.A.; et al. Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time. Cell 2019, 177, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Zegarra-Valdivia, J.A.; Fernandes, J.; Fernandez de Sevilla, M.E.; Trueba-Saiz, A.; Pignatelli, J.; Suda, K.; Martinez-Rachadell, L.; Fernandez, A.M.; Esparza, J.; Vega, M.; et al. Insulin-like growth factor I sensitization rejuvenates sleep patterns in old mice. Geroscience 2022, 44, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, J.; de Sevilla, M.E.F.; Sperber, J.; Horrillo, D.; Medina-Gomez, G.; Aleman, I.T. Insulin-like Growth Factor I Couples Metabolism with Circadian Activity through Hypothalamic Orexin Neurons. Int. J. Mol. Sci. 2022, 23, 4679. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Paulsson, J.F.; Blinder, P.; Burstyn-Cohen, T.; Du, D.; Estepa, G.; Adame, A.; Pham, H.M.; Holzenberger, M.; Kelly, J.W.; et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell 2009, 139, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Gontier, G.; George, C.; Chaker, Z.; Holzenberger, M.; Aid, S. Blocking IGF Signaling in Adult Neurons Alleviates Alzheimer’s Disease Pathology through Amyloid-β Clearance. J. Neurosci. 2015, 35, 11500–11513. [Google Scholar] [CrossRef] [PubMed]
- Biondi, O.; Branchu, J.; Ben Salah, A.; Houdebine, L.; Bertin, L.; Chali, F.; Desseille, C.; Weill, L.; Sanchez, G.; Lancelin, C.; et al. IGF-1R Reduction Triggers Neuroprotective Signaling Pathways in Spinal Muscular Atrophy Mice. J. Neurosci. 2015, 35, 12063–12079. [Google Scholar] [CrossRef]
- De Magalhaes Filho, C.D.; Kappeler, L.; Dupont, J.; Solinc, J.; Villapol, S.; Denis, C.; Nosten-Bertrand, M.; Billard, J.M.; Blaise, A.; Tronche, F.; et al. Deleting IGF-1 receptor from forebrain neurons confers neuroprotection during stroke and upregulates endocrine somatotropin. J. Cereb. Blood Flow. Metab. 2016, 37, 396–412. [Google Scholar] [CrossRef]
- Boucher, J.; Macotela, Y.; Bezy, O.; Mori, M.A.; Kriauciunas, K.; Kahn, C.R. A Kinase-Independent Role for Unoccupied Insulin and IGF-1 Receptors in the Control of Apoptosis. Sci. Signal 2010, 3, ra87. [Google Scholar] [CrossRef]
- Zegarra-Valdivia, J.; Nuñez, A.; Aleman, I.T. Untangling IGF-I signaling in the aging brain. Aging 2023, 15, 599–600. [Google Scholar] [CrossRef]
- Kirkman, M.S.; Briscoe, V.J.; Clark, N.; Florez, H.; Haas, L.B.; Halter, J.B.; Huang, E.S.; Korytkowski, M.T.; Munshi, M.N.; Odegard, P.S.; et al. Diabetes in Older Adults. Diabetes Care 2012, 35, 2650–2664. [Google Scholar] [CrossRef]
- Sandhu, M.S.; Heald, A.H.; Gibson, J.M.; Cruickshank, J.K.; Dunger, D.B.; Wareham, N.J. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: A prospective observational study. Lancet 2002, 359, 1740–1745. [Google Scholar] [CrossRef]
- Dunger, D.B.; Ong, K.K.; Sandhu, M.S. Serum insulin-like growth factor-I levels and potential risk of type 2 diabetes. Horm. Res. 2003, 60 (Suppl. 3), 131–135. [Google Scholar] [CrossRef] [PubMed]
- Irie, F.; Fitzpatrick, A.L.; Lopez, O.L.; Kuller, L.H.; Peila, R.; Newman, A.B.; Launer, L.J. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: The Cardiovascular Health Study Cognition Study. Arch. Neurol. 2008, 65, 89–93. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.G.; Ferreira, S.T. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes 2014, 63, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Risacher, S.L.; Huang, E.; Saykin, A.J.; Alzheimer’s Disease Neuroimaging, I. Type 2 diabetes mellitus is associated with brain atrophy and hypometabolism in the ADNI cohort. Neurology 2016, 87, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Macauley, S.L.; Stanley, M.; Caesar, E.E.; Yamada, S.A.; Raichle, M.E.; Perez, R.; Mahan, T.E.; Sutphen, C.L.; Holtzman, D.M. Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J. Clin. Investig. 2015, 125, 2463–2467. [Google Scholar] [CrossRef]
- Janson, J.; Laedtke, T.; Parisi, J.E.; O’Brien, P.; Petersen, R.C.; Butler, P.C. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 2004, 53, 474–481. [Google Scholar] [CrossRef]
- Ma, Q.L.; Yang, F.; Rosario, E.R.; Ubeda, O.J.; Beech, W.; Gant, D.J.; Chen, P.P.; Hudspeth, B.; Chen, C.; Zhao, Y.; et al. β-Amyloid Oligomers Induce Phosphorylation of Tau and Inactivation of Insulin Receptor Substrate via c-Jun N-Terminal Kinase Signaling: Suppression by Omega-3 Fatty Acids and Curcumin. J. Neurosci. 2009, 29, 9078–9089. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Tong, M.; Daiello, L.A.; Ott, B.R. Early-Stage Alzheimer’s Disease Is Associated with Simultaneous Systemic and Central Nervous System Dysregulation of Insulin-Linked Metabolic Pathways. J. Alzheimer’s Dis. 2019, 68, 657–668. [Google Scholar] [CrossRef]
- Alafuzoff, I.; Aho, L.; Helisalmi, S.; Mannermaa, A.; Soininen, H. Β-amyloid deposition in brains of subjects with diabetes. Neuropathol. Appl. Neurobiol. 2009, 35, 60–68. [Google Scholar] [CrossRef]
- Lee, C.W.; Shih, Y.H.; Wu, S.Y.; Yang, T.; Lin, C.; Kuo, Y.M. Hypoglycemia induces tau hyperphosphorylation. Curr. Alzheimer Res. 2013, 10, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.R.; NM, L.E.S.; Figueiredo, C.P.; Frozza, R.L.; Ledo, J.H.; Beckman, D.; Katashima, C.K.; Razolli, D.; Carvalho, B.M.; Frazao, R.; et al. Alzheimer-associated Aβ oligomers impact the central nervous system to induce peripheral metabolic deregulation. EMBO Mol. Med. 2015, 7, 190–210. [Google Scholar] [CrossRef] [PubMed]
- Goldwaser, E.L.; Acharya, N.K.; Sarkar, A.; Godsey, G.; Nagele, R.G. Breakdown of the Cerebrovasculature and Blood-Brain Barrier: A Mechanistic Link Between Diabetes Mellitus and Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 54, 445–456. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Wang, H.Y.; Capuano, A.W.; Khan, A.; Taïb, B.; Anokye-Danso, F.; Schneider, J.A.; Bennett, D.A.; Ahima, R.S.; Arnold, S.E. Brain Insulin Signaling, Alzheimer Disease Pathology, and Cognitive Function. Ann. Neurol. 2020, 88, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Kodl, C.T.; Seaquist, E.R. Cognitive dysfunction and diabetes mellitus. Endocr. Rev. 2008, 29, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, R.H.; Ma, X.; Fishman, S.; Yang, X.; Atzmon, G.; Vuguin, P.; Einstein, F.H.; Hwang, D.; Cohen, P.; Barzilai, N. Central and opposing effects of IGF-I and IGF-binding protein-3 on systemic insulin action. Diabetes 2006, 55, 2788–2796. [Google Scholar] [CrossRef]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef]
- Grapsa, I.; Mamalaki, E.; Ntanasi, E.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Scarmeas, N.; Yannakoulia, M. Longitudinal Examination of Body Mass Index and Cognitive Function in Older Adults: The HELIAD Study. Nutrients 2023, 15, 1795. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ha, J.; Lee, J.Y.; Kim, E. Weight loss and risk of dementia in individuals with versus without obesity. Alzheimer’s Dement. 2023. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, W.; Lu, M.; Zhang, X.; Zhang, P.; Xin, Z.; Sun, R.; Tian, W.; Cardoso, M.A.; Yang, J.; et al. Relationship between Central Obesity and the incidence of Cognitive Impairment and Dementia from Cohort Studies Involving 5,060,687 Participants. Neurosci. Biobehav. Rev. 2021, 130, 301–313. [Google Scholar] [CrossRef]
- Hoscheidt, S.; Sanderlin, A.H.; Baker, L.D.; Jung, Y.; Lockhart, S.; Kellar, D.; Whitlow, C.T.; Hanson, A.J.; Friedman, S.; Register, T.; et al. Mediterranean and Western diet effects on Alzheimer’s disease biomarkers, cerebral perfusion, and cognition in mid-life: A randomized trial. Alzheimer’s Dement. 2022, 18, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Yusufov, M.; Weyandt, L.L.; Piryatinsky, I. Alzheimer’s disease and diet: A systematic review. Int. J. Neurosci. 2017, 127, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Więckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western diet as a trigger of Alzheimer’s disease: From metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef] [PubMed]
- Venters, H.D.; Tang, Q.; Liu, Q.; VanHoy, R.W.; Dantzer, R.; Kelley, K.W. A new mechanism of neurodegeneration: A proinflammatory cytokine inhibits receptor signaling by a survival peptide. Proc. Natl. Acad. Sci. USA 1999, 96, 9879–9884. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002, 25, 295–301. [Google Scholar] [CrossRef]
- Forbes, D.; Thiessen, E.J.; Blake, C.M.; Forbes, S.C.; Forbes, S. Exercise programs for people with dementia. Cochrane. Database Syst. Rev. 2013, 12, CD006489. [Google Scholar]
- Wolf, S.A.; Kronenberg, G.; Lehmann, K.; Blankenship, A.; Overall, R.; Staufenbiel, M.; Kempermann, G. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer’s disease. Biol. Psychiatry 2006, 60, 1314–1323. [Google Scholar] [CrossRef]
- Carro, E.; Trejo, J.L.; Busiguina, S.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J. Neurosci. 2001, 21, 5678–5684. [Google Scholar] [CrossRef]
- Trejo, J.L.; Carro, E.; Nunez, A.; Torres-Aleman, I. Sedentary life impairs self-reparative processes in the brain: The role of serum insulin-like growth factor-I. Rev. Neurosci. 2002, 13, 365–374. [Google Scholar] [CrossRef]
- Del Ser, T.; Hachinski, V.; Merskey, H.; Munoz, D.G. An autopsy-verified study of the effect of education on degenerative dementia. Brain 1999, 122, 2309–2319. [Google Scholar] [CrossRef]
- Stern, Y.; Albert, S.; Tang, M.X.; Tsai, W.Y. Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology 1999, 53, 1942–1947. [Google Scholar] [CrossRef]
- Stern, Y.; Barulli, D. Cognitive reserve. Handb. Clin. Neurol. 2019, 167, 181–190. [Google Scholar] [PubMed]
- Ngandu, T.; von Strauss, E.; Helkala, E.L.; Winblad, B.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Education and dementia: What lies behind the association? Neurology 2007, 69, 1442–1450. [Google Scholar] [CrossRef]
- Walker, J.M.; Dehkordi, S.K.; Schaffert, J.; Goette, W.; White Iii, C.L.; Richardson, T.E.; Zare, H. The Spectrum of Alzheimer-Type Pathology in Cognitively Normal Individuals. J. Alzheimer’s Dis. 2023, 91, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Seyedsalehi, A.; Warrier, V.; Bethlehem, R.A.I.; Perry, B.I.; Burgess, S.; Murray, G.K. Educational attainment, structural brain reserve and Alzheimer’s disease: A Mendelian randomization analysis. Brain 2023, 146, 2059–2074. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Zhang, H.; Gao, S.; Wang, L.; Wang, T.; Han, Z.; Sun, B.L.; Liu, G. Cognitive performance protects against Alzheimer’s disease independently of educational attainment and intelligence. Mol. Psychiatry 2022, 27, 4297–4306. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.D.; Powell-Braxton, L.; Widmer, H.R.; Valverde, J.; Hefti, F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 1995, 14, 717–730. [Google Scholar] [CrossRef]
- Tu, X.; Jain, A.; Parra Bueno, P.; Decker, H.; Liu, X.; Yasuda, R. Local autocrine plasticity signaling in single dendritic spines by insulin-like growth factors. Sci. Adv. 2023, 9, eadg0666. [Google Scholar] [CrossRef]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Honig, L.S.; Tang, M.X.; Albert, S.; Costa, R.; Luchsinger, J.; Manly, J.; Stern, Y.; Mayeux, R. Stroke and the risk of Alzheimer disease. Arch. Neurol. 2003, 60, 1707–1712. [Google Scholar] [CrossRef]
- Arbel-Ornath, M.; Hudry, E.; Eikermann-Haerter, K.; Hou, S.; Gregory, J.L.; Zhao, L.; Betensky, R.A.; Frosch, M.P.; Greenberg, S.M.; Bacskai, B.J. Interstitial fluid drainage is impaired in ischemic stroke and Alzheimer’s disease mouse models. Acta Neuropathol. 2013, 126, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Ghiso, J.; Frangione, B. Cerebral amyloidosis, amyloid angiopathy, and their relationship to stroke and dementia. J. Alzheimer’s Dis. 2001, 3, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, G.; Qing, H.; Zhou, W.; Dobie, F.; Cai, F.; Staufenbiel, M.; Huang, L.E.; Song, W. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 18727–18732. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.P.; Jiang, M.Q.; Shim, S.S.; Pourkhodadad, S.; Wei, L. Extrasynaptic NMDA receptors in acute and chronic excitotoxicity: Implications for preventive treatments of ischemic stroke and late-onset Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.A.; Valcarcel-Ares, M.N.; Ashpole, N.M. Preclinical and clinical evidence of IGF-1 as a prognostic marker and acute intervention with ischemic stroke. J. Cereb. Blood Flow. Metab. 2021, 41, 2475–2491. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, C.; Dietrich, M.O.; Metzger, F.; Loetscher, H.; Torres-Aleman, I. Disturbed cross talk between insulin-like growth factor I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer’s disease. J. Neurosci. 2007, 27, 824–831. [Google Scholar] [CrossRef]
- Friedrich, N.; Thuesen, B.; Jørgensen, T.; Juul, A.; Spielhagen, C.; Wallaschofksi, H.; Linneberg, A. The association between IGF-I and insulin resistance: A general population study in Danish adults. Diabetes Care 2012, 35, 768–773. [Google Scholar] [CrossRef]
- Zhou, M.; Li, H.; Wang, Y.; Pan, Y.; Wang, Y. Causal effect of insulin resistance on small vessel stroke and Alzheimer’s disease: A Mendelian randomization analysis. Eur. J. Neurol. 2022, 29, 698–706. [Google Scholar] [CrossRef]
- Shalev, A.; Liberzon, I.; Marmar, C. Post-Traumatic Stress Disorder. N. Engl. J. Med. 2017, 376, 2459–2469. [Google Scholar] [CrossRef]
- Greenberg, M.S.; Tanev, K.; Marin, M.F.; Pitman, R.K. Stress, PTSD, and dementia. Alzheimer’s Dement. 2014, 10 (Suppl. 3), S155–S165. [Google Scholar] [CrossRef]
- Kuring, J.K.; Mathias, J.L.; Ward, L. Risk of Dementia in persons who have previously experienced clinically-significant Depression, Anxiety, or PTSD: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2020, 274, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Catania, C.; Sotiropoulos, I.; Silva, R.; Onofri, C.; Breen, K.C.; Sousa, N.; Almeida, O.F.X. The amyloidogenic potential and behavioral correlates of stress. Mol. Psychiatry 2007, 14, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Evans, D.A.; Bienias, J.L.; Mendes De Leon, C.F.; Schneider, J.A.; Bennett, D.A. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology 2003, 61, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Delic, V.; Ratliff, W.A.; Citron, B.A. Sleep Deprivation, a Link Between Post-Traumatic Stress Disorder and Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 79, 1443–1449. [Google Scholar] [CrossRef]
- Weiner, M.W.; Harvey, D.; Landau, S.M.; Veitch, D.P.; Neylan, T.C.; Grafman, J.H.; Aisen, P.S.; Petersen, R.C.; Jack, C.R., Jr.; Tosun, D.; et al. Traumatic brain injury and post-traumatic stress disorder are not associated with Alzheimer’s disease pathology measured with biomarkers. Alzheimer’s Dement. 2022, 19, 884–895. [Google Scholar] [CrossRef]
- Justice, N.J.; Huang, L.; Tian, J.B.; Cole, A.; Pruski, M.; Hunt, A.J.; Flores, R.; Zhu, M.X.; Arenkiel, B.R.; Zheng, H. Posttraumatic Stress Disorder-Like Induction Elevates β-Amyloid Levels, Which Directly Activates Corticotropin-Releasing Factor Neurons to Exacerbate Stress Responses. J. Neurosci. 2015, 35, 2612–2623. [Google Scholar] [CrossRef]
- Raber, J.; Huang, Y.; Ashford, J.W. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol. Aging 2004, 25, 641–650. [Google Scholar] [CrossRef]
- Ferguson, A.C.; Tank, R.; Lyall, L.M.; Ward, J.; Celis-Morales, C.; Strawbridge, R.; Ho, F.; Whelan, C.D.; Gill, J.; Welsh, P.; et al. Alzheimer’s Disease Susceptibility Gene Apolipoprotein E (APOE) and Blood Biomarkers in UK Biobank (N = 395,769). J. Alzheimer’s Dis. 2020, 76, 1541–1551. [Google Scholar] [CrossRef]
- Li, T.; Pappas, C.; Klinedinst, B.; Pollpeter, A.; Larsen, B.; Hoth, N.; Anton, F.; Wang, Q.; Willette, A.A. Associations Between Insulin-Like Growth Factor-1 and Resting-State Functional Connectivity in Cognitively Unimpaired Midlife Adults. J. Alzheimer’s Dis. 2023, 94, S309–S318. [Google Scholar] [CrossRef]
- Wang, W.; Yu, J.T.; Tan, L.; Liu, Q.Y.; Wang, H.F.; Ma, X.Y. Insulin-like growth factor 1 (IGF1) polymorphism is associated with Alzheimer’s disease in Han Chinese. Neurosci. Lett. 2012, 531, 20–23. [Google Scholar] [CrossRef]
- Traversy, M.T.; Vandal, M.; Tremblay, C.; Tournissac, M.; Giguère-Rancourt, A.; Bennett, A.D.; Calon, F. Altered cerebral insulin response in transgenic mice expressing the epsilon-4 allele of the human apolipoprotein E gene. Psychoneuroendocrinology 2017, 77, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Keeney, J.T.; Ibrahimi, S.; Zhao, L. Human ApoE Isoforms Differentially Modulate Glucose and Amyloid Metabolic Pathways in Female Brain: Evidence of the Mechanism of Neuroprotection by ApoE2 and Implications for Alzheimer’s Disease Prevention and Early Intervention. J. Alzheimer’s Dis. 2015, 48, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Ou, S.; Liu, G. Traumatic Brain Injury and Risk of Dementia and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2022, 56, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Dams-O’Connor, K.; Guetta, G.; Hahn-Ketter, A.E.; Fedor, A. Traumatic brain injury as a risk factor for Alzheimer’s disease: Current knowledge and future directions. Neurodegener. Dis. Manag. 2016, 6, 417–429. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Traumatic brain injury and amyloid-β pathology: A link to Alzheimer’s disease? Nat. Rev. Neurosci. 2010, 11, 361–370. [Google Scholar] [CrossRef]
- Mohamed, A.Z.; Nestor, P.J.; Cumming, P.; Nasrallah, F.A. Traumatic brain injury fast-forwards Alzheimer’s pathology: Evidence from amyloid positron emission tomorgraphy imaging. J. Neurol. 2022, 269, 873–884. [Google Scholar] [CrossRef]
- Zheng, P.; Tong, W. IGF-1: An endogenous link between traumatic brain injury and Alzheimer disease? J. Neurosurg. Sci. 2017, 61, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cejudo, J.; Wisniewski, T.; Marmar, C.; Zetterberg, H.; Blennow, K.; de Leon, M.J.; Fossati, S. Traumatic Brain Injury and Alzheimer’s Disease: The Cerebrovascular Link. EBioMedicine 2018, 28, 21–30. [Google Scholar] [CrossRef]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef]
- Kempuraj, D.; Ahmed, M.E.; Selvakumar, G.P.; Thangavel, R.; Dhaliwal, A.S.; Dubova, I.; Mentor, S.; Premkumar, K.; Saeed, D.; Zahoor, H.; et al. Brain Injury-Mediated Neuroinflammatory Response and Alzheimer’s Disease. Neuroscientist 2020, 26, 134–155. [Google Scholar] [CrossRef]
- Shin, M.K.; Vázquez-Rosa, E.; Koh, Y.; Dhar, M.; Chaubey, K.; Cintrón-Pérez, C.J.; Barker, S.; Miller, E.; Franke, K.; Noterman, M.F.; et al. Reducing acetylated tau is neuroprotective in brain injury. Cell 2021, 184, 2715–2732.e23. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Hu, M.; Zhang, J.; Hashem, J.; Chen, C. TDP-43 drives synaptic and cognitive deterioration following traumatic brain injury. Acta Neuropathol. 2022, 144, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Hook, V.; Yoon, M.; Mosier, C.; Ito, G.; Podvin, S.; Head, B.P.; Rissman, R.; O’Donoghue, A.J.; Hook, G. Cathepsin B in neurodegeneration of Alzheimer’s disease, traumatic brain injury, and related brain disorders. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140428. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.H.; Liu, X.; Zhang, Z.; Gu, X.; Yu, S.P.; Keene, C.D.; Cheng, L.; Ye, K. Traumatic brain injury triggers APP and Tau cleavage by delta-secretase, mediating Alzheimer’s disease pathology. Prog. Neurobiol. 2020, 185, 101730. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.S.N.; Jolly, A.; Zimmerman, K.; Bourke, N.J.; Scott, G.; Cole, J.H.; Schott, J.M.; Sharp, D.J. Diffuse axonal injury predicts neurodegeneration after moderate-severe traumatic brain injury. Brain 2020, 143, 3685–3698. [Google Scholar] [CrossRef]
- Brabant, G.; Wallaschofski, H. Normal levels of serum IGF-I: Determinants and validity of current reference ranges. Pituitary 2007, 10, 129–133. [Google Scholar] [CrossRef]
- Harris, T.G.; Strickler, H.D.; Yu, H.; Pollak, M.N.; Monrad, E.S.; Travin, M.I.; Xue, X.; Rohan, T.E.; Kaplan, R.C. Specimen processing time and measurement of total insulin-like growth factor-I (IGF-I), free IGF-I, and IGF binding protein-3 (IGFBP-3). Growth Horm. IGF Res. 2006, 16, 86–92. [Google Scholar] [CrossRef]
- Simstich, S.; Züllig, T.; D’Aurizio, F.; Biasotto, A.; Colao, A.; Isidori, A.M.; Lenzi, A.; Fauler, G.; Köfeler, H.C.; Curcio, F.; et al. The impact of different calibration matrices on the determination of insulin-like growth factor 1 by high-resolution-LC-MS in acromegalic and growth hormone deficient patients. Clin. Biochem. 2023, 114, 95–102. [Google Scholar] [CrossRef]
- Frystyk, J. Utility of Free IGF-I Measurements. Pituitary 2007, 10, 181–187. [Google Scholar] [CrossRef]
- Galle, S.A.; van der Spek, A.; Drent, M.L.; Brugts, M.P.; Scherder, E.J.A.; Janssen, J.; Ikram, M.A.; van Duijn, C.M. Revisiting the Role of Insulin-Like Growth Factor-I Receptor Stimulating Activity and the Apolipoprotein E in Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 20. [Google Scholar] [CrossRef]
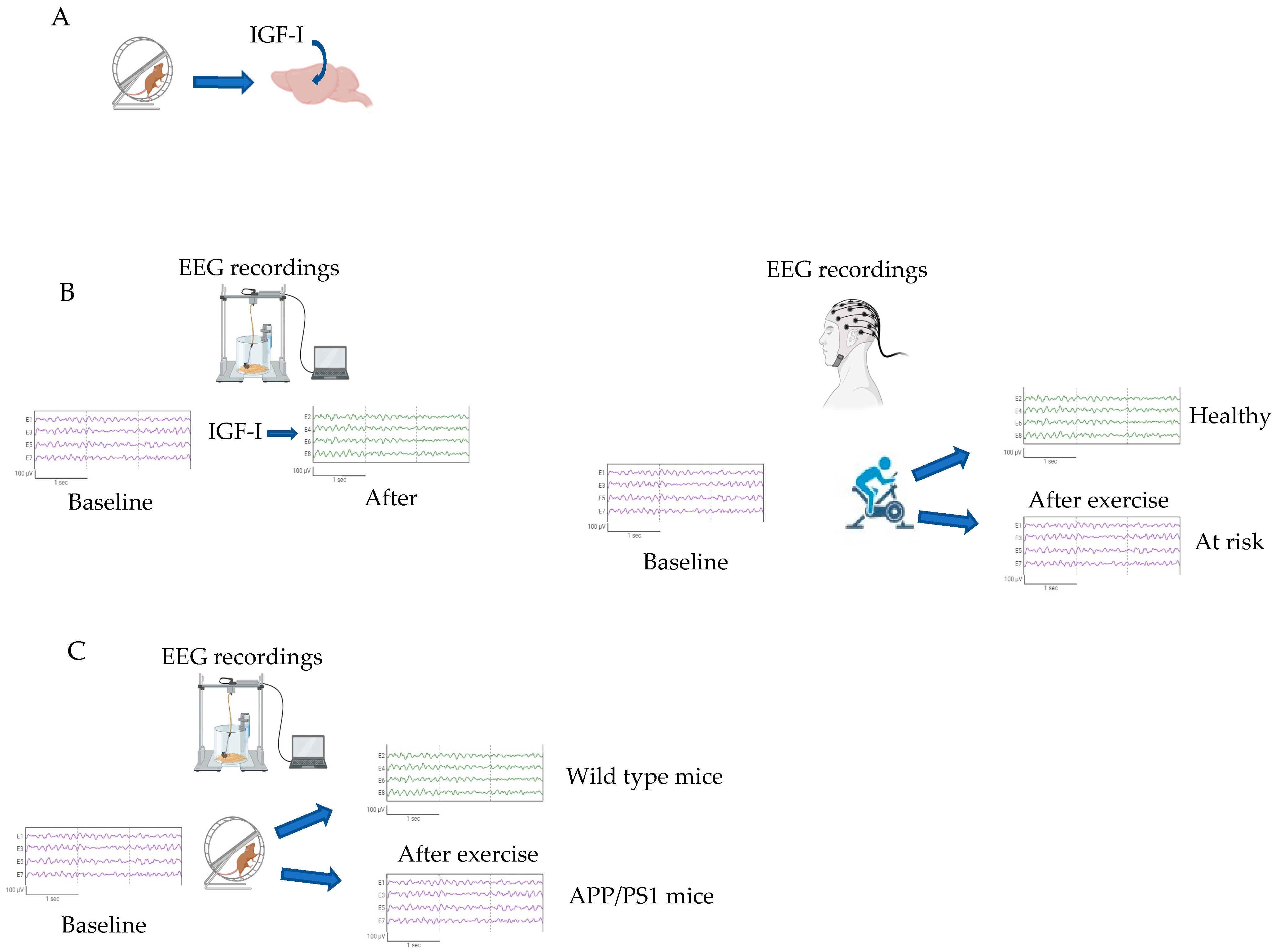
- Miki Stein, A.; Munive, V.; Fernandez, A.M.; Nuñez, A.; Torres Aleman, I. Acute exercise does not modify brain activity and memory performance in APP/PS1 mice. PLoS ONE 2017, 12, e0178247. [Google Scholar] [CrossRef] [PubMed]
- Trueba-Saiz, A.; Cavada, C.; Fernandez, A.M.; Leon, T.; Gonzalez, D.A.; Fortea, O.J.; Lleo, A.; Del, S.T.; Nunez, A.; Torres-Aleman, I. Loss of serum IGF-I input to the brain as an early biomarker of disease onset in Alzheimer mice. Transl. Psychiatry 2013, 3, e330. [Google Scholar] [CrossRef] [PubMed]
- Rollo, J.; Crawford, J.; Hardy, J. A dynamical systems approach for multiscale synthesis of Alzheimer’s pathogenesis. Neuron 2023, 111, 2126–2139. [Google Scholar] [CrossRef] [PubMed]
- Frere, S.; Slutsky, I. Alzheimer’s Disease: From Firing Instability to Homeostasis Network Collapse. Neuron 2018, 97, 32–58. [Google Scholar] [CrossRef]
| Activity | Main References |
|---|---|
| Neurogenesis | [22,34] |
| Re-innervation | [23] |
| Regulation of inflammation | [24,35] |
| Regulation of oxidative stress | [25,36] |
| Neuronal plasticity | [28,37] |
| Cognition | [29] |
| Mood | [30] |
| Energy allocation | [26,31,38] |
| Sleep/wake cycle | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zegarra-Valdivia, J.A.; Pignatelli, J.; Nuñez, A.; Torres Aleman, I. The Role of Insulin-like Growth Factor I in Mechanisms of Resilience and Vulnerability to Sporadic Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 16440. https://doi.org/10.3390/ijms242216440
Zegarra-Valdivia JA, Pignatelli J, Nuñez A, Torres Aleman I. The Role of Insulin-like Growth Factor I in Mechanisms of Resilience and Vulnerability to Sporadic Alzheimer’s Disease. International Journal of Molecular Sciences. 2023; 24(22):16440. https://doi.org/10.3390/ijms242216440
Chicago/Turabian StyleZegarra-Valdivia, Jonathan A., Jaime Pignatelli, Angel Nuñez, and Ignacio Torres Aleman. 2023. "The Role of Insulin-like Growth Factor I in Mechanisms of Resilience and Vulnerability to Sporadic Alzheimer’s Disease" International Journal of Molecular Sciences 24, no. 22: 16440. https://doi.org/10.3390/ijms242216440
APA StyleZegarra-Valdivia, J. A., Pignatelli, J., Nuñez, A., & Torres Aleman, I. (2023). The Role of Insulin-like Growth Factor I in Mechanisms of Resilience and Vulnerability to Sporadic Alzheimer’s Disease. International Journal of Molecular Sciences, 24(22), 16440. https://doi.org/10.3390/ijms242216440








