Glucose Transport and Utilization in the Hippocampus: From Neurophysiology to Diabetes-Related Development of Dementia
Abstract
:1. Introduction
2. The Role of Glucose in Neuronal Function
3. Glucose Supply to the Brain and Glucose Transporters
3.1. Glucose Transporters
3.2. Glucose Transport and Transporters in the Blood–Brain Barrier
3.3. Glucose Transport and Transporters in the Circumventricular Organs (CVOs)
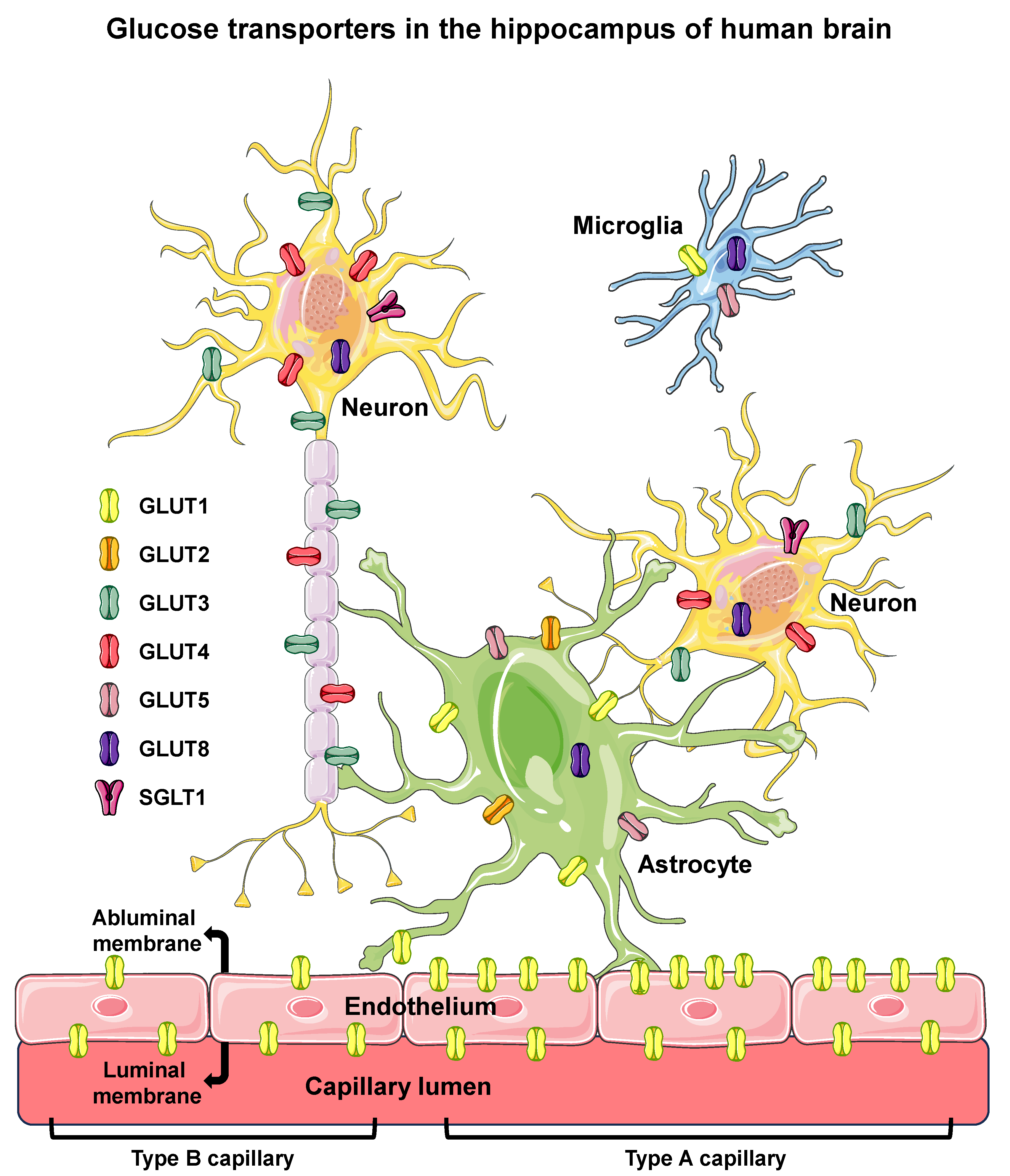
3.4. Glucose Transport and Transporters in the Hippocampus
4. Diabetes Mellitus and Cognitive Impairment
4.1. DM, Hyperglycemia and Hypoglycemia
4.2. Brief Hystory of DM and Cognitive Impairement
4.3. T2D, Hyperglycemia and Dementia
5. Pathophysiological Mechanisms Associated to DM and Dementia
5.1. Inflammation and AGEs
5.2. Insulin Resistance (IR) and Obesity
5.3. BBB Dysfunction
5.4. Common Biomarkers for DM and AD
6. AD, DM, and GLUT1-3 and SGLT1 in the Hippocampus
7. AD, DM, and GLUT4 in the Hippocampus
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-FDG | 2-[18F]fluoro-2-deoxy-D-glucose (substrate for GLUTs) |
| 2-hPG | 2-h post-load glucose |
| 11C-PiB | [11C]-labeled Pittsburgh compound B (marker for amyloid beta accumulation) |
| AB | amyloid-beta |
| ABPP | amyloid beta precursor protein |
| AD | Alzheimer’s disease |
| AGE | advanced glycation end product |
| AMG | [14C]α-methyl D-glucopyranoside |
| AS160 | AKT substrate 160 |
| BAD | Bcl2-associated agonist of cell death |
| BBB | blood–brain barrier |
| BBMEC | bovine brain microvascular endothelial cell |
| BGU | brain glucose utilization |
| CA4 | cornu ammonis 4 area |
| CSF | cerebrospinal fluid |
| CNS | central nervous system |
| CREB/ICER | CRE-binding proteins |
| DHA | dihydroxyacetone |
| DM | diabetes mellitus |
| FOXO1 | forkhead box protein O1 |
| GA | glycated albumin |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GLUT1–GLUT8 | glucose transporter type 1–8 proteins |
| GSK3B | glycogen synthase kinase 3 beta |
| HbA1c | glycated hemoglobin A1c |
| hCMEC/D3 | human cerebral microvascular endothelial cell line/D3 |
| HOMA-IR | homeostatic model assessment for insulin resistance |
| IGF1 | insulin-like growth factor 1 |
| IL6 | interleukin 6 |
| IR | insulin resistance |
| JAK | janus kinase |
| MAPT | microtubule associated protein tau gene (human) |
| Me-4FDG | α-methyl-4-[18F]fluoro-4-deoxy-D-glucopyranoside (substrate for SGLTs) |
| MGO | methylglyoxal |
| MRI | magnetic resonance imaging |
| NFKB-p65 | nuclear factor NF-kappa-B subunit p65 |
| OGTT | oral glucose tolerance test |
| PET | positron emission tomography |
| PI3K | phosphatidylinositol 3-kinase |
| PIP3 | phosphatidylinositol 3,4,5-trisphosphate |
| PKB | protein kinase B (alias AKT) |
| PM | plasma membrane |
| pO2 | partial pressure of oxygen |
| RAGE | advanced glycation end product receptor |
| ROS | reactive oxygen species |
| S100-B | S100 calcium-binding protein B |
| S100b | S100b gene (mouse) |
| SGLT1–2 | sodium/glucose cotransporter types 1–2 |
| Slc2a1–8 | solute carrier family 2 members 1–8 genes (mouse) |
| SLC2A1–8 | solute carrier family 2 members 1–8 genes (human) |
| Slc5a1–2 | solute carrier family 5 members 1–2 genes (mouse) |
| SLC5A1–2 | solute carrier family 5 members 1–2 genes (human) |
| STAT | signal transducer and activator of transcription |
| SVD | small vessel disease |
| TD1 | type 1 diabetes mellitus |
| T2D | type 2 diabetes mellitus |
| TBI | traumatic brain injury |
| TNF | tumor necrosis factor |
| VD | vascular dementia |
References
- Gale, S.A.; Acar, D.; Daffner, K.R. Dementia. Am. J. Med. 2018, 131, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Hugo, J.; Ganguli, M. Dementia and Cognitive Impairment: Epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying neurocognitive disorders: The DSM-5 approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Salvadó, G.; Shekari, M.; Falcon, C.; Operto, G.; Milà-Alomà, M.; Sánchez-Benavides, G.; Cacciaglia, R.; Arenaza-Urquijo, E.; Niñerola-Baizán, A.; Perissinotti, A.; et al. Brain alterations in the early Alzheimer’s continuum with amyloid-β, tau, glial and neurodegeneration CSF markers. Brain Commun. 2022, 4, fcac134. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef]
- Brion, J.P.; Passareiro, H.; Nunez, J.; Flament-Durand, J. Mise en évidence immunologique de la protéine tau au niveau deslésions de dégénérescence neurofibrillaire de la maladie d’Alzheimer. Arch. Biol. 1985, 95, 229–235. [Google Scholar]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Kametani, F.; Hasegawa, M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-beta-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.-M.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M.-Y. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Gulisano, W.; Maugeri, D.; Baltrons, M.A.; Fà, M.; Amato, A.; Palmeri, A.; D’adamio, L.; Grassi, C.; Devanand, D.; Honig, L.S.; et al. Role of Amyloid-β and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J. Alzheimers Dis. 2018, 64, S611–S631. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.F.; Kuhl, D.E.; Hawkins, R.A.; Phelps, M.E.; Cummings, J.L.; Tsai, S.Y. The fluorodeoxyglucose 18F scan in Alzheimer’s disease and multi-infarct dementia. Arch. Neurol. 1983, 40, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.F.; Kuhl, D.E.; Phelps, M.E.; Cummings, J.L.; Tsai, S.Y. Positron emission computed tomography in the diagnosis of dementia. Trans. Am. Neurol. Assoc. 1981, 106, 68–71. [Google Scholar]
- Friedland, R.P.; Jagust, W.J.; Huesman, R.H.; Koss, E.; Knittel, B.; Mathis, C.A.; Ober, B.A.; Mazoyer, B.M.; Budinger, T.F. Regional cerebral glucose transport and utilization in Alzheimer’s disease. Neurology 1989, 39, 1427–1434. [Google Scholar] [CrossRef]
- Nisar, O.; Pervez, H.; Mandalia, B.; Waqas, M.; Sra, H.K. Type 3 Diabetes Mellitus: A Link Between Alzheimer’s Disease and Type 2 Diabetes Mellitus. Cureus 2020, 12, 11. [Google Scholar] [CrossRef]
- Cacciatore, M.; Agata, E.G.; Tripodi, R.; Chiarelli, F. Impact of glucose metabolism on the developing brain. Front. Endocrinol. 2022, 13, 1047545. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Pathogenesis of type 2 diabetes mellitus. Med. Clin. N. Am. 2004, 88, 787–835. [Google Scholar] [CrossRef]
- McEwen, B.S.; Reagan, L.P. Glucose transporter expression in the central nervous system: Relationship to synaptic function. Eur. J. Pharmacol. 2004, 490, 13–24. [Google Scholar] [CrossRef]
- Li, H.; Guglielmetti, C.; Sei, Y.J.; Zilberter, M.; Page, L.M.L.; Shields, L.; Yang, J.; Nguyen, K.; Tiret, B.; Gao, X.; et al. Neurons require glucose uptake and glycolysis in vivo. Cell Rep. 2023, 42, 112335. [Google Scholar] [CrossRef] [PubMed]
- Perantie, D.C.; Koller, J.M.; Weaver, P.M.; Lugar, H.M.; Black, K.J.; White, N.H.; Hershey, T. Prospectively determined impact of type 1 diabetes on brain volume during development. Diabetes 2011, 60, 3006–3014. [Google Scholar] [CrossRef]
- Kowalewski, A.M.; Szylberg, Ł.; Kasperska, A.; Marszałek, A. The diagnosis and management of congenital and adult-onset hyperinsulinism (Nesidioblastosis) literature review. Pol. J. Pathol. 2017, 68, 97–101. [Google Scholar] [CrossRef]
- Pearson-Leary, J.; McNay, E.C. Novel Roles for the Insulin-Regulated Glucose Transporter-4 in Hippocampally Dependent Memory. J. Neurosci. 2016, 36, 11851–11864. [Google Scholar] [CrossRef]
- Tang, M.; Park, S.H.; de Vivo, D.C.; Monani, U.R. Therapeutic strategies for glucose transporter 1 deficiency syndrome. Ann. Clin. Transl. Neurol. 2019, 6, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.C.; Kobayashi, K.; Najdovska, S.; Baker, N.L.; Werther, G.A. Neuronal protection from glucose deprivation via modulation of glucose transport and inhibition of apoptosis: A role for the insulin-like growth factor system. Brain Res. 2004, 1009, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Zhang, Q.; Li, L.; Lai, L.; Zheng, T.; Su, J.; Yang, N.; Yuantao Li, Y. Cytotoxicity study on SH-SY5Y cells cultured at high glucose levels and treated with bupivacaine. Mol. Med. Rep. 2014, 9, 515–520. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, E.D.; Karakus, R.; Aral, A.; Gulbahar, O.; Engin, A. N-Methyl-D aspartate receptor-mediated effect on glucose transporter-3 levels of high glucose exposed-SH-SY5Y dopaminergic neurons. Food Chem. Toxicol. 2017, 109 Pt 1, 465–471. [Google Scholar] [CrossRef]
- Bahniwal, M.; Little, J.P.; Klegeris, A. High Glucose Enhances Neurotoxicity and Inflammatory Cytokine Secretion by Stimulated Human Astrocytes. Curr. Alzheimer Res. 2017, 14, 731–741. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Diabetes Care. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45 (Suppl. S1), S17–S38. [Google Scholar] [CrossRef]
- Hwang, J.J.; Jiang, L.; Hamza, M.; Dai, F.; Belfort-DeAguiar, R.; Cline, G.; Rothman, D.L.; Mason, G.; Sherwin, R.S. The human brain produces fructose from glucose. JCI Insight 2017, 2, e90508. [Google Scholar] [CrossRef] [PubMed]
- Tigchelaar, C.; van Zuylen, M.L.; Hulst, A.H.; Preckel, B.; van Beek, A.P.; Kema, I.P.; Hermanides, J.; Absalom, A.R. Elevated cerebrospinal fluid glucose levels and diabetes mellitus are associated with activation of the neurotoxic polyol pathway. Diabetologia 2022, 65, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Pekkarinen, L.; Kantonen, T.; Rebelos, E.; Latva-Rasku, A.; Dadson, P.; Karjalainen, T.; Bucci, M.; Kalliokoski, K.; Laitinen, K.; Houttu, N.; et al. Obesity risk is associated with brain glucose uptake and insulin resistance. Eur. J. Endocrinol. 2022, 187, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Joost, H.G.; Bell, G.I.; Best, J.D.; Birnbaum, M.J.; Charron, M.J.; Chen, Y.T.; Doege, H.; James, D.E.; Lodish, H.F.; Moley, K.H.; et al. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E974–E976. [Google Scholar] [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef]
- Lizák, B.; Szarka, A.; Kim, Y.; Choi, K.; Németh, C.E.; Marcolongo, P.; Benedetti, A.; Bánhegyi, G.; Margittai, E. Glucose Transport and Transporters in the Endomembranes. Int. J. Mol. Sci. 2019, 20, 5898. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Keating, A.F. Functional properties and genomics of glucose transporters. Curr. Genom. 2007, 8, 113–128. [Google Scholar] [CrossRef]
- Uldry, M.; Thorens, B. The SLC2 family of facilitated hexose and polyol transporters. Pflug. Arch. 2004, 447, 480–489. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in brain in health and disease. Pflug. Arch. 2020, 472, 1299–1343. [Google Scholar] [CrossRef]
- Klip, A.; McGraw, T.E.; James, D.E. Thirty sweet years of GLUT4. J. Biol. Chem. 2019, 294, 11369–11381. [Google Scholar] [CrossRef]
- Bradbury, M.W. The blood-brain barrier. Exp. Physiol. 1993, 78, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Wątroba, M.; Grabowska, A.D.; Szukiewicz, D. Effects of Diabetes Mellitus-Related Dysglycemia on the Functions of Blood-Brain Barrier and the Risk of Dementia. Int. J. Mol. Sci. 2023, 24, 10069. [Google Scholar] [CrossRef] [PubMed]
- Cornford, E.M.; Hyman, S. Localization of brain endothelial luminal and abluminal transporters with immunogold electron microscopy. NeuroRX 2005, 2, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Hegen, H.; Auer, M.; Deisenhammer, F. Serum glucose adjusted cut-off values for normal cerebrospinal fluid/serum glucose ratio: Implications for clinical practice. Clin. Chem. Lab. Med. 2014, 52, 1335–1340. [Google Scholar] [CrossRef]
- Tan, Q.C.; Xing, X.W.; Zhang, J.T.; He, M.W.; Ma, Y.B.; Wu, L.; Wang, X.; Wang, H.F.; Yu, S.Y. Correlation between blood glucose and cerebrospinal fluid glucose levels in patients with differences in glucose metabolism. Front. Neurol. 2023, 14, 1103026. [Google Scholar] [CrossRef]
- Enerson, B.E.; Drewes, L.R. The rat blood-brain barrier transcriptome. J. Cereb. Blood Flow Metab. 2006, 26, 959–973. [Google Scholar] [CrossRef]
- Szablewski, L. Glucose transporters in brain: In health and in Alzheimer’s disease. J. Alzheimers Dis. 2017, 55, 1307–1320. [Google Scholar] [CrossRef]
- Nguyen, T.; Wen, S.; Gong, M.; Yuan, X.; Xu, D.; Wang, C.; Jin, J.; Zhou, L. Dapagliflozin activates neurons in the central nervous system and regulates cardiovascular activity by inhibiting sglt-2 in mice. Diabetes, Metab. Syndr. Obes. Targets Ther. 2020, 13, 2781–2799. [Google Scholar] [CrossRef]
- Wiciński, M.; Wódkiewicz, E.; Górski, K.; Walczak, M.; Malinowski, B. Perspective of SGLT2 inhibition in treatment of conditions connected to neuronal loss: Focus on Alzheimer’s disease and ischemia-related brain injury. Pharmaceuticals 2020, 13, 379. [Google Scholar] [CrossRef]
- Pawlos, A.; Broncel, M.; Woźniak, E.; Gorzelak-Pabiś, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213. [Google Scholar] [CrossRef]
- Mancinetti, F.; Xenos, D.; De Fano, M.; Mazzieri, A.; Porcellati, F.; Boccardi, V.; Mecocci, P. Diabetes-Alzheimer’s connection in older age: SGLT2 inhibitors as promising modulators of disease pathways. Ageing Res. Rev. 2023, 90, 102018. [Google Scholar] [CrossRef] [PubMed]
- Vemula, S.; Roder, K.E.; Yang, T.; Bhat, G.J.; Thekkumkara, T.J.; Abbruscato, T.J. A functional role for sodium dependent glucose transport across the blood-brain barrier during oxygen glucose deprivation. J. Pharmacol. Exp. Ther. 2009, 328, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Sajja, R.K.; Prasad, S.; Cucullo, L. Impact of altered glycaemia on blood-brain barrier endothelium: An in vitro study using the hCMEC/D3 cell line. Fluids Barriers CNS 2014, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Oerter, S.; Förster, C.; Bohnert, M. Validation of sodium/glucose cotransporter proteins in human brain as a potential marker for temporal narrowing of the trauma formation. Int. J. Leg. Med. 2019, 133, 1107–1114. [Google Scholar] [CrossRef]
- Yu, A.S.; Hirayama, B.A.; Timbol, G.; Liu, J.; Basarah, E.; Kepe, V.; Satyamurthy, N.; Huang, S.C.; Wright, E.M.; Barrio, J.R. Functional expression of SGLTs in rat brain. Am. J. Physiol. Cell Physiol. 2010, 299, C1277–C1284. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef]
- Sala-Rabanal, M.; Hirayama, B.A.; Ghezzi, C.; Liu, J.; Huang, S.C.; Kepe, V.; Koepsell, H.; Yu, A.; Powell, D.R.; Bernard Thorens, B.; et al. Revisiting the physiological roles of SGLTs and GLUTs using positron emission tomography in mice. J. Physiol. 2016, 594, 4425–4438. [Google Scholar] [CrossRef]
- Gross, P.M. Circumventricular organ capillaries. Prog. Brain Res. 1992, 91, 219–233. [Google Scholar] [CrossRef]
- Ganong, W.F. Circumventricular organs: Definition and role in the regulation of endocrine and autonomic function. Clin. Exp. Pharmacol. Physiol. 2000, 27, 422–427. [Google Scholar] [CrossRef]
- Miyata, S. New aspects in fenestrated capillary and tissue dynamics in the sensory circumventricular organs of adult brains. Front. Neurosci. 2015, 9, 390. [Google Scholar] [CrossRef]
- Arluison, M.; Quignon, M.; Nguyen, P.; Thorens, B.; Leloup, C.; Penicaud, L. Distribution and anatomical localization of the glucose transporter 2 (GLUT2) in the adult rat brain-an immunohistochemical study. J. Chem. Neuroanat. 2004, 28, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Desilva, S.; Abbruscato, T. The role of glucose transporters in brain disease: Diabetes and Alzheimer’s Disease. Int. J. Mol. Sci. 2012, 13, 12629–12655. [Google Scholar] [CrossRef] [PubMed]
- Uemura, E.; Greenlee, H.W. Insulin regulates neuronal glucose uptake by promoting translocation of glucose transporter GLUT3. Exp. Neurol. 2006, 198, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Benomar, Y.; Naour, N.; Aubourg, A.; Bailleux, V.; Gertler, A.; Djiane, J.; Guerre-Millo, M.; Taouis, M. Insulin and Leptin Induce Glut4 Plasma Membrane Translocation and Glucose Uptake in a Human Neuronal Cell Line by a Phosphatidylinositol 3-Kinase- Dependent Mechanism. Endocrinology 2006, 147, 2550–2556. [Google Scholar] [CrossRef] [PubMed]
- Griffith, C.M.; Macklin, L.N.; Yan Cai, Y.; Sharp, A.A.; Yan, X.X.; Reagan, L.P.; Strader, A.D.; Rose, G.M.; Patrylo, P.R. Impaired Glucose Tolerance and Reduced Plasma Insulin Precede Decreased AKT Phosphorylation and GLUT3 Translocation in the Hippocampus of Old 3xTg-AD Mice. J. Alzheimers Dis. 2019, 68, 809–837. [Google Scholar] [CrossRef] [PubMed]
- Brant, A.M.; Jess, T.J.; Milligan, G.; Brown, C.M.; Gould, G.W. Immunological analysis of glucose transporters expressed in different regions of the rat brain and central nervous system. Biochem. Biophys. Res. Commun. 1993, 192, 1297–1302. [Google Scholar] [CrossRef]
- Leloup, C.; Arluison, M.; Kassis, N.; Lepetit, N.; Cartier, N.; Ferré, P.; Pénicaud, L. Discrete brain areas express the insulin-responsive glucose transporter GLUT4. Brain Res. Mol. Brain Res. 1996, 38, 45–53. [Google Scholar] [CrossRef]
- Ashrafi, G.; Wu, Z.; Farrell, R.J.; Ryan, T.A. GLUT4 Mobilization Supports Energetic Demands of Active Synapses. Neuron 2017, 93, 606–615.e3. [Google Scholar] [CrossRef]
- Rayner, D.V.; Thomas, M.E.; Trayhurn, P. Glucose transporters (GLUTs 1-4) and their mRNAs in regions of the rat brain: Insulin-sensitive transporter expression in the cerebellum. Can. J. Physiol. Pharmacol. 1994, 72, 476–479. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nikami, H.; Morimatsu, M.; Saito, M. Expression and localization of insulin-regulatable glucose transporter (GLUT4) in rat brain. Neurosci. Lett. 1996, 213, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, S.J.; Koehler-Stec, E.M.; Li, K.; Reynolds, T.H.; Clark, R.; Simpson, I.A. GLUT4 glucose transporter expression in rodent brain: Effect of diabetes. Brain Res. 1998, 797, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Grillo, C.A.; Piroli, G.G.; Hendry, R.M.; Reagan, L.P. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res. 2009, 1296, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, F.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.X. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008, 582, 359–364. [Google Scholar] [CrossRef]
- Polito, A.; Brouland, J.P.; Porcher, R.; Sonneville, R.; Siami, S.; Stevens, R.D.; Guidoux, C.; Maxime, V.; de la Grandmaison, G.L.; Chrétien, F.C.; et al. Hyperglycaemia and apoptosis of microglial cells in human septic shock. Crit. Care 2011, 15, R131. [Google Scholar] [CrossRef]
- Yonamine, C.Y.; Passarelli, M.; Suemoto, C.K.; Pasqualucci, C.A.; Jacob-Filho, W.; Alves, V.A.F.; Marie, S.K.N.; Correa-Giannella, M.L.; Britto, L.R.; Machado, U.F. Postmortem Brains from Subjects with Diabetes mellitus display reduced GLUT4 expression and soma area in hippocampal neurons: Potential involvement of inflammation. Cells 2023, 12, 1250. [Google Scholar] [CrossRef]
- Shin, B.C.; McKnight, A.; Devaskar, S.U. Glucose transporter glut8 translocation in neurons is not insulin responsive. J. Neurosci. Res. 2004, 75, 835–844. [Google Scholar] [CrossRef]
- Widmer, M.; Uldry, M.; Thorens, B. Glut8 subcellular localization and absence of translocation to the plasma membrane in pc12 cells and hippocampal neurons. Endocrinology 2005, 146, 4727–4736. [Google Scholar] [CrossRef]
- Poppe, R.; Karbach, U.; Gambaryan, S.; Wiesinger, H.; Lutzenburg, M.; Kraemer, M.; Witte, O.W.; Koepsell, H. Expression of the Na+-D-glucose cotransporter SGLT1 in neurons. J. Neurochem. 1997, 69, 84–94. [Google Scholar] [CrossRef]
- Yu, A.S.; Hirayama, B.A.; Timbol, G.; Liu, J.; Diez-Sampedro, A.; Kepe, V.; Satyamurthy, N.; Huang, S.C.; Wright, E.M.; Barrio, J.R. Regional distribution of SGLT activity in rat brain in vivo. Am. J. Physiol. Cell Physiol. 2013, 304, C240–C247. [Google Scholar] [CrossRef]
- McNay, E.C.; Pearson-Leary, J. GluT4: A central player in hippocampal memory and brain insulin resistance. Exp. Neurol. 2020, 323, 113076. [Google Scholar] [CrossRef]
- Seaquist, E.R.; Anderson, J.; Childs, B.; Cryer, P.; Dagogo-Jack, S.; Fish, L.; Heller, S.R.; Rodriguez, H.; Rosenzweig, J.; Vigersky, R. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013, 36, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- International Hypoglycaemia Study Group. Minimizing Hypoglycemia in Diabetes. Diabetes Care 2015, 38, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Cryer, P.E. Glycemic goals in diabetes: Trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 2014, 63, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Ives, E.R. Mental aberrations in diabetic patients. Bull. Los Angeles Neurol. Soc. 1963, 28, 279–285. [Google Scholar]
- Bale, R.N. Brain damage in diabetes mellitus. Br. J. Psychiatry 1973, 122, 337–341. [Google Scholar] [CrossRef]
- Bianchi, C.; Grandi, G.; Bartoli, M. Recurrent hypoglycemic attacks and Alzheimer’s disease (author’s transl). Pathologica 1978, 70, 571–574. [Google Scholar]
- Bucht, G.; Adolfsson, R.; Lithner, F.; Winblad, B. Changes in blood glucose and insulin secretion in patients with senile dementia of Alzheimer type. Acta Med. Scand. 1983, 213, 387–392. [Google Scholar] [CrossRef]
- Wolf-Klein, G.P.; Siverstone, F.A.; Brod, M.S.; Levy, A.; Foley, C.J.; Termotto, V.; Breuer, J. Are Alzheimer patients healthier? J. Am. Geriatr. Soc. 1988, 36, 219–224. [Google Scholar] [CrossRef]
- Small, G.W.; Rosenthal, M.J. Coexistence of Alzheimer’s disease and diabetes mellitus. J. Am. Geriatr. Soc. 1992, 40, 1075–1076. [Google Scholar] [CrossRef]
- Amiel, A.S. Diabetes and dementia: A causal association? Diabet. Med. 1994, 11, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Northam, E.A.; Anderson, P.J.; Jacobs, R.; Hughes, M.; Warne, G.L.; Werther, G.A. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care 2001, 24, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Husain, K.H.; Sarhan, S.F.; AlKhalifa, H.K.A.A.; Buhasan, A.; Moin, A.S.M.; Butler, A.E. Dementia in Diabetes: The Role of Hypoglycemia. Int. J. Mol. Sci. 2023, 24, 9846. [Google Scholar] [CrossRef]
- Halter, J.B. Alzheimer’s disease and non-insulin-dependent diabetes mellitus: Common features do not make common bedfellows. J. Am. Geriatr. Soc. 1996, 44, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Messier, C.; Ganong, M. Glucose regulation and cognitive functions: Relation to Alzheimer’s disease and diabetes. Behav. Brain Res. 1996, 75, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ott, A.; Stolk, R.P.; Hofman, A.; van Harskamp, F.; Grobbee, D.E.; Breteler, M.M. Association of diabetes mellitus and dementia: The Rotterdam Study. Diabetologia 1996, 39, 1392–1397. [Google Scholar] [CrossRef]
- Ott, A.; Stolk, R.P.; van Harskamp, F.; Pols, H.A.; Hofman, A.; Breteler, M.M. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999, 53, 1937. [Google Scholar] [CrossRef]
- den Heijer, T.; Vermeer, S.E.; Van Dijk, E.J.; Prins, N.D.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 2003, 46, 1604–1610. [Google Scholar] [CrossRef]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-Analysis of Alzheimer’s Disease Risk with Obesity, Diabetes, and Related. Disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef]
- Ohara, T.; Doi, Y.; Ninomiya, T.; Hirakawa, Y.; Hata, J.; Iwaki, T.; Kanba, S.; Kiyohara, Y. Glucose tolerance status and risk of dementia in the community: The Hisayama Study. Neurology 2011, 77, 1126–1134. [Google Scholar] [CrossRef]
- Hirabayashi, N.; Hata, J.; Ohara, T.; Mukai, N.; Nagata, M.; Shibata, M.; Gotoh, S.; Furuta, Y.; Yamashita, F.; Yoshihara, K.; et al. Association Between Diabetes and Hippocampal Atrophy in Elderly Japanese: The Hisayama Study. Diabetes Care 2016, 39, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Ciudin, A.; Simó-Servat, O.; Hernández, C. Cognitive impairment and dementia: A new emerging complication of type 2 diabetes-The diabetologist’s perspective. Acta Diabetol. 2017, 54, 417–424. [Google Scholar] [CrossRef]
- Riederer, P.; Korczyn, A.D.; Ali, S.S.; Bajenaru, O.; Choi, M.S.; Chopp, M.; Dermanovic-Dobrota, V.; Grünblatt, E.; Jellinger, K.A.; Kamal, M.A.; et al. The diabetic brain and cognition. J. Neural Transm. 2017, 124, 1431–1454. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Sato, N. Bidirectional interactions between diabetes and Alzheimer’s disease. Neurochem. Int. 2017, 108, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Holden, R.J.; Mooney, P.A. Interleukin-1 beta: A common cause of Alzheimer’s disease and diabetes mellitus. Med. Hypotheses 1995, 45, 559–571. [Google Scholar] [CrossRef]
- Cunningham, C.; Wilcockson, D.C.; Campion, S.; Lunnon, K.; Perry, V.H. Central and Systemic Endotoxin Challenges Exacerbate the Local Inflammatory Response and Increase Neuronal Death during Chronic Neurodegeneration. J. Neurosci. 2005, 25, 9275–9284. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Beattie, E.C.; Stellwagen, D.; Morishita, D.; Bresnahan, J.C.; Ha, B.K.; Von Zastrow, M.; Beattie, M.S.; Malenka, R.C. Control of synaptic strength by glial TNF-alpha. Science 2002, 295, 2282–2285. [Google Scholar] [CrossRef]
- Clark, I.A.; Alleva, L.M.; Vissel, B. The roles of TNF in brain dysfunction and disease. Pharmacol. Ther. 2010, 128, 519–548. [Google Scholar] [CrossRef]
- Holmes, C.; Cunningham, C.; Zotova, E.; Woolford, J.; Dean, C.; Kerr, S.; Culliford, D.; Perry, V.H. Systemic inflammation and disease progression in Alzheimer disease. Neurology 2009, 73, 768–774. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Roohk, H.V.; Zaidi, A.R.; Patel, D. Glycated albumin (GA) and inflammation: Role of GA as a potential marker of inflammation. Inflamm. Res. 2018, 67, 21–30. [Google Scholar] [CrossRef]
- Smith, M.A.; Sayre, L.M.; Perry, G. Diabetes mellitus and Alzheimer’s disease: Glycation as a biochemical link. Diabetologia 1996, 39, 247. [Google Scholar] [CrossRef]
- Pucci, M.; Aria, F.; Premoli, M.; Maccarinelli, G.; Mastinu, A.; Bonini, S.; Memo, M.; Uberti, D.; Abate, G. Methylglyoxal affects cognitive behaviour and modulates RAGE and Presenilin-1 expression in hippocampus of aged mice. Food Chem. Toxicol. 2021, 158, 112608. [Google Scholar] [CrossRef]
- Wang, J.; Xin, Y.; Chu, T.; Liu, C.; Xu, A. Dexmedetomidine attenuates perioperative neurocognitive disorders by suppressing hippocampal neuroinflammation and HMGB1/RAGE/NF-KB signaling pathway. Biomed. Pharmacother. 2022, 150, 113006. [Google Scholar] [CrossRef]
- Passarelli, M.; Machado, U.F.F. AGEs-Induced and Endoplasmic Reticulum Stress/Inflammation-Mediated Regulation of GLUT4 Expression and Atherogenesis in Diabetes Mellitus. Cells 2021, 11, 104. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Wen, Z.; Yang, Y.; Bu, T.; Bu, X.; Ni, Q. Cognitive dysfunction in diabetes: Abnormal glucose metabolic regulation in the brain. Front. Endocrinol. 2023, 14, 1192602. [Google Scholar] [CrossRef]
- Roberts, R.O.; Knopman, D.S.; Cha, R.H.; Mielke, M.M.; Pankratz, V.S.; Boeve, B.F.; Kantarci, K.; Geda, Y.E.; Jack, C.R.J.r.; Petersen, R.C.; et al. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J. Nucl. Med. 2014, 55, 759–764. [Google Scholar] [CrossRef]
- Rebelos, E.; Rinne, J.O.; Nuutila, P.; Ekblad, L.L. Brain Glucose Metabolism in Health, Obesity, and Cognitive Decline-Does Insulin Have Anything to Do with It? A Narrative Review. J. Clin. Med. 2021, 10, 1532. [Google Scholar] [CrossRef]
- Rhea, E.M.; Leclerc, M.; Yassine, H.N.; Capuano, A.W.; Tong, H.; Petyuk, V.A.; Macauley, S.L.; Fioramonti, X.; Carmichael, O.; Calon, F.; et al. State of the Science on Brain Insulin Resistance and Cognitive Decline Due to Alzheimer’s Disease. Aging Dis. 2023; ahead of print. [Google Scholar] [CrossRef]
- Rivera, E.J.; Goldin, A.; Fulmer, N.; Tavares, R.; Wands, J.R.; de la Monte, S.M. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: Link to brain reductions in acetylcholine. J. Alzheimers Dis. 2005, 8, 247–268. [Google Scholar] [CrossRef]
- de la Monte, S. Type 3 diabetes is sporadic Alzheimer׳s disease: Mini-review. Eur. Neuropsychopharmacol. 2014, 24, 1954–1960. [Google Scholar] [CrossRef]
- Kavanagh, K.; Day, S.M.; Pait, M.C.; Mortiz, W.R.; Newgard, C.B.; Ilkayeva, O.; Mcclain, D.A.; Macauley, S.L. Type-2-Diabetes Alters CSF but Not Plasma Metabolomic and AD Risk Profiles in Vervet Monkeys. Front. Neurosci. 2019, 13, 843. [Google Scholar] [CrossRef]
- Li, W.; Risacher, S.L.; Huang, E.; Saykin, A.J.; Alzheimer’s Disease Neuroimaging Initiative. Type 2 diabetes mellitus is associated with brain atrophy and hypometabolism in the ADNI cohort. Neurology 2016, 87, 595–600. [Google Scholar] [CrossRef]
- Perlmutter, L.S.; Chui, H.C. Microangiopathy, the vascular basement membrane and Alzheimer’s disease: A review. Brain Res. Bull. 1990, 24, 677–686. [Google Scholar] [CrossRef]
- Feinkohl, I.; Price, J.F.; Strachan, M.W.J.; Frier, B.M. The Impact of Diabetes on Cognitive Decline: Potential Vascular, Metabolic, and Psychosocial Risk Factors. Alzheimer’s Res. Ther. 2015, 7, 46. [Google Scholar] [CrossRef]
- Kwa, V.I.H.; van der Sande, J.J.; Stam, J.; Tijmes, N.; Vrooland, J.L. Amsterdam Vascular Medicine Group Retinal Arterial Changes Correlate with Cerebral Small-Vessel Disease. Neurology 2002, 59, 1536–1540. [Google Scholar] [CrossRef]
- Davidson, T.L.; Monnot, A.; Neal, A.U.; Martin, A.A.; Horton, J.J.; Zheng, W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiol. Behav. 2012, 107, 26–33. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef]
- Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Mitschelen, M.; Koller, A.; Szalai, G.; Sonntag, W.E.; Ungvari, Z.; Csiszar, A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: Effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J. Gerontol. A Biol. Med. Sci. 2014, 69, 1212–1226. [Google Scholar] [CrossRef]
- Diniz Pereira, J.; Gomes Fraga, V.; Morais Santos, A.L.; Carvalho, M.D.G.; Caramelli, P.; Braga Gomes, K. Alzheimer’s disease and type 2 diabetes mellitus: A systematic review of proteomic studies. J. Neurochem. 2021, 156, 753–776. [Google Scholar] [CrossRef] [PubMed]
- Simpson, I.A.; Chundu, K.R.; Davies-Hill, T.; Honer, W.G.; Davies, P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease. Ann. Neurol. 1994, 35, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Roltsch, E.; Holcomb, L.; Young, K.A.; Marks, A.; Zimmer, D.B. PSAPP mice exhibit regionally selective reductions in gliosis and plaque deposition in response to S100B ablation. J Neuroinflamm. 2010, 7, 78. [Google Scholar] [CrossRef]
- Marwarha, G.; Ghribi, O. Leptin signaling and Alzheimer’s disease. Am. J. Neurodegener. Dis. 2012, 1, 245–265. [Google Scholar]
- Ceyzériat, K.; Abjean, L.; Sauvage, M.C.; Haim, L.B.; Carole Escartin, C. The complex STATes of astrocyte reactivity: How are they controlled by the JAK-STAT3 pathway? Neuroscience 2016, 330, 205–218. [Google Scholar] [CrossRef]
- Kubis-Kubiak, A.; Dyba, A.; Piwowar, A. The Interplay between Diabetes and Alzheimer’s Disease-In the Hunt for Biomarkers. Int. J. Mol. Sci. 2020, 21, 2744. [Google Scholar] [CrossRef]
- Kumari, S.; Dhapola, R.; Reddy, D.H. Apoptosis in Alzheimer’s disease: Insight into the signaling pathways and therapeutic avenues. Apoptosis 2023, 28, 943–957. [Google Scholar] [CrossRef]
- Dwyer, D.S.; Vannucci, S.J.; Simpson, I.A. Expression, regulation, and functional role of glucose transporters (GLUTs) in brain. Int. Rev. Neurobiol. 2002, 51, 159–188. [Google Scholar] [CrossRef]
- Winocur, G.; Greenwood, C.E.; Piroli, G.G.; Grillo, C.A.; Reznikov, L.R.; Reagan, L.P.; McEwen, B.S. Memory impairment in obese Zucker rats: An investigation of cognitive function in an animal model of insulin resistance and obesity. Behav. Neurosci. 2005, 119, 1389–1395. [Google Scholar] [CrossRef]
- Machado, U.F.; Shimizu, Y.; Saito, M. Decreased glucose transporter (GLUT 4) content in insulin-sensitive tissues of obese aurothioglucose- and monosodium glutamate-treated mice. Horm. Metab. Res. 1993, 25, 462–465. [Google Scholar] [CrossRef]
- Furuya, D.T.; Neri, E.A.; Poletto, A.C.; Anhê, G.F.; Freitas, H.S.; Campello, R.S.; Rebouças, N.A.; Machado, U.F. Identification of nuclear factor-κB sites in the Slc2a4 gene promoter. Mol. Cell. Endocrinol. 2013, 370, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Defo, A.K.; Bakula, V.; Pisaturo, A.P.; Labos, C.; Wing, S.S.; Daskalopoulou, S.S. Diabetes, antidiabetic medications and risk of dementia: A systematic umbrella review and meta-analysis. Diabetes Obes. Metab. 2023; ahead of print. [Google Scholar] [CrossRef]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef] [PubMed]
- Karami, F.; Jamaati, H.; Coleman-Fuller, N.; Zeini, M.S.; Hayes, A.W.; Gholami, M.; Salehirad, M.; Darabi, M.; Motaghinejad, M. Is metformin neuroprotective against diabetes mellitus-induced neurodegeneration? An updated graphical review of molecular basis. Pharmacol. Rep. 2023, 75, 511–543. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yonamine, C.Y.; Michalani, M.L.E.; Moreira, R.J.; Machado, U.F. Glucose Transport and Utilization in the Hippocampus: From Neurophysiology to Diabetes-Related Development of Dementia. Int. J. Mol. Sci. 2023, 24, 16480. https://doi.org/10.3390/ijms242216480
Yonamine CY, Michalani MLE, Moreira RJ, Machado UF. Glucose Transport and Utilization in the Hippocampus: From Neurophysiology to Diabetes-Related Development of Dementia. International Journal of Molecular Sciences. 2023; 24(22):16480. https://doi.org/10.3390/ijms242216480
Chicago/Turabian StyleYonamine, Caio Yogi, Maria Luiza Estimo Michalani, Rafael Junges Moreira, and Ubiratan Fabres Machado. 2023. "Glucose Transport and Utilization in the Hippocampus: From Neurophysiology to Diabetes-Related Development of Dementia" International Journal of Molecular Sciences 24, no. 22: 16480. https://doi.org/10.3390/ijms242216480
APA StyleYonamine, C. Y., Michalani, M. L. E., Moreira, R. J., & Machado, U. F. (2023). Glucose Transport and Utilization in the Hippocampus: From Neurophysiology to Diabetes-Related Development of Dementia. International Journal of Molecular Sciences, 24(22), 16480. https://doi.org/10.3390/ijms242216480





