Protective Effects and Mechanism of Polysaccharides from Edible Medicinal Plants in Alcoholic Liver Injury: A Review
Abstract
:1. Introduction
2. Protective Effects of Polysaccharides from Edible Medicinal Plants on Alcoholic Liver Injury
2.1. Protective Activities of PEMPs against Acute Alcoholic Liver Injury
2.2. Protective Activities of PEMPs against Subacute Alcoholic Liver Injury
2.3. Protective Activities of PEMPs against Subchronic Alcoholic Liver Injury
2.4. Protective Activities of PEMPs against Chronic Alcoholic Liver Injury
3. Mechanism of PEMPs Underlying Protective Effects on ALI
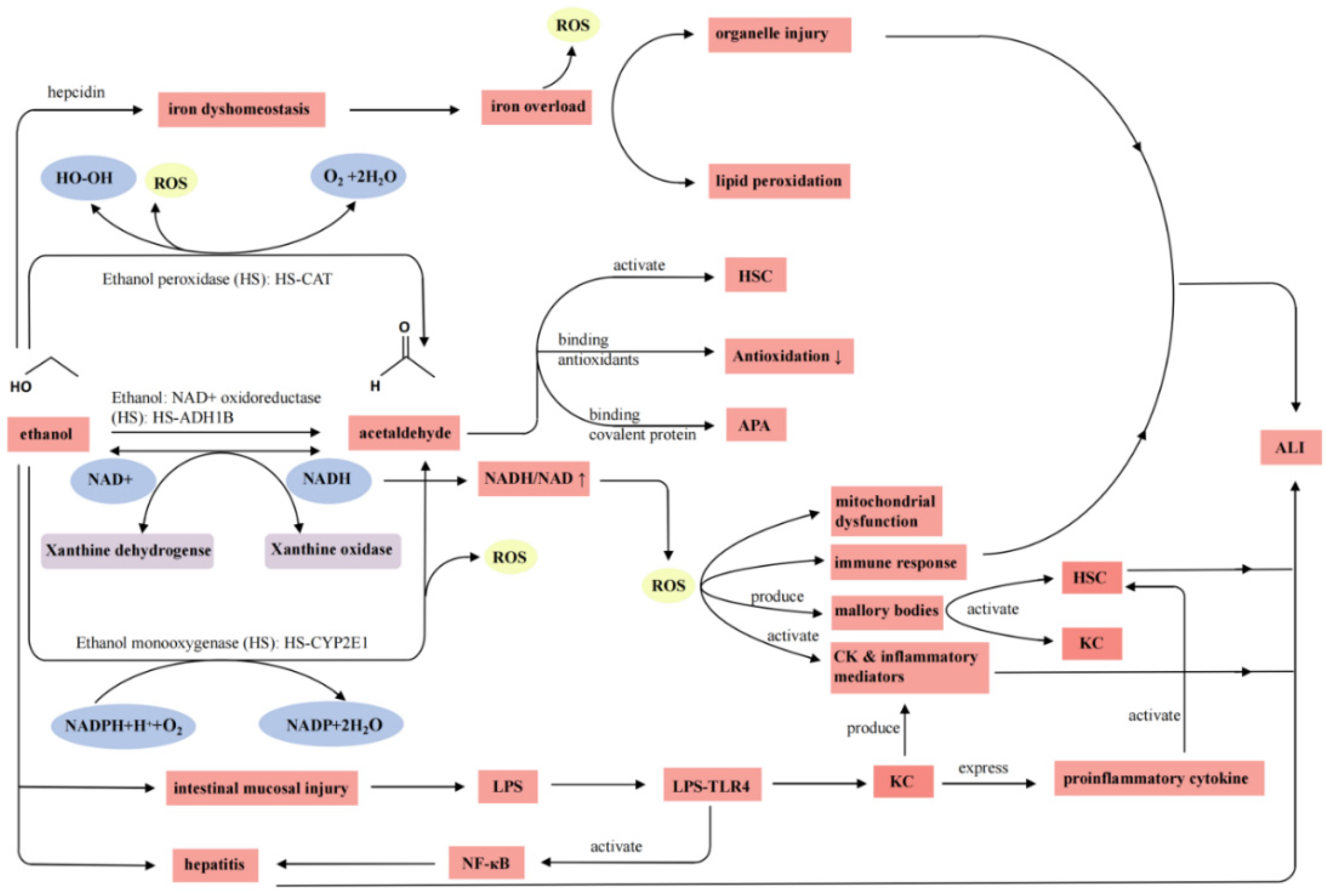
3.1. Pathogenesis of Alcoholic Liver Injury
3.2. Mechanism of Action of PEMPs
3.2.1. Activation of Enzymes Related to Alcohol Metabolism
3.2.2. Attenuation of Damage from Oxidative Stress Caused by Alcohol Intake
3.2.3. Regulation of Cytokines
3.2.4. Inhibition of Apoptosis of Hepatocytes
3.2.5. Improvement of Mitochondrial Function
3.2.6. Regulation of Gut Microbiota
4. Perspectives
4.1. Structure–Activity Relationship (SAR) of PEMPs
4.2. Clarification of Mechanism of Action Using Multi-Omics
4.3. Systematic Investigation of Safety of PEMPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.H.; Zhang, H.F.; Lai, J.H. Alcohol dependence mediated by monoamine neurotransmitters in the central nervous system. Hereditas 2014, 36, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.Y.; Cao, Q.; Yang, F.H.; Zhu, H.Y.; Xu, S.; Chen, Q.; Wang, Z.Y.; Lin, Y.H.; Cinar, R.; Pawlosky, R.J.; et al. Brain ethanol metabolism by astrocytic ALDH2 drives the behavioural effects of ethanol intoxication. Nat. Metab. 2021, 3, 337–351. [Google Scholar] [CrossRef] [PubMed]
- GBD 2020 Alcohol Collaborators. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: A systematic analysis for the global burden of disease study. Lancet 2022, 400, 185–235. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.; Shield, K.D.; Rylett, M.; Hasan, O.S.M.; Probst, C.; Rehm, J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: A modelling study. Lancet 2019, 393, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.E.P.P.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Masarone, M.; Rosato, V.; Dallio, M.; Abenavoli, L.; Federico, A.; Loguercio, C.; Persico, M. Epidemiology and natural history of alcoholic liver disease. Rev. Recent. Clin. Trials. 2016, 11, 167–174. [Google Scholar] [CrossRef]
- Zou, J.; Yang, R.; Feng, R.; Liu, J.; Wan, J.B. Ginsenoside Rk2, a dehydroprotopanaxadiol saponin, alleviates alcoholic liver disease via regulating NLRP3 and NLRP6 inflammasome signaling pathways in mice. J. Pharm. Anal. 2023, 13, 999–1012. [Google Scholar] [CrossRef]
- Zhang, H.F.; Yang, X.H. Asian medicine: Protect rare plants. Nature 2012, 482, 35. [Google Scholar] [CrossRef]
- Song, L.J.; Liu, S.Q.; Zhang, L.; Pan, L.Q.; Xu, L. Polysaccharides from Nitraria retusa fruit: Extraction, purification, structural characterization, and antioxidant activities. Molecules 2023, 28, 1266. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhang, M.Y.; Yang, X.H.; Liang, Q. Application of epimedin C in the prevention and treatment of acute alcoholism. Chinese Patent CN113456660B, 25 October 2022. [Google Scholar]
- Han, C.; Li, Z.; Liu, R.; Zhao, Z.; Wang, Y.; Zuo, X.; Zhang, Y.; Geng, Z.; Huang, H.; Pan, X.; et al. Lonicerae flos polysaccharides improve nonalcoholic fatty liver disease by activating the adenosine 5′-monophosphate-activated protein kinase pathway and reshaping gut microbiota. J. Sci. Food Agric. 2023, 103, 12854. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Jiang, J.; Xing, M.; Cao, Q.; Liang, H.; Ji, A.; Song, S. Advances in oral absorption of natural active polysaccharides. Chem. Life 2019, 39, 605–610. [Google Scholar] [CrossRef]
- Tan, X.; Chen, H.G.; Zhou, X. Study on the activity of Mori Fructus polysaccharides and its derivatives against acute alcoholic liver injury in mice. J. Carbohydr. Chem. 2020, 39, 450–471. [Google Scholar] [CrossRef]
- Bian, L.; Chen, H.G.; Zhou, X. Untargeted lipidomics analysis of Mori Fructus polysaccharide on acute alcoholic liver injury in mice using ultra performance liquid chromatography-quadrupole-orbitrap-high resolution mass spectrometry. Int. Immunopharmacol. 2021, 97, 107521. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, H.F.; Wang, G.Q.; Iia, S.H. Angelica sinensis polysaccharide on mice with alcoholic liver damage morphology observation. J. Chang. Med. Coll. 2016, 30, 256–258. [Google Scholar] [CrossRef]
- Sun, Y.H.; Zhang, H.B. Effects of Ganoderma polysaccharide on liver fat deposits and NLRP3 inflammatory corpuscle expression of mice with acute liver injury. World Chin. Med. 2020, 15, 842–845. [Google Scholar] [CrossRef]
- Ye, L.Y.; Meng, G.L.; Wu, L.Y.; Hao, J.B.; Cheng, B.; Fu, J.S.; Wu, X.P. Preventive effects of the Ganoderma lingzhi fruit-body polysaccharides on the acute alcoholic injury of mice liver based on metabonomics analysis. Mycosystema 2021, 40, 2376–2389. [Google Scholar] [CrossRef]
- Nie, G.; Zhang, Y.; Zhou, Z.; Xu, J.; Wang, H.; Chen, D.; Wang, K. Dynamic evaluation of the protective effect of Dendrobium officinale polysaccharide on acute alcoholic liver injury mice in vitro and in vivo by NIR fluorescence imaging. Anal. Bioanal. Chem. 2021, 413, 5715–5724. [Google Scholar] [CrossRef]
- He, G.F.; Ding, Y.L.; Xu, Q.X.; Chen, W.; Du, G.; Ding, Z.F. Protective effects of Bletilla striata polysaccharides on alcoholic-induced acute liver injury in mice. Chin. J. Hosp. Pharm. 2015, 35, 1658–1661. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, H.Y.; Qian, H. Protective effects of crude aloe polysaccharides and aloin on mice with acute liver injury induced by alcohol. Sci. Technol. Food Ind. 2012, 33, 355–358. [Google Scholar] [CrossRef]
- Xu, B.; Shen, N.; An, Y.; Li, Y.; Li, H.; Zhao, N.X.; Wu, W.N. Effects of Sinomenium acutum polysaccharide on oxidative stress and hepatocyte apoptosis in mice with acute alcoholic liver injury. Chin. Pharm. 2017, 28, 885–888. [Google Scholar] [CrossRef]
- Pan, Y.; Tao, X.; Liang, S.; Yuan, R.S.; Li, H.; Sun, J.H.; Chen, J.G.; Wang, C.M. Ameliorate effect of fractions of Schisandra chinensis acidic polysaccharide on acute alcoholic liver injury in mice. Sci. Technol. Food Ind. 2018, 39, 297–300+305. [Google Scholar] [CrossRef]
- Sun, H.; Yuan, R.S.; Li, H.; Zhang, Y.H.; Shi, J.C.; Chen, J.G.; Wang, C.M. Extraction of polysaccharides from mixed fructus Schisandrae and Radix Astragali and their protective effects on acute liver injury induced by alcohol in mice. Food Sci. 2017, 38, 278–282. [Google Scholar] [CrossRef]
- Wang, K.P.; Xu, J.Y.; Liu, Y.; Cui, Z.; He, Z.H.; Zheng, Z.M.; Huang, X.; Zhang, Y. Self-assembled Angelica sinensis polysaccharide nanoparticles with an instinctive liver-targeting ability as a drug carrier for acute alcoholic liver damage protection. Int. J. Pharm. 2020, 557, 118996. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Yuan, J.; Zhou, Y.M.; Zhang, Y.; Zeng, Y.L.; Liu, M.Z.; Zhao, B.Q. Protective effects of polysaccharide from Bai-shou-wu on alcohol-induced liver injury. Li Shizhen Med. Mater. Med. Res. 2009, 20, 2704–2705. [Google Scholar] [CrossRef]
- Li, Z.M.; Zang, A.M.; Sha, J.Y.; Li, S.S.; Sun, Y.S. Protective effect of extract from America ginseng and Hovenia dulcis Thunb against acute alcohol-induced liver injury in mice. Sci. Technol. Food Ind. 2022, 43, 375–380. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Zhang, Y.; Wang, Y.Y.; Zhang, W.X.; Wang, M.W.; Liu, C.Q.; Peng, D.Y.; Yu, N.J.; Wang, L.; Chen, W.D. Extracts of Poria cocos polysaccharides improves alcoholic liver disease in mice via CYP2E1 and NF-кB inflammatory pathways. Chin. J. Chin. Mter Med. 2022, 47, 134–140. [Google Scholar] [CrossRef]
- Wang, C.X.; Zheng, L.Y.; Liu, S.G.; Guo, X.X.; Qu, Y.; Gao, M.J.; Cui, X.M.; Yang, Y. A novel acidic polysaccharide from the residue of Panax notoginseng and its hepatoprotective effect on alcoholic liver damage in mice. Int. J. Biol. Macromol. 2020, 149, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.X.; Li, S.L.; Li, F.; Pan, L.H.; Zha, X.Q.; Li, D.W.; Luo, J.P. Effects of polysaccharides from six different Dendrobium species against alcohol-induced subacute liver injury in mice. Chin. Pharm. J. 2015, 50, 2117–2123. [Google Scholar] [CrossRef]
- Nie, C.Y.; Wang, H.; Pan, L.H.; Zha, X.Q.; Luo, J.P. Effects of water-soluble polysaccharide from Dendrobium huoshanense against alcohol-induced subacute liver injury. J. Anhui Agric. Sci. 2017, 45, 100–105. [Google Scholar] [CrossRef]
- Wang, X.Y.; Luo, J.P.; Chen, R.; Zha, X.Q.; Wang, H. The effects of daily supplementation of Dendrobium huoshanense polysaccharide on ethanol-induced subacute liver injury in mice by proteomic analysis. Food Funct. 2014, 5, 2020–2035. [Google Scholar] [CrossRef]
- Wang, X.Y.; Luo, J.P.; Chen, R.; Zha, X.Q.; Pan, L.H. Dendrobium huoshanense polysaccharide prevents ethanol-induced liver injury in mice by metabolomic analysis. Int. J. Biol. Macromol. 2015, 78, 354–362. [Google Scholar] [CrossRef]
- Hu, D.K.; Shen, Y.S. Protection of polysaccharide GEP-2 from Gastrodia elata on carbon tetrachloride and alcoholic liver injury in mice. Chin. J. Inf. Tradit. Chin. Med. 2007, 14, 29–31. [Google Scholar] [CrossRef]
- Yan, M.; Teng, C.L.; Tao, H.; Yang, H.B.; Sun, X.H.; Tan, H. Protective effects of Dictyophora rubrovalvata polysaccharide on alcoholic liver injury in rats. Mycosystema 2022, 41, 291–302. [Google Scholar] [CrossRef]
- Li, H.L.; Xie, Z.Y.; Zhang, Y.; Liu, Y.; Niu, A.J.; Liu, Y.Y.; Zhang, L.B.; Guan, L.L. Rosa rugosa polysaccharide attenuates alcoholic liver disease in mice through the gut-liver axis. Food Biosci. 2021, 44, 101385. [Google Scholar] [CrossRef]
- Zhou, B.Y.; Sun, L.; Sui, Z.J.; Meng, L.Y.; Zhang, J.Y.; Gao, F. Protective effect of lentinan on alcoholic liver injury in rats. Chin. J. Publ. Health Eng. 2022, 21, 54–56. [Google Scholar] [CrossRef]
- Wu, Y.L.; Jiang, H.T.; Wang, R.L.; Hua, C. Effect of polysaccharides from Cordyceps militaris stroma against alcohol-induced sub-acute liver injury in male mice. Li Shizhen Med. Mater. Med. Res. 2015, 26, 2600–2605. [Google Scholar] [CrossRef]
- Gu, Y.F.; Zhang, Y.Z.; Chen, H.L.; Li, S.H.; Liu, D.Y. Effect of jujube polysaccharide on alcoholic liver disease rat’s antioxidant function of tissues. J. Anhui Sci. Technol. Univ. 2012, 26, 1–5. [Google Scholar] [CrossRef]
- Wang, Y.C.; Guan, M.; Zhao, X.; Li, X.L. Effects of garlic polysaccharide on alcoholic liver fibrosis and intestinal microflora in mice. Pharm. Biol. 2018, 56, 325–332. [Google Scholar] [CrossRef]
- Cheng, D.; Kong, H. The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules 2011, 16, 2542–2550. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, Y.H.; Liu, Y.X.; Tipoe, G.L.; Xing, F.Y.; So, K.F. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int. J. Biol. Macromol. 2014, 69, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, Y.J. Preventive effects of burdock oligo-saccharide on alcoholic liver injury. Sci. Technol. Food Ind. 2010, 31, 329–331. [Google Scholar] [CrossRef]
- Wei, R.M.; Gao, Y.; Zhong, M.L.; Zhang, K.F. Study on the protective effect of Dicliptera chinensis polysaccharide on rats liver induced by alcohol. Pharm. Clin. Chin. Mater. Med. 2015, 31, 97–99. [Google Scholar] [CrossRef]
- Cui, Y.; Ye, Q.; Wang, H.Y.; Li, Y.C.; Yao, W.R.; Qian, H. Hepatoprotective potential of Aloe vera polysaccharides against chronic alcohol-induced hepatotoxicity in mice. J. Sci. Food Agric. 2014, 94, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Dai, Y.D.; Shu, F.; Gao, M.; Wang, Z.X.; Liu, Y.; Wang, Q. Optimization of extraction technology of polysaccharides from Ganoderma lingzhi mycelium and its protective effect on alcoholic liver injury. Sci. Technol. Food Ind. 2023, 44, 388–396. [Google Scholar] [CrossRef]
- Yan, Y.M.; Wu, W.Q.; Lu, L.; Ren, J.; Mi, J.; Liu, X.; Cao, Y.L. Study on the synergistic protective effect of Lycium barbarum L. polysaccharides and zinc sulfate on chronic alcoholic liver injury in rats. Food Sci. Nutr. 2019, 7, 3435–3442. [Google Scholar] [CrossRef]
- Liu, C.Q.; Chen, J.; Huang, X.S.; Chen, C.T.; Wu, W.Q. Protective effects of garlic polysaccharide on liver injury in mice with chronic alcoholism. J. Jilin Univ. 2012, 38, 23–27+175. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, R.R.; Wan, Y.Y.; Xu, H.; Liang, X.Z. Protective effect and mechanism of polysaccharide from Cinnamomum camphora leaves on chronic alcoholic liver injury in rats. Drug. Clin. 2022, 37, 1182–1188. [Google Scholar] [CrossRef]
- Tao, Y.D.; Tian, H.J.; Li, Y.; Luo, X.Y. Protective effect of Perilla leaf polysaccharides on alcoholic liver injury in mice. Northwest Pharm. J. 2022, 37, 51–56. [Google Scholar] [CrossRef]
- Wu, X.; Fan, X.; Miyata, T.; Kim, A.; Cajigas-Du Ross, C.K.; Ray, S.; Huang, E.; Taiwo, M.; Arya, R.; Wu, J.; et al. Recent advances in understanding of pathogenesis of alcohol-associated liver disease. Annu. Rev. Pathol. 2023, 18, 411–438. [Google Scholar] [CrossRef]
- Liu, H.F.; Zhu, Z.; Su, M.X.; Wang, Q.; Wu, H.; Lai, F.R.; Ma, L.K.; Zeng, C.J.; Zhu, S.M.; Chen, C.M.; et al. Efficacy of polysaccharides as alcoholic liver disease therapeutics: A review. Mod. Food Sci. Technol. 2020, 36, 379–393. [Google Scholar] [CrossRef]
- Joachim, J.H.; Mehta, K.J. Hepcidin in hepatocellular carcinoma. Br. J. Cancer 2022, 127, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhong, W.; Xu, W. Alcohol and the mechanisms of liver disease. J. Gastroenterol. Hepatol. 2023, 38, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Prim. 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Setshedi, M.; Wands, J.R.; Monte, S.M. Acetaldehyde adducts in alcoholic liver disease. Oxid. Med. Cell. Longev. 2010, 3, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.W.; Fouts, D.E.; Brandl, J.; Stärkel, P.; Torralba, M.; Schott, E.; Tsukamoto, H.; Nelson, K.E.; Brenner, D.A.; Schnabl, B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011, 53, 96–105. [Google Scholar] [CrossRef]
- Slevin, E.; Baiocchi, L.; Wu, N.; Ekser, B.; Sato, K.; Lin, E.; Ceci, L.; Chen, L.; Lorenzo, S.R.; Xu, W.; et al. Kupffer cells: Inflammation pathways and cell-cell interactions in alcohol-associated liver disease. Am. J. Pathol. 2020, 190, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Maor, Y.; Nanau, R.M.; Melzer, E.; Mell, H.; Opris, M.; Cohen, L.; Malnick, S. Alcoholic liver disease: Role of cytokines. Biomolecules 2015, 5, 2023–2034. [Google Scholar] [CrossRef]
- Voican, C.S.; Perlemuter, G.; Naveau, S. Mechanisms of the inflammatory reaction implicated in alcoholic hepatitis. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 465–474. [Google Scholar] [CrossRef]
- Albano, E.; Vidali, M. Immune mechanisms in alcoholic liver disease. Genes Nutr. 2010, 5, 141–147. [Google Scholar] [CrossRef]
- Liu, J.H.; Yang, J.W.; Xia, Q.; Xiao, H.P. Alcoholic liver disease pathogenesis research development. Chin. Foreign Med. Res. 2011, 9, 152–153. [Google Scholar] [CrossRef]
- Liang, H.W.; Yang, T.Y.; Teng, C.S.; Lee, Y.J.; Yu, M.H.; Lee, H.J.; Hsu, L.S.; Wang, C.J. Mulberry leaves extract ameliorates alcohol-induced liver damages through reduction of acetaldehyde toxicity and inhibition of apoptosis caused by oxidative stress signals. Int. J. Med. Sci. 2021, 18, 53–64. [Google Scholar] [CrossRef]
- Araújo Júnior, R.F.; Garcia, V.B.; Leitão, R.F.; Brito, G.A.; Miguel, E.d.C.; Guedes, P.M.; de Araújo, A.A. Carvedilol improves inflammatory response, oxidative stress and fibrosis in the alcohol-induced liver injury in rats by regulating Kupffer cells and hepatic stellate cells. PLoS ONE. 2016, 11, e0148868. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.Y.; Zhong, W.; Dong, H.B.; Guo, W.; Sun, X.G.; Zhang, W.L.; Yue, R.C.; Li, T.J.; Griffiths, A.; Ahmadi, A.R.; et al. ATF4 activation promotes hepatic mitochondrial dysfunction by repressing NRF1-TFAM signalling in alcoholic steatohepatitis. Gut 2021, 70, 1933–1945. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, J.B.; Ramos, E.; Nemeth, E.; Ganz, T. Inhibition of hepcidin transcription by growth factors. Hepatology 2012, 56, 291–299. [Google Scholar] [CrossRef]
- Woodard, G.A.; Downey, J.; Hernandez-Boussard, T.; Morton, J.M. Impaired alcohol metabolism after gastric bypass surgery: A case-crossover trial. J. Am. Coll. Surg. 2011, 212, 209–214. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Zhang, T.; Kusumanchi, P.; Han, S.; Yang, Z.; Liangpunsakul, S. Alcohol metabolizing enzymes, microsomal ethanol oxidizing system, cytochrome P450 2E1, catalase, and aldehyde dehydrogenase in alcohol-associated liver disease. Biomedicines 2020, 8, 50. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Yang, X.H.; Duan, M.Y.; Zhang, H.F. Structural characterization of novel fraction EP80 of Epimedium sagittatum polysaccharides and its effects on enzymes related to alcohol metabolism. Nat. Prod. Res. Dev. 2022, 34, 1539–1547. [Google Scholar] [CrossRef]
- Li, Z.Y.; Tang, C.A. Experimental study on effects of Ophiopogonis japonicas polysaccharides on the alleviation of alcoholism and protection of liver. J. Xiangnan Univ. 2017, 19, 20–24. [Google Scholar] [CrossRef]
- Wang, X.; Deng, Q.F.; Chen, H.G.; Zhou, X. Characterization and activity effect on ADH of polysaccharides from Mori Fructus. Chin. J. Chin. Mater. Med. 2017, 42, 2329–2333. [Google Scholar] [CrossRef]
- Cao, L.; Quan, X.B.; Zeng, W.J.; Yang, X.O.; Wang, M.J. Mechanism of hepatocyte apoptosis. J. Cell Death 2016, 9, 19–29. [Google Scholar] [CrossRef]
- Chen, X.N.; Guo, L.B.; Li, Y.H.; Hu, W.S. Research progress on pathogenesis of alcoholic liver diseases and mechanism of its prevention and treatment. Food Mach. 2011, 27, 265–268+274. [Google Scholar] [CrossRef]
- Duan, W.X.; Yang, X.H.; Zhang, H.F.; Feng, J.; Zhang, M.Y. Chemical structure, hypoglycemic activity, and mechanism of action of selenium polysaccharides. Biol. Trace Elem. Res. 2022, 200, 4404–4418. [Google Scholar] [CrossRef]
- Yang, Y.M.; Cho, Y.E.; Hwang, S. Crosstalk between oxidative stress and inflammatory liver injury in the pathogenesis of alcoholic liver disease. Int. J. Mol. Sci. 2022, 23, 774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Guo, M.X.; Li, N.Z.; Zhong, G.Y.; Wang, Y.; Feng, Y.L.; Cheng, H.Y.; Lai, J.L.; Zhu, J.X. Study on the mechanism of action of Tibetan herbal oriental strawberry extract against acute alcoholic liver injury based on multiple signaling pathways. Pharm. Clin. Chin. Mater. Med. 2023, 39, 76–81. [Google Scholar] [CrossRef]
- Sohn, H.; Cooper, M.A. Metabolic regulation of NK cell function: Implications for immunotherapy. Immunometabolism 2023, 5, e00020. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Moteki, H.; Ogihara, M. Role of hepatocyte growth regulators in liver regeneration. Cells 2023, 12, 208. [Google Scholar] [CrossRef]
- Wu, G.S.; Hui, G.Y. Progress of stromal cell-derived factor-1α on tissue reparation and regeneration. Chin. J. Conserv. Dent. 2014, 24, 677–680. [Google Scholar] [CrossRef]
- Shadnoush, M.; Shaker Hosseini, R.; Mehrabi, Y.; Delpisheh, A.; Alipoor, E.; Faghfoori, Z.; Mohammadpour, N.; Zaringhalam Moghadam, J. Probiotic yogurt affects pro- and anti-inflammatory factors in patients with inflammatory bowel disease. Iran. J. Pharm. Res. 2013, 12, 929–936. [Google Scholar] [CrossRef]
- Mai, W.; Liao, Y. Targeting IL-1β in the treatment of atherosclerosis. Front. Immunol. 2020, 11, 589654. [Google Scholar] [CrossRef]
- Chen, X.H. Structural analysis, in vitro digestion and fermentation, and hepatoprotective activity of polysaccharides from ginger stems and leaves. Master’s Thesis, Southwest University, Chongqing, China, 2022. [Google Scholar] [CrossRef]
- Zhao, J.N.; Peng, J.H.; Liu, P.; Hu, Y.Y. Effect of Cordyceps polysaccharides on hepatocyte apoptosis induced by tumor necrosis factor-α. J. Clin. Hepat. 2021, 37, 1368–1372. [Google Scholar] [CrossRef]
- Yang, S.X.; Wang, L.S.; Xie, Z.P.; Zeng, Y.; Xiong, Q.W.; Pei, T.T.; Wei, D.F.; Cheng, W.D. The combination of salidroside and Hedysari Radix Polysaccharide inhibits mitochondrial damage and apoptosis via the PKC/ERK pathway. Evid. Based Complement. Alternat. Med. 2022, 2022, 9475703. [Google Scholar] [CrossRef]
- Xu, Y.N.; Qin, D.X. Advances in molecular mechanisms of mitochondrial damage in alcoholic liver disease. Int. J. Cardiol. 2013, 33, 17–20. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Salari, S.; Baluchnejadmojarad, T.; Roghani, M. Capsaicin protects against septic acute liver injury by attenuation of apoptosis and mitochondrial dysfunction. Heliyon 2023, 9, e14205. [Google Scholar] [CrossRef]
- Huang, J.J.; Bai, Y.M.; Xie, W.T.; Wang, R.M.; Qiu, W.Y.; Zhou, S.L.; Tang, Z.X.; Liao, J.Z.; Su, R.S. Lycium barbarum polysaccharides ameliorate canine acute liver injury by reducing oxidative stress, protecting mitochondrial function, and regulating metabolic pathways. J. Zhejiang Univ. Sci. B. 2023, 24, 157–171. [Google Scholar] [CrossRef]
- Qiu, T.X.; Shi, Y.S.; Wang, R.; Wang, J.L.; Wang, W.J.; Zhu, J.Y.; Wang, W.R.; Wu, Y.; Li, K.; Liu, J.G. Treatment effects of phosphorylated Chrysanthemum indicum polysaccharides on duck viral hepatitis by protecting mitochondrial function from oxidative damage. Vet. Microbiol. 2022, 275, 109600. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Wang, X.X.; Shen, M.Y.; Chen, Y.; Yu, Q.; Yang, J.; Xie, J.H. Combined RNA-seq and molecular biology technology revealed the protective effect of Cyclocarya paliurus polysaccharide on H2O2-induced oxidative damage in L02 cells thought regulating mitochondrial function, oxidative stress and PI3K/Akt and MAPK signaling pathways. Food Res. Int. 2022, 155, 111080. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhang, Y.Q.; Fang, Y.L.; Xu, W.; Zhu, K.D.; Lin, Y. Regulation of SIRT1/AMPK/PGC-1α signaling pathway by Polygonatum polysaccharide improves H2O2-induced oxidative damage in HT22 cells. Chin. J. Mod. Appl. Pharm. 2021, 38, 1952–1957. [Google Scholar] [CrossRef]
- Zhu, L.J.; Xiao, M.M.; Luo, J.G.; Li, S.J.; Liu, W.T.; Wu, J.C.; Song, Z.Y. Polysaccharides from Ostrea rivularis rebuild the balance of gut microbiota to ameliorate non-alcoholic fatty liver disease in ApoE-/- mice. Int. J. Biol. Macromol. 2023, 235, 123853. [Google Scholar] [CrossRef]
- Li, C.M.; Li, W.F.; Yang, H.Y.; Mi, Z.Z.; Tan, S.; Lei, X. Polysaccharides from tumorous stem mustard prevented high fructose diet-induced non-alcoholic fatty liver disease by regulating gut microbiota, hepatic lipid metabolism, and the AKT/FOXO1/MAPK signaling pathway. J. Funct Foods. 2023, 102, 105448. [Google Scholar] [CrossRef]
- Cani, P.D.; Knauf, C. How gut microbes talk to organs: The role of endocrine and nervous routes. Mol. Metab. 2016, 5, 743–752. [Google Scholar] [CrossRef]
- Chen, L.; Tai, W.C.S.; Hsiao, W.L.W. Dietary saponins from four popular herbal tea exert prebiotic-like effects on gut microbiota in C57BL/6 mice. J. Funct. Foods 2015, 17, 892–902. [Google Scholar] [CrossRef]
- Arab, J.P.; Martin-Mateos, R.M.; Shah, V.H. Gut-liver axis, cirrhosis and portal hypertension: The chicken and the egg. Hepatol. Int. 2018, 12, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Chen, Y.C.; Chen, S.J.; Lee, C.H.; Cheng, C.M. Alcohol addiction, gut microbiota, and alcoholism treatment: A review. Int. J. Mol. Sci. 2020, 21, 6413. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.H.; Zhu, H.K.; Xu, W.Q.; Liu, C.; Hu, B.; Guo, Y.H.; Cheng, Y.L.; Qian, H. Echinacea purpurea polysaccharide prepared by fractional precipitation prevents alcoholic liver injury in mice by protecting the intestinal barrier and regulating liver-related pathways. Int. J. Biol. Macromol. 2021, 187, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C. Preventive effect of Auricularia auricula selenium polysaccharide on alcoholic liver injury and its mechanism. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2022. [Google Scholar] [CrossRef]
- Yu, L.H.; Wang, L.L.; Yi, H.X.; Wu, X.J. Beneficial effects of LRP6-CRISPR on prevention of alcohol-related liver injury surpassed fecal microbiota transplant in a rat model. Gut Microbes 2020, 11, 1015–1029. [Google Scholar] [CrossRef]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Ji, X.L.; Guo, J.H.; Cao, T.Z.; Zhang, T.T.; Liu, Y.Q.; Yan, Y.Z. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Wellness 2023, 12, 1969–1980. [Google Scholar] [CrossRef]
- Wang, B.; Yan, L.L.; Guo, S.C.; Wen, L.; Yu, M.L.; Feng, L.; Jia, X.B. Structural elucidation, modification, and structure-activity relationship of polysaccharides in Chinese herbs: A review. Front. Nutr. 2022, 9, 908175. [Google Scholar] [CrossRef]
- Luo, M.C.; Zhang, X.Y.; Wu, J.; Zhao, J.M. Modifications of polysaccharide-based biomaterials under structure-property relationship for biomedical applications. Carbohydr. Polym. 2021, 266, 118097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Su, N.N.; Huang, Q.L.; Zhang, Q.L.; Wang, Y.F.; Li, J.L.; Ye, M. Phosphorylation and antiaging activity of polysaccharide from Trichosanthes peel. J. Food Drug. Anal. 2017, 25, 976–983. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G.L. Antioxidant activities of sulfated pumpkin polysaccharides. Int. J. Biol. Macromol. 2019, 126, 743–746. [Google Scholar] [CrossRef]
- Haas, R.; Zelezniak, A.; Iacovacci, J.; Kamrad, S.; Townsend, S.; Ralser, M. Designing and interpreting ‘multi-omic’ experiments that may change our understanding of biology. Curr. Opin. Syst. Biol. 2017, 6, 37–45. [Google Scholar] [CrossRef]
- Yang, X.H.; Zhang, H.F.; Li, L.; Zhou, X.X.; Liu, Y.C.; Lai, J.H. Proteomic analysis of protective effects of Epimedium flavonoids against ethanol-induced toxicity in retinoic acid-treated SH-SY5Y cells. Molecules 2022, 27, 1026. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, C.; Kia, D.A.; Vandrovcova, J.; Hardy, J.; Wood, N.W.; Lewis, P.A.; Ferrari, R. Genome, transcriptome and proteome: The rise of omics data and their integration in biomedical sciences. Brief. Bioinform. 2018, 19, 286–302. [Google Scholar] [CrossRef]
- Gao, Y.N.; Ye, Q.Y.; Bao, X.Y.; Huang, X.; Wang, J.Q.; Zheng, N. Transcriptomic and proteomic profiling reveals the intestinal immunotoxicity induced by aflatoxin M1 and ochratoxin A. Toxicon 2020, 180, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.V.; Hu, Y.J. Integrative analysis of multi-omics data for discovery and functional studies of complex human diseases. Adv. Genet. 2016, 93, 147–190. [Google Scholar] [CrossRef]
- Demir Karaman, E.; Işık, Z. Multi-omics data analysis identifies prognostic biomarkers across cancers. Med. Sci. 2023, 11, 44. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.; Anyangwe, N.; Carlacci, L.; Casper, S.; Danam, R.P.; Enongene, E.; Erives, G.; Fabricant, D.; Gudi, R.; Hilmas, C.J.; et al. The safety and regulation of natural products used as foods and food ingredients. Toxicol. Sci. 2011, 123, 333–348. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Safety Evaluation of Certain Food Additives. WHO Food Additives Series; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Busch, J.; Allmann, I.; Hölz, H.; Klötzel, M.; Kühn, M.; Mackiw, T.; Riegert, U.; Steinhoff, B. Evaluation of the risk of aflatoxin contamination in fresh medicinal plants. Pharmeur Bio Sci. Notes 2012, 2012, 39–54. [Google Scholar] [PubMed]

| Family | Source of Polysaccharides (Species) | Names of Polysaccharides | Molecular Weight (kDa) | Monosaccharide Composition | Dose (mg/kg) | Bioactivity | Reference |
|---|---|---|---|---|---|---|---|
| Moraceae | Morus alba | MFPA1MFPB1 | / | / | 50 50 | Normalized lipid metabolism; reduced AST, ALT, TG, and MDA levels; increased GSH and SOD levels; and repaired liver injury in mice. | [13] |
| Moraceae | Morus alba | MFPA1 | 177 | Man/Rha/Glc/Xyl = 6.80:7.40:36.60:46.20 | 50 | MFPA1 had the strongest ability to activate ADH in vitro. MFPA1, MFPB1, S-MFPA1, and S-MFPB1 reduced AST, ALT, and TG levels in mouse serum; increased SOD and GSH-Px activities in the liver; and inhibited MDA production. | [14] |
| S-MFPA1 | 364 | 50 | |||||
| MFPA2 | 638 | Man/Rha/GlcA/GalA/Glc/Xyl/Ara = 8.40:7.00:4.90:11.00:25.30:28.50:14.90 | / | ||||
| S-MFPA2 | 731 | / | |||||
| MFPB1 | 165 | Man/Rha/GalA/Glc/Xyl = 9.90:3.60:6.60:43.30:36.50 | 50 | ||||
| S-MFPB1 | 272 | 50 | |||||
| MFPB2 | 380 | Man/Rha/GlcA/GalA/Glc/Xyl/Ara = 2.80:9.50:5.70:6.10:7.40:45.50:23.00 | / | ||||
| S-MFPB2 | 458 | / | |||||
| Orchidaceae | Dendrobium officinale | DOP | 245 | D-Glcp/D-Manp = 1.00:4.18 | 400 | Maintained the relative balance between ROS and antioxidants and improved GSH level in the liver. | [18] |
| Orchidaceae | Bletilla striata | BSPS | / | / | 125, 250, 500 | Reduced oxidative damage and lipid deposition in the liver. High-dose group had the best effect, with increased SOD and GSH activities and decreased ALT and TG levels. | [19] |
| Asphodelaceae | Aloe barbadensis | AP1 AP2 AP3 | / | / | 12.5, 25, 50 | Prolonged drunkenness incubation period shortened sleeping time and waking time of mice. | [20] |
| Menispermaceae | Sinomenium acutum | Sinomenium acutum polysaccharides | / | / | 100, 200, 400 | Improved cell degeneration and necrosis degree of mice in medium-dose and high-dose groups. | [21] |
| Schisandraceae | Schisandra chinensis | SCAP-2 | / | / | 5, 10, 20 | High-dose SCAP-2 decreased ALT and AST activities in serum, increased SOD activity, reduced MDA content in serum and the liver, and reduced TG content in the liver. | [22] |
| Schisandraceae and Fabaceae | Schisandra chinensis and Radix Astragali | ASP | / | / | 100 | Reduced liver index and ALT and AST levels in serum, elevated GSH content, reduced TG and MDA levels in the liver, and improved pathological changes in liver tissues. | [23] |
| Apiaceae | Angelica sinensis | ASP | / | / | 200 | Reduced serum ALT, AST, and TG levels; prevented hepatic steatosis and fat accumulation; and up-regulated hepatic HIF-1α protein expression. | [15] |
| Apiaceae | Angelica sinensis | Cur/ACNPs (Cur-loaded amphiphilic cholesteryl hemisuccinate-Angelica sinensis polysaccharide self-assembled nanoparticles) | / | / | 1.84 | Reduced AST, ALT, and MDA levels; increased GSH content; and increased GSH-Px and SOD activities. | [24] |
| Apocynaceae | Cynanchum bungei | Cynanchum bungei polysaccharides | / | / | 100, 250, 500 | Medium-dose and high-dose polysaccharides increased ALT and AST activities. | [25] |
| Araliaceae and Rhamnaceae | Panax quinquefolius and Hovenia dulcis | AGH | / | / | 100 | Increased serum AST and ALT levels; reduced serum TG and liver MDA levels; increased SOD and GSH-Px activities; enhanced liver GSH level; and inhibited expression of p-ERK, p-JNK, and p-P38. | [26] |
| Polyporaceae | Ganoderma lucidum | Ganoderma lucidum polysaccharides | / | / | 150 | Decreased levels of serum AST, ALT, TG, TC, free fatty acids, IL-1β, IL-6, and TNF-α; inhibited NLRP3 inflammatory corpuscle protein expression in the liver; and alleviated inflammatory response and liver fat deposits. | [16] |
| Polyporaceae | Ganoderma lucidum | GLFPS | / | / | 200 | Inhibited levels of ALT, AST, TG, TC, and ADH in serum and prevented alcoholic liver injury by regulating metabolism of choline, glycerophospholipids, and some ABC transporters. | [17] |
| Family | Source of Polysaccharides (Species) | Names of Polysaccharides | Molecular Weight (kDa) | Monosaccharide Composition | Dose (mg/kg) | Bioactivity | Reference |
|---|---|---|---|---|---|---|---|
| Araliaceae | Panax notoginseng | PNPS-0.5 M | 2600 | Rha/Ara/Xly/Man/Gal = 12.30:33.80:25.80:5.60:22.50 | 100 | Reduced ALT, AST, TG, and MDA levels; elevated the activities of SOD and GSH-Px; and activated Nrf2 signaling as a protective mechanism against Cyp2e1 toxicity. | [28] |
| Orchidaceae | Dendrobium huoshanense | DHP | / | Glc/Man/Gal/Ara = 1.00:0.07:0.11:0.03 | 50, 100, 200 | Among all the Dendrobium polysaccharides, DHP and DOP possessed the highest potential for protecting the liver from hepatotoxicity caused by alcohol intake, which was evidenced as follows: (a) decreased levels of ALT, AST, ALP, TBIL, TC, TG, and LDL-C in serum, as well as TC, TG, MDA, CYP2E1, TNF-α, and IL-1β in hepatic tissues; (b) increased levels of HDL-C in serum and SOD, CAT, GSH-PX, GR, GST, GSH, ADH, and ALDH in hepatic tissues; and (c) ameliorated histopathological changes in hepatic tissues. | [29] |
| Dendrobium officinale | DOP | Glc/Man/Gal/Ara = 1.00:0.13:0.12:0.03 | |||||
| Dendrobium fimbriatum | DFP | Glc/Man/Gal/Ara = 1.00:0.29:0.02:0.01 | |||||
| Dendrobium chrysotoxum | DCP | Glc/Man/Gal = 1.00:0.42:0.04 | |||||
| Dendrobium nobile | DNP | Glc/Man/Gal/Ara/Rha/Xyl = 1.00:0.14:0.10:0.07:0.06:0.04 | |||||
| Dendrobium moniliforme | DMP | Glc/Man/Gal = 1.00:2.25:0.08 | |||||
| Orchidaceae | Dendrobium huoshanense | DHP | / | / | 11, 21, 64 | Suppressed the changes in ALT, AST, ALP, HDL-C, LDL-C, TC, and TG levels in serum; enhanced the activities of ADH, ALDH, SOD, and GSH-Px in the liver; and inhibited the decrease in GSH level and the increase in MDA level in the liver. | [30] |
| Orchidaceae | Dendrobium huoshanense | DHP | / | / | 400 | Decreased the ratio of liver weight to body weight; reduced the levels of serum AST, TG, TBIL, and LDL; alleviated hepatic steatosis; and regulated the expression of Cbs and Ldhd genes. | [31] |
| Orchidaceae | Dendrobium huoshanense | DHP | 22 | Glc/Man/Gal = 2.40:1.00:1.00 | 400 | Alleviated early steatosis and inflammation in liver histology and ameliorated the altered metabolic levels, particularly those involving phosphocholine and L-proline. | [32] |
| Orchidaceae | Gastrodia elata | GEP-2 | / | / | 25, 50, 100 | Reduced the levels of ALT and AST in serum and the content of TG, decreased the level of MDA, and increased the activity of SOD. | [33] |
| Phallaceae | Dictyophora rubrovalvata | DRP | / | / | 100, 200, 400 | Decreased the levels of AST, ALT, and TG; increased the levels of SOD and GSH; decreased the contents of MDA, TNF-α, and IL-6; and improved the pathological phenomena of liver cellular degeneration and necrosis. | [34] |
| Rosaceae | Rosa rugosa | Rosa rugosa polysaccharides | / | / | 100, 300, 500 | Increased the activities of SOD, GSH, and GSH-Px; decreased the contents of NO and MDA; and decreased the serum levels of IL-6, IL-1β, and TNF-α. | [35] |
| Polyporaceae | Poria cocos | PCP | / | / | 50, 200 | Reduced ALT, AST, MDA, IL-6, and TNF-α; potentiated activity of SOD; regulated the expression of CYP2E1; and inhibited TLR4/NF-κB inflammatory signaling pathway. | [27] |
| Omphalotaceae | Lentinula edodes | lentinan | / | / | 750, 1500, 3000 | Decreased MDA and TG, increased GSH content, regulated liver metabolism, and alleviated hepatocyte apoptosis. | [36] |
| Ciavieps purpurea | Cordyceps militaris | CMSP | / | / | 150, 300, 600 | Reduced the pathological damage and inflammatory response of liver tissue, and the pathological improvement was positively correlated with the dose. | [37] |
| Family | Source of Polysaccharides (Species) | Names of Polysaccharides | Molecular Weight (kDa) | Monosaccharide Composition | Dose (mg/kg) | Bioactivity | Reference |
|---|---|---|---|---|---|---|---|
| Rhamnaceae | Ziziphus jujube | Jujube polysaccharides | / | / | 4000, 8000, 16,000 | Increased activities of SOD, CAT, and GSH-Px, but decreased MDA content. | [38] |
| Liliaceae | Allium sativum | Garlic polysaccharides | 10 | Fru/Gal/GalA = 307.00:25.00:32.00 | 150, 250 | Reduced MDA, TC, TG, and LDL levels; increased SOD, GSH-Px, and GSH levels; and inhibited TGF-b1 and TNF-α expression. | [39] |
| Solanaceae | Lycium barbarum | LBPs | / | / | 300 | Elevated GSH, SOD, CAT, and GSH-Px levels, but reduced MDA level. | [40] |
| Asteraceae | Arctium lappa | Arctium lappa polysaccharides | / | / | 100, 300, 900 | Antagonized alcohol-induced hepatic steatosis and reduced TG content in liver tissues. | [42] |
| Acanthaceae | Dicliptera chinensis | Dicliptera chinensis polysaccharides | / | / | 100, 200, 300 | Reduced serum ALT, AST, and MDA levels; elevated GSH-Px and SOD activities; decreased liver TNF-α and IL-6 levels; and alleviated pathological lesions in the liver. | [43] |
| Family | Source of Polysaccharides (Species) | Names of Polysaccharides | Molecular Weight (kDa) | Monosaccharide Composition | Dose (mg/kg) | Bioactivity | Reference |
|---|---|---|---|---|---|---|---|
| Asphodelaceae | Aloe vera | AVGP | / | / | 10, 30 | Attenuated levels of serum aminotransferases and TG; ameliorated histopathological alterations; up-regulated hepatic expression of lipolytic genes (AMPK-α2 and PPAR-α); decreased MDA level; increased GSH and SOD levels; and mitigated alcohol-induced inflammation via reduction in LPS and TNF-α, down-regulation of TLR-4 and MyD88, and up-regulation of IκB-α. | [44] |
| Solanaceae | Lycium barbarum | LBPs | / | / | 250, 500 | Decreased levels of TG, TC, TNF-α, MDA, ALT, AST, and CYP2E1; increased levels of SOD, CAT, GSH-PX, GSH, and ADH; and alleviated liver tissue lesions. | [46] |
| Liliaceae | Allium sativum | garlic polysaccharides | / | / | 100, 150, 200 | Decreased liver index and serum ALT and AST activities, increased GSH and GSH-Px levels, decreased MDA level, and alleviated pathological liver alterations at all doses. Increased body weight and Na+, K+-ATPase activity at high and medium doses. | [47] |
| Lauraceae | Cinnamomum camphora | camphora leaf polysaccharides | / | / | 25.0, 37.5, 50.0 | Decreased levels of IL-1β, IL-6, TNF-α, TG, ALT, and AST; increased GSH level; and improved SIRT1 expression and AMPK-alpha phosphorylation. | [48] |
| Lamiaceae | Perilla frutescens | Perilla frutescens leaf polysaccharides | / | / | 300, 600 | Reduced serum ALT, AST, TG, TC, and LDL levels; increased HDL content; decreased IL-1β, IL-6, TNF-α, and MDA contents in the liver; improved SOD and GSH-Px activities; facilitated expression of p-AMPKα/AMPKα and SIRT1 in the liver; and inhibited expression of SREBP1c. | [49] |
| Polyporaceae | Ganoderma lucidum | SGPs (mycelium polysaccharides) | / | / | 125, 250, 500 | Increased activities of CAT, SOD, and GSH-PX in the liver and serum at medium and high doses; decreased activities of ALT and AST; and reduced contents of MDA, TG, TG, IL-6, IL-1β, and TNF-α at high dose. | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Z.-W.; Yan, T.-Y.; Feng, J.; Zhang, M.-Y.; Han, L.; Zhang, H.-F.; Xiao, Y. Protective Effects and Mechanism of Polysaccharides from Edible Medicinal Plants in Alcoholic Liver Injury: A Review. Int. J. Mol. Sci. 2023, 24, 16530. https://doi.org/10.3390/ijms242216530
Su Z-W, Yan T-Y, Feng J, Zhang M-Y, Han L, Zhang H-F, Xiao Y. Protective Effects and Mechanism of Polysaccharides from Edible Medicinal Plants in Alcoholic Liver Injury: A Review. International Journal of Molecular Sciences. 2023; 24(22):16530. https://doi.org/10.3390/ijms242216530
Chicago/Turabian StyleSu, Zhuo-Wen, Ting-Yu Yan, Jing Feng, Meng-Yuan Zhang, Lei Han, Hua-Feng Zhang, and Ying Xiao. 2023. "Protective Effects and Mechanism of Polysaccharides from Edible Medicinal Plants in Alcoholic Liver Injury: A Review" International Journal of Molecular Sciences 24, no. 22: 16530. https://doi.org/10.3390/ijms242216530
APA StyleSu, Z.-W., Yan, T.-Y., Feng, J., Zhang, M.-Y., Han, L., Zhang, H.-F., & Xiao, Y. (2023). Protective Effects and Mechanism of Polysaccharides from Edible Medicinal Plants in Alcoholic Liver Injury: A Review. International Journal of Molecular Sciences, 24(22), 16530. https://doi.org/10.3390/ijms242216530








