Topical Cellular/Tissue and Molecular Aspects Regarding Nonpharmacological Interventions in Alzheimer’s Disease—A Systematic Review
Abstract
1. Introduction
1.1. Pathology of AD
1.2. Familial and Sporadic AD
2. Methods
3. Results
3.1. Acupuncture
Electroacupuncture
3.2. Cognitive Behavioral Therapy (CBT)
Counseling and Psychoeducation
3.3. Environmental Adjustment
3.4. Exercise/Physical Activity
Music and Dancing
3.5. Information Technology
Virtual Reality (VR)
3.6. Lifestyle Factors
3.6.1. Sensory Practices
3.6.2. Validation Therapy
3.7. Low-Dose Ionizing Radiation (LDIR)
3.8. Mechanical-Based Stimulation
3.9. Photobiomodulation (PBM)
3.10. Reminiscence Therapy (RT)
3.11. Repetitive Transcranial Magnetic Stimulation (rTMS)
3.12. Transcranial Direct Current Stimulation (tDCS)
| Nonpharmacological Interventions in AD | Cellular/Tissue and Molecular Aspects | References |
|---|---|---|
| Acupuncture | Acupuncture in AD potentially modulates neurotransmitters and neuroinflammation, improving ADAS-cog and CIBIC-Plus scores. Data mining aids acupoint selection. | [78,79,80] |
| Electroacupuncture | Electroacupuncture enhances MoCA scores in AD, possibly via cellular mechanisms such as neuroplasticity and molecular pathways like neurotransmitter modulation. | [78] |
| Cognitive behavioral therapy (CBT) | CBT-I may improve cognitive function and potentially delay AD onset through the modulation of Aβ deposition at a molecular level. | [81] |
| Counseling and psychoeducation | The intervention promotes healthy lifestyles and better coping strategies, with a potential cellular impact on BDNF expression, affecting mental health in mild AD. | [82,83,84,85] |
| Environment adjustment | Interventions to combat loneliness may impact regional WM density in the brain cortex, potentially affecting AD development. Activities like aromatherapy with Salvia officinalis could influence acetylcholinesterase levels, offering molecular-level therapeutic potential. | [86,87,88,89,90,91,92,93,94,95,96,97] |
| Exercise/Physical activity | Exercise-based nonpharmacological interventions show multifaceted benefits across cellular and molecular domains, impacting homeostasis, transcription, protein function, and BDNF release. Physical exercise modulates brain metabolic activity, enhances the release of Brain-Derived Neurotrophic Factor (BDNF), and possibly influences monocyte functions, potentially offering a holistic approach to managing AD. Exercise also affects cerebral blood flow and mitochondrial function, although its precise efficacy remains under debate. | [13,20,82,94,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134] |
| Music and dancing | Music-based interventions, including active participation and passive listening, impact neuroplasticity, evoke positive emotions and influence cognitive function in Alzheimer’s disease (AD) patients. These interventions modulate brain structures linked to emotivity and decision-making via dopaminergic circuits and sympathetic arousal. Technological aids like functional MRI and EEG indicate differential neural responses to music, suggesting utility in AD management. Molecular changes, although not fully understood, appear to involve neurotransmitter pathways and neuroconnectivity, particularly in regions like the medial prefrontal cortex and posterior cingulate cortex. | [4,9,13,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149] |
| Information technology | Technology, including mHealth and computer-based cognitive training, empowers Alzheimer’s patients and caregivers by enhancing cognition and psychosocial well-being. These interventions likely modulate neural pathways and cellular functions, although the exact molecular mechanisms remain underexplored. | [87,150,151,152,153,154,155,156,157,158,159,160] |
| Virtual reality (VR) | VR and AR interventions in Alzheimer’s Disease (AD) likely impact neuroplasticity, cognitive function, and motor skills by modulating neural pathways. These immersive technologies may interact with molecular markers associated with cognitive and emotional regulation, although specific mechanisms warrant further study. | [117,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176] |
| Lifestyle factors | Dietary interventions like the Mediterranean and ketogenic diets, as well as exercise, are suggested to modulate cognitive function possibly through anti-inflammatory and antioxidant pathways, impacting cellular and molecular mechanisms relevant to Alzheimer’s Disease. Further studies are needed for mechanistic insights. | [177,178,179,180] |
| Sensory practices | Alternative therapies like aromatherapy and light therapy may modulate neurotransmitter levels and circadian rhythms, potentially ameliorating behavioral symptoms in Alzheimer’s Disease. | [20,143] |
| Validation therapy | Psychosocial interventions may influence neurotransmitter systems and neuronal plasticity, potentially alleviating delusions and hallucinations in dementia patients. | [9,20] |
| Low-dose ionizing radiation (LDIR) | Low-dose ionizing radiation (LDIR) in Alzheimer’s treatment may induce radiation hormesis, enhancing DNA repair, gene expression, antioxidant defense, and anti-inflammatory actions, while potentially improving synaptic and myelin integrity. | [181] |
| Mechanical-based stimulation. | Whole-body vibration (WBV), transcranial ultrasound stimulation (TUSS), and auditory stimulation (AS) may impact neurotrophic and neurotransmission pathways. WBV enhances musculoskeletal and hormonal systems, TUSS affects cerebral circuitry and neuroplasticity, while AS modulates gamma brain waves. | [145,182,183,184,185] |
| Photobiomodulation (PBM) | Low-level LASER therapy (LLLT) in Alzheimer’s Disease (AD) mainly targets mitochondrial function, modulating cytochrome c oxidase (CCO) and exerting antioxidant effects. It also impacts mitochondrial fission/fusion and neuroinflammation. | [186,187] |
| Reminiscence therapy (RT) | Reminiscence therapy (RT) in AD focuses on stimulating remote memory. While not directly cellular or molecular, EEG signals suggest its neurophysiological relevance, impacting cognition and mood. | [9,10,30,82,123,170,188,189,190,191,192,193,194,195,196,197] |
| Repetitive transcranial magnetic stimulation (rTMS) | Repetitive transcranial magnetic stimulation (rTMS) in AD shows promise at the cellular and molecular levels by reducing Aβ peptides, counteracting tau hyperphosphorylation, and modulating ApoE expression. It also influences BDNF levels and GABAergic synaptic strength, potentially rectifying imbalanced neural inhibition–excitation dynamics. | [179,180,187,198,199,200] |
| Transcranial direct current stimulation (tDCS) | Transcranial direct current stimulation (tDCS) in Alzheimer’s Disease (AD) shows polarity-dependent effects, enhancing cognition and combating Aβ peptide deposits. It improves cerebral circulation, synaptic plasticity, and NMDA receptor activity, while also exerting antineuroinflammatory effects. | [187,202,203,204] |
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs, Population Division. World Population Ageing 2015 (ST/ESA/SER.A/390); Economic and Social Affairs United Nations: New York, NY, USA, 2015. [Google Scholar]
- United Nations Department of Economic and Social Affairs, Population Division. World Population Ageing 2020 Highlights: Living Arrangements of Older Persons (ST/ESA/SER.A/451); Economic and Social Affairs United Nations: New York, NY, USA, 2020; pp. 1–47. ISBN 978-92-1-005193-4. Available online: https://digitallibrary.un.org/record/3898412 (accessed on 28 August 2023).
- 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021, 17, 327–406. [CrossRef] [PubMed]
- Miculas, D.C.; Negru, P.A.; Bungau, S.G.; Behl, T.; Hassan, S.S.U.; Tit, D.M. Pharmacotherapy Evolution in Alzheimer’s Disease: Current Framework and Relevant Directions. Cells 2023, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Schwertner, E.; Pereira, J.B.; Xu, H.; Secnik, J.; Winblad, B.; Eriksdotter, M.; Nägga, K.; Religa, D. Behavioral and Psychological Symptoms of Dementia in Different Dementia Disorders: A Large-Scale Study of 10,000 Individuals. J. Alzheimer’s Dis. 2022, 87, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.; Levine, H.; Randhawa, P.; Park, J. Technology-based group exercise interventions for people living with dementia or mild cognitive impairment: A scoping review protocol. BMJ Open 2022, 12, e055990. [Google Scholar] [CrossRef] [PubMed]
- Pinyopornpanish, K.; Soontornpun, A.; Wongpakaran, T.; Wongpakaran, N.; Tanprawate, S.; Pinyopornpanish, K.; Nadsasarn, A.; Pinyopornpanish, M. Impact of behavioral and psychological symptoms of Alzheimer’s disease on caregiver outcomes. Sci. Rep. 2022, 12, 14138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fu, X.; Han, R.; Liu, X.; Zhao, X.; Wei, J. Neuroprotective Effect of HIIT against GFAP Hypertrophy through Mitochondrial Dynamics in APP/PS1 Mice. Oxidative Med. Cell. Longev. 2022, 2022, 1764589. [Google Scholar] [CrossRef]
- Agüera-Ortiz, L.; Babulal, G.M.; Bruneau, M.-A.; Creese, B.; D’antonio, F.; Fischer, C.E.; Gatchel, J.R.; Ismail, Z.; Kumar, S.; McGeown, W.J.; et al. Psychosis as a Treatment Target in Dementia: A Roadmap for Designing Interventions. J. Alzheimer’s Dis. 2022, 88, 1203–1228. [Google Scholar] [CrossRef]
- Nair, P.; Barrado-Martín, Y.; Anantapong, K.; Moore, K.; Smith, C.; Sampson, E.; Manthorpe, J.; Walters, K.; Davies, N. Experiences of Carers and People with Dementia from Ethnic Minority Groups Managing Eating and Drinking at Home in the United Kingdom. Nutrients 2022, 14, 2395. [Google Scholar] [CrossRef]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimer’s Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Mroczko, B. New Possibilities in the Therapeutic Approach to Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 8902. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.L.; Kuller, L.H. Epidemiology of aging and associated cognitive disorders: Prevalence and incidence of Alzheimer’s disease and other dementias. In Handbook of Clinical Neurology, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 167, pp. 139–148. [Google Scholar] [CrossRef]
- Rebeck, G.W.; Reiter, J.S.; Strickland, D.K.; Hyman, B.T. Apolipoprotein E in sporadic Alzheimer’s disease: Allelic variation and receptor interactions. Neuron 1993, 11, 575–580. [Google Scholar] [CrossRef]
- Silva, M.V.F.; Loures, C.d.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M.d.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Houben, S.; Bonnechère, B. The Impact of COVID-19 Infection on Cognitive Function and the Implication for Rehabilitation: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 7748. [Google Scholar] [CrossRef] [PubMed]
- Dagnino, A.P.A.; Campos, M.M. Chronic Pain in the Elderly: Mechanisms and Perspectives. Front. Hum. Neurosci. 2022, 16, 736688. [Google Scholar] [CrossRef]
- Das, R.; Paul, S.; Mourya, G.K.; Kumar, N.; Hussain, M. Recent Trends and Practices Toward Assessment and Rehabilitation of Neurodegenerative Disorders: Insights from Human Gait. Front. Neurosci. 2022, 16, 859298. [Google Scholar] [CrossRef]
- Warren, A. Behavioral and Psychological Symptoms of Dementia as a Means of Communication: Considerations for Reducing Stigma and Promoting Person-Centered Care. Front. Psychol. 2022, 13, 875246. [Google Scholar] [CrossRef]
- Oba, H.; Kobayashi, R.; Kawakatsu, S.; Suzuki, K.; Otani, K.; Ihara, K. Non-pharmacological Approaches to Apathy and Depression: A Scoping Review of Mild Cognitive Impairment and Dementia. Front. Psychol. 2022, 13, 815913. [Google Scholar] [CrossRef]
- Li, X.; Feng, X.; Sun, X.; Hou, N.; Han, F.; Liu, Y. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2019. Front. Aging Neurosci. 2022, 14, 937486. [Google Scholar] [CrossRef]
- Wong, W. Economic burden of Alzheimer disease and managed care considerations. Am. J. Manag. Care 2020, 26, S177–S183. [Google Scholar] [CrossRef]
- Attems, J.; Jellinger, K.; Thal, D.R.; Van Nostrand, W. Review: Sporadic cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol. 2011, 37, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.A.; Härtig, W.; Kacza, J.; Schliebs, R.; Weller, R.O.; Nicoll, J.A.; Carare, R.O. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 2011, 121, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Keable, A.; Fenna, K.; Yuen, H.M.; Johnston, D.A.; Smyth, N.R.; Smith, C.; Salman, R.A.-S.; Samarasekera, N.; Nicoll, J.A.; Attems, J.; et al. Deposition of amyloid β in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 1037–1046. [Google Scholar] [CrossRef]
- Myers, N.; Pasquini, L.; Göttler, J.; Grimmer, T.; Koch, K.; Ortner, M.; Neitzel, J.; Mühlau, M.; Förster, S.; Kurz, A.; et al. Within-patient correspondence of amyloid-β and intrinsic network connectivity in Alzheimer’s disease. Brain 2014, 137, 2052–2064. [Google Scholar] [CrossRef]
- Hawkes, C.A.; Sullivan, P.M.; Hands, S.; Weller, R.O.; Nicoll, J.A.R.; Carare, R.O. Disruption of arterial perivascular drainage of amyloid-β from the brains of mice expressing the human APOE ε4 allele. PLoS ONE 2012, 7, e41636. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.A.; Gentleman, S.M.; Nicoll, J.A.; Carare, R.O. Prenatal high-fat diet alters the cerebrovasculature and clearance of β-amyloid in adult offspring. J. Pathol. 2015, 235, 619–631. [Google Scholar] [CrossRef]
- Jopowicz, A.; Wiśniowska, J.; Tarnacka, B. Cognitive and Physical Intervention in Metals’ Dysfunction and Neurodegeneration. Brain Sci. 2022, 12, 345. [Google Scholar] [CrossRef]
- Ren, R.; Qi, J.; Lin, S.; Liu, X.; Yin, P.; Wang, Z.; Tang, R.; Wang, J.; Huang, Q.; Li, J.; et al. The China Alzheimer Report 2022. Gen. Psychiatry 2022, 35, e100751. [Google Scholar] [CrossRef]
- Jacquens, A.; Needham, E.J.; Zanier, E.R.; Degos, V.; Gressens, P.; Menon, D. Neuro-Inflammation Modulation and Post-Traumatic Brain Injury Lesions: From Bench to Bed-Side. Int. J. Mol. Sci. 2022, 23, 11193. [Google Scholar] [CrossRef]
- Brandt, L.; Liu, S.; Heim, C.; Heinz, A. The effects of social isolation stress and discrimination on mental health. Transl. Psychiatry 2022, 12, 398. [Google Scholar] [CrossRef]
- Chen, L.; Ru, Q.; Xiong, Q.; Yang, J.; Xu, G.; Wu, Y. Potential Effects of Nrf2 in Exercise Intervention of Neurotoxicity Caused by Methamphetamine Oxidative Stress. Oxidative Med. Cell. Longev. 2022, 2022, 4445734. [Google Scholar] [CrossRef]
- Nijakowski, K.; Gruszczyński, D.; Łaganowski, K.; Furmańczak, J.; Brożek, A.; Nowicki, M.; Formanowicz, D.; Surdacka, A. Salivary Morning Cortisol as a Potential Predictor for High Academic Stress Level in Dental Students: A Preliminary Study. Int. J. Environ. Res. Public Health 2022, 19, 3132. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, H.; Li, Z.; Ye, W.; Liu, Z.; Gao, J.; Wang, Y.; Li, X.; Zhang, L.; Alenina, N.; et al. Oxidative RNA Damage in the Pathogenesis and Treatment of Type 2 Diabetes. Front. Physiol. 2022, 13, 725919. [Google Scholar] [CrossRef] [PubMed]
- Shohag, S.; Akhter, S.; Islam, S.; Sarker, T.; Sifat, M.K.; Rahman, M.; Islam, R.; Sharma, R. Perspectives on the Molecular Mediators of Oxidative Stress and Antioxidant Strategies in the Context of Neuroprotection and Neurolongevity: An Extensive Review. Oxidative Med. Cell. Longev. 2022, 2022, 7743705. [Google Scholar] [CrossRef] [PubMed]
- Anghelescu, A.; Onose, G.; Popescu, C.; Băilă, M.; Stoica, S.I.; Postoiu, R.; Brumă, E.; Petcu, I.R.; Ciobanu, V.; Munteanu, C. Parkinson’s Disease and SARS-CoV-2 Infection: Particularities of Molecular and Cellular Mechanisms Regarding Pathogenesis and Treatment. Biomedicines 2022, 26, 1000. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, Y.-M.; Lin, M.-M.; Wang, Y.; Zhang, X.; Liang, L.; He, X. P2X7Rs: New therapeutic targets for osteoporosis. Purinergic Signal. 2023, 19, 207–219. [Google Scholar] [CrossRef]
- Thal, D.R.; von Arnim, C.; Griffin, W.S.T.; Yamaguchi, H.; Mrak, R.E.; Attems, J.; Upadhaya, A.R. Pathology of clinical and preclinical Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263 (Suppl. S2), 137–145. [Google Scholar] [CrossRef]
- Theofilas, P.; Ehrenberg, A.J.; Nguy, A.; Thackrey, J.M.; Dunlop, S.; Mejia, M.B.; Alho, A.T.; Leite, R.E.P.; Rodriguez, R.D.; Suemoto, C.K.; et al. Probing the correlation of neuronal loss, neurofibrillary tangles, and cell death markers across the Alzheimer’s disease Braak stages: A quantitative study in humans. Neurobiol. Aging 2018, 61, 1–12. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, J.H.; Rubinsztein, D.C. Tau degradation: The ubiquitin–proteasome system versus the autophagy-lysosome system. Prog. Neurobiol. 2013, 105, 49–59. [Google Scholar] [CrossRef]
- Hampel, H.; Hu, Y.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; et al. The amyloid-β pathway in Alzheimer’s disease: A plain language summary. Neurodegener. Dis. Manag. 2023, 13, 141–149. [Google Scholar] [CrossRef]
- LaDu, M.J.; Falduto, M.T.; Manelli, A.M.; Reardon, C.A.; Getz, G.S.; Frail, D.E. Isoform-specific binding of apolipoprotein E to β-amyloid. J. Biol. Chem. 1994, 269, 23403–23406. [Google Scholar] [CrossRef] [PubMed]
- Attems, J. Sporadic cerebral amyloid angiopathy: Pathology, clinical implications, and possible pathomechanisms. Acta Neuropathol. 2005, 110, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Mohs, R.C.; Rogers, H.; Fillenbaum, G.; Heyman, A. Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol. Bull. 1988, 24, 641–652. [Google Scholar] [PubMed]
- Alafuzoff, I.; Arzberger, T.; Al-Sarraj, S.; Bodi, I.; Bogdanovic, N.; Braak, H.; Bugiani, O.; Del-Tredici, K.; Ferrer, I.; Gelpi, E.; et al. Staging of Neurofibrillary Pathology in Alzheimer’s Disease: A Study of the BrainNet Europe Consortium. Brain Pathol. 2008, 18, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Attems, J.; Ewers, M. Spreading of amyloid, tau, and microvascular pathology in alzheimer’s disease: Findings from neuropathological and neuroimaging studies. J. Alzheimer’s Dis. 2014, 42, S421–S429. [Google Scholar] [CrossRef]
- Röcken, C.; Saeger, W.; Linke, R. Gastrointestinal amyloid deposits in old age: Report on 110 Consecutive Autopsical Patients and 98 Retrospective Bioptic Specimens. Pathol.-Res. Pract. 1994, 190, 641–649. [Google Scholar] [CrossRef]
- Deane, R.; Wu, Z.; Sagare, A.; Davis, J.; Du Yan, S.; Hamm, K.; Xu, F.; Parisi, M.; LaRue, B.; Hu, H.W.; et al. LRP/Amyloid-Peptide Interaction Mediates Differential Brain Efflux of A Isoforms. Neuron 2004, 43, 333–344. [Google Scholar] [CrossRef]
- Soontornniyomkij, V.; Choi, C.; Pomakian, J.; Vinters, H.V. High-definition characterization of cerebral β-amyloid angiopathy in Alzheimer’s disease. Hum. Pathol. 2010, 41, 1601–1608. [Google Scholar] [CrossRef]
- Vekrellis, K.; Ye, Z.; Qiu, W.Q.; Walsh, D.; Hartley, D.; Chesneau, V.; Rosner, M.R.; Selkoe, D.J. Neurons regulate extracellular levels of amyloid β-protein via proteolysis by insulin-degrading enzyme. J. Neurosci. 2000, 20, 1657–1665. [Google Scholar] [CrossRef]
- Jäkel, L.; Van Nostrand, W.E.; Nicoll, J.A.R.; Werring, D.J.; Verbeek, M.M. Animal models of cerebral amyloid angiopathy. Clin. Sci. 2017, 131, 2469–2488. [Google Scholar] [CrossRef]
- Miners, J.S.; Jones, R.; Love, S. Differential changes in Aβ42 and Aβ40 with age. J. Alzheimer’s Dis. 2014, 40, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Yamaguchi, H.; Saido, T.C.; Thal, D.R. Capillary CAA and perivascular Aβ-deposition: Two distinct features of Alzheimer’s disease pathology. J. Neurol. Sci. 2010, 299, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Mumford, P.; Tosh, J.; Anderle, S.; Wikberg, E.G.; Lau, G.; Noy, S.; Cleverley, K.; Saito, T.; Saido, T.C.; Yu, E.; et al. Genetic Mapping of APP and Amyloid-β Biology Modulation by Trisomy 21. J. Neurosci. 2022, 42, 6453–6468. [Google Scholar] [CrossRef]
- Nunan, J.; Small, D.H. Regulation of APP cleavage by α-, β- and γ-secretases. FEBS Lett. 2000, 483, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Strickland, M.R.; Soranno, A.; Holtzman, D.M. Apolipoprotein E: Structural Insights and Links to Alzheimer Disease Pathogenesis. Neuron 2021, 109, 205–221. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, D.; Peng, X.; Zhang, Q.; Jia, J.; Crutcher, K.A. Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J. Neurotrauma 2008, 25, 279–290. [Google Scholar] [CrossRef]
- Khalil, Y.A.; Rabès, J.-P.; Boileau, C.; Varret, M. APOE gene variants in primary dyslipidemia. Atherosclerosis 2021, 328, 11–22. [Google Scholar] [CrossRef]
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 2020, 581, 71–76. [Google Scholar] [CrossRef]
- Wojtas, A.; Kang, S.S.; Bu, G.; Carare, R.O.; Fryer, J.D. Loss of Clusterin Shifts Amyloid Deposition To the Cerebrovasculature Via Disruption of Perivascular Drainage Pathways. Alzheimer’s Dement. 2017, 13, P312. [Google Scholar] [CrossRef][Green Version]
- Ishii, K.; Lippa, C.; Tomiyama, T.; Miyatake, F.; Ozawa, K.; Tamaoka, A.; Hasegawa, T.; Fraser, P.E.; Shoji, S.; Nee, L.E.; et al. Distinguishable effects of Presenilin-1 and APP717 mutations on amyloid plaque deposition. Neurobiol. Aging 2001, 22, 367–376. [Google Scholar] [CrossRef]
- Citron, M.; Westaway, D.; Xia, W.; Carlson, G.; Diehl, T.; Levesque, G.; Johnson-Wood, K.; Lee, M.; Seubert, P.; Davis, A.; et al. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat. Med. 1997, 3, 67–72. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B. Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007, 8, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, F.; Liu, S.; Battaglia, F.; Walter, S.; Mathews, P.M.; Arancio, O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann. Neurol. 2004, 55, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Revesz, T.; Holton, J.L.; Lashley, T.; Plant, G.; Frangione, B.; Rostagno, A.; Ghiso, J. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 2009, 118, 115–130. [Google Scholar] [CrossRef]
- Albargothy, N.J.; Johnston, D.A.; Sharp, M.M.; Weller, R.O.; Verma, A.; Hawkes, C.A.; Carare, R.O. Convective influx/glymphatic system: Tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. 2018, 136, 139–152. [Google Scholar] [CrossRef]
- Li, F.; Hearn, M.; Bennett, L.E. The role of microbial infection in the pathogenesis of Alzheimer’s disease and the opportunity for protection by anti-microbial peptides. Crit. Rev. Microbiol. 2021, 47, 240–253. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.-G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. (BBA)-Mol. Basis Dis. 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Vitali, M.; Fontana, M.; De Giorgi, A.; Marotta, D.; Crucianelli, S.; Antonucci, A.; Protano, C. Natural Mineral Water and Diuresis: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 5527. [Google Scholar] [CrossRef]
- Onose, G.; Popescu, N.; Munteanu, C.; Ciobanu, V.; Sporea, C.; Mirea, M.-D.; Daia, C.; Andone, I.; Spînu, A.; Mirea, A. Mobile mechatronic/robotic orthotic devices to assist-rehabilitate neuromotor impairments in the upper limb: A systematic and synthetic review. Front. Neurosci. 2018, 12, 577. [Google Scholar] [CrossRef]
- Anghelescu, A.; Firan, F.C.; Onose, G.; Munteanu, C.; Trandafir, A.-I.; Ciobanu, I.; Gheorghița, Ș.; Ciobanu, V. PRISMA Systematic Literature Review, including with Meta-Analysis vs. Chatbot/GPT (AI) regarding Current Scientific Data on the Main Effects of the Calf Blood Deproteinized Hemoderivative Medicine (Actovegin) in Ischemic Stroke. Biomedicines 2023, 11, 1623. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Oh, J.; Kim, S.-Y.; Shin, J.; Kim, S.; Roh, C. Impact of Digital Device, Exercise, and Music Intervention Programs on the Cognition and Depression of the Elderly in South Korea: A Meta-Regression Analysis. Int. J. Environ. Res. Public Health 2022, 19, 4036. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, N.; Bader, L.R. Consensus development methods: Considerations for national and global frameworks and policy development. Res. Soc. Adm. Pharm. 2022, 18, 2222–2229. [Google Scholar] [CrossRef] [PubMed]
- Onose, G.; Anghelescu, A.; Blendea, C.D.; Ciobanu, V.; Daia, C.O.; Firan, F.C.; Munteanu, C.; Oprea, M.; Spinu, A.; Popescu, C. Non-invasive, non-pharmacological/bio-technological interventions towards neurorestoration upshot after ischemic stroke, in adults—Systematic, synthetic, literature review. Front. Biosci. 2021, 26, 1204–1239. [Google Scholar]
- Wang, J.; Zhu, X.; Li, Y.; Zhang, P.; Wang, T.; Li, M. Jiedu-Yizhi Formula Improves Cognitive Impairment in an A β 25-35-Induced Rat Model of Alzheimer’s Disease by Inhibiting Pyroptosis. Evid.-Based Complement. Altern. Med. 2022, 2022, 6091671. [Google Scholar]
- Wang, Y.-F.; Chen, W.-Y.; Lee, C.-T.; Shen, Y.-Y.; Lan, C.-C.; Liu, G.-T.; Kuo, C.-Y.; Chen, M.-L.; Hsieh, P.-C. Combinations of scalp acupuncture location for the treatment of post-stroke hemiparesis: A systematic review and Apriori algorithm-based association rule analysis. Front. Neurosci. 2022, 16, 956854. [Google Scholar] [CrossRef]
- Chaochao, Y.; Li, W.; Lihong, K.; Feng, S.; Chaoyang, M.; Yanjun, D.; Hua, Z. Acupoint combinations used for treatment of Alzheimer’s disease: A data mining analysis. J. Tradit. Chin. Med. 2018, 38, 943–952. [Google Scholar] [CrossRef]
- Carvalhas-Almeida, C.; Cavadas, C.; Álvaro, A.R. The impact of insomnia on frailty and the hallmarks of aging. Aging Clin. Exp. Res. 2023, 35, 253–269. [Google Scholar] [CrossRef]
- Rostamzadeh, A.; Kahlert, A.; Kalthegener, F.; Jessen, F. Psychotherapeutic interventions in individuals at risk for Alzheimer’s dementia: A systematic review. Alzheimer’s Res. Ther. 2022, 14, 18. [Google Scholar] [CrossRef]
- Waldorff, F.B.; Buss, D.V.; Eckermann, A.; Rasmussen, M.L.H.; Keiding, N.; Rishøj, S.; Siersma, V.; Sørensen, J.; Sørensen, L.V.; Vogel, A.; et al. Efficacy of psychosocial intervention in patients with mild Alzheimer’s disease: The multicentre, rater blinded, randomised Danish Alzheimer Intervention Study (DAISY). BMJ 2012, 345, 345. [Google Scholar] [CrossRef]
- Loi, S.M.; Flynn, L.; Cadwallader, C.; Stretton-Smith, P.; Bryant, C.; Baker, F.A. Music and Psychology & Social Connections Program: Protocol for a Novel Intervention for Dyads Affected by Younger-Onset Dementia. Brain Sci. 2022, 12, 503. [Google Scholar] [PubMed]
- Miao, Z.; Wang, Y.; Sun, Z. The relationships between stress, mental disorders, and epigenetic regulation of BDNF. Int. J. Mol. Sci. 2020, 21, 1375. [Google Scholar] [CrossRef] [PubMed]
- Beadle, J.N.; Gifford, A.; Heller, A. A Narrative Review of Loneliness and Brain Health in Older Adults: Implications of COVID-19. Curr. Behav. Neurosci. Rep. 2022, 9, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Döring, N.; Conde, M.; Brandenburg, K.; Broll, W.; Gross, H.-M.; Werner, S.; Raake, A. Can Communication Technologies Reduce Loneliness and Social Isolation in Older People? A Scoping Review of Reviews. Int. J. Environ. Res. Public Health 2022, 19, 11310. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.; Peplau, L.A.; Ferguson, M.L. Developing a Measure of Loneliness. J. Pers. Assess. 1978, 42, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.; Peplau, L.A.; Cutrona, C.E. The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. J. Pers. Soc. Psychol. 1980, 39, 472–480. [Google Scholar] [CrossRef]
- Nakagawa, S.; Takeuchi, H.; Taki, Y.; Nouchi, R.; Sekiguchi, A.; Kotozaki, Y.; Miyauchi, C.M.; Iizuka, K.; Yokoyama, R.; Shinada, T.; et al. White matter structures associated with loneliness in young adults. Sci. Rep. 2015, 5, 17001. [Google Scholar] [CrossRef]
- Hoang, P.; King, J.A.; Moore, S.; Moore, K.; Reich, K.; Sidhu, H.; Tan, C.V.; Whaley, C.; McMillan, J. Interventions Associated with Reduced Loneliness and Social Isolation in Older Adults: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2236676. [Google Scholar] [CrossRef]
- Chirico, I.; Ottoboni, G.; Giebel, C.; Pappadà, A.; Valente, M.; Degli Esposti, V.; Gabbay, M.; Chattat, R. COVID-19 and community-based care services: Experiences of people living with dementia and their informal carers in Italy. Health Soc. Care Community 2022, 30, e3128–e3137. [Google Scholar] [CrossRef]
- Nayak, S.; Coleman, P.L.; Ladányi, E.; Nitin, R.; Gustavson, D.E.; Fisher, S.E.; Magne, C.L.; Gordon, R.L. The Musical Abilities, Pleiotropy, Language, and Environment (MAPLE) Framework for Understanding Musicality-Language Links Across the Lifespan. Neurobiol. Lang. 2022, 3, 615–664. [Google Scholar] [CrossRef]
- Arias-Casais, N.; Thiyagarajan, J.A.; Perracini, M.R.; Park, E.; Block, L.V.D.; Sumi, Y.; Sadana, R.; Banerjee, A.; Han, Z.-A. What long-term care interventions have been published between 2010 and 2020? Results of a WHO scoping review identifying long-term care interventions for older people around the world. BMJ Open 2022, 12, e054492. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Mot, M.D.; Gavrilaș, S.; Lupitu, A.I.; Moisa, C.; Chambre, D.; Tit, D.M.; Bogdan, M.A.; Bodescu, A.M.; Copolovici, L.; Copolovici, D.M.; et al. Salvia officinalis L. Essential Oil: Characterization, Antioxidant Properties, and the Effects of Aromatherapy in Adult Patients. Antioxidants 2022, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Miroddi, M.; Navarra, M.; Quattropani, M.C.; Calapai, F.; Gangemi, S.; Calapai, G. Systematic review of clinical trials assessing pharmacological properties of salvia species on memory, cognitive impairment and Alzheimer’s disease. CNS Neurosci. Ther. 2014, 20, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Renke, M.B.; Marcinkowska, A.B.; Kujach, S.; Winklewski, P.J. A Systematic Review of the Impact of Physical Exercise-Induced Increased Resting Cerebral Blood Flow on Cognitive Functions. Front. Aging Neurosci. 2022, 14, 803332. [Google Scholar] [CrossRef]
- Grusdat, N.P.; Stäuber, A.; Tolkmitt, M.; Schnabel, J.; Schubotz, B.; Wright, P.R.; Schulz, H. Routine cancer treatments and their impact on physical function, symptoms of cancer-related fatigue, anxiety, and depression. Support. Care Cancer 2022, 30, 3733–3744. [Google Scholar] [CrossRef]
- Abedpoor, N.; Taghian, F.; Hajibabaie, F. Cross Brain–Gut Analysis Highlighted Hub Genes and LncRNA Networks Differentially Modified During Leucine Consumption and Endurance Exercise in Mice with Depression-like Behaviors. Mol. Neurobiol. 2022, 59, 4106–4123. [Google Scholar] [CrossRef]
- Jemna, D.-V.; David, M.; Depret, M.-H.; Ancelot, L. Physical activity and healthcare utilization in France: Evidence from the European Health Interview Survey (EHIS) 2014. BMC Public Health 2022, 22, 1355. [Google Scholar] [CrossRef]
- Ding, M.; Li, H.; Zheng, L. Drosophila exercise, an emerging model bridging the fields of exercise and aging in human. Front. Cell Dev. Biol. 2022, 10, 966531. [Google Scholar] [CrossRef]
- Meng, Q.; Lin, M.-S.; Tzeng, I.-S. Relationship Between Exercise and Alzheimer’s Disease: A Narrative Literature Review. Front. Neurosci. 2020, 14, 131. [Google Scholar] [CrossRef]
- López-Ortiz, S.; Pinto-Fraga, J.; Valenzuela, P.L.; Martín-Hernández, J.; Seisdedos, M.M.; García-López, O.; Toschi, N.; Di Giuliano, F.; Garaci, F.; Mercuri, N.B.; et al. Physical Exercise and Alzheimer’ s Disease: Effects on Pathophysiological Molecular Pathways of the Disease. Int. J. Mol. Sci. 2021, 22, 2897. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-W.; Tzeng, H.-Y.; Chu, C.-M.; Lan, H.-Y.; Chiang, H.-H. A Novel Intensity-Based Approach to Increasing Prefrontal Cerebral Oxygenation by Walking Exercise. J. Pers. Med. 2022, 12, 510. [Google Scholar] [CrossRef] [PubMed]
- Salzman, T.; Sarquis-Adamson, Y.; Son, S.; Montero-Odasso, M.; Fraser, S. Associations of Multidomain Interventions with Improvements in Cognition in Mild Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e226744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, H.; Luo, Q.; Cui, S. The effect of physical exercise on circulating brain-derived neurotrophic factor in healthy subjects: A meta-analysis of randomized controlled trials. Brain Behav. 2022, 12, e2544. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Sanabria, L.C.; Cavero-Redondo, I.; Martínez-Vizcaino, V.; Cano-Gutierrez, C.A.; Álvarez-Bueno, C. Effect of multicomponent exercise in cognitive impairment: A systematic review and meta-analysis. BMC Geriatr. 2022, 22, 617. [Google Scholar] [CrossRef]
- Stoica, S.I.; Bleotu, C.; Ciobanu, V.; Ionescu, A.M.; Albadi, I.; Onose, G.; Munteanu, C. Considerations about Hypoxic Changes in Neuraxis Tissue Injuries and Recovery. Biomedicines 2022, 10, 481. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Z.; Zhu, L.; Huang, G.; Li, B.; Chen, C.; Huang, J.; Ma, F.; Liu, T.C. Effects of different physical activities on brain-derived neurotrophic factor: A systematic review and bayesian network meta-analysis. Front. Aging Neurosci. 2022, 14, 981002. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Luo, Z.; Chen, X.; Wang, Y.; Qi, B.; Lin, J.; Lin, W.-W.; Sun, C.; Zhou, Y.; et al. Exercise Modifies the Transcriptional Regulatory Features of Monocytes in Alzheimer’s Patients: A Multi-Omics Integration Analysis Based on Single Cell Technology. Front. Aging Neurosci. 2022, 14, 881488. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Lin, S. Irisin: A bridge between exercise and neurological diseases. Heliyon 2022, 8, e12352. [Google Scholar] [CrossRef]
- Gubert, C.; Gasparotto, J.; Morais, L.H. Convergent pathways of the gut microbiota–brain axis and neurodegenerative disorders. Gastroenterol. Rep. 2022, 10, goac017. [Google Scholar] [CrossRef]
- Cámara-Calmaestra, R.; Martínez-Amat, A.; Aibar-Almazán, A.; Hita-Contreras, F.; de Miguel Hernando, N.; Achalandabaso-Ochoa, A. Effectiveness of Physical Exercise on Alzheimer’s disease. A Systematic Review. J. Prev. Alzheimer’s Dis. 2022, 9, 601–616. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.P.B.; Padovez, R.d.F.C.M.; Serrão, P.R.M.d.S.; de Noronha, M.A.; Cezar, N.O.d.C.; de Andrade, L.P. Effectiveness of physical exercise at improving functional capacity in older adults living with Alzheimer’s disease: A systematic review of randomized controlled trials. Disabil. Rehabil. 2023, 45, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Cardona, M.I.; Weißenborn, M.; Zöllinger, I.; Kroeber, E.S.; Bauer, A.; Luppa, M.; Pabst, A.; Czock, D.; König, H.H.; Wiese, B.; et al. Physical Activity Determinants in Older German Adults at Increased Dementia Risk with Multimorbidity: Baseline Results of the AgeWell.de Study. Int. J. Environ. Res. Public Health 2022, 19, 3164. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.; Mehrabi, S.; Li, Y.; Basharat, A.; Middleton, L.E.; Cao, S.; Barnett-Cowan, M.; Boger, J. Immersive Virtual Reality Exergames for Persons Living with Dementia: User-Centered Design Study as a Multistakeholder Team During the COVID-19 Pandemic. JMIR Serious Games 2022, 10, e29987. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, M.; Bao, D.; Zhou, J. Effects of Traditional Chinese Exercises on Cognitive Function in Older Adults with Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Front. Hum. Neurosci. 2022, 16, 849530. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, R.; Feng, Y.; Cheng, L. Molecular mechanisms of exercise contributing to tissue regeneration. Signal Transduct. Target. Ther. 2022, 7, 383. [Google Scholar] [CrossRef]
- Li, K.; Cui, C.; Zhang, H.; Jia, L.; Li, R.; Hu, H.-Y. Exploration of combined physical activity and music for patients with Alzheimer’s disease: A systematic review. Front. Aging Neurosci. 2022, 14, 962475. [Google Scholar] [CrossRef]
- Janzen, T.B.; Koshimori, Y.; Richard, N.M.; Thaut, M.H. Rhythm and Music-Based Interventions in Motor Rehabilitation: Current Evidence and Future Perspectives. Front. Hum. Neurosci. 2022, 15, 789467. [Google Scholar] [CrossRef]
- Pitkänen, A.; Alanen, H.-M.; Kampman, O.; Suontaka-Jamalainen, K.; Leinonen, E. Implementing physical exercise and music interventions for patients suffering from dementia on an acute psychogeriatric inpatient ward. Nord. J. Psychiatry 2019, 73, 401–408. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, C.; Yang, L.; Mei, X.; Wei, X.; Kuang, J.; Zhou, K.; Xu, M. Corrigendum: Longitudinal Association Between Depressive Symptoms and Cognitive Function Among Older Adults: A Parallel Latent Growth Curve Modeling Approach. Int. J. Public Health 2022, 67, 1605525. [Google Scholar] [CrossRef]
- So, K.-F.; Li, A.; Liang, Y.-Y.; Zhang, L.-D.; Luo, X.; Wu, L.-L.; Chen, Z.-W.; Wei, G.-H.; Zhang, K.-Q.; Du, Z.-A.; et al. All roads lead to Rome—A review of the potential mechanisms by which exerkines exhibit neuroprotective effects in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1210–1227. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Rotariu, M.; Turnea, M.-A.; Anghelescu, A.; Albadi, I.; Dogaru, G.; Silișteanu, S.C.; Ionescu, E.V.; Firan, F.C.; Ionescu, A.M.; et al. Topical Reappraisal of Molecular Pharmacological Approaches to Endothelial Dysfunction in Diabetes Mellitus Angiopathy. Curr. Issues Mol. Biol. 2022, 44, 3378–3397. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Su, Q.W.; Sheng, Z.R.; Weng, Q.Y.; Niu, Y.F.; Zhou, H.D.; Liu, C.B. Effectiveness of Physical Activity Interventions on Cognition, Neuropsychiatric Symptoms, and Quality of Life of Alzheimer’s Disease: An Update of a Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022, 14, 155. [Google Scholar] [CrossRef]
- Steichele, K.; Keefer, A.; Dietzel, N.; Graessel, E.; Prokosch, H.-U.; Kolominsky-Rabas, P.L. The effects of exercise programs on cognition, activities of daily living, and neuropsychiatric symptoms in community-dwelling people with dementia—A systematic review. Alzheimer’s Res. Ther. 2022, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Kouloutbani, K.; Venetsanou, F.; Markati, A.; Karteroliotis, K.E.; Politis, A. The effectiveness of physical exercise interventions in the management of neuropsychiatric symptoms in dementia patients: A systematic review. Int. Psychogeriatr. 2021, 34, 177–190. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, X.; Cai, Y.; Wan, Q. The cerebral changes induced by exercise interventions in people with mild cognitive impairment and Alzheimer’s disease: A systematic review. Arch. Gerontol. Geriatr. 2022, 98, 104547. [Google Scholar] [CrossRef]
- Qiu, Y.; Fernández-García, B.; Lehmann, H.I.; Li, G.; Kroemer, G.; López-Otín, C.; Xiao, J. Exercise sustains the hallmarks of health. J. Sport Health Sci. 2023, 12, 8–35. [Google Scholar] [CrossRef]
- Dauwan, M.; Begemann, M.J.H.; Slot, M.I.E.; Lee, E.H.M.; Scheltens, P.; Sommer, I.E.C. Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: A transdiagnostic systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2021, 268, 1222–1246. [Google Scholar] [CrossRef]
- Vita, A.; Gaebel, W.; Mucci, A.; Sachs, G.; Barlati, S.; Giordano, G.M.; Nibbio, G.; Nordentoft, M.; Wykes, T.; Galderisi, S. European Psychiatric Association guidance on treatment of cognitive impairment in schizophrenia. Eur. Psychiatry 2022, 65, e58. [Google Scholar] [CrossRef]
- Su, K.; Yuan, J.; Liu, H.; Luo, M.; Li, Q.; Liu, S.; Feng, X. The Comparative Effectiveness of Traditional Chinese Medicine Exercise Therapies in Elderly People with Mild Cognitive Impairment: A Systematic Review and Network Meta-Analysis. Front. Neurol. 2022, 13, 775190. [Google Scholar] [CrossRef]
- Li, W.; Weng, L.; Xiang, Q.; Fan, T. Trends in Research on Traditional Chinese Health Exercises for Improving Cognitive Function: A Bibliometric Analysis of the Literature from 2001 to 2020. Front. Public Health 2022, 9, 794836. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhu, F.; Li, Z.; Che, D.; Li, L.; Zhang, L.; Zhong, Y.; Luo, B.; Wu, X. Research Hotspots and Trends in Music Therapy Intervention for Patients with Dementia: A Bibliometrics and Visual Analysis of Papers Published from 2010 to 2021. Front. Psychiatry 2022, 13, 860758. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Persaud, S.; Patel, M.; Popard, P.; Colverson, A.; Doré, S. Effects of Sound Interventions on the Permeability of the Blood–Brain Barrier and Meningeal Lymphatic Clearance. Brain Sci. 2022, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.; Nouchi, R.; Dinet, J.; Cheng, C.-H.; Husebø, B.S. The Effect of Music-Based Intervention on General Cognitive and Executive Functions, and Episodic Memory in People with Mild Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis of Recent Randomized Controlled Trials. Healthcare 2022, 10, 1462. [Google Scholar] [CrossRef]
- Qiu, L.; Zhong, Y.; Xie, Q.; He, Z.; Wang, X.; Chen, Y.; Zhan, C.A.A.; Pan, J. Multi-Modal Integration of EEG-fNIRS for Characterization of Brain Activity Evoked by Preferred Music. Front. Neurorobot. 2022, 16, 823435. [Google Scholar] [CrossRef]
- Chu, H.; Moon, S.; Park, J.; Bak, S.; Ko, Y.; Youn, B.-Y. The Use of Artificial Intelligence in Complementary and Alternative Medicine: A Systematic Scoping Review. Front. Pharmacol. 2022, 13, 826044. [Google Scholar] [CrossRef]
- Quinci, M.A.; Belden, A.; Goutama, V.; Gong, D.; Hanser, S.; Donovan, N.J.; Geddes, M.; Loui, P. Longitudinal changes in auditory and reward systems following receptive music-based intervention in older adults. Sci. Rep. 2022, 12, 11517. [Google Scholar] [CrossRef]
- Hofbauer, L.M.; Ross, S.D.; Rodriguez, F.S. Music-based interventions for community-dwelling people with dementia: A systematic review. Health Soc. Care Community 2022, 30, 2186–2201. [Google Scholar] [CrossRef]
- Simmons-Stern, N.R.; Budson, A.E.; Ally, B.A. Music as a memory enhancer in patients with Alzheimer’s disease. Neuropsychologia 2010, 48, 3164–3167. [Google Scholar] [CrossRef]
- Pardo-Moreno, T.; González-Acedo, A.; Rivas-Domínguez, A.; García-Morales, V.; García-Cozar, F.J.; Ramos-Rodríguez, J.J.; Melguizo-Rodríguez, L. Therapeutic Approach to Alzheimer’s Disease: Current Treatments and New Perspectives. Pharmaceutics 2022, 14, 1117. [Google Scholar] [CrossRef]
- Gassner, L.; Geretsegger, M.; Mayer-Ferbas, J. Effectiveness of music therapy for autism spectrum disorder, dementia, depression, insomnia and schizophrenia: Update of systematic reviews. Eur. J. Public Health 2022, 32, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Matziorinis, A.M.; Koelsch, S. The promise of music therapy for Alzheimer’s disease: A review. Ann. N. Y. Acad. Sci. 2022, 1516, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, J.-K.; Yeo, M.S. Dual-Task-Based Music Therapy to Improve Executive Functioning of Elderly Patients with Early Stage Alzheimer’s Disease: A Multiple Case Study. Int. J. Environ. Res. Public Health 2022, 19, 11940. [Google Scholar] [CrossRef] [PubMed]
- Flo, B.K.; Matziorinis, A.M.; Skouras, S.; Sudmann, T.T.; Gold, C.; Koelsch, S. Study protocol for the Alzheimer and music therapy study: An RCT to compare the efficacy of music therapy and physical activity on brain plasticity, depressive symptoms, and cognitive decline, in a population with and at risk for Alzheimer’s disease. PLoS ONE 2022, 17, e0270682. [Google Scholar] [CrossRef]
- Kim, C.K.; Sachdev, P.S.; Braidy, N. Recent Neurotherapeutic Strategies to Promote Healthy Brain Aging: Are we there yet? Aging Dis. 2022, 13, 175–214. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, Y.; Guo, C.; Qi, M.; Xiao, M.; Wu, H.; Ma, J.; Zhong, Q.; Ding, H.; Zhou, Q.; et al. Effect of 3-Month Aerobic Dance on Hippocampal Volume and Cognition in Elderly People with Amnestic Mild Cognitive Impairment: A Randomized Controlled Trial. Front. Aging Neurosci. 2022, 14, 771413. [Google Scholar] [CrossRef]
- Cammisuli, D.M.; Cipriani, G.; Castelnuovo, G. Technological Solutions for Diagnosis, Management and Treatment of Alzheimer’s Disease-Related Symptoms: A Structured Review of the Recent Scientific Literature. Int. J. Environ. Res. Public Health 2022, 19, 3122. [Google Scholar] [CrossRef]
- Garnett, A.; Northwood, M.; Ting, J.; Sangrar, R. mHealth Interventions to Support Caregivers of Older Adults: Equity-Focused Systematic Review. JMIR Aging 2022, 5, e33085. [Google Scholar] [CrossRef]
- World Health Organization. mHealth: New horizons for Health through Mobile Technologies. Observatory 2011, 3, 66–71. Available online: https://www.afro.who.int/publications/mhealth-new-horizons-health-through-mobile-technologie (accessed on 28 August 2023).
- Diaz Baquero, A.A.; Franco-Martín, M.A.; Parra Vidales, E.; Toribio-Guzmán, J.M.; Bueno-Aguado, Y.; Martinez Abad, F.; Perea Bartolomé, M.V.; Asl, A.M.; van der Roest, H.G. The Effectiveness of GRADIOR: A Neuropsychological Rehabilitation Program for People with Mild Cognitive Impairment and Mild Dementia. Results of a Randomized Controlled Trial After 4 and 12 Months of Treatment. J. Alzheimer’s Dis. 2022, 86, 711–727. [Google Scholar] [CrossRef]
- Li, R.; Geng, J.; Yang, R.; Ge, Y.; Hesketh, T. Effectiveness of Computerized Cognitive Training in Delaying Cognitive Function Decline in People with Mild Cognitive Impairment: Systematic Review and Meta-analysis. J. Med. Internet Res. 2022, 24, e38624. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.R.O.; Boersma, P.; Ettema, T.P.; Aëgerter, L.; Gobbens, R.; Stek, M.L.; Dröes, R.-M. Known in the nursing home: Development and evaluation of a digital person-centered artistic photo-activity intervention to promote social interaction between residents with dementia, and their formal and informal carers. BMC Geriatr. 2022, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Stargatt, J.; Bhar, S.; Bhowmik, J.; Al Mahmud, A. Digital Storytelling for Health-Related Outcomes in Older Adults: Systematic Review. J. Med. Internet Res. 2022, 24, e28113. [Google Scholar] [CrossRef] [PubMed]
- Filoteo, J.V.; Cox, E.M.; Split, M.; Gross, M.; Culjat, M.; Keene, D. Evaluation of ReminX as a Behavioral Intervention for Mild to Moderate Dementia. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3314–3317. [Google Scholar]
- Shaughnessy, L.; Brunton, S.; Chepke, C.; Farmer, J.G.; Rosenzweig, A.S.; Grossberg, G. Using Telemedicine to Assess and Manage Psychosis in Neurodegenerative Diseases in Long-Term Care. J. Am. Med. Dir. Assoc. 2022, 23, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Fadzil, N.H.M.; Shahar, S.; Rajikan, R.; Singh, D.K.A.; Ludin, A.F.M.; Subramaniam, P.; Ibrahim, N.; Vanoh, D.; Ali, N.M. A Scoping Review for Usage of Telerehabilitation among Older Adults with Mild Cognitive Impairment or Cognitive Frailty. Int. J. Environ. Res. Public Health 2022, 19, 4000. [Google Scholar] [CrossRef]
- Talbot, C.V.; Briggs, P. The use of digital technologies by people with mild-to-moderate dementia during the COVID-19 pandemic: A positive technology perspective. Dementia 2022, 21, 1363–1380. [Google Scholar] [CrossRef]
- Onose, G.; Morcov, M.V.; Sporea, C.; Mirea, A.; Ciobanu, V. Augmentation and Rehabilitation with Active Orthotic Devices. In Modern Approaches to Augmentation of Brain Function; Opris, I., Lebedev, M.A., Casanova, M.F., Eds.; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-54563-5. [Google Scholar] [CrossRef]
- Gauthier, C.; Arel, J.; Brosseau, R.; Hicks, A.L.; Gagnon, D.H. Reliability and minimal detectable change of a new treadmill-based progressive workload incremental test to measure cardiorespiratory fitness in manual wheelchair users. J. Spinal Cord Med. 2017, 40, 759–767. [Google Scholar] [CrossRef]
- Philippe, T.J.; Sikder, N.; Jackson, A.; Koblanski, M.E.; Liow, E.; Pilarinos, A.; Vasarhelyi, K. Digital Health Interventions for Delivery of Mental Health Care: Systematic and Comprehensive Meta-Review. JMIR Ment. Health 2022, 9, e35159. [Google Scholar] [CrossRef]
- Usmani, S.S.; Sharath, M.; Mehendale, M. Future of mental health in the metaverse. Gen. Psychiatry 2022, 35, e100825. [Google Scholar] [CrossRef]
- Cavedoni, S.; Cipresso, P.; Mancuso, V.; Bruni, F.; Pedroli, E. Virtual reality for the assessment and rehabilitation of neglect: Where are we now? A 6-year review update. Virtual Real. 2022, 26, 1663–1704. [Google Scholar] [CrossRef]
- Moon, H.-J.; Han, S. Perspective: Present and Future of Virtual Reality for Neurological Disorders. Brain Sci. 2022, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, Y.; Zhou, F.; Mao, R.; Fei, F. Systematic Bibliometric Analysis of Research Hotspots and Trends on the Application of Virtual Reality in Nursing. Front. Public Health 2022, 10, 906715. [Google Scholar] [CrossRef] [PubMed]
- Clay, F.; Howett, D.; FitzGerald, J.; Fletcher, P.; Chan, D.; Price, A. Use of Immersive Virtual Reality in the Assessment and Treatment of Alzheimer’s Disease: A Systematic Review. J. Alzheimer’s Dis. 2020, 75, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Chung, O.S.; Robinson, T.; Johnson, A.M.; Dowling, N.L.; Ng, C.H.; Yücel, M.; Segrave, R.A. Implementation of Therapeutic Virtual Reality into Psychiatric Care: Clinicians’ and Service Managers’ Perspectives. Front. Psychiatry 2022, 12, 791123. [Google Scholar] [CrossRef]
- Kalantari, S.; Xu, T.B.; Mostafavi, A.; Lee, A.; Barankevich, R.; Boot, W.R.; Czaja, S.J. Using a Nature-Based Virtual Reality Environment for Improving Mood States and Cognitive Engagement in Older Adults: A Mixed-Method Feasibility Study. Innov. Aging 2022, 6, igac015. [Google Scholar] [CrossRef]
- Scott, H.; Griffin, C.; Coggins, W.; Elberson, B.; Abdeldayem, M.; Virmani, T.; Larson-Prior, L.J.; Petersen, E. Virtual Reality in the Neurosciences: Current Practice and Future Directions. Front. Surg. 2022, 8, 807195. [Google Scholar] [CrossRef]
- Rizzuto, D.; Melis, R.J.F.; Angleman, S.; Qiu, C.; Marengoni, A. Effect of Chronic Diseases and Multimorbidity on Survival and Functioning in Elderly Adults. J. Am. Geriatr. Soc. 2017, 65, 1056–1060. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Cerasa, A.; Ciancarelli, I.; Pignolo, L.; Tonin, P.; Iosa, M.; Morone, G. The Arrival of the Metaverse in Neurorehabilitation: Fact, Fake or Vision? Biomedicines 2022, 10, 2602. [Google Scholar] [CrossRef]
- Shaikh, T.A.; Dar, T.R.; Sofi, S. A data-centric artificial intelligent and extended reality technology in smart healthcare systems. Soc. Netw. Anal. Min. 2022, 12, 122. [Google Scholar] [CrossRef]
- Chaze, F.; Hayden, L.; Azevedo, A.; Kamath, A.; Bucko, D.; Kashlan, Y.; Dube, M.; De Paula, J.; Jackson, A.; Reyna, C.; et al. Virtual reality and well-being in older adults: Results from a pilot implementation of virtual reality in long-term care. J. Rehabil. Assist. Technol. Eng. 2022, 9, 205566832110723. [Google Scholar] [CrossRef]
- Michmizos, K.P.; Krebs, H.I. Serious games for the pediatric anklebot. In Proceedings of the 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 1710–1714. [Google Scholar]
- Ruiz-Muelle, A.; López-Rodríguez, M.M. Dance for People with Alzheimer’s Disease: A Systematic Review. Curr. Alzheimer Res. 2019, 16, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Masood, W.; Annamaraju, P.; Khan Suheb, M.Z.; Uppaluri, K.R. Ketogenic Diet. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https//www.ncbi.nlm.nih.gov/books/NBK499830/ (accessed on 28 August 2023).
- Arora, S.; Santiago, J.A.; Bernstein, M.; Potashkin, J.A. Diet and lifestyle impact the development and progression of Alzheimer’s dementia. Front. Nutr. 2023, 10, 1213223. [Google Scholar] [CrossRef] [PubMed]
- Jebelli, J.; Hamper, M.C.; Van Quelef, D.; Caraballo, D.; Hartmann, J.; Kumi-Diaka, J. The Potential Therapeutic Effects of Low-Dose Ionizing Radiation in Alzheimer’s Disease. Cureus 2022, 14, e23461. [Google Scholar] [CrossRef]
- Monteiro, F.; Sotiropoulos, I.; Carvalho, Ó.; Sousa, N.; Silva, F.S. Multi-mechanical waves against Alzheimer’s disease pathology: A systematic review. Transl. Neurodegener. 2021, 10, 36. [Google Scholar] [CrossRef]
- van Heuvelen, M.J.G.; Rittweger, J.; Judex, S.; Sañudo, B.; Seixas, A.; Fuermaier, A.B.M.; Tucha, O.; Nyakas, C.; Marín, P.J.; Taiar, R.; et al. Reporting guidelines for whole-body vibration studies in humans, animals and cell cultures: A consensus statement from an international group of experts. Biology 2021, 10, 965. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Romagnoli, C.; Annino, G. Effectiveness of focal muscle vibration on hemiplegic upper extremity spasticity in individuals with stroke: A systematic review. NeuroRehabilitation 2019, 45, 471–481. [Google Scholar] [CrossRef]
- Oroszi, T.; de Boer, S.F.; Nyakas, C.; Schoemaker, R.G.; van der Zee, E.A. Chronic whole body vibration ameliorates hippocampal neuroinflammation, anxiety-like behavior, memory functions and motor performance in aged male rats dose dependently. Sci. Rep. 2022, 12, 9020. [Google Scholar] [CrossRef]
- Xie, X.; Shu, R.; Yu, C.; Fu, Z.; Li, Z. Mammalian AKT, the Emerging Roles on Mitochondrial Function in Diseases. Aging Dis. 2022, 13, 157–174. [Google Scholar] [CrossRef]
- Wu, C.; Feng, S.; Zhu, L.; Yang, L.; Liu, T.C.-Y.; Duan, R. Therapeutic non-invasive brain treatments in Alzheimer’s disease: Recent advances and challenges. Inflamm. Regen. 2022, 42, 31. [Google Scholar] [CrossRef]
- Ries, J.D. A framework for rehabilitation for older adults living with dementia. Arch. Physiother. 2022, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Su, H.; Zhang, S.; Huang, Y. Reminiscence therapy-based care program serves as an optional nursing modality in alleviating anxiety and depression, improving quality of life in surgical prostate cancer patients. Int. Urol. Nephrol. 2022, 54, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Rentz, C.A. Reminiscence. A supportive intervention for the person with Alzheimer’s disease. J. Psychosoc. Nurs. Ment. Health Serv. 1995, 33, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, P.E.G.; Davidson, P.M.; Mejilla, J.L.; Rodney, T.W. Reminiscence therapy for older adults with Alzheimer’s disease: A literature review. Int. J. Ment. Health Nurs. 2020, 29, 364–371. [Google Scholar] [CrossRef]
- Woods, B.; O’Philbin, L.; Farrell, E.M.; Spector, A.E.; Orrell, M. Reminiscence Therapy for Dementia. Cochrane Database Syst. Rev. 2018, 3, CD001120. [Google Scholar] [CrossRef]
- Jiang, L.; Siriaraya, P.; Choi, D.; Kuwahara, N. Emotion Recognition Using Electroencephalography Signals of Older People for Reminiscence Therapy. Front. Physiol. 2022, 12, 823013. [Google Scholar] [CrossRef]
- Nebot, À.; Domènech, S.; Albino-Pires, N.; Mugica, F.; Benali, A.; Porta, X.; Nebot, O.; Santos, P.M. LONG-REMI: An AI-Based Technological Application to Promote Healthy Mental Longevity Grounded in Reminiscence Therapy. Int. J. Environ. Res. Public Health 2022, 19, 5997. [Google Scholar] [CrossRef]
- Ray, A.; Bhardwaj, A.; Malik, Y.K.; Singh, S.; Gupta, R. Artificial intelligence and Psychiatry: An overview. Asian J. Psychiatry 2022, 70, 103021. [Google Scholar] [CrossRef]
- Pérez-Sáez, E.; Justo-Henriques, S.I.; Apóstolo, J.L.A. Multicenter randomized controlled trial of the effects of individual reminiscence therapy on cognition, depression and quality of life: Analysis of a sample of older adults with Alzheimer’s disease and vascular dementia. Clin. Neuropsychol. 2022, 36, 1975–1996. [Google Scholar] [CrossRef]
- Huang, L.-C.; Yang, Y.-H. The Long-term Effects of Immersive Virtual Reality Reminiscence in People with Dementia: Longitudinal Observational Study. JMIR Serious Games 2022, 10, e36720. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef] [PubMed]
- Weiler, M.; Stieger, K.C.; Long, J.M.; Rapp, P.R. Transcranial magnetic stimulation in Alzheimer’s disease: Are we ready? eNeuro 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Somaa, F.A.; de Graaf, T.A.; Sack, A.T. Transcranial Magnetic Stimulation in the Treatment of Neurological Diseases. Front. Neurol. 2022, 13, 793253. [Google Scholar] [CrossRef] [PubMed]
- Murer, M.; Yan, Q.; Raisman-Vozari, R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol. 2001, 63, 71–124. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S.J.; Cicchetti, F. Cellular and molecular mechanisms of action of transcranial direct current stimulation: Evidence from in vitro and in vivo models. Int. J. Neuropsychopharmacol. 2015, 18, pyu047. [Google Scholar] [CrossRef]
- Peruzzotti-Jametti, L.; Cambiaghi, M.; Bacigaluppi, M.; Gallizioli, M.; Gaude, E.; Mari, S.; Sandrone, S.; Cursi, M.; Teneud, L.; Comi, G.; et al. Safety and efficacy of transcranial direct current stimulation in acute experimental ischemic stroke. Stroke 2013, 44, 3166–3174. [Google Scholar] [CrossRef]
- Onose, G.; Anghelescu, A.; Blendea, D.; Ciobanu, V.; Daia, C.; Firan, F.C.; Oprea, M.; Spinu, A.; Popescu, C.; Ionescu, A.; et al. Cellular and Molecular Targets for Non-Invasive, Non-Pharmacological Therapeutic/Rehabilitative Interventions in Acute Ischemic Stroke. Int. J. Mol. Sci. 2022, 23, 907. [Google Scholar] [CrossRef]
- Zhao, Z. Analysis of Health and Longevity in the Oldest-Old Population—A Health Capital Approach. In Healthy Longevity in China; The Springer Series on Demographic Methods and Population Analysis; Springer: Dordrecht, The Netherland, 2008; Volume 20, pp. 157–176. [Google Scholar]
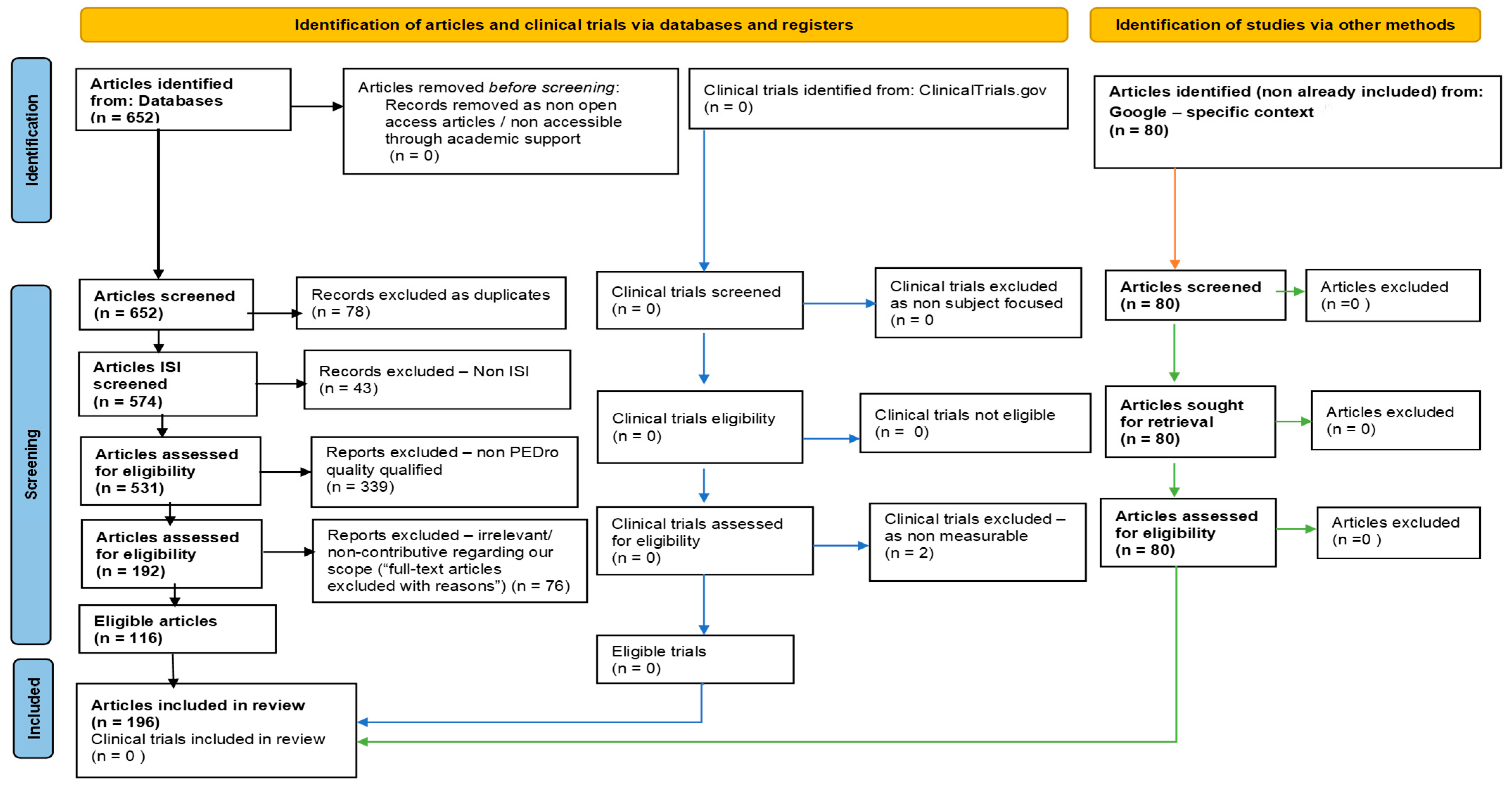

| Keywords | Elsevier | PubMed | PMC | PEDro | Total |
|---|---|---|---|---|---|
| “Alzheimer’s disease” + “Video game therapy” | 0 | 0 | 0 | 0 | 0 |
| “Alzheimer’s disease” + “Augmented reality therapy” | 0 | 0 | 0 | 0 | 0 |
| “Alzheimer’s disease” + “Virtual reality therapy” | 0 | 0 | 19 | 0 | 19 |
| “Alzheimer’s disease” + “Serious games therapy” | 0 | 0 | 0 | 0 | 0 |
| “Alzheimer’s disease” + “Reminiscence therapy” | 1 | 6 | 96 | 0 | 103 |
| “Alzheimer’s disease” + “Music therapy” | 0 | 16 | 249 | 0 | 265 |
| “Alzheimer’s disease” + “Dancing therapy” | 0 | 0 | 0 | 0 | 0 |
| “Alzheimer’s disease” + “Exercise therapy” | 2 | 22 | 241 | 0 | 265 |
| Total | 3 | 44 | 605 | 0 | 652 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aurelian, S.; Ciobanu, A.; Cărare, R.; Stoica, S.-I.; Anghelescu, A.; Ciobanu, V.; Onose, G.; Munteanu, C.; Popescu, C.; Andone, I.; et al. Topical Cellular/Tissue and Molecular Aspects Regarding Nonpharmacological Interventions in Alzheimer’s Disease—A Systematic Review. Int. J. Mol. Sci. 2023, 24, 16533. https://doi.org/10.3390/ijms242216533
Aurelian S, Ciobanu A, Cărare R, Stoica S-I, Anghelescu A, Ciobanu V, Onose G, Munteanu C, Popescu C, Andone I, et al. Topical Cellular/Tissue and Molecular Aspects Regarding Nonpharmacological Interventions in Alzheimer’s Disease—A Systematic Review. International Journal of Molecular Sciences. 2023; 24(22):16533. https://doi.org/10.3390/ijms242216533
Chicago/Turabian StyleAurelian, Sorina, Adela Ciobanu, Roxana Cărare, Simona-Isabelle Stoica, Aurelian Anghelescu, Vlad Ciobanu, Gelu Onose, Constantin Munteanu, Cristina Popescu, Ioana Andone, and et al. 2023. "Topical Cellular/Tissue and Molecular Aspects Regarding Nonpharmacological Interventions in Alzheimer’s Disease—A Systematic Review" International Journal of Molecular Sciences 24, no. 22: 16533. https://doi.org/10.3390/ijms242216533
APA StyleAurelian, S., Ciobanu, A., Cărare, R., Stoica, S.-I., Anghelescu, A., Ciobanu, V., Onose, G., Munteanu, C., Popescu, C., Andone, I., Spînu, A., Firan, C., Cazacu, I. S., Trandafir, A.-I., Băilă, M., Postoiu, R.-L., & Zamfirescu, A. (2023). Topical Cellular/Tissue and Molecular Aspects Regarding Nonpharmacological Interventions in Alzheimer’s Disease—A Systematic Review. International Journal of Molecular Sciences, 24(22), 16533. https://doi.org/10.3390/ijms242216533









