An Overview of the Potential of Food-Based Carbon Dots for Biomedical Applications
Abstract
:1. Introduction
2. Food-Based CDs
2.1. CDs from Processed Food and Beverages
| Food Groups | Food Source | Purification | Type/Size (nm) | Yield (%) | Quantum Yield (%) | Toxic Evaluation | Ref. |
|---|---|---|---|---|---|---|---|
| Complex processed foods | Pizza | Ethanol for 12 h, then dialyzed (0.5 kDa) | CNPs/2.6–4.1 | NA | 2.1 | In vitro >1 mg/mL, 6 h (Caco-2 cells) In vivo >100 mg/mL, 48 h (C. elegans) | [49] |
| Burger meat (beef) | Ethanol for 12 h, then dialyzed (3.5 kDa) | CDs/0.9–54.8 | NA | 23.3 | In vitro >3.2 mg/mL, 12 h (MO cells) In vivo >3.2 mg/mL, 12 h (bean) | [50] | |
| Canned yellow croaker | Ethanol for 12 h, then dialyzed (1 kDa) | CDs/1.8–5.8 | 0.3 (w/w) | 9.7 | In vitro >0.25 mg/mL, 12 h (HepG2 cells) | [51] | |
| Commercial beverages | Nescafé® coffee | Size exclusion (Sephadex G-25) | CDs/3.0–6.0 | 2.0 (w/w) | 5.5 | In vitro >20 mg/mL, 24 h (CHO cells)/>1.5 mg/mL, 24 h (SMMC-7721 cells) In vivo >1000 mg/g, 56 h (guppy fish) | [39] |
| ILLY® coffee | Dialyzed (14 kDa) | CQDs/2.0–7.0 | NA | NA | NA | [52] | |
| Cola | Size exclusion (Sephadex G-25) | CNPs/3.9–5.5 | 3.0 (w/v) | NA | In vitro >20.0 mg/mL, 24 h (CHO cells) In vivo >2000 mg/g, 24 h (mice) | [53] | |
| Beverages | Size exclusion (Sephadex G-25) | CDs/2.8–39.1 | 2.0–5.0 (w/w) | 1.5–11.9 | In vitro >20 mg/mL, 24 h (CHO cells)/>10 mg/mL, 24 h (Tca-8113 cells) In vivo >40 mg/mL, 6 h (onion) | [54] | |
| Fermented food products | Beer | Size exclusion (macroporous resin) | CDs/0.9–4.1 | NA | 1.4–3.9 | In vitro >5 mg/mL, 4 h (MC3T3-E1 cells) In vivo >2000 mg/kg, 24 h (mice) | [37] |
| Tsingtao® beer | Size exclusion (Sephadex G-25) | CDs/1.0–5.0 | 1.2 (w/v) | 7.4 | In vitro >50 mg/mL, 48 h (MCF-7 cells) | [38] | |
| Bread | Methanol for 10 min, then dialyzed (1 kDa) | CNPs/21.4–33.6 | NA | 1.2 | In vitro >2 µg/mL, 24 h (HeLa cells) | [55] | |
| Bread | Methanol for 1 h, then dialyzed (1 kDa) | CNs/5.0–20.0 | NA | NA | In vitro >400 µg/mL, 48 h (hMSCs cells) | [56] | |
| Breadcrumbs | Ethanol for 12 h, then dialyzed (3.5 kDa) | CDs/2.2–3.2 | 0.013 (w/v) | 1.8 | NA | [48] | |
| Vinegar | Size exclusion (macroporous resin) | CNPs/1.2–6.2 | 1.5 (w/v) | 5.7 | NA | [57] | |
| Vinegar | Ethanol for 12 h, then dialyzed (1 kDa) | CNPs/142.6–281.2 | NA | NA | In vitro 100 µg/mL, 24 h (Caco-2 cells) | [58] | |
| Soybean sauce | Ethanol for 12 h, then dialyzed (1 kDa) | CNPs/298.5–398.2 | NA | NA | In vitro 100 µg/mL, 24 h (Caco-2 cells) | [58] | |
| Tofu wastewater | Ultrasonic shock for 5 min, then centrifuged | CDs/2.0–10.0 | NA | NA | NA | [59] | |
| Flavor enhancers | Caramels | Methanol for 10 min, then dialyzed (1 kDa) | CNPs/2.8–5.8 | NA | 0.6 | NA | [55] |
| Jaggery | Methanol for 10 min, then dialyzed (1 kDa) | CNPs/12.8–27.8 | NA | 0.6 | In vitro >2 µg/mL, 24 h (HeLa cells) | [55] | |
| Honey | Dialyzed for 48 h, treatment with acetonitrile, then lyophilized | CDs/1.7–4.7 | 1.5 (w/w) | 1.6 | NA | [60] |
2.2. CDs Synthesized from Raw Food or Edible Plants
| Food Groups | Food Source | Synthetic Method | Types/Size (nm) | Quantum Yield (%) | Toxic Evaluation | Potential Biomedical Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Raw meat | Lamb | Oven heating (280 °C for 15–45 min) | CDs/2.6–4.1 | 10 | In vitro >4 mg/mL, 24 h (PCl12 cells) | Protein adsorption | [26] |
| Lamb | Oven heating (200–300 °C for 30 min), then extraction by ethanol for 24 h | CDs/1.7–2.8 | 6–45 | In vitro >2 mg/mL, 7 h (HepG2 cells) | Scavenging ROS | [27] | |
| Beef | Oven heating (280 °C for 30 min), then extraction by ethanol for 30 min | CDs/1.0–4.0 | NA | In vitro >1 mg/mL, 12 h (NRK cells) | Protein adsorption | [28] | |
| Beef broth | Oven heating (117 °C for 30–70 min), then extraction by ethanol for 40 min | CNPs/2.4–5.4 | 2.0–2.5 | In vitro >10 mg/mL, 24 h (NRK cells) | Carrier for zinc | [29] | |
| Duck | Oven heating (200–300 °C for 30 min), then extraction by ethanol for 1 h | CDs/1.5–3.2 | 10.5–38.0 | In vitro >4 mg/mL, 36 h (PC12 cells) In vivo >15 mg/mL, 24 h (C. elegans) | In vivo C. elegans bio-imaging | [30] | |
| Duck | Oven heating (170 °C for 1 h) then extract by ethanol for 1 h | CNPs/0.7–2.3 | 4.4 | NA | Protein adsorption | [31] | |
| Chicken | Oven heating (150–300 °C for 1 h) then extraction by ethanol for 36 h | CDs/1.5–20.4 | 6.5–17.9 | In vitro >4 mg/mL, 24 h (HepG2 cells) In vivo >2 g/kg, 20 h (mice) | Dopamine sensing | [32] | |
| Pike eel | Oven heating (160–300 °C for 30 min), then extraction by ethanol for 24 h | CNs/1.8–4.3 | 80.2 | In vitro >20 mg/mL, 24 h (MC3T3-E1 cells) | In vitro bio-imaging | [33] | |
| Atlantic salmon | Oven heating (200 °C for 10–60 min), then extraction by ethanol for 2 h | CQDs/1.9–4.1 | 2.2–12.1 | In vitro >6 mg/mL, 6 h (NRK cells) In vivo >2 g/kg, 24 h (mice) | In vivo mice bio-imaging | [34] | |
| Mackerel | Oven heating (230 °C for 40 min), then extraction by ethanol for 2 h | CDs/0.9–3.5 | 12.0 | NA | Scavenging ROS | [35] | |
| Spanish Mackerel | Grill-heating (230 °C for 30 min) and then extraction by 10% methanol for 2 h | CDs/2.9–3.0 | NA | NA | Protein adsorption | [36] | |
| Processed food | Breadcrumbs | Oven heating (180 °C with cooking oil) then extraction by petroleum ether for overnight | CDs/2.6–4.0 | 1.0 | NA | Protein adsorption | [48] |
| Flavor enhancers | Grounded spice of cinnamon, red chili, turmeric and black pepper | Hydrothermal (200 °C for 12 h) | CDs/10.3–15.0 | NA | In vitro >2.0 mg/mL, 24 h (HK2 cells) | In vitro bio-imaging/Anticancer | [118] |
| Milk | Commercial cow milk | Hydrothermal (190–200 °C for 1–8 h) | CDs/ 0.5–4.0 | NA | In vitro >0.4 mg/mL, 24 h (HT22 cells) | Scavenging ROS | [73] |
| Commercial fat-free cow milk | Hydrothermal (180 °C for 2 h) | CDs/ 2.0–4.0 | 12 | In vitro >1 mg/mL, 24 h (U87 cells) | In vitro bio-imaging | [74] | |
| Cow yogurt | Microwave (800 W for 30 min) | CDs/1.4–9.5 | 1.5 | In vitro >7.1 mg/mL, 100 h (MCF-7 and CoN cells) | In vitro bio-imaging | [75] | |
| Fruits | Kiwi, Avocado, or Pear | Hydrothermal (200 °C for 12 h) | CDs/4.0–4.5 | 20–35 | In vitro >1.2 mg/mL, 72 h (HK-2 cells)/>2.2 mg/mL, 72 h (Caco-2 cells) In vivo >64 mg/mL, 80 h (zebra fish embryo) | In vivo zebrafish bio-imaging/Anticancer | [76] |
| Mango | Hydrothermal (100 °C for 1 h, in H2SO4; 80 °C for 15 min, in H3PO4; 80 °C for 30 min, in H3PO4), then adjusted to pH 7.0 with NaOH | CNPs/5.0–10.0, 5.0–10.0, or 10.0–14.0 | 3.9, 1.6, or 0.5 | In vitro >5 mg/mL, 24 h (A549 cells) In vivo >5 mg/kg, 24 h (mice) | In vivo mice bio-imaging | [77] | |
| Sapodilla fruits | Hydrothermal (100 °C for 1 h, in H2SO4; 80 °C for 15 min, in H3PO4; 80 °C for 30 min, in H3PO4), then adjusted to pH 7.0 with NaOH | CDs/1.6–2.2, 2.2–3.6, or 3.3–5.8 | 5.7, 7.9, or 5.2 | In vitro >300 µg/mL, 15 h (HeLa cells) | In vivo bacterial/Fungal bio-imaging | [78] | |
| Cherry plum juice | Hydrothermal (200 °C for 20 h) | CDs/1.0–8.0 | NA | In vitro >500 µg/mL, 24 h (HepG2 cells) | In vitro bio-imaging | [79] | |
| Lemon juice | Hydrothermal (120 °C for 3 h) | CQDs/2.0–4.5 | 9.0 | NA | In vivo plant bio-imaging (onion epidermal cells) | [80] | |
| Tomato juice | Hydrothermal (160 °C for 3 h) | CDs/2.4–3.6 | NA | In vitro >100 µg/mL, 96 h (A549, and Human dermal fibroblasts cells) | Scavenging ROS | [81] | |
| Watermelon juice/Orange juice/Lemon juice/Cantaloupe juice/Red plum juice/Green plum juice/Carrot juice/Red pitaya juice/White pitaya juice | Hydrothermal (180 °C for 4 h) | CDs/1.6–5.6 | 13–25 | In vitro >1 mg/mL, 4 h (RAW 264.7 cells) In vivo >2 mg/kg, 5 h (zebrafish eleutheroembryo)/3.2 mg/kg, 6 h (zebrafish eleuthero-embryo) | In vivo zebrafish bio-imaging (ROS sensing) | [82] | |
| Edible plants | Linseed (seeds) | Hydrothermal (180 °C for 12 h) | CDs/4.0–8.0 | 14.2 | In vitro >200 µg/mL, 24 h (MCF-7 cells) | In vitro bio-imaging | [83] |
| Peanuts (seeds) | Hydrothermal (250 °C for 6 h) | CDs/2.0–8.0 | 7.9 | In vitro >1 mg/mL, 24 h (MCF-7 cells) | In vitro bio-imaging | [84] | |
| Wheat bran (seeds) | Hydrothermal (180 °C for 3 h) | CDs/ca. 4.9 | 33.2 | In vitro >6 mg/mL, 24 h (SH-SY5Y cells) | Drug carrier (amoxicillin; antibiotic) | [85] | |
| Forsythia (dried fruit powder) + Urea + Ethanolamine | Microwave (300 W for 2 min, repeat 3 times) | CQDs/1.8–3.6 | NA | NA | Antifungal | [86] | |
| Rose (flower petals) + thymol | Powder carbonization (180 °C for 6 h) and decorate with thymol | CDs/5.0–6.0 | NA | In vivo >10 mg/kg, 144 h (rats) | Immuno-modulatory effect | [64] | |
| Phellodendri chinensis (Cortex) | Powder carbonization (400 °C for 1 h) | CDs/0.5–3.6 | 5.6 | In vitro >39 µg/mL, 24 h (L02, 293T, and RAW 264.7 cells) In vivo >0.86 mg/kg, 7 days (mice) | Immuno-modulatory effect | [65] | |
| Cabbage (leaves) | Hydrothermal (140 °C for 5 h) | CQDs/2.0–8.0 | 16.5 | In vitro >700 µg/mL, 24 h (HaCaT cells) | In vitro bio-imaging | [87] | |
| Chinese mugwort (leaves) | Purified fume particulate matter | CDs/3.0–7.0 | NA | In vitro >150 µg/mL, 24 h (HEK 293T cells) | Antibacterial | [88] | |
| Coriander (leaves) | Hydrothermal (240 °C for 4 h) | CDs/1.5–3.0 | 6.48 | In vitro >1 mg/mL, 12 h (A549 and L-132 cells) | Scavenging ROS/In vitro bio-imaging | [89] | |
| Ginkgo (leaves) | Hydrothermal (200 °C for 10 h) | CQDs/2.0–4.0 | 22.8 | NA | Disease detection in mouse serum | [90] | |
| Green chiretta (leaf extract) | Hydrothermal (160 °C for 8 h) | CDs/8.0–11.0 | 15.1 | In vitro >700 µg/mL, 24 h (MCF-7 cells) | Scavenging ROS/In vitro bio-imaging/Antibacterial/Anticancer | [91] | |
| Henna (leaves) | Hydrothermal (180 °C for 12 h) | CDs/2.7–7.8 | 28.7 (Rhodamine B) | NA | Antibacterial/Anticancer drug sensing | [92] | |
| Holy basil (leaves) | Hydrothermal (180 °C for 4 h) | CDs/1.0–4.0 | 9.3 | In vitro >200 mg/mL, 24 h (MDA-MB-648 cells) | In vitro bio-imaging | [93] | |
| Pakchoi (leaves) | Hydrothermal (150 °C for 12 h) | CDs/1.0–3.0 | 37.5 | In vitro >2 mg/mL, 24 h (HeLa cells) | In vitro bio-imaging | [94] | |
| Rosemary (leaves) | Hydrothermal (140–200 °C for 6–12 h) | CDs/11.5–20.7 | NA | NA | Antibacterial | [95] | |
| Spinach (leaves) | Hydrothermal (150 °C for 6 h) | CDs/3.0–11.0 | 15.3 | In vitro >200 µg/mL, 24 h (A549 cells) In vivo >2 mg/mL, 24 h (mice) | In vivo tumor imaging in mice | [96] | |
| Tea tree (leaves) | Hydrothermal (220 °C for 3 h) | CDs/1.7–5.0 | 4.9 | In vitro >4 mg/mL, 24 h (HepG2 cells) | In vitro bio-imaging | [97] | |
| Tea tree /Osmanthus/Milk vetch (leaves) | Hydrothermal (200 °C for 2 h) | CDs/3.0–18.0 | NA | In vitro >1 mg/mL, 24 h (293T cells) | Antibacterial | [98] | |
| Escallion (stem) | Hydrothermal (220 °C for 3 h) | CDs/ca. 4.22 | 10.5 | In vitro >200 µg/mL, 24 h (MCF-7 and K562 cells) | In vitro bio-imaging | [99] | |
| Garlic (bulb) | Hydrothermal (180 °C for 10 h) | CDs/ca. 3.6 | 6.8 | NA | In vitro bio-imaging | [100] | |
| Ginger (rhizome) | Hydrothermal (300 °C for 20 min) | CDs/3.5–5.1 | 13.4 | In vitro >2.8 mg/mL, 24 h (A549, MDA-MB-231, and FL83B cells)/>1.4 mg/mL, 24 h (HeLa cells)/>0.4 mg/mL, 24 h (HepG2 cells) | Anticancer | [101] | |
| Konjac (bulb) | Powder carbonization (470 °C for 1.5 h) | CDs/ca. 3.4 | 13.0 | In vitro >150 mg/mL, 12 h (HeLa cells) | In vitro bio-imaging | [102] | |
| Rhei radix (rhizome) | Powder carbonization (350 °C for 1 h) | CDs/1.4–4.5 | NA | In vitro >200 µg/mL, 24 h (RAW 264.7 cells) | Immuno-modulatory effect | [66] | |
| Turmeric (rhizome) | Hydrothermal (180 °C for 10 h) | CDs/1.5–4.0 | NA | In vitro >200 µg/mL, 24 h (PC3 cells) | Antibacterial effects | [103] | |
| Turmeric (rhizome) + Ammonium persulfate | Hydrothermal (200 °C for 6 h) | CDs/9.4–11.8 | NA | In vitro >1 mg/mL, 72 h (L929 cells) | Antibacterial effects/Scavenging ROS | [104] | |
| Yam (stem tuber) | Hydrothermal (200 °C for 2 h) | CDs/1.5–4.0 | 9.3 | NA | Anticancer drug sensing | [105] | |
| Beetroot (root) | Hydrothermal (160 °C for 8 h) | CDs/<5.0 | 11.6 | In vitro >2.5 µg/mL, 24 h (HEK-293 cells) | Anticancer/Scavenging ROS | [106] | |
| Carrot (root) | Hydrothermal (170 °C for 12 h) | CDs/ca. 2.3 | 7.6 | In vitro >2 mg/mL, 24 h (MCF-7 cells) | Drug carrier (mitomycin; anticancer) | [107] | |
| Rose-heart radish (root) | Hydrothermal (180 °C for 3 h) | CDs/1.2–6.0 | 13.6 | In vitro >500 µg/mL, 3 h (SiHa cells) | In vitro bio-imaging | [108] | |
| Sweet potato (root) | Hydrothermal (180 °C for 18 h) | CDs/2.5–5.5 | 8.6 | In vitro >150 µg/mL, 24 h (HeLa, HepG2 cells) | In vitro bio-imaging | [109] | |
| Oyster mushroom (Sporocarp) | Hydrothermal (120 °C for 4 h; dissolved in 5% H2SO4) | CDs/5.0–18.0 | NA | In vitro >25 µg/mL, 24 h (HEK 293 cells) | Antibacterial/Anticancer | [110] | |
| Water chestnut (bulb) + Onion (bulb) | Hydrothermal (180 °C for 4 h) | CDs/2.0–4.0 | 12.0 | In vitro >300 µg/mL, 24 h (T24 cells) | In vivo bio-imaging and quantification of coenzyme A (pig liver) | [111] | |
| Natural flavor enhancers | Guar gum (Seed endosperm) | Microwave (400 W for 30 min) | CDs/19.2–31.1 | 7.5 | In vivo >1 mg/mL, 1 h (China rose leaf) | In vivo plant bio-imaging (China rose leaf guard cells) | [112] |
| Honey + Garlic (bulb)+ Ammonia | Hydrothermal (200 °C for 6 h) | CQDs/4.0–13.0 | 4.2 | NA | Antibacterial | [113] |
2.3. CDs Synthesized from Dietary Compounds
| Precursor | Synthetic Method | Type/Size (nm) | Yield (%) | Quantum Yield (%) | Toxic Evaluation | Potential Biomedical Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Ammonium citrate/Spermidine | Powder carbonization (180 °C for 2 h and 260 °C for 2 h) | CDs/3.8–5.4 | 50.8 | 2.8 | In vitro >50 mg/mL, 24 h (HEK-293T, MCF-7, A549, HeLa, and HaCaT cells) In vivo >50 mg/mL, 12 days (mice) | Antibacterial/Wound healing | [153] |
| Citric acid + Diethyl-enetriamine | Powder carbonization (170 °C for 3 h in a nitrogen atmosphere) | CDs/5.0–8.0 | NA | 25.5 | In vitro >100 μM, 24 h (A2780 cells) In vivo >100 μM, 14 days (mice) | In vivo tumor image in mice/Drug carrier (cisplatin; anticancer) | [154] |
| Curcumin | Powder carbonization (180 °C for 2 h) | CQDs/ 4.2–5.2 | 10.0–25.0 (w/w) | 0.3 | In vitro >50 mg/mL, 24 h (RD cells) In vivo >25 mg/kg, 15 days (mice) | Antivirus | [119] |
| Curcumin | Powder carbonization (180 °C for 2 h) | CQDs/ ca. 4.8 | NA | NA | In vitro >100 mg/mL, 24 h (BHK-21 cells) | Antivirus | [120] |
| Folic acid | Powder carbonization (140 °C for 6 h) | CDs/1.0–1.6 | NA | NA | In vitro >200 μg/mL, 72 h (chondrocytes and macrophages) In vivo >2 mg/kg, 6 weeks (mice) | Immuno-modulatory | [121] |
| Glutamic acid | Powder carbonization (210 °C for ~1 min) | GQDs/3.4–5.9 | NA | 54.5 (NaOH) | In vitro >10 mg/mL, 1 h (MH-S cells) In vivo >25 mg/mL, 1 h (mice) | In vivo bioimage in mice | [122] |
| Hesperidin | Powder carbonization (250 °C for 2 h) | CPDs/46.7–60.1 | NA | NA | In vitro >500 μg/mL, 72 h (RD cells) In vivo >25 mg/kg, 9 days (mice) | Antivirus | [123] |
| Spermidine | Powder carbonization (270 °C for 3 h) | CQDs/ ca. 6.0 | NA | 2.0–4.3 | In vitro >200 mg/mL, 24 h (RCK cells) | Antibacterial | [124] |
| Spermidine | Powder carbonization (270 °C for 3 h) | CQDs/ ca. 6.0 | NA | 2.0–4.3 | In vitro >200 mg/mL, 24 h (RCK cells) | Antivirus | [125] |
| Spermine + Dopamine | Powder carbonization (250 °C for 2 h) | CQDs/ ca. 10.0 | 11.4 | 4.3 | In vitro >100 μg/mL 24 h (SIRC cells) In vivo >200 μg/mL, 14 days (rabbit) | Antibacterial | [155] |
| Citric acid/boronic acids | Powder carbonization (250 °C for 0.5 h) and then mix with the boronic acid solution | CQDs/5.4–7.0 | N.A | N.A | In vitro >600 μg/mL, 24 h (MOLT-4 cells) | Antivirus | [156] |
| Lysine | Powder carbonization (270 °C for 3 h) | CNGs/120.0–510.0 | 66.5 | 8.1 | In vitro >50 μg/mL, 24 h (BHK-21 and Vero cells) In vivo >30 μg/mL, 7 days (chicken embryo) | Antivirus | [126] |
| Lysine | Powder carbonization (270 °C for 3 h) | CNGs/118.9–178.7 | 66.5 | 8.1 | In vitro >100 μg/mL, 24 h (HUVEC, RD, HepG2, HaCaT, and HEK-293T cells) | Antibacterial | [127] |
| Lysine | Powder carbonization (270 °C for 3 h) | CNGs/118.9–178.7 | 66.5 | 8.1 | In vivo 50 μg/mL, 96 h (zebrafish embryos)/10 μg/mL, 96 h (zebrafish eleutheroembryo)/0.5 μg/mL, 90 days (adult zebrafish)/2000 mg/kg, 48 h (guinea pigs)/2000 mg/kg, 72 h (rabbit)/2000 mg/kg, 14 days (rats) | In vivo bioimage in zebrafish | [128] |
| Lysine or Arginine | Powder carbonization (240 °C for 3 h) | CQDs/2.0–7.0 | NA | NA | In vitro >1 mg/mL, 24 h (NIH-3T3, BMSCs, and HUVECs cells) In vivo >2 mg/mL, 5 days (mice) | Antibacterial/Scavenging ROS/Promoting tissue repair in mice | [129] |
| Quercetin | Powder carbonization (270 °C for 2 h) then dissolved in sodium phosphate buffer (pH 12) | CNGs/326.9–423.3 | 78 | <1 | In vitro >1 mg/mL, 24 h (MDCK cells) In vivo >500 μg/mL, 14 days (mice) | Antivirus | [130] |
| Quercetin + Lysine | Powder carbonization (270 °C for 3 h) | CNGs/44.8–235.2 | 17.5 | 3.3 | In vitro >100 μg/mL, 24 h (SIRC cells) In vivo >50 μg/mL, 28 days (rabbit) | Antibacterial/Scavenging ROS/Anti-inflammatory effects | [17] |
| Sodium alginate + Ammonium sulfite | Powder carbonization (180 °C for 3 h) | CNGs/116.0–183.0 | 31.2 | 13.0 | In vitro >1 mg/mL, 24 h (MDCK cells) In vivo >500 μg/mL, 14 days (mice) | Antivirus/Scavenging ROS/Anti-inflammatory effects | [157] |
| Sorbitan monolaurate | Powder carbonization (230 °C for 3 h) then dissolved in ethanol | VCDs/390–430 | NA | NA | NA | Enzyme and nanomaterial carrier/Cholesterol detection in serum | [21] |
| Asparagine | Microwave (180 °C for 15 min) | CDs/ca. 1.4 | NA | <1 | In vitro >800 μg/mL, 24 h (HeLa cells) | In vitro bioimage | [131] |
| Casein (milk protein) | Microwave (450 W for 30 min; heating for 2 min and then pausing for 15 s) | CDs/ca. 1.6 | NA | 18.7 | In vivo >200 μg/mL, 10 min (spinach leaf) | In vivo plant bio-imaging (spinach guard and epidermal cells) | [132] |
| Chitosan | Microwave (700 W for 9.5 min) | CDs/2.7–6.5 | 6.4 | 6.4 | NA | In vitro bioimage | [133] |
| Citric acid + Cysteine | Microwave (140 °C for 25 min) | CQDs/0.9–1.0 | NA | 91.2 | In vivo ca. 1 mL/mice, 3 h (mice) | Drug carrier (insulin)/In vivo glycemic control | [134] |
| Citric acid + Poly-ethyleneimine | Microwave (1150 W for 3 min) then mixed with locked nucleic acid (LNA) | CDs/ca. 3.7 | NA | NA | In vitro >1 μg/mL, 3 days (KMM, BC3, BCP1, BCBL1, and BJAB cells) In vivo >50 μg/mice, 3 weeks (mice) | Antivirus | [135] |
| Citric acid + RNase A enzyme | Microwave (700 W for 3–5 min) | CDs/ca. 4.0 | NA | 24.2 | In vitro >3 mg/mL, 24 h (MGC-803 cells) In vivo >5 mg/mL, 24 h (mice) | In vivo tumor imaging in mice | [136] |
| Citric acid + Tryptophan | Microwave (700 W for 3 min) | CDs/ca. 2.6 | NA | 20.6 | In vitro >400 μg/mL, 24 h (MGC-803 cells) | In vitro bioimage/Drug carrier (siRNA) | [137] |
| Citric acid + Urea | Microwave (800 W for 15 min) | CDs/1.0–5.5 | NA | NA | NA | Antibacterial | [138] |
| Citric acid + Urea | SPMA (6 kW for 5 min) | GQDs/ 3.0–20.0 | ca. 40 | NA | In vitro >50 μg/mL, 72 h (H171 cells) | Antivirus | [139] |
| Citric acid + Urea | Microwave (650 W for 4–5 min), then powder carbonization (60 °C for 1 h) | CDs/2.0–6.0 | NA | 36.0 | In vitro >100 μg/mL, 96 h, (HepG2 and HL-7702 cells) In vivo >500 μg/mL, 14 days (mice) | Drug carrier (doxorubicin; anticancer)/In vivo tumor imaging in mice | [140] |
| Glucose + Arginine | Microwave (700 W for 10 min) | CDs/1.0–7.0 | NA | 12.7 | In vitro >200 μg/mL, 24 h (MEFs cells) | In vitro bioimage/Drug carrier (circular DNA)/Chondrogenic differentiation | [141] |
| Microcrystalline cellulose | Alkaline hydrolysis (90 °C for 2 h), then infrared-assisted heating (125 °C for 6 h) | CQDs/6.7–12.5 | NA | NA | NA | Antibacterial/Anticancer | [148] |
| Boronic acid derivatives | Hydrothermal (160 °C for 8 h) | CQDs/8.9–9.5 | NA | 0.05 | In vitro >100 μg/mL, 8 h (Huh-7 cells) | Antivirus | [158] |
| Ciprofloxacin (antibiotic) | Hydrothermal (200 °C for 4 h) | CDs/4.7–6.8 | NA | 25.3 | NA | Antibacterial | [159] |
| Citric acid + amino acid (Arg, Cys, Glu, Gly, His, Leu, Phe, and Tyr) | Hydrothermal (180 °C for 12 h; dissolved in formamide) | CDs/3.0–6.0 | NA | 25.5–62.1 | In vitro >100 μg/mL, 24 h (HeLa cells) | In vitro bioimage | [160] |
| Citric acid + Curcumin | Hydrothermal (180 °C for 1 h) | CQDs/1.2–1.8 | NA | 3.6 | In vitro >250 μg/mL, 18 h (RAW 264.7 cells) | Antivirus | [161] |
| Citric acid + Branched poly-ethyleneimine | Hydrothermal (200 °C for 12 h) | CQDs/2.0–8.0 | NA | NA | In vitro >500 μg/mL, 72 h (L929 cells) | Antibacterial | [162] |
| Citric acid + Curcumin | Hydrothermal (180 °C for 24 h) | CDs/1.5–2.5 | NA | 30 | In vitro >250 μg/mL, 48 h (RAW 264.7, HK-2, and HPMCs cells) | Antibacterial | [163] |
| Citric acid + Ethyl-enediamine/ampicillin (antibiotic) | Hydrothermal (250 °C for 4 h) coupled with ampicillin conjugation | CDs/ca. 34.0–54.0 | 60 | 19 | In vitro >200 μg/mL, 24 h (HeLa cells) | Antibacterial | [164] |
| Vit C + PEG-diamine | Hydrothermal (180 °C for 1 h) | CDs/4.7 | NA | NA | In vitro >250 μg/mL, 48 h (PK-15 and MARC-145 cells) | Antivirus | [165] |
| Caffeic acid | Hydrothermal (200 °C for 6 h) | CQDs/1.5–2.5 | 10.2 | NA | In vitro >10 mg/mL, 12 h (HeLa cells) | Antibacterial/Antivirus | [142] |
| Carrageenan or Pullulan | Alkaline hydrolysis (90 °C for 2 h), then hydrothermal (210 °C for 6 h) | CQDs/ ca. 3.1 or ca. 4.2 | NA | NA | In vitro >1000 or >500 μg/mL, 24 h (Vero E6 cells) | Antivirus/Anticancer | [143] |
| Chlorogenic acid + Caffeic acid + Quinic acid | Hydrothermal (230 °C for 2 h) | CQDs/5.0–10.0 | NA | NA | In vitro >100 μg/mL, 24 h (L02 cells) In vivo >200 mg/kg, 90 min (mice) | Anticancer/GSH oxidase-like activity/Scavenging ROS | [52] |
| Folic acid | Hydrothermal (180 °C for 2 h) | CDs/3.0–11.0 | NA | 23.0 | In vitro >1 mg/mL, 3 h (U87 cells) | In vitro bioimage | [144] |
| Fucoidan | Hydrothermal (200 °C for 12 h) | CDs/4.0–10.0 | NA | NA | In vitro >1 mg/mL, 3 h (MC3T3-E1 cells) | Antibacterial | [145] |
| Glucose, Vit C, or Fructose | Hydrothermal (200 °C for 12 h) | CDs/ca. 9.0–10.0 | 34/56/29 (w/w) | 1.8/1.5/0.3 | In vitro >1000/>250/<1 μg/mL, 96 h (HeLa cells) | Drug carrier (doxorubicin) | [146] |
| Glucose + Ethylenediamine | Hydrothermal (200 °C for 4 h) | CDs/1.0–3.0 | NA | NA | In vivo >2.5 mg/mL, 3 h (zebrafish embryos)/>1.5 mg/mL, 10 h (zebrafish eleuthero-embryos) | In vivo bio-imaging in zebrafish embryos and eleuthero-embryos | [166] |
| Glucose + Glutamic acid | Hydrothermal (125 °C for 30 min, then 200 °C for 20 min; dissolved in NaOH) | CDs/ca. 2.0 | 29.8 | NA | In vitro >1000 μg/mL, 48 h (HeLa cells) | Drug and fluorescent dye carrier (doxorubicin; anticancer)/In vitro bioimage | [167] |
| Glucose + Aspartic acid | Hydrothermal (125 °C for 30 min, then 200 °C for 20 min; dissolved in NaOH) | CDs/1.8–2.7 | 34.5 | 7.5 | In vitro >500 μg/mL, 48 h (L929 and C6 cells) In vivo >200 mg/kg, 90 min (mice) | In vivo tumor image in mice | [168] |
| Glycyrrhizic acid | Hydrothermal (180 °C for 7 h; dissolved in NaOH) | CQDs/ ca. 11.4 | NA | 1.4 | In vitro >450 μg/mL, 48 h (MRC 145 cells) In vivo >200 mg/kg, 90 min (mice) | Antivirus | [143] |
| Sorbitol + Ethyl-enediamine | Hydrothermal (180 °C for 5 h) | CDs/ca. 5.0 | NA | 8.9 | In vitro >1000 μg/mL, 24 h (MCF-7 cells) | In vitro bioimage | [160] |
| Vitamin C | Hydrothermal (180 °C for 4 h) | CDs/ca. 9.0 | NA | NA | In vitro >1 mg/mL, 48 h (NIH-3T3 cells) In vivo >1 mg/mL, 48 h (fungus) | Fluorescent dye carrier/In vivo bioimaging in fungus Candida albicans | [149] |
| Triolein | Hydrothermal (220 °C for 3 days), then dissolved in NaOH | CDsomes/80.0–100.0 | ca. 30 | 4.1 | In vitro >300 μg/mL, 24 h (HaCaT cells) In vivo >100 μg/mL, 12 days (mice) | Antibacterial/Controllable ROS induction/Wound healing | [150] |
| Triolein | Hydrothermal (220 °C for 3 days), then dissolved in NaOH | CDsomes/80.0–100.0 | NA | 1.0 | In vitro >300 μg/mL, 48 h (HeLa cells) | In vitro bioimage | [20] |
| Triolein | Hydrothermal (220 °C for 3 days), then dissolved in NaOH | CDsomes/80.0–100.0 | 68 | NA | In vitro >400 μg/mL, 24 h (NIH-3T3 cells) | Anticancer/Controlable ROS induction | [151] |
| Citric acid + Glutathione | Oil bath (200 °C) | CDs/2.5–3.0 | NA | 80.3 | In vitro >3 mg/mL, 24 h (A549 cells) | In vitro bioimage | [169] |
| Vitamin C | Electrolysis (0.1 A for 3 weeks) | CDs/3.0–6.0 | NA | ca. 30 | NA | Antibacterial/Antifungal | [170] |
3. Biomedical Applications of Food-Based CDs
3.1. Bio-Imaging Applications
3.2. Antibacterial Activity
| Precursor | Type/Size (nm) | Zeta-Potential (mV) | Target Bacteria | MIC90 /ZOI > 10 mm | Antibacterial Mechanism | Ref. |
|---|---|---|---|---|---|---|
| Chinese mugwort (leaves) | CDs/3.0–7.0 | NA | E. coli and S. aureus | 150.0 µg/mL | Inhibition of cell wall synthesis | [78] |
| Green chiretta (leaf extract) | CDs/8.0–11.0 | −3.7 | S. aureus and K. pneumonia (multi-drug resistant clinically isolated strains) | 9.6 mg/mL | NA | [81] |
| Henna (leaves) | CDs/2.7–7.8 | −39.0 | E. coli and S. aureus | 5.0 mg/mL | NA | [82] |
| Rosemary (leaves) | CDs/11.5–20.7 | NA | S. aureus, B. subtilis, Bacillus cereus, E. coli, S. typhimurium, and C. albicans | 12.0 µg/mL | NA | [95] |
| Tea tree, Osmanthus, or Milk vetch (leaves) | CDs/5.0–18.0 | ca. −20 | E. coli and S. aureus | 1.0 mg/mL | Cationic effects on bacterial membrane | [98] |
| Turmeric (rhizome) | CDs/1.5–4.0 | −7.5 | E. coli, K. pneumoniae, S. aureus, and S. epidermidis | 250.0–1000.0 µg/mL | ROS generation | [103] |
| Turmeric (rhizome) + Ammonium persulfate | CDs/9.4–11.8 | −17.2 | E. coli and L. monocytogenes | NA | ROS generation | [104] |
| Oyster mushroom (Sporocarp) | CDs/2.5–5.5 | NA | S. aureus, K. pneumoniae, and P. aeruginosa | 30.0 µg/mL | ROS generation/Bacterial cell wall damage | [110] |
| Honey + Garlic | CQDs/4.0–13.0 | NA | E. coli, S. aureus, and P. aeruginosa | 10.0 µg/mL | Cationic effects on bacterial membrane/ROS generation | [113] |
| Lysine | CNGs/118.9–178.7 | +21.1 | E. coli, PHBV-producing E. coli, CRAB, S. epidermidis, S. aureus, and MRSA | 0.6–10.0 μg/mL | Bacterial cell wall damage/Cationic effects on bacterial membrane/ROS generation | [127] |
| Lysine or Arginine | CQDs/2.0–7.0 | +30.8 or +15.7 | E. coli and S. aureus | 16.0–31.3 or 62.5 μg/mL | Cationic effects on bacterial membrane/ROS generation | [129] |
| Quercetin + Lysine | CNGs/44.8–235.2 | +24.2 | E. coli, S. enterica, P. aeruginosa, S. aureus, and MRSA | 0.1–0.9 μg/mL | Bacterial cell wall damage/Cationic effects on bacterial membrane | [17] |
| Spermidine | CQDs/ ca. 6.0 | +45.4 | S. aureus, MRSA, E. coli., P. aeruginosa, and S. Entertidis | 2.0–4.0 µg/mL | Cationic effects on bacterial membrane | [125] |
| Ammonium citrate/Spermidine | CDs/3.8–5.4 | +60.6 | E. coli, S. enterica, P. aeruginosa, S. aureus, and MRSA | 0.9 µg/mL | Cationic effects on bacterial membrane | [153] |
| Spermine + Dopamine | CQDs/ ca. 10 | +31.0 | S. aureus, MRSA, E. coli., P. aeruginosa, and S. entertidis | 2.0–8.0 µg/mL | Cationic effects on the bacterial membrane/Biofilm inhibition | [155] |
| Ciprofloxacin (antibiotic) | CDs/4.7–6.8 | NA | E. coli and S. aureus | 0.025–1.0 µg/mL | NA | [159] |
| Citric acid + Curcumin | CDs/1.5–2.5 | −15.1 | E. coli, S. aureus, P. aeruginosa, and B. subtilis | 375.0–500.0 µg/mL | Cationic effects on bacterial membrane/Biofilm inhibition | [163] |
| Citric acid + Ethylenediamine/Ampicillin (antibiotic) | CDs/ca. 1.3 | −8.0 | E. coli, S. aureus, P. aeruginosa, and B. subtilis | 25.0–200.0 µg/mL | Cationic effects on bacterial membrane/ROS generation | [153] |
| Citric acid + Branched poly-ethyleneimine | CQDs/2.0–8.0 | ca. +15 | S. aureus | 500.0 µg/mL | Cationic effects on bacterial membrane/Biofilm inhibition | [162] |
| Caffeic acid | CQDs/1.5–2.5 | NA | S. aureus, M. luteus, and B. cereus | 5.0–10.0 mg/mL | Cationic effects on bacterial membrane | [142] |
| Fucoidan | CDs/4.0–10.0 | −15.8 | E. faecalis | 3.0 mg/mL | ROS generation/Biofilm inhibition | [147] |
| Citric acid + Urea | CDs/1.0–5.5 | −11.6 | MRSA and VISA | 0.6 µg/mL | NA | [138] |
| Microcrystalline cellulose | CQDs/5.4–10.2 | ca.−10 | E. coli and S. aureus | 100.0–350.0 µg/mL | ROS generation/Bacterial cell wall damage | [148] |
| Triolein | CDsomes/80.0–100.0 | −31.4 | S. aureus, MRSA, E. coli., and P. aeruginosa, | 1.7–2.5 μg/mL for Gram-positive bacteria; 104.1–112.4 μg/mL for Gram-negative bacteria | Light-triggered ROS generation | [150] |
| Vitamin C | CDs/3.0–6.0 | −20.0 | S. aureus, B. subtilis, Bacillus sp. WL-6, and E. coli | 50.0–75.0 µg/mL | ROS generation | [170] |
3.3. Antifungal Properties
3.4. Antivirus Activity
| Precursor | Type/Size (nm) | Target Virus | Toxicity (CC50) | Antiviral Effects (EC50) | Antiviral Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Citric acid/Boronic acids | CQDs/5.4–7.0 | HIV | >600.0 μg/mL | 4.7–9.4 μg/mL | Prevent viral attachment | [156] |
| Curcumin | CQDs/4.2–5.2 | EV71 | 452.0 μg/mL | 0.2 μg/mL | Prevent viral attachment/Inhibition of viral replication | [119] |
| Curcumin | CQDs/ ca. 4.8 | JEV | >100.0 μg/mL | 0.9 μg/mL | Prevent viral attachment | [120] |
| Hesperidin | CPDs/46.7–60.1 | EV71 | 773.0 μg/mL | 17.7 μg/mL | Prevent viral attachment/Inhibition of viral replication and translation/Inhibition of viral release/Alleviation of virus-induced oxidation | [123] |
| Lysine | CNGs/120.0–510.0 | IBV (poultry-affecting coronavirus), BEFV (cow-affecting virus), and PRV (pig-affecting virus) | >50.0 μg/mL | <5.0 μg/mL | Prevent viral attachment | [126] |
| Quercetin | CNGs/326.9–423.3 | IAVs | >600.0 μg/mL | 0.7 μg/mL | Prevent viral attachment/Alleviation of virus-induced oxidation and inflammation | [130] |
| Sodium alginate + Ammonium sulfite | CNGs/116.0–183.0 | IAVs | >1.0 mg/mL | ca. 250.0 μg/mL | Prevent viral attachment/Inhibition of viral invasion/Alleviation of virus-induced oxidation and inflammation | [157] |
| Spermidine | CQDs/ ca. 6.0 | WSSV (shrimp-affecting virus) | NA | ca. 1.0 μg/mL | Prevent viral attachment/Activation of the immune system | [125] |
| Vitamin C + PEG-diamine | CDs/4.7 | PRRSV (pig-affecting coronavirus) | >250.0 μg/mL | 125.0 μg/mL | Inducement of immune defense responses | [165] |
| Boronic acid derivatives | CQDs/8.9–9.5 | HCoV | >100.0 μg/mL | 2.0–20.0 μg/mL | Inhibition of the interaction between host cells and viruses/Inhibition of viral replication | [158] |
| Caffeic acid | CQDs/1.5–2.5 | vB-Eos-IME167, T4, and VMY22 | >10.0 mg/mL | ca. 2.5 mg/mL | Prevent viral attachment | [142] |
| Carrageenan or pullulan | CQDs/ ca. 3.1 or ca. 4.2 | MERS-CoV | 2.0–4.0 μg/mL | ca. 2.5 μg/mL | Prevent viral attachment/Inhibition of viral replication | [143] |
| Citric acid + Curcumin | CQDs/1.2–1.8 | PEDV (pig-affecting virus) | >250.0 μg/mL | ca. 60.0 μg/mL | Prevent viral attachment/Block viral invasion/Inhibition of viral replication/Inhibition of viral release/Alleviation of virus-induced oxidation and inflammation/Stimulation of interferon production | [161] |
| Glycyrrhizic acid | CQDs/ ca. 11.4 | PEDV and PRRSV | >900.0 μg/mL | ca. 300.0 μg/mL | Prevent viral invasion/Inhibition of viral replication/Stimulation of interferon production/Alleviation of virus-induced oxidation | [147] |
| Citric acid + Urea | GQDs/ 3.0–20.0 | feline coronavirus (cat-affecting coronavirus) and EV71 | >50.0 μg/mL | ca. 5.0 μg/mL | Prevent viral attachment | [139] |
| Citric acid + Poly-ethyleneimine | CDs/ca. 3.7 | KSHV and EBV | 5.0 μg/mL | <5.0 μg/mL | Inhibition of viral replication | [136] |
3.5. Anticancer Activity
| Precursor | Type/Size (nm) | Cell Strain; Cancer Type | Anticancer Effects (EC50) | Anticancer Mechanisms | Ref. |
|---|---|---|---|---|---|
| Kiwi, Avocado, or Pear | CDs/4.0–4.5 | Caco-2 (colon cancer)/HK-2 (kidney cancer) | In vitro 2.2–3.2 mg/mL, 72 h (Caco-2 cells)/1.3–2.0 mg/mL, 72 h (HK-2 cells) | NA | [76] |
| Green chiretta (leaf extract) | CDs/8.0–11.0 | MCF-7 (breast cancer) | In vitro 2 mg/mL, 24 h (MCF-7 cells) | NA | [91] |
| Ginger (rhizome) | CDs/3.5–5.1 | A549 (Lung cancer)/MDA-MB-231 (breast cancer)/HeLa (cervical cancer)/HepG2 (liver cancer)/FL83B (liver cancer) | In vitro >2.8 mg/mL, 24 h (A549 cells, FL83B cells, and MDA-MB-231 cells)/>0.35 mg/mL, 24 h (HeLa cells)/>1.4 mg/mL, 24 h (HepG2 cells) In vivo 440 μg/mice, 16 days, 97% reduction (nude mice; HepG2 cells) | Apoptosis promotion | [101] |
| Beetroot (root) | CDs/<5.0 | MCF-7 (breast cancer)/HepG2 (liver cancer) | In vitro 2.7 μg/mL, 24 h (MCF-7 cells) 2.1 μg/mL, 24 h (HepG2 cells) | NA | [106] |
| Carrot (root) | CDs/ ca. 2.3 | MCF-7 (breast cancer) | In vitro >1 mg/mL, 24 h (MCF-7 cells) | Anticancer drug delivery (mitomycin) | [107] |
| Grounded spice of cinnamon, red chili, turmeric or black pepper | CDs/1.0–10.0 | LN-229 (brain cancer) | In vitro >1–2 mg/mL, 24 h (LN-229 cells; expect for cinnamon CDs) | NA | [118] |
| Oyster mushroom (Sporocarp) | CDs/5.0–18.0 | MDA-MB-231 (breast cancer) | In vitro 3.34 μg/mL; 24 h (MDA-MB-231 cells) | Apoptosis promotion | [110] |
| Carrageenan or pullulan | CQDs/ ca. 3.1 or ca. 4.2 | MDA-MB-231 (breast cancer) | In vitro ca. 1000 μg/mL; 48 h (MDA-MB-231 cells) | Apoptosis promotion | [143] |
| Citric acid + Urea | CDs/2.0–6.0 | HepG2 (liver cancer)/HeLa (cervical cancer)/MCF-7 (breast cancer) | In vitro <2.5 μg/mL (doxorubicin), 48 h (HepG2, HeLa, and MCF-7 cells) In vivo 10 mg/mL, 72 h, 50% reduction (HepG2 tumor-bearing mice) | Anticancer drugs delivery (doxorubicin; pH-dependence release) | [140] |
| Citric acid + Tryptophan | CDs/ ca. 2.6 | MGC-083 (gastric cancer) | In vitro <1 μM (siRNA), 48 h (MGC-083 cells) | Anticancer drug delivery (siRNA)/Apoptosis promotion | [137] |
| Citric acid + Diethyl-enetriamine | CDs/5.0–8.0 | A2780 and U14 (ovarian cancer) | In vitro <11.4 μM (cisplatin), 2 h (A2780 cells) In vivo 1.5 mg/mL, 14 days, ~85% reduction (U14 tumor-bearing mice) | Anticancer drugs delivery (cisplatin; pH-dependence release) | [138] |
| Chlorogenic acid + Caffeic acid + Quinic acid | CQDs/5.0–10.0 | HepG2 (liver cancer) | In vitro <50 μg/mL, 24 h (HepG2 cells) In vivo 25 mg/kg, 12 days, ~80% reduction (HepG2 tumor-bearing mice) | Ferroptosis promotion/ROS induction/Promoting immune cell infiltration | [165] |
| Glucose + Glutamic acid | CDs/ ca. 2.0 | HeLa (cervical cancer) | In vitro <0.5 μg/mL (doxorubicin), 48 h (HeLa cells) | Anticancer drugs delivery (doxorubicin; pH-dependence release) | [167] |
| Microcrystalline cellulose | CQDs/5.4–12.5 | HepG2 (liver cancer) | In vitro 378.2–482.5 μg/mL, 24 h (HepG2 cells) | Apoptosis promotion/ROS induction | [132] |
| Triolein | CDsomes | Tramp-C1 (prostate cancer) | In vitro <200 μg/mL, 24 h (Tramp-C1 cells) | ROS induction (photocatalytic activity) | [136] |
3.6. Immunomodulatory Functions
| Precursor | Type/Size (nm) | Treatment | Effective Dose | Autoimmune Diseases/Model | Immunomodulatory Mechanism | Ref. |
|---|---|---|---|---|---|---|
| Rose petals/thymol | CDs/5.0–6.0 | Oral administration | 2 mg/kg | Rheumatoid arthritis/FCA-induced arthritic rats | Drug carrier (thymol) | [64] |
| Phellodendri chinensis cortex | CDs/0.5–3.6 | Oral administration | 220 μg/kg/day | Psoriasis/IMQ-induced psoriasis-like skin mouse model | Prevent M1 transition of macrophages/Activation of M2 macrophages | [65] |
| Rhei radix (rhizome) | CDs/1.4–4.5 | Oral administration | 60 μg/kg/day | Ulcerative colitis/DSS-induced ulcerative colitis mouse model | Inhibition of inflammatory cytokine/Increase antioxidant protein expression level | [66] |
| Citric acid + Cysteine | CQDs/0.9–1.0 | Oral administration | 1 mL CQDs solution with 50 IU insulin | Type I diabetes/AOAC standard diet-induced diabetic mice | Drug carrier (insulin) | [134] |
| Folic acid | CDs/1.0–1.6 | Intra-articular injection | 2 mg/mL CDs twice per week 6 consecutive weeks. | Osteoarthritis/ACLT mouse model | Inhibition of inflammatory cytokine/Prevent M1 transition of macrophages/Activation of M2 macrophages | [121] |
4. Safety Assessment of Food-Based CDs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Pang, Y.; Zhou, H. The Interaction between Nanoparticles and Immune System: Application in the Treatment of Inflammatory Diseases. J. Nanobiotechnol. 2022, 20, 127. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-Based Therapeutics for Antibiotic-Resistant Bacterial Infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, G.; Zhao, L.; Lai, K.W. Carbon Nanomaterial-Based Biosensors: A Review of Design and Applications. IEEE Nanotechnol. Mag. 2019, 13, 4–14. [Google Scholar] [CrossRef]
- Wang, B.; Lu, S. The Light of Carbon Dots: From Mechanism to Applications. Matter 2022, 5, 110–149. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.C.; Wang, H.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Tao, S.; Zhu, S.; Feng, T.; Xia, C.; Song, Y.; Yang, B. The Polymeric Characteristics and Photoluminescence Mechanism in Polymer Carbon Dots: A Review. Mater. Today Chem. 2017, 6, 13–25. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.; Zhang, H.; Yu, J. Carbon Dots-in-Matrix Boosting Intriguing Luminescence Properties and Applications. Small 2019, 15, 1805504. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent Advances in Graphene Quantum Dots for Sensing. Mater. Today 2013, 16, 442–443. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Carolina, C.C.; Valcárcel, M. Semiconductor and Carbon-Based Fluorescent Nanodots: The Need for Consistency. Chem. Commun. 2016, 52, 1311–1326. [Google Scholar] [CrossRef]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-warthan, A.A.; Tremel, W. Graphene Based Metal and Metal Oxide Nanocomposites: Synthesis, Properties and Their Applications. J. Mater. Chem. A 2015, 37, 18753–18808. [Google Scholar] [CrossRef]
- Yang, Z.-C.; Wang, M.; Yong, A.-M.; Wong, S.-Y.; Zhang, X.-H.; Tan, H.; Chang, A.-Y.; Li, X.; Wang, J. Intrinsically Fluorescent Carbon Dots with Tunable Emission Derived from Hydrothermal Treatment of Glucose in the Presence of Monopotassium Phosphate. Chem. Commun. 2011, 47, 11615–11617. [Google Scholar] [CrossRef]
- Kwon, W.; Lee, G.; Do, S.; Joo, T.; Rhee, S.W. Size-Controlled Soft-Template Synthesis of Carbon Nanodots toward Versatile Photoactive Materials. Small 2014, 10, 506–513. [Google Scholar] [CrossRef]
- Nguyen, V.; Si, J.; Yan, L.; Hou, X. Direct Demonstration of Photoluminescence Originated from Surface Functional Groups in Carbon Nanodots. Carbon 2016, 108, 268–273. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Wang, S.-W.; Mao, J.-Y.; Chang, H.-T.; Harroun, S.G.; Lin, H.-J.; Huang, C.-C.; Lai, J.-Y. Carbonized Nanogels for Simultaneous Antibacterial and Antioxidant Treatment of Bacterial Keratitis. Chem. Eng. J. 2021, 411, 128469. [Google Scholar] [CrossRef]
- Mao, J.-Y.; Unnikrishnan, B.; Chu, H.-W.; Harroun, S.G.; Chen, Y.-R.; Wu, A.-T.; Chang, H.-T.; Lin, H.-J.; Huang, C.-C. Thermally Driven Formation of Polyphenolic Carbonized Nanogels with High Anticoagulant Activity from Polysaccharides. Biomater. Sci. 2021, 9, 4679–4690. [Google Scholar] [CrossRef]
- Chen, T.-H.; Chang, H.-T. Stable and Photoswitchable Carbon-Dot Liposome. ACS Appl. Mater. Interfaces 2017, 9, 44259–44263. [Google Scholar] [CrossRef]
- Wu, R.-S.; Lin, Y.-S.; Nain, A.; Unnikrishnan, B.; Lin, Y.-F.; Yang, C.-R.; Chen, T.-H.; Huang, Y.-F.; Huang, C.-C.; Chang, H.-T. Evaluation of Chemotherapeutic Response in Living Cells Using Subcellular Organelle–Selective Amphipathic Carbon Dots. Biosens. Bioelectron. 2022, 211, 114362. [Google Scholar] [CrossRef]
- Wu, R.-S.; Yang, C.-R.; Huang, Y.-F.; Huang, C.-C.; Chen, Y.-L.; Chang, H.-T. Ratiometric Fluorescence Probe of Vesicle-like Carbon Dots and Gold Clusters for Quantitation of Cholesterol. Chemosensors 2022, 10, 160. [Google Scholar] [CrossRef]
- Anand, A.; Jian, H.-J.; Huang, H.-H.; Hean, L.E.; Li, Y.-J.; Lai, J.-Y.; Chou, H.-D.; Kang, Y.-C.; Wu, W.-C.; Lai, C.-C.; et al. Anti-Angiogenic Carbon Nanovesicles Loaded with Bevacizumab for the Treatment of Age-Related Macular Degeneration. Carbon 2023, 201, 362–370. [Google Scholar] [CrossRef]
- Goswami, A.D.; Trivedi, D.H.; Jadhav, N.L.; Pinjari, D.V. Sustainable and Green Synthesis of Carbon Nanomaterials: A Review. J. Environ. Chem. Eng. 2021, 9, 106118. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Karuppasamy, K.; Lee, S.-J.; Shwetharani, R.; Kim, H.-S.; Pasha, S.K.K.; Ashokkumar, M.; Choi, M.-Y. Fundamentals and Comprehensive Insights on Pulsed Laser Synthesis of Advanced Materials for Diverse Photo- and Electrocatalytic Applications. Light Sci. Appl. 2022, 11, 250. [Google Scholar] [CrossRef]
- Grossmann, L.; Hinrichs, J.; Weiss, J. Technologies for Sustainable Heat Generation in Food Processing. Compr. Rev. Food Sci. 2022, 21, 4971–5003. [Google Scholar] [CrossRef]
- Zhang, L.; Na, X.; Lai, B.; Song, Y.; Wang, H.; Tan, M. Effects of Fluorescent Carbon Dots from the Baked Lamb on Energy and Lipid Metabolism. Food Chem. 2021, 338, 127832. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Y.; Na, X.; Bi, J.; Liu, S.; Zhang, L.; Tan, M. Fluorescent Carbon Dots in Baked Lamb: Formation, Cytotoxicity and Scavenging Capability to Free Radicals. Food Chem. 2019, 286, 405–412. [Google Scholar] [CrossRef]
- Liu, K.; Song, Y.; Tan, M. Toxicity Alleviation of Carbon Dots from Roast Beef after the Formation of Protein Coronas with Human Serum Albumin. J. Agric. Food Chem. 2020, 68, 9789–9795. [Google Scholar] [CrossRef]
- Geng, J.; Song, X.; Zhang, X.; Tie, S.; Cao, L.; Tan, M. Hydrophilic Food-Borne Nanoparticles from Beef Broth as Novel Nanocarriers for Zinc. J. Agric. Food Chem. 2019, 67, 6995–7004. [Google Scholar] [CrossRef]
- Cong, S.; Liu, K.; Qiao, F.; Song, Y.; Tan, M. Biocompatible Fluorescent Carbon Dots Derived from Roast Duck for in Vitro Cellular and in vivo C. elegans Bio-Imaging. Methods 2019, 168, 76–83. [Google Scholar] [CrossRef]
- Cong, S.; Bi, J.; Song, X.; Yu, C.; Tan, M. Ultrasmall Fluorescent Nanoparticles Derived from Roast Duck: Their Physicochemical Characteristics and Interaction with Human Serum Albumin. Food Funct. 2018, 9, 2490–2495. [Google Scholar] [CrossRef]
- Song, X.; Wang, H.; Zhang, R.; Yu, C.; Tan, M. Bio-Distribution and Interaction with Dopamine of Fluorescent Nanodots from Roasted Chicken. Food Funct. 2018, 9, 6227–6235. [Google Scholar] [CrossRef]
- Bi, J.; Li, Y.; Wang, H.; Song, Y.; Cong, S.; Yu, C.; Zhu, B.-W.; Tan, M. Presence and Formation Mechanism of Foodborne Carbonaceous Nanostructures from Roasted Pike Eel (Muraenesox cinereus). J. Agric. Food Chem. 2018, 66, 2862–2869. [Google Scholar] [CrossRef]
- Song, Y.; Wu, Y.; Wang, H.; Liu, S.; Song, L.; Li, S.; Tan, M. Carbon Quantum Dots from Roasted Atlantic Salmon (Salmo salar L.): Formation, Biodistribution and Cytotoxicity. Food Chem. 2019, 293, 387–395. [Google Scholar] [CrossRef]
- Li, J.; Cao, L.; Li, D.; Yu, C.; Tan, M. Carbon Dots from Roasted Mackerel (Scomberomorus niphonius) for Free Radical Scavenging. LWT 2019, 111, 588–593. [Google Scholar] [CrossRef]
- Cui, G.; Song, Y.; Liu, K.; Tan, M. Interaction of Carbon Dots from Grilled Spanish Mackerel with Human Serum Albumin, γ-Globulin and Fibrinogen. Foods 2021, 10, 2336. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Song, Y.; Zhu, B.-W.; Tan, M. Universal Existence of Fluorescent Carbon Dots in Beer and Assessment of Their Potential Toxicity. Nanotoxicology 2019, 13, 160–173. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, H.; Wu, H.; Wang, B.; Zhao, H.; Tan, M. Fluorescent Carbon Dots from Beer for Breast Cancer Cell Imaging and Drug Delivery. Anal. Methods 2015, 7, 8911–8917. [Google Scholar] [CrossRef]
- Jiang, C.; Wu, H.; Song, X.; Ma, X.; Wang, J.; Tan, M. Presence of Photoluminescent Carbon Dots in Nescafe® Original Instant Coffee: Applications to Bioimaging. Talanta 2014, 127, 68–74. [Google Scholar] [CrossRef]
- Liu, B.; Guo, S.; Fan, X.; Gong, X. Carbon Quantum Dot Preparation and Application to Detecting Active Ingredients in Traditional Chinese Medicine. Acupunct. Herba. Med. 2021, 1, 81–89. [Google Scholar] [CrossRef]
- Luo, W.-K.; Zhang, L.-L.; Yang, Z.-Y.; Guo, X.-H.; Wu, Y.; Zhang, W.; Luo, J.-K.; Tang, T.; Wang, Y. Herbal Medicine Derived Carbon Dots: Synthesis and Applications in Therapeutics, Bioimaging and Sensing. J. Nanobiotechnol. 2021, 19, 320. [Google Scholar] [CrossRef]
- Hassoun, A.; Aït-Kaddour, A.; Sahar, A.; Cozzolino, D. Monitoring Thermal Treatments Applied to Meat Using Traditional Methods and Spectroscopic Techniques: A Review of Advances over the Last Decade. Food Bioproc. Technol. 2021, 14, 195–208. [Google Scholar] [CrossRef]
- Balakrishnan, T.; Ang, W.-L.; Mahmoudi, E.; Mohammad, A.W.; Sambudi, N.S. Formation Mechanism and Application Potential of Carbon Dots Synthesized from Palm Kernel Shell via Microwave Assisted Method. Carbon Resour. Convers. 2022, 5, 116–150. [Google Scholar] [CrossRef]
- Papaioannou, N.; Titirici, M.-M.; Mahmoudi, E.; Sapelkin, A. Investigating the Effect of Reaction Time on Carbon Dot Formation, Structure, and Optical Properties. ACS Omega 2019, 4, 21658–21665. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem. Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Sruthi, N.U.; Premjit, Y.; Pandiselvam, R.; Kothakota, A.; Ramesh, S.V. An Overview of Conventional and Emerging Techniques of Roasting: Effect on Food Bioactive Signatures. Food Chem. 2021, 348, 129088. [Google Scholar] [CrossRef]
- Rani, L.; Kumar, M.; Kaushik, D.; Kaur, J.; Kumar, A.; Oz, F.; Proestos, C.; Oz, E. A Review on the Frying Process: Methods, Models and Their Mechanism and Application in the Food Industry. Food Res. Int. 2023, 172, 113176. [Google Scholar] [CrossRef]
- Yin, J.; Liu, K.; Yuan, S.; Guo, Y.; Yu, H.; Cheng, Y.; Xie, Y.; Qian, H.; Yao, W. Carbon Dots in Breadcrumbs: Effect of Frying on Them and Interaction with Human Serum Albumin. Food Chem. 2023, 424, 136371. [Google Scholar] [CrossRef]
- Cong, S.; Wang, N.; Wang, K.; Wu, Y.; Li, D.; Song, Y.; Prakash, S.; Tan, M. Fluorescent Nanoparticles in the Popular Pizza: Properties, Biodistribution and Cytotoxicity. Food Funct. 2019, 10, 2408–2416. [Google Scholar] [CrossRef]
- Li, Y.; Bi, J.; Liu, S.; Wang, H.; Yu, C.; Li, D.; Zhu, B.-W.; Tan, M. Presence and Formation of Fluorescence Carbon Dots in a Grilled Hamburger. Food Funct. 2017, 8, 2558–2565. [Google Scholar] [CrossRef]
- Li, D.; Na, X.; Zhou, W.; Wang, C.; Li, Y.; Zhu, B.-W.; Tan, M. Adverse Effects of Fluorescent Carbon Dots from Canned Yellow Croaker on Cellular Respiration and Glycolysis. Food Funct. 2019, 10, 1123–1131. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, M.-M.; Luo, Q.-W.; Zhang, Y.-C.; Liu, T.-T.; Yang, Z.; Liao, M.; Tu, P.; Zeng, K.-W. Carbon Quantum Dots-Based Nanozyme from Coffee Induces Cancer Cell Ferroptosis to Activate Antitumor Immunity. ACS Nano 2022, 16, 9228–9239. [Google Scholar] [CrossRef]
- Li, S.; Jiang, C.; Wang, H.; Cong, S.; Tan, M. Fluorescent Nanoparticles Present in Coca-Cola and Pepsi-Cola: Physiochemical Properties, Cytotoxicity, Biodistribution and Digestion Studies. Nanotoxicology 2018, 12, 49–62. [Google Scholar] [CrossRef]
- Liao, H.; Jiang, C.; Liu, W.; Vera, J.M.; Seni, O.D.; Demera, K.; Yu, C.; Tan, M. Fluorescent Nanoparticles from Several Commercial Beverages: Their Properties and Potential Application for Bioimaging. J. Agric. Food Chem. 2015, 63, 8527–8533. [Google Scholar] [CrossRef]
- Sk, M.P.; Jaiswal, A.; Paul, A.; Ghosh, S.S.; Chattopadhyay, A. Presence of Amorphous Carbon Nanoparticles in Food Caramels. Sci. Rep. 2012, 383, 2. [Google Scholar] [CrossRef]
- Al-Hadi, A.M.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. The Presence of Carbon Nanostructures in Bakery Products Induces Metabolic Stress in Human Mesenchymal Stem Cells through CYP1A and P53 Gene Expression. Environ. Toxicol. Pharmacol. 2016, 41, 103–112. [Google Scholar] [CrossRef]
- Cao, L.; Song, X.; Song, Y.; Bi, J.; Cong, S.; Yu, C.; Tan, M. Fluorescent Nanoparticles from Mature Vinegar: Their Properties and Interaction with Dopamine. Food Funct. 2017, 8, 4744–4751. [Google Scholar] [CrossRef]
- Jiang, S.; Shi, Y.; Li, M.; Xiong, L.; Sun, Q. Characterization of Maillard Reaction Products Micro/Nano-Particles Present in Fermented Soybean Sauce and Vinegar. Sci. Rep. 2019, 9, 11285. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Xiao, Y.; Tang, J.; Liang, C.; Li, F.; Dong, H.; Xu, W. A Simple Approach for Synthesizing of Fluorescent Carbon Quantum Dots from Tofu Wastewater. Nanoscale Res. Lett. 2017, 12, 611. [Google Scholar] [CrossRef]
- Mandani, S.; Dey, D.; Sharma, B.; Sarma, T.K. Natural Occurrence of Fluorescent Carbon Dots in Honey. Carbon 2017, 119, 569–572. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Shiha, A.M.; Mahrous, H.; Mohammed, A.B.A. Green Synthesis of Chitosan Nanoparticles, Optimization, Characterization and Antibacterial Efficacy against Multi Drug Resistant Biofilm-Forming Acinetobacter baumannii. Sci. Rep. 2022, 12, 19869. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Saber, W.I.A.; Zweil, A.M.; Bashir, S.I. An Innovative Green Synthesis Approach of Chitosan Nanoparticles and Their Inhibitory Activity against Phytopathogenic Botrytis Cinerea on Strawberry Leaves. Sci. Rep. 2022, 12, 3515. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K.S. Phytosynthesis of Nanoparticles: Concept, Controversy and Application. Nanoscale Res. Lett. 2014, 9, 229. [Google Scholar] [CrossRef]
- Murugesan, S.; Srinivasan, V.; Lakshmanan, D.K.; Venkateswaran, M.R.; Jayabal, S.; Muthukumar Nadar, M.S.A.; Kathiravan, A.; Asha Jhonsi, M.; Thilagar, S.; Periyasamy, S. Evaluation of the Anti-Rheumatic Properties of Thymol Using Carbon Dots as Nanocarriers on FCA Induced Arthritic Rats. Food Funct. 2021, 12, 5038–5050. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, J.; Hu, J.; Luo, J.; Zhang, Y.; Lu, F.; Kong, H.; Qu, H.; Zhao, Y. Green Phellodendri chinensis Cortex-Based Carbon Dots for Ameliorating Imiquimod-Induced Psoriasis-Like Inflammation in Mice. J. Nanobiotechnol. 2021, 19, 105. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Zhao, Y.; Bai, X.; Chen, Y.; Liu, Y.; Zhang, Y.; Kong, H.; Qu, H.; Zhao, Y. The Rhei radix Rhizoma-Based Carbon Dots Ameliorates Dextran Sodium Sulphate-Induced Ulcerative Colitis in Mice. Artif. Cells Nanomed. Biotechnol. 2023, 51, 180–191. [Google Scholar] [CrossRef]
- Ðorđević, L.; Arcudi, F.; Cacioppo, M.; Prato, M.A. Multifunctional Chemical Toolbox to Engineer Carbon Dots for Biomedical and Energy Applications. Nat. Nanotechnol. 2022, 17, 112–130. [Google Scholar] [CrossRef]
- Tepliakov, N.V.; Kundelev, E.V.; Khavlyuk, P.D.; Xiong, Y.; Leonov, M.Y.; Zhu, W.; Baranov, A.V.; Fedorov, A.V.; Rogach, A.L.; Rukhlenko, I.D. Sp2–Sp3-Hybridized Atomic Domains Determine Optical Features of Carbon Dots. ACS Nano 2019, 13, 10737–10744. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, M.; Liu, Y.; Feng, X.Z.; Yin, X.B.; He, X.W.; Zhang, Y.K. Nitrogen-Doped Carbon Dots: A Facile and General Preparation Method, Photoluminescence Investigation, and Imaging Applications. Chem. Eur. J. 2013, 19, 2276–2283. [Google Scholar] [CrossRef]
- Havigh, S.R.; Chenari, M.H. A Comprehensive Study on the Effect of Carbonization Temperature on the Physical and Chemical Properties of Carbon Fibers. Sci. Rep. 2022, 12, 10704. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuel Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Arpia, A.A.; Chen, W.-H.; Lam, S.S.; Rousset, P.; de Luna, M.D.G. Sustainable Biofuel and Bioenergy Production from Biomass Waste Residues Using Microwave-Assisted Heating: A Comprehensive Review. Chem. Eng. J. 2021, 403, 126233. [Google Scholar] [CrossRef]
- Shabbir, H.; Wojtaszek, K.; Rutkowski, B.; Csapó, E.; Bednarski, M.; Adamiec, A.; Głuch-Lutwin, M.; Mordyl, B.; Druciarek, J.; Kotańska, M.; et al. Milk-Derived Carbon Quantum Dots: Study of Biological and Chemical Properties Provides Evidence of Toxicity. Molecules 2022, 27, 8728. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.S. Green Synthesis of Luminescent Nitrogen-Doped Carbon Dots from Milk and Its Imaging Application. Anal. Chem. 2014, 86, 8902–8905. [Google Scholar] [CrossRef]
- Dinç, S.; Kara, M.; Demirel Kars, M.; Aykül, F.; Çiçekci, H.; Akkuş, M. Biocompatible Yogurt Carbon Dots: Evaluation of Utilization for Medical Applications. Appl. Phys. A 2017, 123, 572. [Google Scholar] [CrossRef]
- Dias, C.; Vasimalai, N.; Sárria, M.P.; Pinheiro, I.; Vilas-Boas, V.; Peixoto, J.; Espiña, B. Biocompatibility and Bioimaging Potential of Fruit-Based Carbon Dots. Nanomaterials 2019, 9, 199. [Google Scholar] [CrossRef]
- Jeong, C.J.; Roy, A.K.; Kim, S.H.; Lee, J.-E.; Jeong, J.H.; In, I.; Park, S.Y. Fluorescent Carbon Nanoparticles Derived from Natural Materials of Mango Fruit for Bio-Imaging Probes. Nanoscale 2014, 6, 15196–15202. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Park, T.J.; Kailasa, S.K. Green Synthesis of Multi-Color Emissive Carbon Dots from Manilkara zapota Fruits for Bioimaging of Bacterial and Fungal Cells. J. Photochem. Photobiol. B Biol. 2019, 191, 150–155. [Google Scholar] [CrossRef]
- Ma, H.P.; Sun, C.C.; Xue, G.H.; Wu, G.L.; Zhang, X.H.; Han, X.; Qi, X.H.; Lv, X.; Sun, H.J.; Zhang, J.B. Facile Synthesis of Fluorescent Carbon Dots from Prunus cerasifera Fruits for Fluorescent Ink, Fe3+ Ion Detection and Cell Imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 213, 281–287. [Google Scholar] [CrossRef]
- He, M.; Zhang, J.; Wang, H.; Kong, Y.; Xiao, Y.; Xu, W. Material and Optical Properties of Fluorescent Carbon Quantum Dots Fabricated from Lemon Juice via Hydrothermal Reaction. Nanoscale Res. Lett. 2018, 13, 175. [Google Scholar] [CrossRef]
- Rodríguez-Varillas, S.; Fontanil, T.; Obaya, Á.J.; Fernández-González, A.; Murru, C.; Badía-Laíño, R. Biocompatibility and Antioxidant Capabilities of Carbon Dots Obtained from Tomato (Solanum lycopersicum). Appl. Sci. 2022, 12, 773. [Google Scholar] [CrossRef]
- Zhu, W.S.; Gao, X.F.; Yu, X.K.; Li, Q.S.; Zhou, Y.; Qiu, H.; Xing, B.G.; Zhang, Z.J. Screening of Multifunctional Fruit Carbon Dots for Fluorescent Labeling and Sensing in Living Immune Cells and Zebrafishes. Mikrochim. Acta 2022, 189, 223. [Google Scholar] [CrossRef]
- Song, Y.; Yan, X.; Li, Z.H.; Qu, L.B.; Zhu, C.Z.; Ye, R.F.; Li, S.Q.; Du, D.; Lin, Y.H. Highly Photoluminescent Carbon Dots Derived from Linseed and Their Applications in Cellular Imaging and Sensing. J. Mater. Chem. B 2018, 6, 3181–3187. [Google Scholar] [CrossRef]
- Roshni, V.; Misra, S.; Santra, M.K.; Ottoor, D. One Pot Green Synthesis of C-dots from Groundnuts and Its Application as Cr(VI) Sensor and in vitro Bioimaging Agent. J. Photochem. Photobiol. A 2019, 373, 28–36. [Google Scholar] [CrossRef]
- John, T.S.; Yadav, P.K.; Kumar, D.; Singh, S.K.; Hasan, S.H. Highly Fluorescent Carbon Dots from Wheat Bran as A Novel Drug Delivery System for Bacterial Inhibition. Luminescence 2020, 35, 913–923. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Ren, S.; Hu, Z.; Wang, Y. One-Pot Synthesis of Forsythia@carbon Quantum Dots with Natural Anti-Wood Rot Fungus Activity. Mater. Des. 2021, 206, 109800. [Google Scholar] [CrossRef]
- Alam, A.M.; Park, B.Y.; Ghouri, Z.K.; Park, M.; Kim, H.Y. Synthesis of Carbon Quantum Dots from Cabbage with Down- and Up-Conversion Photoluminescence Properties: Excellent Imaging Agent for Biomedical Applications. Green Chem. 2015, 17, 3791–3797. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Ma, Y.; Wang, B.; Shao, M.; Huang, H.; Liu, Y.; Kang, Z. Selective Inactivation of Gram-Negative Bacteria by Carbon Dots Derived from Natural Biomass: Artemisia argyi Leaves. J. Mater. Chem. B 2020, 8, 2666–2672. [Google Scholar] [CrossRef]
- Sachdev, A.; Gopinath, P. Green Synthesis of Multifunctional Carbon Dots from Coriander Leaves and Their Potential Application as Antioxidants, Sensors and Bioimaging Agents. Analyst 2015, 140, 4260–4269. [Google Scholar] [CrossRef]
- Jiang, X.H.; Qin, D.M.; Mo, G.C.; Feng, J.S.; Yu, C.H.; Mo, W.M.; Deng, B.Y. Ginkgo Leaf-Based Synthesis of Nitrogen-Doped Carbon Quantum Dots for Highly Sensitive Detection of Salazosulfapyridine in Mouse Plasma. J. Pharm. Biomed. Anal. 2019, 164, 514–519. [Google Scholar] [CrossRef]
- Naik, G.G.; Alam, M.B.; Pandey, V.; Mohapatra, D.; Dubey, P.K.; Parmar, A.S.; Sahu, A.N. Multi-Functional Carbon Dots from an Ayurvedic Medicinal Plant for Cancer Cell Bioimaging Applications. J. Fluoresc. 2020, 30, 407–418. [Google Scholar] [CrossRef]
- Shahshahanipour, M.; Rezaei, B.; Ensafi, A.A.; Etemadifar, Z. An Ancient Plant for the Synthesis of a Novel Carbon Dot and Its Applications as an Antibacterial Agent and Probe for Sensing of an Anti-Cancer Drug. Mater. Sci. Eng. C 2019, 98, 826–833. [Google Scholar] [CrossRef]
- Kumar, A.; Chowdhuri, A.R.; Laha, D.; Mahto, T.K.; Karmakar, P.; Sahu, S.K. Green Synthesis of Carbon Dots from Ocimum Sanctum for Effective Fluorescent Sensing of Pb2+ Ions and Live Cell Imaging. Sens. Actuators. B Chem. 2017, 242, 679–686. [Google Scholar] [CrossRef]
- Niu, X.; Liu, G.; Li, L.; Fu, Z.; Xu, H.; Cui, F. Green and Economical Synthesis of Nitrogen-Doped Carbon Dots from Vegetables for Sensing and Imaging Applications. RSC Adv. 2015, 5, 95223–95229. [Google Scholar] [CrossRef]
- Eskalen, H.; Çeşme, M.; Kerli, S.; Özğan, Ş. Green Synthesis of Water-Soluble Fluorescent Carbon Dots from Rosemary Leaves: Applications in Food Storage Capacity, Fingerprint Detection, and Antibacterial Activity. J. Chem. Res. 2021, 45, 428–435. [Google Scholar] [CrossRef]
- Li, L.; Zhang, R.; Lu, C.; Sun, J.; Wang, L.; Qu, B.; Li, T.; Liu, Y.; Li, S. In Situ Synthesis of NIR-Light Emitting Carbon Dots Derived from Spinach for Bio-Imaging Applications. J. Mater. Chem. B 2017, 5, 7328–7334. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, B.; Li, X.; Zhang, G.; Zhang, Y.; Dong, C.; Shuang, S. Green-Fluorescent Nitrogen-Doped Carbon Nanodots for Biological Imaging and Paper-Based Sensing. Anal. Methods 2017, 9, 2197–2204. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, M.; Wang, H.; Wang, B.; Huang, H.; Liu, Y.; Kang, Z. N-Doped Carbon Dots Derived from Leaves with Low Toxicity via Damaging Cytomembrane for Broad-Spectrum Antibacterial Activity. Mater. Today Commun. 2020, 24, 101222. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, B.; Liu, Y.; Liu, Z.; Zhang, H.; Zhang, S.; Chang, J.B.; Lu, S. Green Synthesis of Nitrogen and Sulfur Co-Doped Carbon Dots from Allium fistulosum for Cell Imaging. New J. Chem. 2019, 43, 718–723. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Weng, B.; Wang, B.; Li, C. Facile Synthesis of Nitrogen and Sulfur Co-Doped Carbon Dots and Application for Fe (III) Ions Detection and Cell Imaging. Sens. Actuators B Chem. 2016, 223, 689–696. [Google Scholar] [CrossRef]
- Li, C.-L.; Ou, C.-M.; Huang, C.-C.; Wu, W.-C.; Chen, Y.-P.; Lin, T.-E.; Ho, L.-C.; Wang, C.-W.; Shih, C.-C.; Zhou, H.-C.; et al. Carbon Dots Prepared from Ginger Exhibiting Efficient Inhibition of Human Hepatocellular Carcinoma Cells. J. Mater. Chem. B 2014, 2, 4564. [Google Scholar] [CrossRef]
- Teng, X.; Ma, C.; Ge, C.; Yan, M.; Yang, J.; Zhang, Y.; Morais, P.C.; Bi, H. Green Synthesis of Nitrogen-Doped Carbon Dots from Konjac Flour with “Off–On” Fluorescence by Fe3+ and L-Lysine for Bioimaging. J. Mater. Chem. B 2014, 2, 4631. [Google Scholar] [CrossRef]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Luong, J.H.T.; Gedanken, A. Green Synthesis of Multifunctional Carbon Dots with Antibacterial Activities. Nanomaterials 2021, 11, 369. [Google Scholar] [CrossRef]
- Roy, S.; Ezati, P.; Rhim, J.-W.; Molaei, R. Preparation of Turmeric-Derived Sulfur-Functionalized Carbon Dots: Antibacterial and Antioxidant Activity. J. Mater. Sci. 2022, 57, 2941–2952. [Google Scholar] [CrossRef]
- Li, Z.; Ni, Y.; Kokot, S. A New Fluorescent Nitrogen-Doped Carbon Dot System Modified by the Fluorophore-Labeled ssDNA for the Analysis of 6-Mercaptopurine and Hg (II). Biosens. Bioelectron. 2015, 74, 91–97. [Google Scholar] [CrossRef]
- Smrithi, S.P.; Kottam, N.; Muktha, H.; Mahule, A.M.; Chamarti, K.; Vismaya, V.; Sharath, R. Carbon Dots Derived from Beta Vulgaris: Evaluation of Its Potential as Antioxidant and Anticancer Agent. Nanotechnology 2022, 33, 045403. [Google Scholar] [CrossRef]
- D’souza, S.L.; Chettiar, S.S.; Koduru, J.R.; Kailasa, S.K. Synthesis of Fluorescent Carbon Dots Using Daucus carota subsp. sativus Roots for Mitomycin Drug Delivery. Optik 2018, 158, 893–900. [Google Scholar] [CrossRef]
- Liu, W.; Diao, H.; Chang, H.; Wang, H.; Li, T.; Wei, W. Green Synthesis of Carbon Dots from Rose-Heart Radish and Application for Fe3+ Detection and Cell Imaging. Sens. Actuators B Chem. 2017, 241, 190–198. [Google Scholar] [CrossRef]
- Shen, J.; Shang, S.; Chen, X.; Wang, D.; Cai, Y. Facile Synthesis of Fluorescence Carbon Dots from Sweet Potato for Fe3+ Sensing and Cell Imaging. Mater. Sci. Eng. C 2017, 76, 856–864. [Google Scholar] [CrossRef]
- Boobalan, T.; Sethupathi, M.; Sengottuvelan, N.; Kumar, P.; Balaji, P.; Gulyás, B.; Padmanabhan, P.; Selvan, S.T.; Arun, A. Mushroom-Derived Carbon Dots for Toxic Metal Ion Detection and as Antibacterial and Anticancer Agents. ACS Appl. Nano Mater. 2020, 3, 5910–5919. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Li, X.; Liu, R.; Lin, L.; Zhao, S. Green Preparation of S and N Co-Doped Carbon Dots from Water Chestnut and Onion as Well as Their Use as An Off–On Fluorescent Probe for the Quantification and Imaging of Coenzyme A. ACS Sustain. Chem. Eng. 2017, 5, 4992–5000. [Google Scholar] [CrossRef]
- Bajpai, S.K.; D’Souza, A.; Suhail, B. Carbon Dots from Guar Gum: Synthesis, Characterization and Preliminary in Vivo Application in Plant Cells. Mater. Sci. Eng. C 2019, 241, 92–99. [Google Scholar] [CrossRef]
- Surendran, P.; Lakshmanan, A.; Priya, S.S.; Balakrishnan, K.; Rameshkumar, P.; Kannan, K.; Geetha, P.; Hegde, T.A.; Vinitha, G. Bioinspired Fluorescence Carbon Quantum Dots Extracted from Natural Honey: Efficient Material for Photonic and Antibacterial Applications. Nano-Struct. Nano-Objects 2020, 24, 100589. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, G.; van der Mei, H.C.; Shi, L.; Busscher, H.J.; Ren, Y. Synergy between “Probiotic” Carbon Quantum Dots and Ciprofloxacin in Eradicating Infectious Biofilms and Their Biosafety in Mice. Pharmaceutics 2021, 13, 1809. [Google Scholar] [CrossRef]
- Plácido, J.; Bustamante-López, S.; Meissner, K.E.; Kelly, D.E.; Kelly, S.L. Microalgae Biochar-Derived Carbon Dots and Their Application in Heavy Metal Sensing in Aqueous Systems. Sci. Total Environ. 2019, 656, 531–539. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Ding, Y.; Hua, J.; Tang, B.; Ji, X.; Zhang, Q.; Wei, Y.; Qin, K.; Li, B. Bacteria-Derived Fluorescent Carbon Dots for Highly Selective Detection of p-Nitrophenol and Bioimaging. Analyst 2019, 144, 5497–5503. [Google Scholar] [CrossRef]
- Feng, W.; Ao, H.; Peng, C.; Yan, D. Gut Microbiota, A New Frontier to Understand Traditional Chinese Medicines. Pharmacol. Res. 2019, 142, 176–191. [Google Scholar] [CrossRef]
- Vasimalai, N.; Vilas-Boas, V.; Gallo, J.; Cerqueira, M.F.; Menéndez-Miranda, M.; Costa-Fernández, J.M.; Diéguez, L.; Espiña, B.; Fernández-Argüelles, M.T. Green Synthesis of Fluorescent Carbon Dots from Spices for In Vitro Imaging and Tumour Cell Growth Inhibition. Beilstein J. Nanotechnol. 2018, 9, 530–544. [Google Scholar] [CrossRef]
- Lin, C.-J.; Chang, L.; Chu, H.-W.; Lin, H.-J.; Chang, P.-C.; Wang, R.Y.L.; Unnikrishnan, B.; Mao, J.-Y.; Chen, S.-Y.; Huang, C.-C. High Amplification of the Antiviral Activity of Curcumin through Transformation into Carbon Quantum Dots. Small 2019, 15, 1902641. [Google Scholar] [CrossRef]
- Chen, H.-H.; Lin, C.-J.; Anand, A.; Lin, H.-J.; Lin, H.-Y.; Mao, J.-Y.; Wang, P.-H.; Tseng, Y.J.; Tzou, W.-S.; Huang, C.-C.; et al. Development of Antiviral Carbon Quantum Dots That Target the Japanese Encephalitis Virus Envelope Protein. J. Biol Chem. 2022, 298, 101957. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Q.; Qin, X.; Liu, Z.; Li, Z.; Zhong, X.; Xia, L.; He, J.; Fang, B. Carbon Dots Derived from Folic Acid Attenuates Osteoarthritis by Protecting Chondrocytes through NF-ΚB/MAPK Pathway and Reprogramming Macrophages. J. Nanobiotechnol. 2022, 20, 469. [Google Scholar] [CrossRef]
- Wu, X.; Tian, F.; Wang, W.; Chen, J.; Wu, M.; Zhao, J.-X. Fabrication of Highly Fluorescent Graphene Quantum Dots Using L-Glutamic Acid for in vitro/in vivo Imaging and Sensing. J. Mater. Chem. C 2013, 1, 4676–4684. [Google Scholar] [CrossRef]
- Lin, C.-J.; Unnikrishnan, B.; Lehman, C.W.; Wang, P.-H.; Tseng, Y.J.; Harroun, S.G.; Lin, S.-C.; Huang, C.-C. Exploring Molecular Moieties on Carbonized Polymer Dots from Flavonoid Glycosides with Activity against Enterovirus A71. Carbon 2022, 192, 285–294. [Google Scholar] [CrossRef]
- Jian, H.-J.; Wu, R.-S.; Lin, T.-Y.; Li, Y.-J.; Lin, H.-J.; Harroun, S.G.; Lai, J.-Y.; Huang, C.-C. Super-Cationic Carbon Quantum Dots Synthesized from Spermidine as an Eye Drop Formulation for Topical Treatment of Bacterial Keratitis. ACS Nano 2017, 11, 6703–6716. [Google Scholar] [CrossRef]
- Huang, H.-T.; Lin, H.-J.; Huang, H.-J.; Huang, C.-C.; Lin, J.H.-Y.; Chen, L.-L. Synthesis and Evaluation of Polyamine Carbon Quantum Dots (CQDs) in Litopenaeus Vannamei as A Therapeutic Agent against WSSV. Sci. Rep. 2020, 10, 7343. [Google Scholar] [CrossRef]
- Chou, D.-L.; Mao, J.-Y.; Anand, A.; Lin, H.-J.; Lin, J.H.-Y.; Tseng, C.-P.; Huang, C.-C.; Wang, H.-Y. Carbonized Lysine-Nanogels Protect against Infectious Bronchitis Virus. Int. J. Mol. Sci. 2021, 22, 5415. [Google Scholar] [CrossRef]
- Mao, J.-Y.; Miscevic, D.; Unnikrishnan, B.; Chu, H.-W.; Chou, C.P.; Chang, L.; Lin, H.-J.; Huang, C.-C. Carbon Nanogels Exert Multipronged Attack on Resistant Bacteria and Strongly Constrain Resistance Evolution. J. Colloid Interface Sci. 2022, 608, 1813–1826. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Yen, S.-C.; Kang, C.-H.; Chung, C.-Y.; Hsu, M.-C.; Wang, C.-Y.; Lin, J.H.-Y.; Huang, C.-C.; Lin, H.-J. How to Evaluate the Potential Toxicity of Therapeutic Carbon Nanomaterials? A Comprehensive Study of Carbonized Nanogels with Multiple Animal Toxicity Test Models. J. Hazard. Mater. 2022, 429, 128337. [Google Scholar] [CrossRef]
- Li, P.; Han, F.; Cao, W.; Zhang, G.; Li, J.; Zhou, J.; Gong, X.; Turnbull, G.; Shu, W.; Xia, L.; et al. Carbon Quantum Dots Derived from Lysine and Arginine Simultaneously Scavenge Bacteria and Promote Tissue Repair. Appl. Mater. Today 2020, 19, 100601. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Zeng, Y.-T.; Lin, C.-J.; Harroun, S.G.; Anand, A.; Chang, L.; Wu, C.-J.; Lin, H.-J.; Huang, C.-C. Partial Carbonization of Quercetin Boosts the Antiviral Activity against H1N1 Influenza A Virus. J. Colloid Interface Sci. 2022, 622, 481–493. [Google Scholar] [CrossRef]
- Wang, X.; Gao, T.; Yang, M.; Zhao, J.; Jiang, F.-L.; Liu, Y. Microwave-Assisted Synthesis, Characterization, Cell Imaging of Fluorescent Carbon Dots Using L-Asparagine as Precursor. New J. Chem. 2019, 43, 3323–3331. [Google Scholar] [CrossRef]
- Bajpai, S.K.; D’Souza, A.; Suhail, B. Blue Light-Emitting Carbon Dots (CDs) from A Milk Protein and Their Interaction with Spinacia oleracea Leaf Cells. Int. Nano Lett. 2019, 9, 203–212. [Google Scholar] [CrossRef]
- Xiao, D.; Yuan, D.; He, H.; Lu, J. Microwave-Assisted One-Step Green Synthesis of Amino-Functionalized Fluorescent Carbon Nitride Dots from Chitosan. Luminescence 2013, 28, 612–615. [Google Scholar] [CrossRef]
- Camlik, G.; Ozakca, I.; Bilakaya, B.; Ozcelikay, A.T.; Velaro, A.J.; Wasnik, S.; Degim, I.T. Development of Composite Carbon Quantum Dots-Insulin Formulation for Oral Administration. J. Drug Deliv. Sci. Technol. 2022, 76, 103833. [Google Scholar] [CrossRef]
- Ju, E.; Li, T.; Liu, Z.; da Silva, S.R.; Wei, S.; Zhang, X.; Wang, X.; Gao, S.-J. Specific Inhibition of Viral MicroRNAs by Carbon Dots-Mediated Delivery of Locked Nucleic Acids for Therapy of Virus-Induced Cancer. ACS Nano 2020, 14, 476–487. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Shen, G.; Zhang, C.; Li, C.; Ji, W.; Wang, C.; Cui, D. A Multifunctional Ribonuclease A-Conjugated Carbon Dot Cluster Nanosystem for Synchronous Cancer Imaging and Therapy. Nanoscale Res. Lett. 2014, 9, 397. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Shen, G.; Liu, H.; Fu, H.; Cui, D. Fluorescent Carbon Dots as An Efficient siRNA Nanocarrier for Its Interference Therapy in Gastric Cancer Cells. J. Nanobiotechnol. 2014, 12, 58. [Google Scholar] [CrossRef]
- Kung, J.-C.; Tseng, I.-T.; Chien, C.-S.; Lin, S.-H.; Wang, C.-C.; Shih, C.-J. Microwave Assisted Synthesis of Negative-Charge Carbon Dots with Potential Antibacterial Activity against Multi-Drug Resistant Bacteria. RSC Adv. 2020, 10, 41202–41208. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Gu, S.; Gandomi, Y.A.; Fu, C.-C.; Sung, P.-Y.; Juang, R.-S.; Chen, C.-C. Employing Functionalized Graphene Quantum Dots to Combat Coronavirus and Enterovirus. J. Colloid Interface Sci. 2023, 630, 1–10. [Google Scholar] [CrossRef]
- Zeng, Q.; Shao, D.; He, X.; Ren, Z.; Ji, W.; Shan, C.; Qu, S.; Li, J.; Chenb, L.; Li, Q. Carbon Dots as A Trackable Drug Delivery Carrier for Localized Cancer Therapy in vivo. J. Mater. Chem. B 2016, 4, 5119–5126. [Google Scholar] [CrossRef]
- Cao, X.; Wang, J.; Deng, W.; Chen, J.; Wang, Y.; Zhou, J.; Du, P.; Xu, W.; Wang, Q.; Wang, Q.; et al. Photoluminescent Cationic Carbon Dots as Efficient Non-Viral Delivery of Plasmid SOX9 and Chondrogenesis of Fibroblasts. Sci. Rep. 2018, 8, 7057. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, K.; Zheng, X.; Luo, Q.; Zhang, Q.; Ji, X.; Wei, Y. Synthesis of Carbon Dots with Antiphage Activity Using Caffeic Acid. Anal. Methods 2021, 13, 5165–5172. [Google Scholar] [CrossRef]
- Emam, H.E.; Ahmed, H.B. Antitumor/Antiviral Carbon Quantum Dots Based on Carrageenan and Pullulan. Int. J. Biol. Macromol. 2021, 170, 688–700. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.; Jain, A.; Zhou, H.-S. Aqueous-Phase Synthesis of Highly Luminescent, Nitrogen-Doped Carbon Dots and Their Application as Bioimaging Agents. Langmuir 2014, 30, 14270–14275. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, H.; Mei, L.; Dou, K.; Jiang, Y.; Sun, Z.; Wang, S.; Hasanin, M.S.; Deng, J.; Zhou, Q. Fucoidan-Derived Carbon Dots against Enterococcus Faecalis Biofilm and Infected Dentinal Tubules for the Treatment of Persistent Endodontic Infections. J. Nanobiotechnol. 2022, 20, 321. [Google Scholar] [CrossRef]
- Cailotto, S.; Amadio, E.; Facchin, M.; Selva, M.; Pontoglio, E.; Rizzolio, F.; Riello, P.; Toffoli, G.; Benedetti, A.; Perosa, A. Carbon Dots from Sugars and Ascorbic Acid: Role of the Precursors on Morphology, Properties, Toxicity, and Drug Uptake. ACS Med. Chem. Lett. 2018, 9, 832–837. [Google Scholar] [CrossRef]
- Tong, T.; Hu, H.; Zhou, J.; Deng, S.; Zhang, X.; Tang, W.; Fang, L.; Xiao, S.; Liang, J. Glycyrrhizic-Acid-Based Carbon Dots with High Antiviral Activity by Multisite Inhibition Mechanisms. Small 2020, 16, 1906206. [Google Scholar] [CrossRef]
- Mogharbel, A.T.; Abu-Melha, S.; Hameed, A.; Attar, R.M.S.; Alrefaei, A.F.; Almahri, A.; El-Metwaly, N. Anticancer and Microbicide Action of Carbon Quantum Dots Derived from Microcrystalline Cellulose: Hydrothermal versus Infrared Assisted Techniques. Arab. J. Chem. 2023, 16, 104419. [Google Scholar] [CrossRef]
- Malik, M.; Chand, P.; Prasad, T. L-Ascorbic Acid Carbon Dots: As Ros Scavenger and Biocompatible Nano-Probe for High Contrast Fluorescence Imaging. SPAST Abs. 2021, 1, 1. [Google Scholar]
- Wei, S.-C.; Amit, N.; Lin, Y.-F.; Wu, R.-S.; Srivastava, P.; Chang, L.; Huang, Y.-F.; Chang, H.-T.; Chuang, K.-T.; Huang, C.-C. Light Triggered Programmable States of Carbon Dot Liposomes Accelerate Chronic Wound Healing via Photocatalytic Cascade Reaction. Carbon 2023, 201, 952–961. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Lin, Y.-S.; Huang, T.-Y.; Wei, S.-C.; Wu, R.-S.; Huang, C.-C.; Huang, Y.-F.; Chang, H.-T. Photoswitchable Carbon-Dot Liposomes Mediate Catalytic Cascade Reactions for Amplified Dynamic Treatment of Tumor Cells. J. Colloid Interface Sci. 2022, 628, 717–725. [Google Scholar] [CrossRef]
- Yu, J.; Jeon, Y.-R.; Kim, Y.-H.; Jung, E.-B.; Choi, S.-J. Characterization and Determination of Nanoparticles in Commercial Processed Foods. Foods 2021, 10, 2020. [Google Scholar] [CrossRef]
- Li, Y.-J.; Harroun, S.G.; Su, Y.-C.; Huang, C.-F.; Unnikrishnan, B.; Lin, H.-J.; Lin, C.-H.; Huang, C.-C. Synthesis of Self-Assembled Spermidine-Carbon Quantum Dots Effective against Multidrug-Resistant Bacteria. Adv. Healthc. Mater. 2016, 5, 2545–2554. [Google Scholar] [CrossRef]
- Feng, T.; Ai, X.; An, G.; Yang, P.; Zhao, Y. Charge-Convertible Carbon Dots for Imaging-Guided Drug Delivery with Enhanced in Vivo Cancer Therapeutic Efficiency. ACS Nano. 2016, 10, 4410–4420. [Google Scholar] [CrossRef]
- Jian, H.-J.; Yu, J.; Li, Y.-J.; Unnikrishnan, B.; Huang, Y.-F.; Luo, L.-J.; Hui-Kang Ma, D.; Harroun, S.G.; Chang, H.-T.; Lin, H.-J.; et al. Highly Adhesive Carbon Quantum Dots from Biogenic Amines for Prevention of Biofilm Formation. Chem. Eng. J. 2020, 386, 123913. [Google Scholar] [CrossRef]
- Fahmi, M.Z.; Sukmayani, W.; Khairunisa, S.Q.; Witaningrum, A.M.; Indriati, D.W.; Matondang, M.Q.Y.; Chang, J.-Y.; Kotaki, T.; Kameoka, M. Design of Boronic Acid-Attributed Carbon Dots on Inhibits HIV-1 Entry. RSC Adv. 2016, 6, 92996–93002. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Luo, K.-L.; Mao, J.-Y.; Lin, C.-J.; Wang, C.-Y.; Panny, L.; Chen, S.-Y.; Lin, S.-C.; Huang, C.-C.; Harroun, S.G.; et al. In Situ Derived Sulfated/Sulfonated Carbon Nanogels with Multi-Protective Effects against Influenza A Virus. Chem. Eng. J. 2023, 458, 141429. [Google Scholar] [CrossRef]
- Łoczechin, A.; Séron, K.; Barras, A.; Giovanelli, E.; Belouzard, S.; Chen, Y.-T.; Metzler-Nolte, N.; Boukherroub, R.; Dubuisson, J.; Szunerits, S. Functional Carbon Quantum Dots as Medical Countermeasures to Human Coronavirus. ACS Appl. Mater. Interfaces 2019, 11, 42964–42974. [Google Scholar] [CrossRef]
- Hou, P.; Yang, T.; Liu, H.; Li, Y.F.; Huang, C.Z. An Active Structure Preservation Method for Developing Functional Graphitic Carbon Dots as An Effective Antibacterial Agent and A Sensitive PH and Al (III) Nanosensor. Nanoscale 2017, 9, 17334–17341. [Google Scholar] [CrossRef]
- Pandit, S.; Behera, P.; Sahoo, J.; De, M. In Situ Synthesis of Amino Acid Functionalized Carbon Dots with Tunable Properties and Their Biological Applications. ACS Appl. Bio Mater. 2019, 2, 3393–3403. [Google Scholar] [CrossRef]
- Ting, D.; Dong, N.; Fang, L.; Lu, J.; Bi, J.; Xiao, S.; Han, H. Multisite Inhibitors for Enteric Coronavirus: Antiviral Cationic Carbon Dots Based on Curcumin. ACS Appl. Nano Mater. 2018, 1, 5451–5459. [Google Scholar] [CrossRef]
- Quan, K.-C.; Hou, J.-P.; Zhang, Z.-X.; Ren, Y.-J.; Peterson, B.W.; Flemming, H.C.; Mayer, C.; Busscher, H.J.; van der Mei, H.C. Water in Bacterial Biofilms: Pores and Channels, Storage and Transport Functions. Crit. Rev. Microbiol. 2022, 48, 283–302. [Google Scholar] [CrossRef]
- Lu, F.; Ma, Y.; Wang, H.; Zhang, M.; Wang, B.; Zhang, Y.; Huang, H.; Liao, F.; Liu, Y.; Kang, Z. Water-Solvable Carbon Dots Derived from Curcumin and Citric Acid with Enhanced Broad-Spectrum Antibacterial and Antibiofilm Activity. Mater. Today Commun. 2021, 26, 102000. [Google Scholar] [CrossRef]
- Jijie, R.; Barras, A.; Bouckaert, J.; Dumitrascu, N.; Szunerits, S.; Boukherroub, R. Enhanced Antibacterial Activity of Carbon Dots Functionalized with Ampicillin Combined with Visible Light Triggered Photodynamic Effects. Colloids Surf. B Biointerfaces 2018, 170, 347–354. [Google Scholar] [CrossRef]
- Du, T.; Liang, J.; Dong, N.; Liu, L.; Fang, L.; Xiao, S.; Han, H. Carbon Dots as Inhibitors of Virus by Activation of Type I Interferon Response. Carbon 2016, 110, 278–285. [Google Scholar] [CrossRef]
- Kang, Y.-F.; Li, Y.-H.; Fang, Y.-W.; Xu, Y.; Wei, X.-M.; Yin, X.-B. Carbon Quantum Dots for Zebrafish Fluorescence Imaging. Sci. Rep. 2015, 5, 11835. [Google Scholar] [CrossRef]
- Sun, T.-T.; Zheng, M.; Xie, Z.-G.; Jing, X.-B. Supramolecular Hybrids of Carbon Dots with Doxorubicin: Synthesis, Stability and Cellular Trafficking. Mater. Chem. Front. 2017, 1, 354–360. [Google Scholar] [CrossRef]
- Zheng, M.; Ruan, S.; Liu, S.; Sun, T.; Qu, D.; Zhao, H.; Xie, Z.; Gao, H.; Jing, X.; Sun, Z. Self-Targeting Fluorescent Carbon Dots for Diagnosis of Brain Cancer Cells. ACS Nano 2015, 9, 11455–11461. [Google Scholar] [CrossRef]
- Zhuo, Y.; Miao, H.; Zhong, D.; Zhu, S.-S.; Yang, X.-M. One-Step Synthesis of High Quantum-Yield and Excitation-Independent Emission Carbon Dots for Cell Imaging. Mater. Lett. 2015, 139, 197–200. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Song, Y.; Zhang, M.; Wang, H.; Lu, F.; Huang, H.; Liu, Y.; Dai, X.; Gu, Z.; et al. Degradable Carbon Dots with Broad-Spectrum Antibacterial Activity. ACS Appl. Mater. Interfaces 2018, 10, 26936–26946. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Primaver, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular Uptake and Retention of Nanoparticles: Insights on Particle Properties and Interaction with Cellular Components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of Nanoparticle Cellular Uptake, Intracellular Trafficking, and Kinetics in Nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Wang, C.; Wu, C.; Zhou, X.; Han, T.; Xin, X.; Wu, J.; Zhang, J.; Guo, S. Enhancing Cell Nucleus Accumulation and DNA Cleavage Activity of Anti-Cancer Drug via Graphene Quantum Dots. Sci. Rep. 2013, 3, 2852. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Chen, Y.-J.; Kang, C.-H.; Lin, H.-Y.; Huang, C.-C.; Hsu, P.-H.; Lin, H.-J. Toxic or Not Toxic, That Is the Carbon Quantum Dot’s Question: A Comprehensive Evaluation with Zebrafish Embryo, Eleutheroembryo, and Adult Models. Polymers 2021, 13, 1598. [Google Scholar] [CrossRef]
- Geng, B.; Hu, J.; Li, Y.; Feng, S.; Pan, D.; Feng, L.; Shen, L. Near-Infrared Phosphorescent Carbon Dots for Sonodynamic Precision Tumor Therapy. Nat. Commun. 2022, 13, 5735. [Google Scholar] [CrossRef]
- Stan, D.; Enciu, A.-M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural Compounds with Antimicrobial and Antiviral Effect and Nanocarriers Used for Their Transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus Biofilm: An Emerging Battleground in Microbial Communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Li, P.; Yang, X.; Zhang, X.; Pan, J.; Tang, W.; Cao, W.; Zhou, J.; Gong, X.; Xing, X. Surface Chemistry-Dependent Antibacterial and Antibiofilm Activities of Polyamine-Functionalized Carbon Quantum Dots. J. Mater. Sci. 2020, 55, 16744–16757. [Google Scholar] [CrossRef]
- Ghorai, S.; Banik, S.P.; Verma, D.; Chowdhury, S.; Mukherjee, S.; Khowala, S. Fungal Biotechnology in Food and Feed Processing. Food Res. Int. 2009, 42, 577–587. [Google Scholar] [CrossRef]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. Mbio 2020, 11, e00449-20. [Google Scholar] [CrossRef]
- Kanafani, Z.A.; Perfect, J.R. Resistance to Antifungal Agents: Mechanisms and Clinical Impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Zhong, X.-J.; Zhao, Y.-S.; Rajoka, M.S.R.; Hashmi, M.H.; Zhai, P.; Song, X. Antifungal Natural Products and Their Derivatives: A Review of Their Activity and Mechanism of Actions. Pharmacol. Res. Mod. Chin. 2023, 7, 100262. [Google Scholar] [CrossRef]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant Derived Substances with Anti-Cancer Activity: From Folklore to Practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef]
- Rouse, B.T.; Sehrawat, S. Immunity and Immunopathology to Viruses: What Decides the Outcome? Nat. Rev. Immunol. 2010, 10, 514–526. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv. Healthc. Mater. 2020, 9, e1901223. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.-S.; Xiong, L.; Wang, W.-J.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.-Q.; et al. Small Molecules in Targeted Cancer Therapy: Advances, Challenges, and Future Perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Xu, Y.-L.; Wen, Z.-S.; Xu, Z.-R. Chitosan Nanoparticles Inhibit the Growth of Human Hepatocellular Carcinoma Xenografts through An Antiangiogenic Mechanism. Anticancer Res. 2009, 29, 5103–5109. [Google Scholar]
- Chen, X.; Li, J.-B.; Kang, R.; Klionsky, D.J.; Tang, D.-L. Ferroptosis: Machinery and Regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Faria, M.; Björnmalm, M.; Thurecht, K.J.; Kent, S.J.; Parton, R.G.; Kavallaris, M.; Johnston, A.P.R.; Gooding, J.; Corrie, S.R.; Boyd, B.J.; et al. Minimum Information Reporting in Bio–Nano Experimental Literature. Nat. Nanotechnol. 2018, 13, 777–785. [Google Scholar] [CrossRef]
- Fan, J.; Claudel, M.; Ronzani, C.; Arezki, Y.; Lebeau, L.; Pons, F. Physicochemical Characteristics That Affect Carbon Dot Safety: Lessons from A Comprehensive Study on A Nanoparticle Library. Int. J. Pharm. 2019, 569, 118521. [Google Scholar] [CrossRef]
- Truskewycz, A.; Yin, H.; Halberg, N.; Lai, D.T.H.; Ball, A.S.; Truong, V.K.; Rybicka, A.M.; Cole, I. Carbon Dot Therapeutic Platforms: Administration, Distribution, Metabolism, Excretion, Toxicity, and Therapeutic Potential. Small 2022, 18, 2106342. [Google Scholar] [CrossRef]
- Liang, X.; Wang, H.; Zhu, Y.; Zhang, R.; Cogger, V.C.; Liu, X.; Xu, Z.-P.; Grice, J.E.; Roberts, M.S. Short- and Long-Term Tracking of Anionic Ultrasmall Nanoparticles in Kidney. ACS Nano 2016, 10, 387–395. [Google Scholar] [CrossRef]
- Tsoi, K.M.; MacParland, S.A.; Ma, X.-Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of Hard-Nanomaterial Clearance by the Liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef]
- Girardi, F.A.; Bruch, G.E.; Peixoto, C.S.; Dal Bosco, L.; Sahoo, S.K.; Gonçalves, C.O.F.; Santos, A.P.; Furtado, C.A.; Fantini, C.; Barros, D.M. Toxicity of Single-Wall Carbon Nanotubes Functionalized with Polyethylene Glycol in Zebrafish (Danio rerio) Embryos. J. Appl. Toxicol. 2017, 37, 214–221. [Google Scholar] [CrossRef]
- Andón, F.T.; Kapralov, A.A.; Yanamala, N.; Feng, W.-H.; Baygan, A.; Chambers, B.J.; Hultenby, K.; Ye, F.; Toprak, M.S.; Brandner, B.D.; et al. Biodegradation of Single-Walled Carbon Nanotubes by Eosinophil Peroxidase. Small 2013, 9, 2721–2729. [Google Scholar] [CrossRef]
- Kagan, V.E.; Konduru, N.V.; Feng, W.-H.; Allen, B.L.; Conroy, J.; Volkov, Y.; Vlasova, I.I.; Belikova, N.A.; Yanamala, N.; Kapralov, A.; et al. Carbon Nanotubes Degraded by Neutrophil Myeloperoxidase Induce Less Pulmonary Inflammation. Nat. Nanotechnol. 2010, 5, 354–359. [Google Scholar] [CrossRef]
- Kurapati, R.; Russier, J.; Squillaci, M.A.; Treossi, E.; Ménard-Moyon, C.; Rio-Castillo, A.E.D.; Vazquez, E.; Samorì, P.; Palermo, V.; Bianco, A. Dispersibility-Dependent Biodegradation of Graphene Oxide by Myeloperoxidase. Small 2015, 11, 3985–3994. [Google Scholar] [CrossRef]
- Martín, C.; Jun, G.; Schurhammer, R.; Reina, G.; Chen, P.; Bianco, A.; Ménard-Moyon, C. Enzymatic Degradation of Graphene Quantum Dots by Human Peroxidases. Small 2019, 15, e1905405. [Google Scholar] [CrossRef]
- Srivastava, I.; Sar, D.; Mukherjee, P.; Schwartz-Duval, A.S.; Huang, Z.-L.; Jaramillo, C.; Civantos, A.; Tripathi, I.; Allain, J.P.; Bhargava, R.; et al. Enzyme-Catalysed Biodegradation of Carbon Dots Follows Sequential Oxidation in A Time Dependent Manner. Nanoscale 2019, 11, 8226–8236. [Google Scholar] [CrossRef]
- Martín, C.; Kostarelos, K.; Prato, M.; Bianco, A. Biocompatibility and Biodegradability of 2D Materials: Graphene and Beyond. Chem. Commun. 2019, 55, 5540–5546. [Google Scholar] [CrossRef]
- Mendonça, M.C.P.; Rizoli, C.; Ávila, D.S.; Amorim, M.J.B.; de Jesus, M.B. Nanomaterials in the Environment: Perspectives on in Vivo Terrestrial Toxicity Testing. Front. Environ. Sci. 2017, 5, 71. [Google Scholar] [CrossRef]
- Ema, M.; Kobayashi, N.; Naya, M.; Hanai, S.; Nakanishi, J. Reproductive and Developmental Toxicity Studies of Manufactured Nanomaterials. Reprod. Toxicol. 2010, 30, 343–352. [Google Scholar] [CrossRef]
- Ema, M.; Okuda, H.; Gamo, M.; Honda, K. A Review of Reproductive and Developmental Toxicity of Silver Nanoparticles in Laboratory Animals. Reprod. Toxicol. 2017, 67, 149–164. [Google Scholar] [CrossRef]
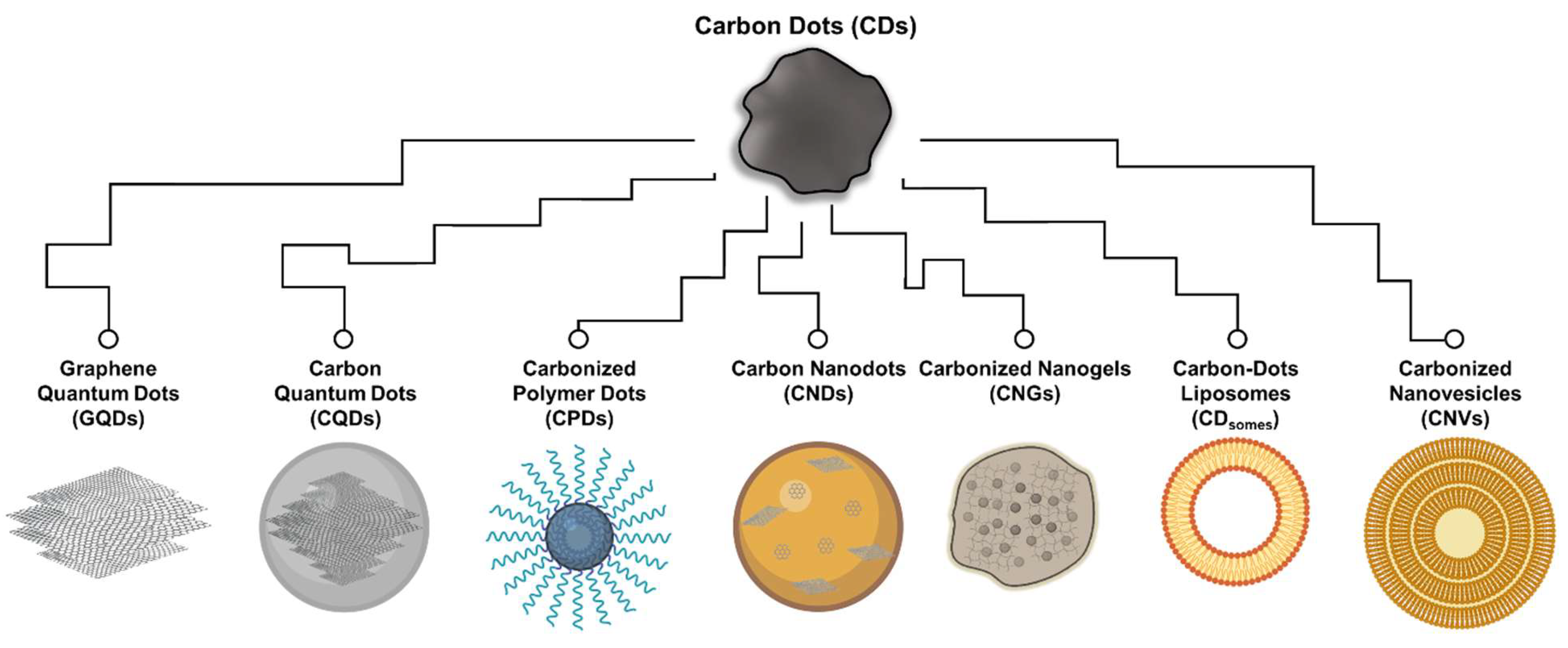






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-Y.; Ndraha, N.; Wu, R.-S.; Liu, H.-Y.; Lin, S.-W.; Yang, K.-M.; Lin, H.-Y. An Overview of the Potential of Food-Based Carbon Dots for Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 16579. https://doi.org/10.3390/ijms242316579
Wang C-Y, Ndraha N, Wu R-S, Liu H-Y, Lin S-W, Yang K-M, Lin H-Y. An Overview of the Potential of Food-Based Carbon Dots for Biomedical Applications. International Journal of Molecular Sciences. 2023; 24(23):16579. https://doi.org/10.3390/ijms242316579
Chicago/Turabian StyleWang, Chen-Yow, Nodali Ndraha, Ren-Siang Wu, Hsin-Yun Liu, Sin-Wei Lin, Kuang-Min Yang, and Hung-Yun Lin. 2023. "An Overview of the Potential of Food-Based Carbon Dots for Biomedical Applications" International Journal of Molecular Sciences 24, no. 23: 16579. https://doi.org/10.3390/ijms242316579
APA StyleWang, C. -Y., Ndraha, N., Wu, R. -S., Liu, H. -Y., Lin, S. -W., Yang, K. -M., & Lin, H. -Y. (2023). An Overview of the Potential of Food-Based Carbon Dots for Biomedical Applications. International Journal of Molecular Sciences, 24(23), 16579. https://doi.org/10.3390/ijms242316579







