TRAIL-Sensitizing Effects of Flavonoids in Cancer
Abstract
1. Introduction
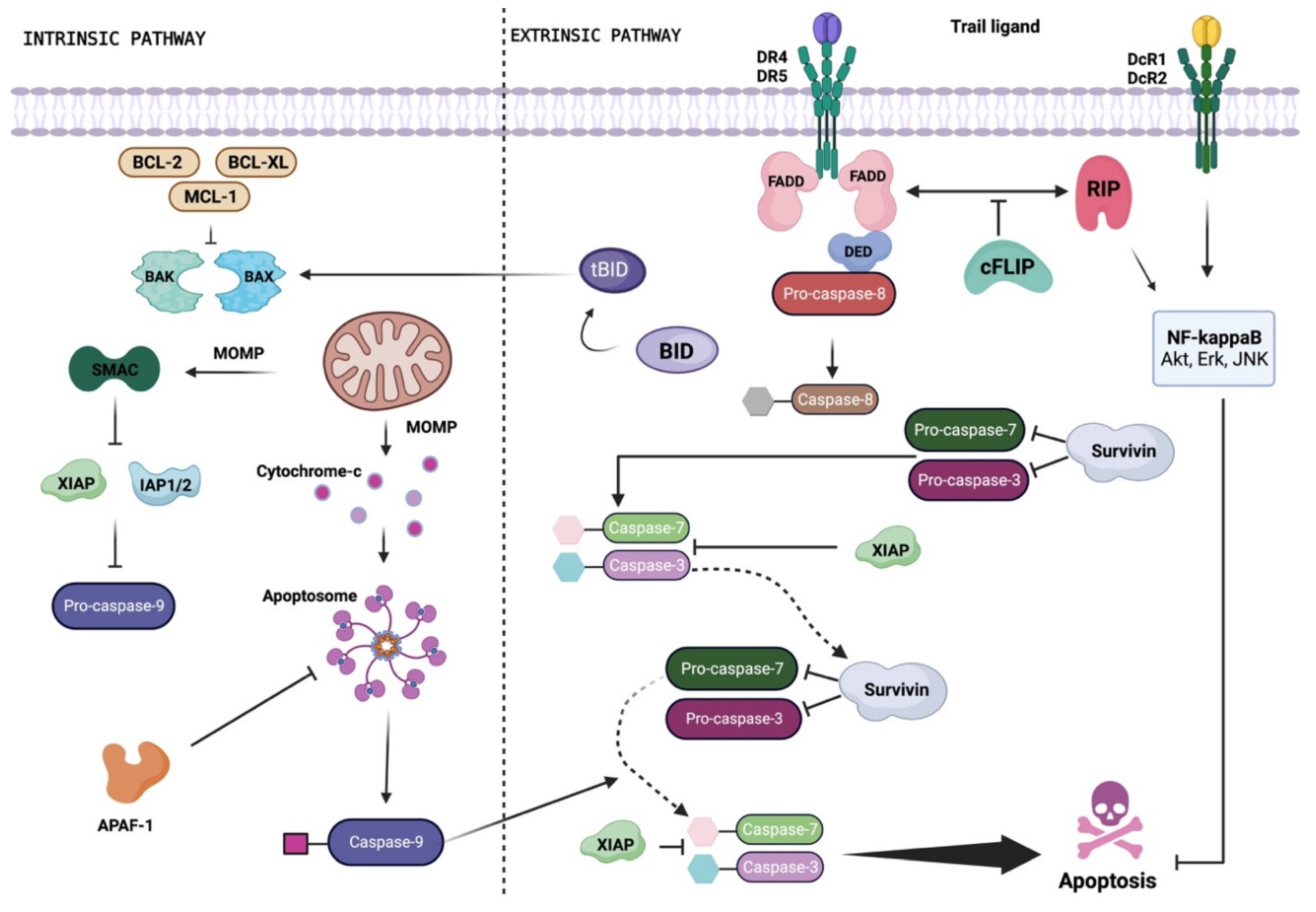
2. Apoptosis Signaling Pathways
TRAIL as Potential Candidate for Cancer Treatment: Benefits and Drawbacks
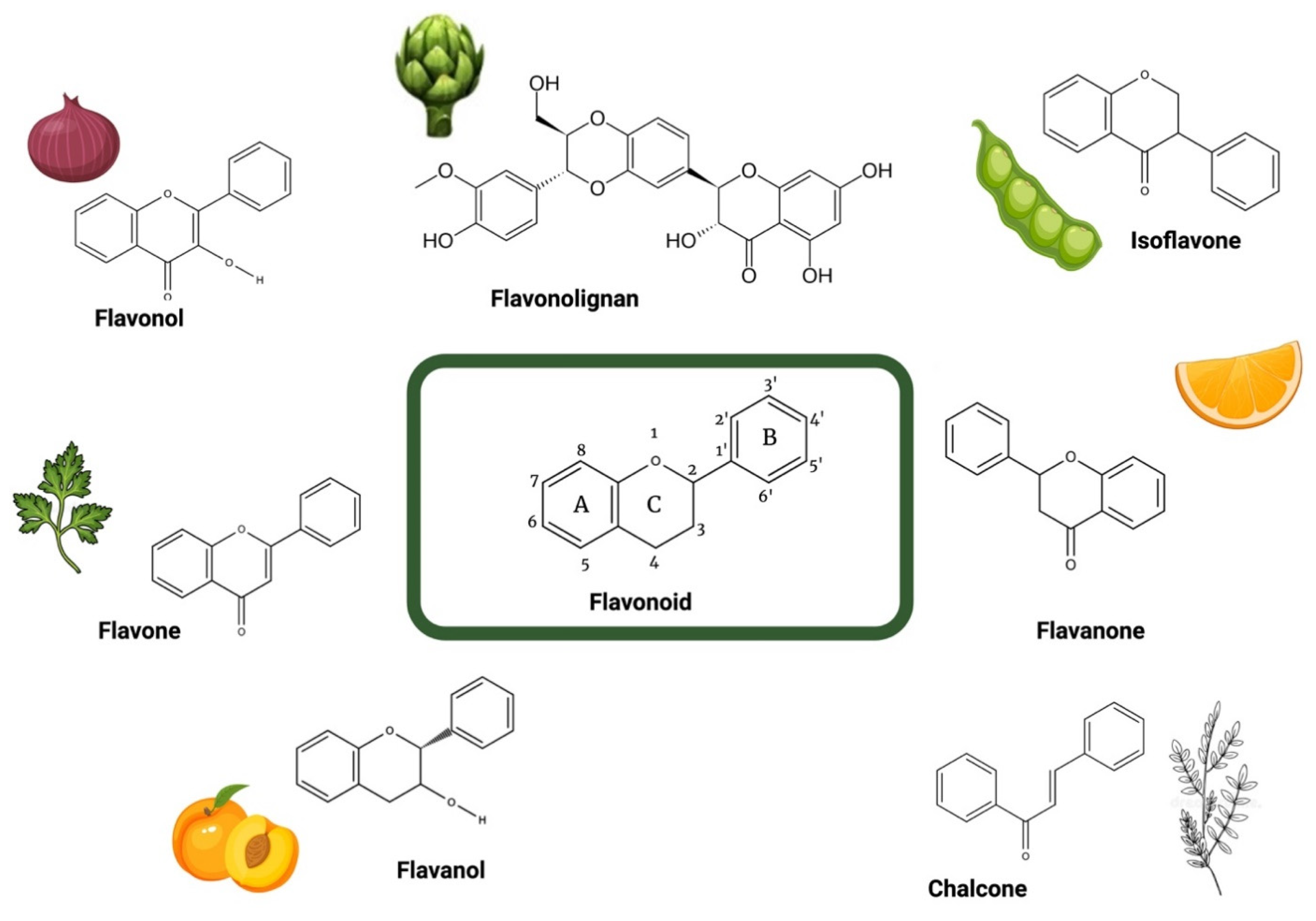
3. Medicinal Plants and Flavonoids
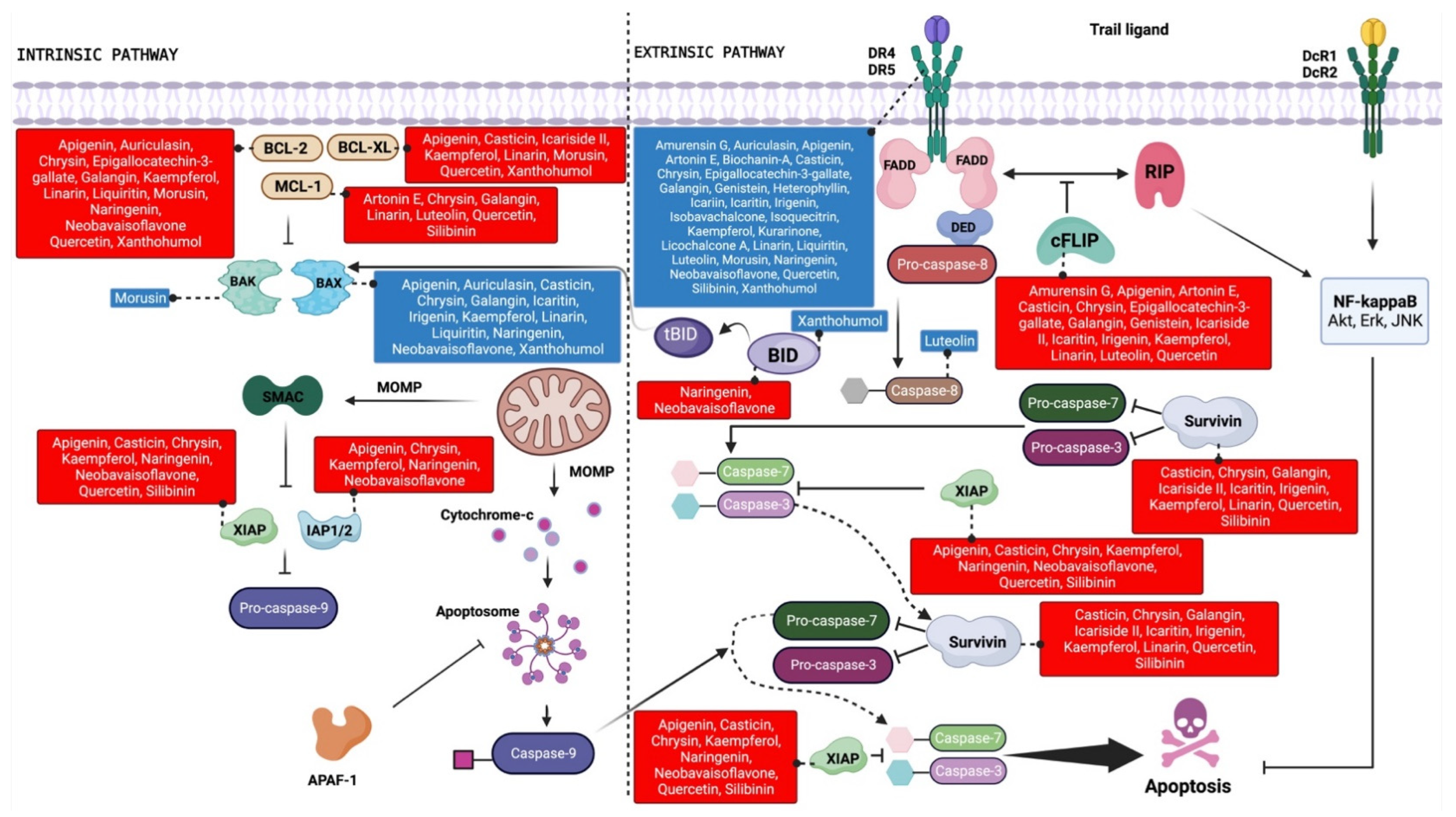
4. TRAIL-Sensitizing Effects of Flavonoids in Cancer
4.1. Flavones
4.1.1. Apigenin
4.1.2. Luteolin
4.1.3. Chrysin
4.1.4. Other Flavones
4.2. Flavonols
4.2.1. Quercetin
4.2.2. Kaempferol
4.2.3. Other Flavonols
4.3. Isoflavones
4.4. Other Flavonoids
4.5. Prenylflavonoids
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.; Denlinger, N.; Yang, Y. Recent Advances and Challenges in Cancer Immunotherapy. Cancers 2022, 14, 3972. [Google Scholar] [CrossRef] [PubMed]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Green, D.R. The Death Receptor Pathway of Apoptosis. Cold Spring Harb. Perspect. Biol. 2022, 14, a041053. [Google Scholar] [CrossRef]
- Green, D.R. Caspase Activation and Inhibition. Cold Spring Harb. Perspect. Biol. 2022, 14, a041020. [Google Scholar] [CrossRef]
- LeBlanc, H.N.; Ashkenazi, A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003, 10, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, V.; Yamniuk, A.P.; Lawrence, E.J.; Yong, W.; Schneeweis, L.A.; Cheng, L.; Murdock, M.; Corbett, M.J.; Doyle, M.L.; Sheriff, S. The structure of the death receptor 4-TNF-related apoptosis-inducing ligand (DR4-TRAIL) complex. Acta Crystallogr. F Struct. Biol. Commun. 2015, 71 Pt 10, 1273–1281. [Google Scholar] [CrossRef]
- Truneh, A.; Sharma, S.; Silverman, C.; Khandekar, S.; Reddy, M.P.; Deen, K.C.; McLaughlin, M.M.; Srinivasula, S.M.; Livi, G.P.; Marshall, L.A.; et al. Temperature-sensitive differential affinity of TRAIL for its receptors. DR5 is the highest affinity receptor. J. Biol. Chem. 2000, 275, 23319–23325. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.S.; Kim, M.S.; Choi, Y.H.; Sung, B.J.; Shin, N.K.; Shin, H.C.; Sung, Y.C.; Oh, B.H. 2.8 A resolution crystal structure of human TRAIL, a cytokine with selective antitumor activity. Immunity 1999, 11, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Lalaoui, N.; Morizot, A.; Schneider, P.; Solary, E.; Micheau, O. Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol. Cell Biol. 2006, 26, 7046–7055. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Huang, Z.; Chen, X.; Zhang, B. The roles of osteoprotegerin in cancer, far beyond a bone player. Cell Death Discov. 2022, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O.; Shirley, S.; Dufour, F. Death receptors as targets in cancer. Br. J. Pharmacol. 2013, 169, 1723–1744. [Google Scholar] [CrossRef]
- Willms, A.; Schittek, H.; Rahn, S.; Sosna, J.; Mert, U.; Adam, D.; Trauzold, A. Impact of p53 status on TRAIL-mediated apoptotic and non-apoptotic signaling in cancer cells. PLoS ONE 2019, 14, e0214847. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005, 12, 228–237. [Google Scholar] [CrossRef]
- Deng, D.; Shah, K. TRAIL of Hope Meeting Resistance in Cancer. Trends Cancer 2020, 6, 989–1001. [Google Scholar] [CrossRef]
- de Miguel, D.; Lemke, J.; Anel, A.; Walczak, H.; Martinez-Lostao, L. Onto better TRAILs for cancer treatment. Cell Death Differ. 2016, 23, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Araruna, M.E.; Serafim, C.; Alves Junior, E.; Hiruma-Lima, C.; Diniz, M.; Batista, L. Intestinal Anti-Inflammatory Activity of Terpenes in Experimental Models (2010–2020): A Review. Molecules 2020, 25, 5430. [Google Scholar] [CrossRef] [PubMed]
- Getachew, S.; Medhin, G.; Asres, A.; Abebe, G.; Ameni, G. Traditional medicinal plants used in the treatment of tuberculosis in Ethiopia: A systematic review. Heliyon 2022, 8, e09478. [Google Scholar] [CrossRef] [PubMed]
- Li, F.S.; Weng, J.K. Demystifying traditional herbal medicine with modern approach. Nat. Plants 2017, 3, 17109. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Gaobotse, G.; Venkataraman, S.; Brown, P.D.; Masisi, K.; Kwape, T.E.; Nkwe, D.O.; Rantong, G.; Makhzoum, A. The use of African medicinal plants in cancer management. Front. Pharmacol. 2023, 14, 1122388. [Google Scholar] [CrossRef]
- Xiang, Y.; Guo, Z.; Zhu, P.; Chen, J.; Huang, Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med. 2019, 8, 1958–1975. [Google Scholar] [CrossRef]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef] [PubMed]
- Bojic, M.; Males, Z.; Antolic, A.; Babic, I.; Tomicic, M. Antithrombotic activity of flavonoids and polyphenols rich plant species. Acta Pharm. 2019, 69, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Lei, L.; Zhou, Y.; Ye, F.; Zhao, G. Dietary Flavonoids and the Risk of Colorectal Cancer: An Updated Meta-Analysis of Epidemiological Studies. Nutrients 2018, 10, 950. [Google Scholar] [CrossRef] [PubMed]
- Fardoun, M.M.; Maaliki, D.; Halabi, N.; Iratni, R.; Bitto, A.; Baydoun, E.; Eid, A.H. Flavonoids in adipose tissue inflammation and atherosclerosis: One arrow, two targets. Clin. Sci. 2020, 134, 1403–1432. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Watanabe, T.; Sueoka, E.; Rawangkan, A.; Suganuma, M. Cancer Prevention with Green Tea and Its Principal Constituent, EGCG: From Early Investigations to Current Focus on Human Cancer Stem Cells. Mol. Cells 2018, 41, 73–82. [Google Scholar] [PubMed]
- Maaliki, D.; Shaito, A.A.; Pintus, G.; El-Yazbi, A.; Eid, A.H. Flavonoids in hypertension: A brief review of the underlying mechanisms. Curr. Opin. Pharmacol. 2019, 45, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef]
- Rossi, M.; Bosetti, C.; Negri, E.; Lagiou, P.; La Vecchia, C. Flavonoids, proanthocyanidins, and cancer risk: A network of case-control studies from Italy. Nutr. Cancer 2010, 62, 871–877. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Zha, D.; Cai, F.; Zhang, W.; He, Y.; Huang, Q.; Zhuang, H.; Hua, Z.C. Apigenin potentiates TRAIL therapy of non-small cell lung cancer via upregulating DR4/DR5 expression in a p53-dependent manner. Sci. Rep. 2016, 6, 35468. [Google Scholar] [CrossRef]
- Voss, O.H.; Arango, D.; Tossey, J.C.; Villalona Calero, M.A.; Doseff, A.I. Splicing reprogramming of TRAIL/DISC-components sensitizes lung cancer cells to TRAIL-mediated apoptosis. Cell Death Dis. 2021, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Oishi, M.; Iizumi, Y.; Taniguchi, T.; Goi, W.; Miki, T.; Sakai, T. Apigenin sensitizes prostate cancer cells to Apo2L/TRAIL by targeting adenine nucleotide translocase-2. PLoS ONE 2013, 8, e55922. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Yu, J.S.; Yang, M.; Kim, A.K. Sub-toxic dose of apigenin sensitizes HepG2 cells to TRAIL through ERK-dependent up-regulation of TRAIL receptor DR5. Mol. Cells 2013, 35, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.H.; Molagoda, I.M.N.; Choi, Y.H.; Park, C.; Moon, D.O.; Kim, G.Y. Apigenin promotes TRAIL-mediated apoptosis regardless of ROS generation. Food Chem. Toxicol. 2018, 111, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, Q.; Zheng, X.; Sun, H.; Zhou, Y.; Li, D.; Lin, Y.; Wang, X. Luteolin enhances TNF-related apoptosis-inducing ligand’s anticancer activity in a lung cancer xenograft mouse model. Biochem. Biophys. Res. Commun. 2012, 417, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.C.; Li, J.R.; Kuan, Y.H.; Raung, S.L.; Wang, C.C.; Hung, Y.Y.; Pan, P.H.; Lu, H.C.; Chen, C.J. Luteolin sensitizes human 786-O renal cell carcinoma cells to TRAIL-induced apoptosis. Life Sci. 2014, 100, 110–117. [Google Scholar] [CrossRef]
- Nazim, U.M.; Park, S.Y. Luteolin sensitizes human liver cancer cells to TRAIL-induced apoptosis via autophagy and JNK-mediated death receptor 5 upregulation. Int. J. Oncol. 2019, 54, 665–672. [Google Scholar] [CrossRef]
- Wu, B.; Xiong, J.; Zhou, Y.; Wu, Y.; Song, Y.; Wang, N.; Chen, L.; Zhang, J. Luteolin enhances TRAIL sensitivity in non-small cell lung cancer cells through increasing DR5 expression and Drp1-mediated mitochondrial fission. Arch. Biochem. Biophys. 2020, 692, 108539. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, T.; Cai, X.; Yang, J.; Lu, W.; Hu, C.; Wang, Z.; Wang, X.; Cao, P. 5,7-Dihydroxyflavone Enhances the Apoptosis-Inducing Potential of TRAIL in Human Tumor Cells via Regulation of Apoptosis-Related Proteins. Evid. Based Complement. Alternat Med. 2013, 2013, 434709. [Google Scholar] [CrossRef]
- Yang, J.F.; Cao, J.G.; Tian, L.; Liu, F. 5,7-Dimethoxyflavone sensitizes TRAIL-induced apoptosis through DR5 upregulation in hepatocellular carcinoma cells. Cancer Chemother. Pharmacol. 2012, 69, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Lirdprapamongkol, K.; Sakurai, H.; Abdelhamed, S.; Yokoyama, S.; Athikomkulchai, S.; Viriyaroj, A.; Awale, S.; Ruchirawat, S.; Svasti, J.; Saiki, I. Chrysin overcomes TRAIL resistance of cancer cells through Mcl-1 downregulation by inhibiting STAT3 phosphorylation. Int. J. Oncol. 2013, 43, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Polier, G.; Kohler, R.; Giaisi, M.; Krammer, P.H.; Li-Weber, M. Wogonin and related natural flavones overcome tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein resistance of tumors by down-regulation of c-FLIP protein and up-regulation of TRAIL receptor 2 expression. J. Biol. Chem. 2012, 287, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Bronikowska, J.; Szliszka, E.; Kostrzewa-Suslow, E.; Jaworska, D.; Czuba, Z.P.; Bednarski, P.; Krol, W. Novel Structurally Related Flavones Augment Cell Death Induced by rhsTRAIL. Int. J. Mol. Sci. 2017, 18, 1211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Wang, R.; Zheng, X.; Li, N.; Li, H.; Cao, X.; Zhou, B.; Lin, Y.; Yang, L. Baicalin potentiates TRAIL-induced apoptosis through p38 MAPK activation and intracellular reactive oxygen species production. Mol. Med. Rep. 2017, 16, 8549–8555. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Gao, C.C.; Yang, X.Z.; Wu, S.N.; Wang, H.G.; Zhang, J.L.; Yan, W.; Ma, T.H. Combining TRAIL and liquiritin exerts synergistic effects against human gastric cancer cells and xenograft in nude mice through potentiating apoptosis and ROS generation. Biomed. Pharmacother. 2017, 93, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.F.; Sun, X.K.; Lan, Y.; Han, C.; Zhang, Y.D.; Chen, G. Linarin sensitizes tumor necrosis factor-related apoptosis (TRAIL)-induced ligand-triggered apoptosis in human glioma cells and in xenograft nude mice. Biomed. Pharmacother. 2017, 95, 1607–1618. [Google Scholar] [CrossRef]
- Szliszka, E.; Helewski, K.J.; Mizgala, E.; Krol, W. The dietary flavonol fisetin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells. Int. J. Oncol. 2011, 39, 771–779. [Google Scholar]
- Han, M.A.; Lee, D.H.; Woo, S.M.; Seo, B.R.; Min, K.J.; Kim, S.; Park, J.W.; Kim, S.H.; Choi, Y.H.; Kwon, T.K. Galangin sensitizes TRAIL-induced apoptosis through down-regulation of anti-apoptotic proteins in renal carcinoma Caki cells. Sci. Rep. 2016, 6, 18642. [Google Scholar] [CrossRef]
- Song, W.; Yan, C.Y.; Zhou, Q.Q.; Zhen, L.L. Galangin potentiates human breast cancer to apoptosis induced by TRAIL through activating AMPK. Biomed. Pharmacother. 2017, 89, 845–856. [Google Scholar] [CrossRef]
- Jacquemin, G.; Granci, V.; Gallouet, A.S.; Lalaoui, N.; Morle, A.; Iessi, E.; Morizot, A.; Garrido, C.; Guillaudeux, T.; Micheau, O. Quercetin-mediated Mcl-1 and survivin downregulation restores TRAIL-induced apoptosis in non-Hodgkin’s lymphoma B cells. Haematologica 2012, 97, 38–46. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.J.; Choi, K.C.; Son, J. Quercetin sensitizes pancreatic cancer cells to TRAIL-induced apoptosis through JNK-mediated cFLIP turnover. Int. J. Biochem. Cell Biol. 2016, 78, 327–334. [Google Scholar] [CrossRef]
- Yi, L.; Zongyuan, Y.; Cheng, G.; Lingyun, Z.; Guilian, Y.; Wei, G. Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Sci. 2014, 105, 520–527. [Google Scholar] [CrossRef]
- Manouchehri, J.M.; Turner, K.A.; Kalafatis, M. TRAIL-Induced Apoptosis in TRAIL-Resistant Breast Carcinoma Through Quercetin Cotreatment. Breast Cancer 2018, 12, 1178223417749855. [Google Scholar] [CrossRef]
- Turner, K.A.; Manouchehri, J.M.; Kalafatis, M. Sensitization of recombinant human tumor necrosis factor-related apoptosis-inducing ligand-resistant malignant melanomas by quercetin. Melanoma Res. 2018, 28, 277–285. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, B.; Wang, Y.; Ding, H. Kaempferol Sensitizes Human Ovarian Cancer Cells-OVCAR-3 and SKOV-3 to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)-Induced Apoptosis via JNK/ERK-CHOP Pathway and Up-Regulation of Death Receptors 4 and 5. Med. Sci. Monit. 2017, 23, 5096–5105. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Naimi, A.; Hagh, M.F.; Saraei, R.; Marofi, F.; Solali, S. Kaempferol Improves TRAIL-Mediated Apoptosis in Leukemia MOLT-4 Cells by the Inhibition of Anti-apoptotic Proteins and Promotion of Death Receptors Expression. Anticancer. Agents Med. Chem. 2019, 19, 1835–1845. [Google Scholar] [CrossRef]
- Tang, S.Y.; Zhong, M.Z.; Yuan, G.J.; Hou, S.P.; Yin, L.L.; Jiang, H.; Yu, Z. Casticin, a flavonoid, potentiates TRAIL-induced apoptosis through modulation of anti-apoptotic proteins and death receptor 5 in colon cancer cells. Oncol. Rep. 2013, 29, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, M.J.; Kim, D.W.; Kang, C.D.; Kim, S.H. Amurensin G enhances the susceptibility to tumor necrosis factor-related apoptosis-inducing ligand-mediated cytotoxicity of cancer stem-like cells of HCT-15 cells. Cancer Sci. 2013, 104, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Bandil, K.; Proksch, P.; Murugiyan, K.; Bharadwaj, M. Effects of A. marina-Derived Isoquercitrin on TNF-Related Apoptosis-Inducing Ligand Receptor (TRAIL-R) Expression and Apoptosis Induction in Cervical Cancer Cells. Appl. Biochem. Biotechnol. 2017, 182, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Nazim, U.M.; Park, S.Y. Genistein enhances TRAIL-induced cancer cell death via inactivation of autophagic flux. Oncol. Rep. 2015, 34, 2692–2698. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, B.; Shin, S.J.; Kwon, S.H.; Cha, S.D.; Lee, H.G.; Bae, I.; Cho, C.H. The synergistic apoptotic interaction of Indole-3-Carbinol and Genistein with TRAIL on endometrial cancer cells. J. Korean Med. Sci. 2013, 28, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Mertas, A.; Paradysz, A.; Krol, W. The dietary isoflavone biochanin-A sensitizes prostate cancer cells to TRAIL-induced apoptosis. Urol. Oncol. 2013, 31, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Choi, W.I.; Ko, H.; So, Y.; Kang, K.S.; Kim, I.; Kim, K.; Yoon, H.G.; Kim, T.J.; Choi, K.C. Neobavaisoflavone sensitizes apoptosis via the inhibition of metastasis in TRAIL-resistant human glioma U373MG cells. Life Sci. 2014, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gao, C.C.; Pan, Z.G.; Zhou, C.W. Irigenin sensitizes TRAIL-induced apoptosis via enhancing pro-apoptotic molecules in gastric cancer cells. Biochem. Biophys. Res. Commun. 2018, 496, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Jaworska, D.; Ksek, M.; Czuba, Z.P.; Krol, W. Targeting death receptor TRAIL-R2 by chalcones for TRAIL-induced apoptosis in cancer cells. Int. J. Mol. Sci. 2012, 13, 15343–15359. [Google Scholar] [CrossRef] [PubMed]
- Kauntz, H.; Bousserouel, S.; Gosse, F.; Raul, F. The flavonolignan silibinin potentiates TRAIL-induced apoptosis in human colon adenocarcinoma and in derived TRAIL-resistant metastatic cells. Apoptosis 2012, 17, 797–809. [Google Scholar] [CrossRef]
- Wei, R.; Zhu, G.; Jia, N.; Yang, W. Epigallocatechin-3-gallate Sensitizes Human 786-O Renal Cell Carcinoma Cells to TRAIL-Induced Apoptosis. Cell Biochem. Biophys. 2015, 72, 157–164. [Google Scholar] [CrossRef]
- Kwon, O.S.; Jung, J.H.; Shin, E.A.; Park, J.E.; Park, W.Y.; Kim, S.H. Epigallocatechin-3-Gallate Induces Apoptosis as a TRAIL Sensitizer via Activation of Caspase 8 and Death Receptor 5 in Human Colon Cancer Cells. Biomedicines 2020, 8, 84. [Google Scholar] [CrossRef]
- Song, T.; Zhang, M.; Wu, J.; Chen, F.; Wang, Y.; Ma, Y.; Dai, Z. Glioma progression is suppressed by Naringenin and APO2L combination therapy via the activation of apoptosis in vitro and in vivo. Investig. New Drugs 2020, 38, 1743–1754. [Google Scholar] [CrossRef]
- Han, H.; Xu, B.; Hou, P.; Jiang, C.; Liu, L.; Tang, M.; Yang, X.; Zhang, Y.; Liu, Y. Icaritin Sensitizes Human Glioblastoma Cells to TRAIL-Induced Apoptosis. Cell Biochem. Biophys. 2015, 72, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Ha, I.J.; Park, S.Y.; Choi, M.; Lim, S.L.; Kim, S.H.; Lee, J.H.; Ahn, K.S.; Yun, M.; Lee, S.G. Morusin Induces TRAIL Sensitization by Regulating EGFR and DR5 in Human Glioblastoma Cells. J. Nat. Prod. 2016, 79, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Engelsgjerd, S.; Kunnimalaiyaan, S.; Kandil, E.; Gamblin, T.C.; Kunnimalaiyaan, M. Xanthohumol increases death receptor 5 expression and enhances apoptosis with the TNF-related apoptosis-inducing ligand in neuroblastoma cell lines. PLoS ONE 2019, 14, e0213776. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, T.; Toume, K.; Arai, M.A.; Koyano, T.; Kowithayakorn, T.; Ishibashi, M. Prenylflavonoids isolated from Artocarpus champeden with TRAIL-resistance overcoming activity. Phytochemistry 2013, 96, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Toume, K.; Habu, T.; Arai, M.A.; Koyano, T.; Kowithayakorn, T.; Ishibashi, M. Prenylated flavonoids and resveratrol derivatives isolated from Artocarpus communis with the ability to overcome TRAIL resistance. J. Nat. Prod. 2015, 78, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cao, A.; Wang, L.; Wu, D. Kurarinone Synergizes TRAIL-Induced Apoptosis in Gastric Cancer Cells. Cell Biochem. Biophys. 2015, 72, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Seo, O.W.; Kim, J.H.; Lee, K.S.; Lee, K.S.; Kim, J.H.; Won, M.H.; Ha, K.S.; Kwon, Y.G.; Kim, Y.M. Kurarinone promotes TRAIL-induced apoptosis by inhibiting NF-kappaB-dependent cFLIP expression in HeLa cells. Exp. Mol. Med. 2012, 44, 653–664. [Google Scholar] [CrossRef]
- Du, J.; Wu, J.; Fu, X.; Tse, A.K.; Li, T.; Su, T.; Yu, Z.L. Icariside II overcomes TRAIL resistance of melanoma cells through ROS-mediated downregulation of STAT3/cFLIP signaling. Oncotarget 2016, 7, 52218–52229. [Google Scholar] [CrossRef][Green Version]
- Cho, H.D.; Gu, I.A.; Won, Y.S.; Moon, K.D.; Park, K.H.; Seo, K.I. Auriculasin sensitizes primary prostate cancer cells to TRAIL-mediated apoptosis through up-regulation of the DR5-dependent pathway. Food Chem. Toxicol. 2019, 126, 223–232. [Google Scholar] [CrossRef]
- Kim, B.; Seo, J.H.; Lee, K.Y.; Park, B. Icariin sensitizes human colon cancer cells to TRAIL-induced apoptosis via ERK-mediated upregulation of death receptors. Int. J. Oncol. 2020, 56, 821–834. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2020, 11, 592654. [Google Scholar] [CrossRef] [PubMed]
- Manouchehri, J.M.; Kalafatis, M. Sensitization of rhTRAIL-resistant Triple-negative Breast Carcinoma Through Silibinin Co-Treatment. Anticancer. Res. 2017, 37, 6593–6599. [Google Scholar] [PubMed]
- Razeghian, E.; Suksatan, W.; Sulaiman Rahman, H.; Bokov, D.O.; Abdelbasset, W.K.; Hassanzadeh, A.; Marofi, F.; Yazdanifar, M.; Jarahian, M. Harnessing TRAIL-Induced Apoptosis Pathway for Cancer Immunotherapy and Associated Challenges. Front. Immunol. 2021, 12, 699746. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, D.J.; Forero-Torres, A.; LoBuglio, A.F. TRAIL-receptor antibodies as a potential cancer treatment. Future Oncol. 2007, 3, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W.; Frew, A.J.; Smyth, M.J. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat. Rev. Cancer 2008, 8, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Snajdauf, M.; Havlova, K.; Vachtenheim, J., Jr.; Ozaniak, A.; Lischke, R.; Bartunkova, J.; Smrz, D.; Strizova, Z. The TRAIL in the Treatment of Human Cancer: An Update on Clinical Trials. Front. Mol. Biosci. 2021, 8, 628332. [Google Scholar] [CrossRef] [PubMed]
- Laudisi, F.; Pacifico, T.; Maresca, C.; Luiz-Ferreira, A.; Antonelli, S.; Ortenzi, A.; Colantoni, A.; Di Grazia, A.; Franze, E.; Colella, M.; et al. Rafoxanide sensitizes colorectal cancer cells to TRAIL-mediated apoptosis. Biomed. Pharmacother. 2022, 155, 113794. [Google Scholar] [CrossRef]
- Safa, A.R.; Pollok, K.E. Targeting the Anti-Apoptotic Protein c-FLIP for Cancer Therapy. Cancers 2011, 3, 1639–1671. [Google Scholar] [CrossRef]
- Fulda, S.; Vucic, D. Targeting IAP proteins for therapeutic intervention in cancer. Nat. Rev. Drug Discov. 2012, 11, 109–124. [Google Scholar] [CrossRef]
- Garg, H.; Suri, P.; Gupta, J.C.; Talwar, G.P.; Dubey, S. Survivin: A unique target for tumor therapy. Cancer Cell Int. 2016, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Costa, M. XIAP’s Profile in Human Cancer. Biomolecules 2020, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 protein family: Attractive targets for cancer therapy. Apoptosis 2023, 28, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Cardoso Alves, L.; Corazza, N.; Micheau, O.; Krebs, P. The multifaceted role of TRAIL signaling in cancer and immunity. FEBS J. 2021, 288, 5530–5554. [Google Scholar] [CrossRef] [PubMed]
- Bugel, S.M.; Bonventre, J.A.; Tanguay, R.L. Comparative Developmental Toxicity of Flavonoids Using an Integrative Zebrafish System. Toxicol. Sci. 2016, 154, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Skibola, C.F.; Smith, M.T. Potential health impacts of excessive flavonoid intake. Free Radic. Biol. Med. 2000, 29, 375–383. [Google Scholar] [CrossRef]
- Bisol, A.; de Campos, P.S.; Lamers, M.L. Flavonoids as anticancer therapies: A systematic review of clinical trials. Phytother. Res. 2020, 34, 568–582. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Brockmueller, A.; Zhai, K.; Abdellatif, B.; Siddiqui, M.; Biringer, K.; Kudela, E.; Pec, M.; et al. Flavonoids as an effective sensitizer for anti-cancer therapy: Insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 2021, 12, 155–176. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Aatif, M.; Ahmad, A. Current Understanding of Flavonoids in Cancer Therapy and Prevention. Metabolites 2023, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Costa, D.; Sousa, A. Flavonoids-Based Delivery Systems towards Cancer Therapies. Bioengineering 2022, 9, 197. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Donovan, J.L. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic. Res. 2004, 38, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Chakraborty, P.; Bhattacharya, H.; Singh, S.K.; Dua, K.; Dey, A.; Jha, N.K. Recent advances in flavonoid-based nanocarriers as an emerging drug delivery approach for cancer chemotherapy. Drug Discov. Today 2023, 28, 103409. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Daniel-da-Silva, A.L.; Oliveira, H. Drug Delivery Systems and Flavonoids: Current Knowledge in Melanoma Treatment and Future Perspectives. Micromachines 2022, 13, 1838. [Google Scholar] [CrossRef]
- Ronka, S.; Kowalczyk, A.; Baczynska, D.; Zolnierczyk, A.K. Pluronics-Based Drug Delivery Systems for Flavonoids Anticancer Treatment. Gels 2023, 9, 143. [Google Scholar] [CrossRef]
| Compound (Class) | Cancer Type | Mechanism(s) of Action | Concentration/s | Reference |
|---|---|---|---|---|
| Apigenin (Flavone) | NSCLC | ↑Bax, ↑Bad, ↑DR4/5, ↓Bcl-2, ↓Bcl-xL, ↓c-FLIP | 10–40 µM | [41] |
| NSCLC, primary patient-derived lung cancer cells | ↑DR5, ↓c-FLIP | 10–50 µM | [42] | |
| Prostate cancer | ↑DR5 | 5–20 µmol/L | [43] | |
| HCC | ↑DR4/5 | 2–30 µg/mL | [44] | |
| HCC | ↑DR5, ↓Bcl-2, ↓IAP-1/2, ↓XIAP | 15–20 µM | [45] | |
| Luteolin (Flavone) | Lung cancer, cervical cancer | ↑Caspase-8 | 5 µM | [46] |
| RCC | ↑DR4/5, ↓c-FLIP, ↓Mcl-1 | 10 µM | [47] | |
| HCC | ↑DR5 | 5–20 µM | [48] | |
| NSCLC | ↑DR5 | 5–40 µM | [49] | |
| Chrysin (Flavone) | HCC | ↑Bax, ↓Bcl-2, ↓IAP-1/2, ↓Mcl-1, ↓Survivin, ↓XIAP | 10–40 µM | [50] |
| HCC | ↑DR5 | 2.5–10 µmol/L | [51] | |
| Cervical cancer, lung cancer | ↓Mcl-1 | 60–100 µM | [52] | |
| CRC, HCC, breast cancer, ATL, melanoma, pancreatic carcinoma | ↑DR5, ↓c-FLIP, ↓Mcl-1, | 50–100 µM | [53] | |
| 7-Hydroxyflavone (Flavone) | CRC | - | 50–100 µM | [54] |
| Baicalin (Flavone) | Lung cancer | - | 50–100 µM | [55] |
| Liquiritin (Flavone) | Gastric adenocarcinoma | ↑Bax, ↑DR4/5, ↓Bcl-2 | 25–200 µM | [56] |
| Linarin (Flavone) | Glioma | ↑Bax, ↑DR4/5, ↓Bcl-2, ↓Bcl-xL, ↓c-FLIP, ↓Mcl-1, ↓Survivin | 5 µM | [57] |
| Fisetin (Flavone) | Prostate cancer | - | 10–50 µM | [58] |
| Galangin (Flavone) | RCC | ↓Bcl-2, ↓c-FLIP, ↓Mcl-1, ↓Survivin, | 30 µM | [59] |
| Breast cancer | ↑Bax, ↑DR4, ↓Bcl-2 | 20–40 µM | [60] | |
| Quercetin (Flavonol) | Non-Hodgkin’s B-lymphoma | ↓Mcl-1, ↓Survivin | 20 µM | [61] |
| Pancreatic cancer | ↓c-FLIP | 30–90 µM | [62] | |
| Ovarian cancer | ↑DR5, ↓Bcl-2, ↓Bcl-xL, ↓Survivin, ↓XIAP | 50–200 µM | [63] | |
| Breast cancer | ↑DR5, ↓c-FLIP | 12.5–50 µM | [64] | |
| Melanoma | ↑DR4/5, ↓ c-FLIP | 25–50 µM | [65] | |
| Kaempferol (Flavonol) | Ovarian cancer | ↑Bax, ↑DR4/5, ↓Bcl-2, ↓Bcl-xL, ↓c-FLIP, ↓Survivin, ↓XIAP | 25–100 µM | [66] |
| Acute lymphoblastic leukemia | ↑DR4/5, ↓c-FLIP, ↓IAP-1/2, ↓XIAP | 95 µM | [67] | |
| Casticin (Flavonol) | CRC | ↑Bax, ↑DR5, ↓Bcl-xL, ↓c-FLIP, ↓Survivin, ↓XIAP | 1–3 µmol/L | [68] |
| Amurensin G (Flavonol) | CRC | ↑DR4/5, ↓c-FLIP | 1–5 µM | [69] |
| Isoquecitrin (Flavonol) | Cervical cancer | ↑DR4/5 | 100–1400 µM | [70] |
| Genistein (Isoflavone) | Lung cancer | - | 10–40 µM | [71] |
| Endometrial cancer | ↑DR4/5, ↓c-FLIP | 10–50 µM | [72] | |
| Biochanin-A (Isoflavone) | Prostate cancer | ↑DR5 | 100 µM | [73] |
| Neobavaisoflavone (Isoflavone) | Glioma | ↑Bax, ↑DR5 ↓Bid, ↓Bcl-2, ↓IAP-3, ↓XIAP | 5–30 µM | [74] |
| Irigenin (Isoflavone) | Gastric adenocarcinoma | ↑Bax, ↑DR5, ↓Bcl-2, ↓c-FLIP, ↓Survivin | 5–10 µM | [75] |
| Isobavachalcone (Chalcone) | Cervical cancer | ↑DR5 | 25–50 µM | [76] |
| Licochalcone A (Chalcone) | Cervical cancer | ↑DR5 | 25–50 µM | [76] |
| Silibinin (Flavonolignan) | CRC | ↑DR4/5, ↓Mcl-1, ↓XIAP | 100–300 µM | [77] |
| Breast cancer | ↑DR4/5, ↓Survivin | 25–50 µM | [64] | |
| Epigallocatechin-3-gallate (Flavanol) | RCC | ↓Bcl-2, ↓c-FLIP, ↓Mcl-1 | 50 µg/mL | [78] |
| CRC | ↑DR5 | 20–60 µM | [79] | |
| Naringenin (Flavanone) | Glioma | ↑Bax, ↑DR5 ↓Bid, ↓Bcl-2, ↓IAP-3, ↓XIAP | 160 µM | [80] |
| Icaritin (Prenylflavonoid) | Glioblastoma | ↑Bax, ↑DR5, ↓Bcl-2, ↓c-FLIP, ↓Survivin | 10–20 µM | [81] |
| Morusin (Prenylflavonoid) | Glioma | ↑Bak, ↑Bad, ↑DR4/5, ↓Bcl-2, ↓Bcl-xL | 2.5–5 µM | [82] |
| Xanthohumol (Prenylflavonoid) | Cervical cancer | ↑DR5 | 25–50 µM | [76] |
| Neuroblastoma | ↑Bax, ↑Bid, ↑DR5 ↓Bcl-2, ↓Bcl-xL | 7.5 µM | [83] | |
| Heterophyllin (Prenylflavonoid) | Gastric adenocarcinoma | ↑DR4/5 | 4 µM | [84] |
| Artonin E (Prenylflavonoid) | Gastric adenocarcinoma | ↑DR5 | 3 µM | [85] |
| Kurarinone (Prenylflavonoid) | Gastric adenocarcinoma | ↓c-FLIP, ↓Mcl-1 | 5 µM | [86] |
| Cervical cancer | ↑DR5 | 5 µM | [87] | |
| Icariside II (Prenylflavonoid) | Melanoma | ↓Bcl-xL, ↓c-FLIP, ↓Survivin | 20 µM | [88] |
| Auriculasin (Prenylflavonoid) | Prostate cancer | ↑Bax, ↑DR5, ↓Bcl-2 | 2.5–10 µM | [89] |
| Icariin (Prenylflavonoid) | CRC | ↑DR4/5 | 1–10 µM | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luiz-Ferreira, A.; Pacifico, T.; Cruz, Á.C.; Laudisi, F.; Monteleone, G.; Stolfi, C. TRAIL-Sensitizing Effects of Flavonoids in Cancer. Int. J. Mol. Sci. 2023, 24, 16596. https://doi.org/10.3390/ijms242316596
Luiz-Ferreira A, Pacifico T, Cruz ÁC, Laudisi F, Monteleone G, Stolfi C. TRAIL-Sensitizing Effects of Flavonoids in Cancer. International Journal of Molecular Sciences. 2023; 24(23):16596. https://doi.org/10.3390/ijms242316596
Chicago/Turabian StyleLuiz-Ferreira, Anderson, Teresa Pacifico, Álefe Cardoso Cruz, Federica Laudisi, Giovanni Monteleone, and Carmine Stolfi. 2023. "TRAIL-Sensitizing Effects of Flavonoids in Cancer" International Journal of Molecular Sciences 24, no. 23: 16596. https://doi.org/10.3390/ijms242316596
APA StyleLuiz-Ferreira, A., Pacifico, T., Cruz, Á. C., Laudisi, F., Monteleone, G., & Stolfi, C. (2023). TRAIL-Sensitizing Effects of Flavonoids in Cancer. International Journal of Molecular Sciences, 24(23), 16596. https://doi.org/10.3390/ijms242316596








