Animal Models in Eye Research: Focus on Corneal Pathologies
Abstract
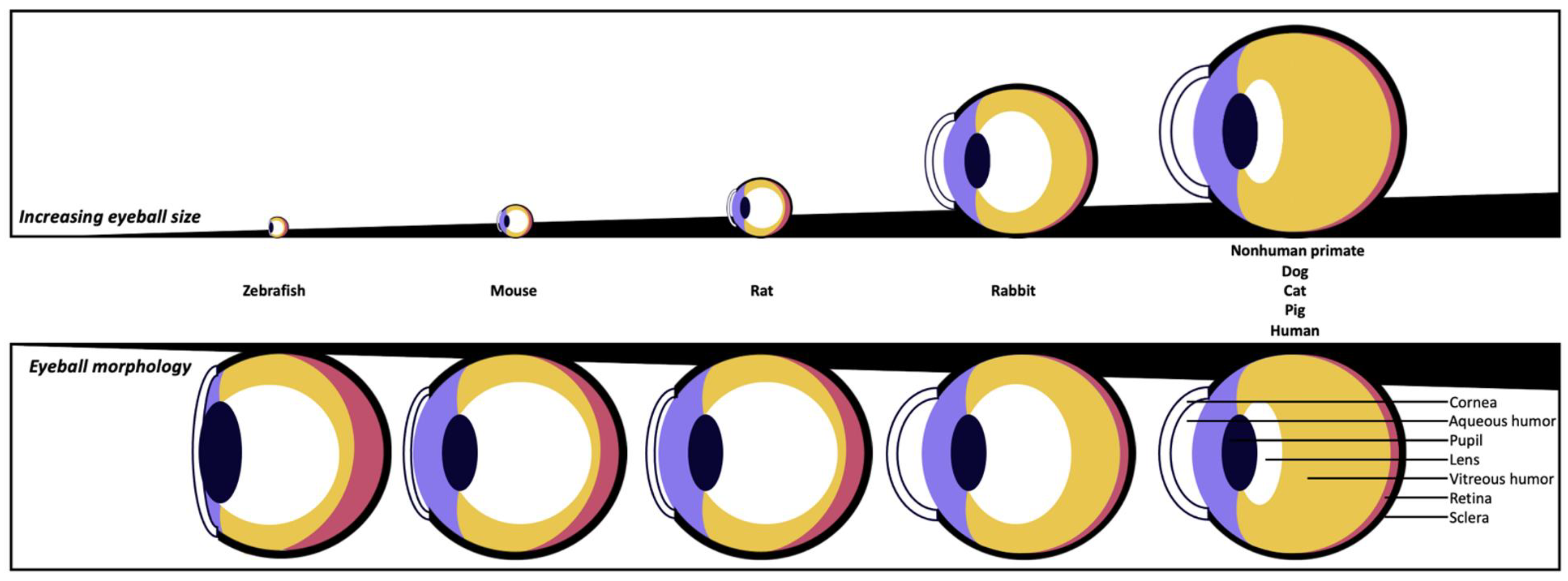
:1. Animal Models in Ocular Research
| Species | Zebrafish | Mouse | Rat | Rabbit | Nonhuman Primate | Dog | Cat | Pig | Human |
|---|---|---|---|---|---|---|---|---|---|
| Average eye dimension in volume (cm3) | 0.0035 | 0.025 | 0.1 | 2.6 | 4.0 | 4.5 | 5.1 | 6.5 | 7.2 |
| Average eye dimension (axial length in mm) | 2 | 3.4 | 6.0 | 17.1 | 19.8 | 20.5 | 21.4 | 23.9 | 24 |
| Corneal horizontal diameter (µm) | 2–2.5 | 3.1 | 5.1 | 13.4 | 10.2–11.4 | 13–17 | 15.5–18 | 14.5–16.5 | 11.8 |
| Average central corneal thickness (µm) | 16–20 | 90–130 | 160–200 | 350–400 | 420–460 | 500–600 | 545–650 | 600–1100 | 505–560 |
| Cornea shape | Flat | Flat | Flat | Dome | Dome | Dome | Dome | Dome | Dome |
| Bowman’s membrane | Yes | No | No | No | Yes | No | No | Less developed or absent | Yes |
| Average tear volume (µL) | / | 0.06–0.2 | 4.6 | 5–7.5 | / | 65 | 32 | / | 7–12 |
| Average tear turnover rate (%/min) | / | 5 | / | 6.5 | / | 12 | 11 | / | 15 |
| Time between eye blinks | / | 5 min | 5 min | 6 min | 6 s | 10–20 s | 18 s | 20–30 s | 5 s |
| Nictitating membranes | No | Yes (non-functional) | Yes (non-functional) | Yes | No | Yes | Yes | Yes | No |
| Average aqueous humor volume (µL) | / | 6 | 14 | 287 | 100–120 | 770 | 820–850 | 260–300 | 200–310 |
| Lens size (axial length in mm) | 1.0 | 2.2 | 3.8 | 6.4–7.9 | 3–4 | 6.7 | 7.7 | 7.4–7.8 | 4 |
| Lens shape | Spheroid | Spheroid | Spheroid | ≈Spheroid | Ellipsoid | ≈Spheroid | ≈Spheroid | ≈Spheroid | Ellipsoid |
| Space taken by lens in eyeball | Very High | Very High | Very High | High | Low | Medium | Medium | Medium | Low |
| Fovea | No | No | No | No | Yes | No | No | Non-functional | Yes |
| References | [18,19,20,21,22,23] | [3,24,25,26,27] | [3,25,27,28,29] | [3,25,27,30,31,32,33] | [3,25,26,34,35] | [3,25,27,36,37,38,39,40,41] | [3,25,35,41,42,43,44] | [3,25,35,45,46,47,48] | [3,18,19,23,25,26,27,34,47,49,50] |
1.1. Mouse Models
1.2. Rabbit Models
1.3. Nonhuman Primate Models
1.4. Porcine Models
1.5. Feline Models
1.6. Canine Models
1.7. Zebrafish Models
2. Focus on the Most Used Animal Models in Corneal Pathologies
2.1. Dry Eye Diseases
2.1.1. Pathology
2.1.2. Animal Models
Mouse Models
Rabbit Models
2.2. Ocular Herpes (Herpetic Keratitis)
2.2.1. Pathology
2.2.2. Animal Models
Mouse Models
Rabbit Models
2.3. Corneal Repair and Transplantation
2.3.1. Pathology
2.3.2. Animal Models
Animal Models for Corneal Wounds

Animal Models for Corneal Transplantation
2.4. Corneal Neovascularization
2.4.1. Pathology
2.4.2. Animal Models
2.5. Corneal Dystrophy
2.5.1. Pathology
2.5.2. Animal Models for Fuchs Endothelial Corneal Dystrophy
2.6. Diabetic Keratopathy
2.6.1. Pathology
2.6.2. Animal Models
Induction of Diabetes in Animal Models
Animal Models for the Treatment of Diabetic Keratopathy
2.7. Keratoconus
2.7.1. Pathology
2.7.2. Animal Models
2.8. Development of Therapeutic Devices Requiring Animal Models
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeiss, C.J. Translational models of ocular disease. Vet. Ophthalmol. 2013, 16, 15–33. [Google Scholar] [CrossRef]
- Chan, C.-C. (Ed.) Animal Models of Ophthalmic Diseases; Springer International Publishing: Cham, Switzerland, 2015; p. 152. [Google Scholar] [CrossRef]
- Vézina, M. Comparative ocular anatomy in commonly used laboratory animals. In Assessing Ocular Toxicology in Laboratory Animals; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–21. [Google Scholar]
- Tsonis, P.A. Animal Models in Eye Research; Academic Press: Cambridge, MA, USA, 2011; p. 214. [Google Scholar]
- Bouhenni, R.A.; Dunmire, J.; Sewell, A.; Edward, D.P. Animal models of glaucoma. BioMed Res. Int. 2012, 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.C.; Vorontsova, I.; Martis, R.M.; Donaldson, P.J. Chapter 4—Animal Models in Cataract Research. In Animal Models for the Study of Human Disease, 2nd ed.; Conn, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 103–116. [Google Scholar]
- Liu, C.H.; Wang, Z.; Sun, Y.; Chen, J. Animal models of ocular angiogenesis: From development to pathologies. FASEB J. 2017, 31, 4665–4681. [Google Scholar] [CrossRef] [PubMed]
- Dewi, P.A.S.; Sitompul, R.; Pawitan, J.; Naroeni, A. Animal model of diabetic keratopathy. ARSHI Vet. Lett. 2019, 3, 57–58. [Google Scholar] [CrossRef]
- Quiroz, J.; Yazdanyar, A. Animal models of diabetic retinopathy. Ann. Transl. Med. 2021, 9, 1272. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990-2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [PubMed]
- Porth, J.M.; Deiotte, E.; Dunn, M.; Bashshur, R. A Review of the Literature on the Global Epidemiology of Corneal Blindness. Cornea 2019, 38, 1602–1609. [Google Scholar] [CrossRef]
- Guérin, L.-P.; Le-Bel, G.; Desjardins, P.; Couture, C.; Gillard, E.; Boisselier, É.; Bazin, R.; Germain, L.; Guérin, S.L. The Human Tissue-Engineered Cornea (hTEC): Recent Progress. Int. J. Mol. Sci. 2021, 22, 1291. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Deshmukh, R.; Ting, D.S.W.; Ang, M. Big data in corneal diseases and cataract: Current applications and future directions. Front. Big Data 2023, 6, 1017420–1017434. [Google Scholar] [CrossRef]
- Cursiefen, C. Immune privilege and angiogenic privilege of the cornea. Immune Response Eye 2007, 92, 50–57. [Google Scholar]
- Merindano, M.D.; Costa, J.; Canals, M.; Potau, J.; Ruano, D. A comparative study of Bowman’s layer in some mammals: Relationships with other constituent corneal structures. Eur. J. Anat. 2003, 6, 133–140. [Google Scholar]
- Espana, E.M.; Birk, D.E. Composition, structure and function of the corneal stroma. Exp. Eye Res. 2020, 198, 108137. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Bowman’s layer in the cornea– structure and function and regeneration. Exp. Eye Res. 2020, 195, 108033. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, J.; Jacobson, G.; Gueven, N. Zebrafish—On the move towards ophthalmological research. Eye 2014, 28, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Luo, Y. Zebrafish Model in Ophthalmology to Study Disease Mechanism and Drug Discovery. Pharmaceuticals 2021, 14, 716. [Google Scholar] [CrossRef]
- Dahm, R.; Schonthaler, H.B.; Soehn, A.S.; Van Marle, J.; Vrensen, G.F. Development and adult morphology of the eye lens in the zebrafish. Exp. Eye Res. 2007, 85, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Divakar Rao, K.; Verma, Y.; Patel, H.; Gupta, P. Non-invasive ophthalmic imaging of adult zebrafish eye using optical coherence tomography. Curr. Sci. 2006, 90, 1506–1510. [Google Scholar]
- Wiggenhauser, L.M.; Kohl, K.; Dietrich, N.; Hammes, H.-P.; Kroll, J. Studying diabetes through the eyes of a fish: Microdissection, visualization, and analysis of the adult tg (fli: EGFP) zebrafish retinal vasculature. J. Vis. Exp. 2017, 130, e56674. [Google Scholar]
- Zhang, M.; Sun, S.; Wang, L.; Wang, X.; Chen, T.; Chen, Z.; Jiang, Y. Zonular defects in loxl1-deficient zebrafish. Clin. Exp. Ophthalmol. 2022, 50, 62–73. [Google Scholar] [CrossRef]
- Clough, J.; Parikh, C.; Edelhauser, H. Anterior chamber, lens and globe volumes in balb/c and c57/bL6 mice. Investig. Ophthalmol. Vis. Sci. 2003, 44, 648. [Google Scholar]
- Thomasy, S.M.; Eaton, J.S.; Timberlake, M.J.; Miller, P.E.; Murphy, C.J. Species differences in the geometry of the anterior segment differentially affect anterior chamber cell scoring systems in laboratory animals. J. Ocul. Pharmacol. Ther. 2016, 32, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.A.; Conley, S.M.; Makkia, R.; Watson, J.N.; Han, Z.; Cooper, M.J.; Naash, M.I. DNA nanoparticles are safe and nontoxic in non-human primate eyes. Int. J. Nanomed. 2018, 13, 1361–1379. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, L.; Mochel, J.P. An eye on the dog as the scientist’s best friend for translational research in ophthalmology: Focus on the ocular surface. Med. Res. Rev. 2020, 40, 2566–2604. [Google Scholar] [CrossRef]
- Hughes, A. A schematic eye for the rat. Vis. Res. 1979, 19, 569–588. [Google Scholar] [CrossRef] [PubMed]
- Lozano, D.C.; Twa, M.D. Development of a rat schematic eye from in vivo biometry and the correction of lateral magnification in SD-OCT imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6446–6455. [Google Scholar] [CrossRef]
- Chrai, S.S.; Patton, T.F.; Mehta, A.; Robinson, J.R. Lacrimal and instilled fluid dynamics in rabbit eyes. J. Pharm. Sci. 1973, 62, 1112–1121. [Google Scholar] [CrossRef]
- Barathi, A.; Thu, M.K.; Beuerman, R.W. Dimensional growth of the rabbit eye. Cells Tissues Organs 2002, 171, 276–285. [Google Scholar] [CrossRef]
- Axer-Siegel, R.; Bourla, D.; Kremer, I.; Weinberger, D.; Snir, M. Effect of peripheral retinal ablation with cryotherapy versus diode laser photocoagulation on axial length in the growing rabbit eye. Br. J. Ophthalmol. 2006, 90, 491–495. [Google Scholar] [CrossRef]
- Toni, M.C.; Meirelles, A.É.W.B.; Gava, F.N.; Camacho, A.A.; Laus, J.L.; Canola, J.C. Rabbits’ eye globe sonographic biometry. Vet. Ophthalmol. 2010, 13, 384–386. [Google Scholar] [CrossRef]
- Augusteyn, R.C.; Maceo Heilman, B.; Ho, A.; Parel, J.-M. Nonhuman Primate Ocular Biometry. Investig. Ophthalmol. Vis. Sci. 2016, 57, 105–114. [Google Scholar] [CrossRef]
- Barnett, B.P.; Jun, A.S. Corneal Endothelial Cell Transplantation: Animal Models. In Corneal Regeneration; Springer International Publishing: Cham, Switzerland, 2019; pp. 437–454. [Google Scholar]
- Williams, D.L. Lens morphometry determined by B-mode ultrasonography of the normal and cataractous canine lens. Vet. Ophthalmol. 2004, 7, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Tuntivanich, N.; Petersen-Jones, S.M.; Steibel, J.P.; Johnson, C.; Forcier, J.Q. Postnatal development of canine axial globe length measured by B-scan ultrasonography. Vet. Ophthalmol. 2007, 10, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Alario, A.F.; Pirie, C.G. Central corneal thickness measurements in normal dogs: A comparison between ultrasound pachymetry and optical coherence tomography. Vet. Ophthalmol. 2014, 17, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Martín-Suárez, E.; Molleda, C.; Tardón, R.; Galán, A.; Gallardo, J.; Molleda, J. Diurnal variations of central corneal thickness and intraocular pressure in dogs from 8: 00 am to 8: 00 pm. Can. Vet. J. 2014, 55, 361. [Google Scholar] [PubMed]
- Barbé, C.; Harran, N.; Goulle, F. Analysis of factors of variation in lens equatorial length and axial globe length measurements using ultrasonography in dogs with cataract. Rev. Vet. Clin. 2017, 52, 33–40. [Google Scholar] [CrossRef]
- Sebbag, L.; Allbaugh, R.A.; Wehrman, R.F.; Uhl, L.K.; Ben-Shlomo, G.; Chen, T.; Mochel, J.P. Fluorophotometric Assessment of Tear Volume and Turnover Rate in Healthy Dogs and Cats. J. Ocul. Pharmacol. Ther. 2019, 35, 497–502. [Google Scholar] [CrossRef]
- Moodie, K.; Hashizume, N.; Houston, D.; Hoopes, P.; Demidenko, E.; Trembly, B.; Davidson, M. Postnatal development of corneal curvature and thickness in the cat. Vet. Ophthalmol. 2001, 4, 267–272. [Google Scholar] [CrossRef]
- Konrade, K.A.; Hoffman, A.R.; Ramey, K.L.; Goldenberg, R.B.; Lehenbauer, T.W. Refractive states of eyes and associations between ametropia and age, breed, and axial globe length in domestic cats. Am. J. Vet. Res. 2012, 73, 279–284. [Google Scholar] [CrossRef]
- Mirshahi, A.; Shafigh, S.; Azizzadeh, M. Ultrasonographic biometry of the normal eye of the Persian cat. Aust. Vet. J. 2014, 92, 246–249. [Google Scholar] [CrossRef]
- McMenamin, P.G.; Steptoe, R.J. Normal anatomy of the aqueous humour outflow system in the domestic pig eye. J. Anat. 1991, 178, 65. [Google Scholar]
- Reilly, M.A.; Hamilton, P.D.; Perry, G.; Ravi, N. Comparison of the behavior of natural and refilled porcine lenses in a robotic lens stretcher. Exp. Eye Res. 2009, 88, 483–494. [Google Scholar] [CrossRef]
- Sanchez, I.; Martin, R.; Ussa, F.; Fernandez-Bueno, I. The parameters of the porcine eyeball. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 475–482. [Google Scholar] [CrossRef]
- Kling, S.; Hafezi, F. Corneal biomechanics–a review. Ophthalmic Physiol. Opt. 2017, 37, 240–252. [Google Scholar] [CrossRef]
- Tomlinson, A.; Khanal, S. Assessment of tear film dynamics: Quantification approach. Ocul. Surf. 2005, 3, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Kapnisis, K.; Van Doormaal, M.; Ethier, C.R. Modeling aqueous humor collection from the human eye. J. Biomech. 2009, 42, 2454–2457. [Google Scholar] [CrossRef] [PubMed]
- Formisano, N.; van der Putten, C.; Grant, R.; Sahin, G.; Truckenmüller, R.K.; Bouten, C.V.; Kurniawan, N.A.; Giselbrecht, S. Mechanical properties of bioengineered corneal stroma. Adv. Healthc. Mater. 2021, 10, 2100972. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, N.; Brown, S. The mouse ascending: Perspectives for human-disease models. Nat. Cell Biol. 2007, 9, 993–999. [Google Scholar] [CrossRef]
- Justice, M.J.; Dhillon, P. Using the mouse to model human disease: Increasing validity and reproducibility. Dis. Models Mech. 2016, 9, 101–103. [Google Scholar] [CrossRef]
- McDowell, C.M.; Kizhatil, K.; Elliott, M.H.; Overby, D.R.; van Batenburg-Sherwood, J.; Millar, J.C.; Kuehn, M.H.; Zode, G.; Acott, T.S.; Anderson, M.G.; et al. Consensus Recommendation for Mouse Models of Ocular Hypertension to Study Aqueous Humor Outflow and Its Mechanisms. Investig. Ophthalmol. Vis. Sci. 2022, 63, 12. [Google Scholar] [CrossRef]
- Fini, M.E.; Jeong, S.; Gong, H.; Martinez-Carrasco, R.; Laver, N.M.; Hijikata, M.; Keicho, N.; Argüeso, P. Membrane-associated mucins of the ocular surface new genes, new protein functions and new biological roles in human and mouse. Prog. Retin. Eye Res. 2020, 75, 100777. [Google Scholar] [CrossRef]
- Perlman, R.L. Mouse models of human disease: An evolutionary perspective. Evol. Med. Public Health 2016, 2016, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Webre, J.M.; Hill, J.M.; Nolan, N.M.; Clement, C.; McFerrin, H.E.; Bhattacharjee, P.S.; Hsia, V.; Neumann, D.M.; Foster, T.P.; Lukiw, W.J. Rabbit and mouse models of HSV-1 latency, reactivation, and recurrent eye diseases. J. Biomed. Biotechnol. 2012, 2012, 18. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Knouse, J.A.; Hernon, K.M. Rabbit models for studying human infectious diseases. Comp. Med. 2015, 65, 499–507. [Google Scholar]
- Portal, C.; Gouyer, V.; Gottrand, F.; Desseyn, J.-L. Ocular mucins in dry eye disease. Exp. Eye Res. 2019, 186, 107724. [Google Scholar] [CrossRef]
- Fini, M.E.; Stramer, B.M. How the Cornea Heals: Cornea-specific Repair Mechanisms Affecting Surgical Outcomes. Cornea 2005, 24, S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Ohnishi, Y.; Ooshima, A.; Liu, C.-Y.; Kao, W.W.-Y. Epithelial Repair: Roles of Extracellular Matrix. Cornea 2002, 21, S23–S29. [Google Scholar] [CrossRef]
- Barabino, S.; Dana, M.R. Animal Models of Dry Eye: A Critical Assessment of Opportunities and Limitations. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; Shen, L.; Chen, L.; Rashid, S.; Rolando, M.; Dana, M.R. The Controlled-Environment Chamber: A New Mouse Model of Dry Eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2766–2771. [Google Scholar] [CrossRef]
- Stern, M.E.; Pflugfelder, S.C. What we have learned from animal models of dry eye. Int. Ophthalmol. Clin. 2017, 57, 109. [Google Scholar] [CrossRef]
- Bauskar, A.; Mack, W.J.; Mauris, J.; Argüeso, P.; Heur, M.; Nagel, B.A.; Kolar, G.R.; Gleave, M.E.; Nakamura, T.; Kinoshita, S.; et al. Clusterin seals the ocular surface barrier in mouse dry eye. PLoS ONE 2015, 10, e0138958. [Google Scholar] [CrossRef]
- Huang, W.; Tourmouzis, K.; Perry, H.; Honkanen, R.A.; Rigas, B. Animal models of dry eye disease: Useful, varied and evolving (Review). Exp. Ther. Med. 2021, 22, 1394. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T. Inflammatory Response in Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES192–DES199. [Google Scholar] [CrossRef]
- Qin, D.Y.; Wang, L.X.; Deng, Y.P. Transgenic dry eye mouse models: Powerful tools to study dry eye disease. Int. J. Ophthalmol. 2022, 15, 635–645. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507. [Google Scholar] [CrossRef]
- Zernii, E.Y.; Baksheeva, V.E.; Iomdina, E.N.; Averina, O.A.; Permyakov, S.E.; Philippov, P.P.; Zamyatnin, A.A.; Senin, I.I. Rabbit models of ocular diseases: New relevance for classical approaches. CNS Neurol. Disord.-Drug Targets Former. Curr. Drug Targets-CNS Neurol. Disord. 2016, 15, 267–291. [Google Scholar] [CrossRef]
- Matsuhisa, F.; Kitajima, S.; Nishijima, K.; Akiyoshi, T.; Morimoto, M.; Fan, J. Transgenic rabbit models: Now and the future. Appl. Sci. 2020, 10, 7416. [Google Scholar] [CrossRef]
- Graur, D.; Duret, L.; Gouy, M. Phylogenetic position of the order Lagomorpha (rabbits, hares and allies). Nature 1996, 379, 333–335. [Google Scholar] [CrossRef]
- Fontanesi, L. Rabbit genetic resources can provide several animal models to explain at the genetic level the diversity of morphological and physiological relevant traits. Appl. Sci. 2021, 11, 373. [Google Scholar] [CrossRef]
- York, M.; Steiling, W. A critical review of the assessment of eye irritation potential using the Draize rabbit eye test. In Journal of Applied Toxicology: An International Forum Devoted to Research and Methods Emphasizing Direct Clinical, Industrial and Environmental Applications; John Wiley & Sons, Ltd.: Chichester, UK, 1998; pp. 233–240. [Google Scholar]
- Barabino, S.; Chen, W.; Dana, M.R. Tear film and ocular surface tests in animal models of dry eye: Uses and limitations. Exp. Eye Res. 2004, 79, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Korb, D.R.; Greiner, J.V.; Glonek, T.; Whalen, A.; Hearn, S.L.; Esway, J.E.; Leahy, C.D. Human and rabbit lipid layer and interference pattern observations. Lacrimal Gland. Tear Film. Dry. Eye Syndr. 2 Basic. Sci. Clin. Relev. 1998, 305–308. [Google Scholar] [CrossRef]
- Honkanen, R.A.; Huang, L.; Xie, G.; Rigas, B. Phosphosulindac is efficacious in an improved concanavalin A-based rabbit model of chronic dry eye disease. Transl. Res. 2018, 198, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, R.A.; Huang, L.; Rigas, B. A Rabbit Model of Aqueous-Deficient Dry Eye Disease Induced by Concanavalin A Injection into the Lacrimal Glands: Application to Drug Efficacy Studies. J. Vis. Exp. 2020, 155, e59631. [Google Scholar]
- Short, B.G. Safety evaluation of ocular drug delivery formulations: Techniques and practical considerations. Toxicol. Pathol. 2008, 36, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Morrison, P.W.; Khutoryanskiy, V.V. Advances in ophthalmic drug delivery. Ther. Deliv. 2014, 5, 1297–1315. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Rogel-Gaillard, C.; Spina, D.; Fontanesi, L.; de Almeida, A.M. The rabbit as an experimental and production animal: From genomics to proteomics. Curr. Protein Pept. Sci. 2014, 15, 134–145. [Google Scholar] [CrossRef]
- Meade, M.L.; Shiyanov, P.; Schlager, J.J. Enhanced detection method for corneal protein identification using shotgun proteomics. Proteome Sci. 2009, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Chen, J.; Wu, W.; Wei, W.; Yu, Y.; Feng, Y. Comparison of the rabbit and human corneal endothelial proteomes regarding proliferative capacity. Exp. Eye Res. 2021, 209, 108629. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W.; Huang, L.; Barathi, A.; Foo, Y.H.; Li, S.F.Y.; Chew, F.T.; Tan, D. Proteomic analysis of rabbit tear fluid: Defensin levels after an experimental corneal wound are correlated to wound closure. Proteomics 2007, 7, 3194–3206. [Google Scholar] [CrossRef]
- Qiu, X.; Gong, L.; Sun, X.; Guo, J.; Chodara, A.M. Efficacy of acupuncture and identification of tear protein expression changes using iTRAQ quantitative proteomics in rabbits. Curr. Eye Res. 2011, 36, 886–894. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Liu, Y.; Challa, P.; Gonzalez, P.; Liu, Y. Proteomic analysis of regenerated rabbit lenses reveal crystallin expression characteristic of adult rabbits. Mol. Vis. 2008, 14, 2404. [Google Scholar]
- Stastna, M.; Behrens, A.; McDonnell, P.J.; Van Eyk, J.E. Analysis of protein composition of rabbit aqueous humor following two different cataract surgery incision procedures using 2-DE and LC-MS/MS. Proteome Sci. 2011, 9, 8. [Google Scholar] [CrossRef]
- Edward, D.P.; Bouhenni, R. Anterior segment alterations and comparative aqueous humor proteomics in the buphthalmic rabbit (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc. 2011, 109, 66. [Google Scholar] [PubMed]
- Moshiri, A.; Chen, R.; Kim, S.; Harris, R.A.; Li, Y.; Raveendran, M.; Davis, S.; Liang, Q.; Pomerantz, O.; Wang, J.; et al. A nonhuman primate model of inherited retinal disease. J. Clin. Investig. 2019, 129, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Picaud, S.; Dalkara, D.; Marazova, K.; Goureau, O.; Roska, B.; Sahel, J.-A. The primate model for understanding and restoring vision. Proc. Natl. Acad. Sci. USA 2019, 116, 26280–26287. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Chu, C.; Wang, F.; Niu, Y. CRISPR/Cas9-mediated genome editing in nonhuman primates. Dis. Models Mech. 2019, 12, dmm039982. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Zhu, S.; Liu, H.; Chen, K.; Zhu, Y.; Li, Z.; Yang, C.; Pan, L.; Li, R.; et al. Design, methodology, and preliminary results of the non-human primates eye study. BMC Ophthalmol. 2023, 23, 53. [Google Scholar] [CrossRef]
- Chatfield, K.; Morton, D. The use of non-human primates in research. In Ethics Dumping: Case Studies From North-South Research Collaborations; Springer International Publishing: Cham, Switzerland, 2018; pp. 81–90. [Google Scholar]
- Gibbs, R.A.; Rogers, J.; Katze, M.G.; Bumgarner, R.; Weinstock, G.M.; Mardis, E.R.; Remington, K.A.; Strausberg, R.L.; Venter, J.C.; Wilson, R.K. Evolutionary and biomedical insights from the rhesus macaque genome. Science 2007, 316, 222–234. [Google Scholar] [CrossRef]
- Seah, I.; Liu, Z.; Wong, D.S.L.; Wong, W.; Holder, G.E.; Barathi, V.A.; Lingam, G.; Su, X.; Stanzel, B.V. Retinal pigment epithelium transplantation in a non-human primate model for degenerative retinal diseases. J. Vis. Exp. 2021, 172, e62638. [Google Scholar]
- Sivak, J.G. The role of the lens in refractive development of the eye: Animal models of ametropia. Exp. Eye Res. 2008, 87, 3–8. [Google Scholar] [CrossRef]
- Braundmeier, A.G.; Fazleabas, A.T. The non-human primate model of endometriosis: Research and implications for fecundity. Mol. Hum. Reprod. 2009, 15, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, C.F. The non-human primate experimental glaucoma model. Exp. Eye Res. 2015, 141, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Penha, F.M.; Rodrigues, E.B.; Maia, M.; Furlani, B.A.; Regatieri, C.; Melo, G.B.; Magalhães, O., Jr.; Manzano, R.; Farah, M.E. Retinal and ocular toxicity in ocular application of drugs and chemicals–part II: Retinal toxicity of current and new drugs. Ophthalmic Res. 2010, 44, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Williams, G.; Downs, J.C.; Sigal, I.A.; Roberts, M.D.; Thompson, H.; Burgoyne, C.F. Posterior (outward) migration of the lamina cribrosa and early cupping in monkey experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7109–7121. [Google Scholar] [CrossRef]
- Crespo-Moral, M.; García-Posadas, L.; López-García, A.; Diebold, Y. Histological and immunohistochemical characterization of the porcine ocular surface. PLoS ONE 2020, 15, e0227732. [Google Scholar] [CrossRef]
- Menduni, F.; Davies, L.N.; Madrid-Costa, D.; Fratini, A.; Wolffsohn, J.S. Characterisation of the porcine eyeball as an in-vitro model for dry eye. Contact Lens Anterior Eye 2018, 41, 13–17. [Google Scholar] [CrossRef]
- Olsen, T.W.; Sanderson, S.; Feng, X.; Hubbard, W.C. Porcine sclera: Thickness and surface area. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2529–2532. [Google Scholar]
- Hendrickson, A.; Hicks, D. Distribution and density of medium-and short-wavelength selective cones in the domestic pig retina. Exp. Eye Res. 2002, 74, 435–444. [Google Scholar] [CrossRef]
- Peynshaert, K.; Devoldere, J.; De Smedt, S.C.; Remaut, K. In vitro and ex vivo models to study drug delivery barriers in the posterior segment of the eye. Adv. Drug Deliv. Rev. 2018, 126, 44–57. [Google Scholar] [CrossRef]
- Lee, G.A.; Chiang, M.Y.M.; Shah, P. Pig eye trabeculectomy—A wet-lab teaching model. Eye 2006, 20, 32–37. [Google Scholar] [CrossRef]
- Fallano, K.; Bussel, I.; Kagemann, L.; Lathrop, K.L.; Loewen, N. Training strategies and outcomes of ab interno trabeculectomy with the trabectome. F1000Research 2017, 6. [Google Scholar] [CrossRef]
- Brunette, I.; Rosolen, S.G.; Carrier, M.; Abderrahman, M.; Nada, O.; Germain, L.; Proulx, S. Comparison of the pig and feline models for full thickness corneal transplantation. Vet. Ophthalmol. 2011, 14, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Schründer, S.; Gärtner, S.; Keipert, S.; Hartmann, C.; Pleyer, U. Ocular drug permeation following experimental excimer laser treatment on the isolated pig eye. J. Ocul. Pharmacol. Ther. 2002, 18, 177–183. [Google Scholar] [CrossRef]
- Proulx, S.; Bourget, J.-M.; Gagnon, N.; Martel, S.; Deschambeault, A.; Carrier, P.; Giasson, C.J.; Auger, F.A.; Brunette, I.; Germain, L. Optimization of culture conditions for porcine corneal endothelial cells. Mol. Vis. 2007, 13, 524–533. [Google Scholar]
- Vijayasekaran, S.; McAllister, I.L.; Morgan, W.H.; Mendis, K.R.; McMenamin, P.G.; Cringle, S.J.; Yu, D.-Y. Intravitreal triamcinolone acetonide induced changes in the anterior segment in a pig model of branch retinal vein occlusion. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 215–222. [Google Scholar] [CrossRef]
- Ekser, B.; Ezzelarab, M.; Hara, H.; van der Windt, D.J.; Wijkstrom, M.; Bottino, R.; Trucco, M.; Cooper, D.K. Clinical xenotransplantation: The next medical revolution? Lancet 2012, 379, 672–683. [Google Scholar] [PubMed]
- Boulze Pankert, M.; Goyer, B.; Zaguia, F.; Bareille, M.; Perron, M.-C.; Liu, X.; Cameron, J.D.; Proulx, S.; Brunette, I. Biocompatibility and Functionality of a Tissue-Engineered Living Corneal Stroma Transplanted in the Feline Eye. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6908–6920. [Google Scholar] [CrossRef]
- Bostan, C.; Thériault, M.; Forget, K.J.; Doyon, C.; Cameron, J.D.; Proulx, S.; Brunette, I. In Vivo Functionality of a Corneal Endothelium Transplanted by Cell-Injection Therapy in a Feline Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1620–1634. [Google Scholar] [CrossRef]
- Hara, H.; Koike, N.; Long, C.; Piluek, J.; Roh, D.S.; SundarRaj, N.; Funderburgh, J.L.; Mizuguchi, Y.; Isse, K.; Phelps, C.J.; et al. Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5278–5286. [Google Scholar] [CrossRef]
- Narfström, K.; Deckman, K.H.; Menotti-Raymond, M. Cats: A gold mine for ophthalmology. Annu. Rev. Anim. Biosci. 2013, 1, 157–177. [Google Scholar] [CrossRef]
- Meekins, J.M.; Rankin, A.J.; Samuelson, D.A. Ophthalmic Anatomy. Vet. Ophthalmol. 2021, 1, 41–123. [Google Scholar]
- Ohno, K.; Nelson, L.R.; Mitooka, K.; Bourne, W.M. Transplantation of cryopreserved human corneas in a xenograft model. Cryobiology 2002, 44, 142–149. [Google Scholar] [CrossRef]
- Ohno, K.; Mitooka, K.; Nelson, L.R.; Hodge, D.O.; Bourne, W.M. Keratocyte activation and apoptosis in transplanted human corneas in a xenograft model. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1025–1031. [Google Scholar]
- Menotti-Raymond, M.; Deckman, K.H.; David, V.; Myrkalo, J.; O’Brien, S.J.; Narfström, K. Mutation discovered in a feline model of human congenital retinal blinding disease. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2852–2859. [Google Scholar] [CrossRef] [PubMed]
- Ellinwood, N.; Deckman, K.; Zhao, Z.; Rutz-Mendicino, M.; Jens, J.; David, V.; Kuehn, M.; O’Brien, S.; Menotti-Raymond, M.; McLellan, G. Candidate gene analysis of a feline model of primary congenital glaucoma implicates LTBP2 as the causative locus. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6390. [Google Scholar]
- Dietrich, J.; Schrader, S. Towards lacrimal gland regeneration: Current concepts and experimental approaches. Curr. Eye Res. 2020, 45, 230–240. [Google Scholar] [CrossRef]
- Schrader, S.; Mircheff, A.K.; Geerling, G. Animal models of dry eye. Surg. Dry. Eye 2008, 41, 298–312. [Google Scholar]
- de Souza, R.G.; de Paiva, C.S.; Alves, M.R. Age-related autoimmune changes in lacrimal glands. Immune Netw. 2019, 19, e3. [Google Scholar] [CrossRef] [PubMed]
- Bentley, E.; Murphy, C.J.; Li, F.; Carlsson, D.J.; Griffith, M. Biosynthetic corneal substitute implantation in dogs. Cornea 2010, 29, 910–916. [Google Scholar] [CrossRef]
- Bentley, E.; Abrams, G.A.; Covitz, D.; Cook, C.S.; Fischer, C.A.; Hacker, D.; Stuhr, C.M.; Reid, T.W.; Murphy, C.J. Morphology and immunohistochemistry of spontaneous chronic corneal epithelial defects (SCCED) in dogs. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2262–2269. [Google Scholar]
- Bibliowicz, J.; Tittle, R.K.; Gross, J.M. Chapter 7—Toward a Better Understanding of Human Eye Disease: Insights from the Zebrafish, Danio rerio. In Progress in Molecular Biology and Translational Science; Chang, K.T., Min, K.-T., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 100, pp. 287–330. [Google Scholar]
- Gestri, G.; Link, B.A.; Neuhauss, S.C.F. The visual system of zebrafish and its use to model human ocular Diseases. Dev. Neurobiol. 2012, 72, 302–327. [Google Scholar] [CrossRef]
- Blanco-Sánchez, B.; Clément, A.; Phillips, J.B.; Westerfield, M. Chapter 16—Zebrafish models of human eye and inner ear diseases. In Methods in Cell Biology; Detrich, H.W., Westerfield, M., Zon, L.I., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 138, pp. 415–467. [Google Scholar]
- Simonetti, R.B.; Marques, L.S.; Streit, D.P., Jr.; Oberst, E.R. Zebrafish (Danio rerio): The future of animal model in biomedical research. J. FisheriesSciences.com 2015, 9, 39. [Google Scholar]
- Bilotta, J.; Saszik, S.; DeLorenzo, A.S.; Hardesty, H.R. Establishing and maintaining a low-cost zebrafish breeding and behavioral research facility. Behav. Res. Methods Instrum. Comput. 1999, 31, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Cavodeassi, F.; Wilson, S.W. Looking to the future of zebrafish as a model to understand the genetic basis of eye disease. Hum. Genet. 2019, 138, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Fadool, J.M.; Dowling, J.E. Zebrafish: A model system for the study of eye genetics. Prog. Retin. Eye Res. 2008, 27, 89–110. [Google Scholar] [CrossRef]
- Soules, K.A.; Link, B.A. Morphogenesis of the anterior segment in the zebrafish eye. BMC Dev. Biol. 2005, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.C.; Yee, R.W.; Norcom, E.; Burgess, H.; Avanesov, A.S.; Barrish, J.P.; Malicki, J. The zebrafish cornea: Structure and development. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4341–4348. [Google Scholar] [CrossRef]
- Greiling, T.M.; Clark, J.I. The transparent lens and cornea in the mouse and zebra fish eye. Semin. Cell Dev. Biol. 2008, 19, 94–99. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Deeti, S.; O’Farrell, S.; Kennedy, B.N. Early safety assessment of human oculotoxic drugs using the zebrafish visualmotor response. J. Pharmacol. Toxicol. Methods 2014, 69, 1–8. [Google Scholar] [CrossRef]
- Ichinohe, S.; Igarashi, T.; Nakajima, D.; Ono, M.; Takahashi, H. Symptoms of dry eye disease and personality traits. PLoS ONE 2016, 11, e0166838. [Google Scholar] [CrossRef]
- Kojima, T.; Dogru, M.; Kawashima, M.; Nakamura, S.; Tsubota, K. Advances in the diagnosis and treatment of dry eye. Prog. Retin. Eye Res. 2020, 78, 100842. [Google Scholar] [CrossRef]
- Jain, M. Dry Eye Syndrome: Emerging Challenge in Ophthalmology. J. Nutr. 2009, 5–15. [Google Scholar]
- Wagh, V.D.; Apar, D.U.; Surana, S.J. Animal Models of Dry Eye Disease-A Review. J. Pharm. Sci. Res. 2012, 4, 1758. [Google Scholar]
- Baudouin, C.; Rolando, M.; Del Castillo, J.M.B.; Messmer, E.M.; Figueiredo, F.C.; Irkec, M.; Van Setten, G.; Labetoulle, M. Reconsidering the central role of mucins in dry eye and ocular surface diseases. Prog. Retin. Eye Res. 2019, 71, 68–87. [Google Scholar] [CrossRef]
- Vickers, L.A.; Gupta, P.K. The future of dry eye treatment: A glance into the therapeutic pipeline. Ophthalmol. Ther. 2015, 4, 69–78. [Google Scholar] [CrossRef]
- Szczotka-Flynn, L.B.; Maguire, M.G.; Ying, G.-s.; Lin, M.C.; Bunya, V.Y.; Dana, R.; Asbell, P.A.; Assessment, D.E. Impact of dry eye on visual acuity and contrast sensitivity: Dry eye assessment and management study. Optom. Vis. Sci. 2019, 96, 387–396. [Google Scholar] [CrossRef]
- Kawashima, M.; Uchino, M.; Yokoi, N.; Uchino, Y.; Dogru, M.; Komuro, A.; Sonomura, Y.; Kato, H.; Kinoshita, S.; Mimura, M.; et al. Associations between Subjective Happiness and Dry Eye Disease: A New Perspective from the Osaka Study. PLoS ONE 2015, 10, e0123299. [Google Scholar] [CrossRef]
- Sano, K.; Kawashima, M.; Imada, T.; Suzuki, T.; Nakamura, S.; Mimura, M.; Tanaka, K.F.; Tsubota, K. Enriched environment alleviates stress-induced dry-eye through the BDNF axis. Sci. Rep. 2019, 9, 3422. [Google Scholar] [CrossRef]
- Prusky, G.T.; West, P.W.R.; Douglas, R.M. Behavioral assessment of visual acuity in mice and rats. Vis. Res. 2000, 40, 2201–2209. [Google Scholar] [CrossRef]
- Baudouin, C. The pathology of dry eye. Surv. Ophthalmol. 2001, 45, S211–S220. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Solomon, A.; Stern, M.E. The diagnosis and management of dry eye: A twenty-five–year review. Cornea 2000, 19, 644–649. [Google Scholar] [CrossRef]
- Han, S.B.; Liu, Y.-C.; Mohamed-Noriega, K.; Tong, L.; Mehta, J.S. Objective imaging diagnostics for dry eye disease. J. Ophthalmol. 2020, 2020, 11. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J. Tfos dews ii epidemiology report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef]
- Clayton, J.A. Dry eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef]
- Barabino, S.; Benitez-Del-Castillo, J.; Fuchsluger, T.; Labetoulle, M.; Malachkova, N.; Meloni, M.; Paaske Utheim, T.; Rolando, M. Dry eye disease treatment: The role of tear substitutes, their future, and an updated classification. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8642–8652. [Google Scholar]
- Chang, Y.-A.; Wu, Y.-Y.; Lin, C.-T.; Kawasumi, M.; Wu, C.-H.; Kao, S.-Y.; Yang, Y.-P.; Hsu, C.-C.; Hung, K.-F.; Sun, Y.-C. Animal models of dry eye: Their strengths and limitations for studying human dry eye disease. J. Chin. Med. Assoc. 2021, 84, 459–464. [Google Scholar] [CrossRef]
- Guzmán, M.; Keitelman, I.; Sabbione, F.; Trevani, A.S.; Giordano, M.N.; Galletti, J.G. Desiccating stress-induced disruption of ocular surface immune tolerance drives dry eye disease. Clin. Exp. Immunol. 2016, 184, 248–256. [Google Scholar] [CrossRef]
- Gong, L.; Guan, Y.; Cho, W.; Li, B.; Pan, L.; Yang, Z.; Wu, M.; Yang, Z.; Chauhan, S.K.; Zeng, W. A new non-human primate model of desiccating stress-induced dry eye disease. Sci. Rep. 2022, 12, 7957. [Google Scholar] [CrossRef]
- Honkanen, R.; Huang, W.; Huang, L.; Kaplowitz, K.; Weissbart, S.; Rigas, B. A new rabbit model of chronic dry eye disease induced by complete surgical dacryoadenectomy. Curr. Eye Res. 2019, 44, 863–872. [Google Scholar] [CrossRef]
- Zhu, Z.; Stevenson, D.; Schechter, J.E.; Mircheff, A.K.; Atkinson, R.; Trousdale, M.D. Lacrimal histopathology and ocular surface disease in a rabbit model of autoimmune dacryoadenitis. Cornea 2003, 22, 25–32. [Google Scholar] [CrossRef]
- Culp, D.J.; Latchney, L.R.; Fallon, M.A.; Denny, P.A.; Denny, P.C.; Couwenhoven, R.I.; Chuang, S. The gene encoding mouse Muc19: cDNA, genomic organization and relationship to Smgc. Physiol. Genom. 2004, 19, 303–318. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Liu, Z.; Monroy, D.; Li, D.Q.; Carvajal, M.E.; Price–Schiavi, S.A.; Idris, N.; Solomon, A.; Perez, A.; Carraway, K.L. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1316–1326. [Google Scholar]
- Gipson, I.K. Distribution of mucins at the ocular surface. Exp. Eye Res. 2004, 78, 379–388. [Google Scholar] [CrossRef]
- Marko, C.K.; Tisdale, A.S.; Spurr-Michaud, S.; Evans, C.; Gipson, I.K. The ocular surface phenotype of Muc5ac and Muc5b null mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 291–300. [Google Scholar] [CrossRef]
- Menon, B.B.; Kaiser-Marko, C.; Spurr-Michaud, S.; Tisdale, A.S.; Gipson, I.K. Suppression of Toll-like receptor-mediated innate immune responses at the ocular surface by the membrane-associated mucins MUC1 and MUC16. Mucosal Immunol. 2015, 8, 1000–1008. [Google Scholar] [CrossRef]
- Kim, C.-S.; Jo, K.; Lee, I.-S.; Kim, J. Topical application of apricot kernel extract improves dry eye symptoms in a unilateral exorbital lacrimal gland excision mouse. Nutrients 2016, 8, 750. [Google Scholar] [CrossRef]
- Gipson, I.K.; Spurr-Michaud, S.; Tisdale, A. Human conjunctival goblet cells express the membrane associated mucin MUC16: Localization to mucin granules. Exp. Eye Res. 2016, 145, 230–234. [Google Scholar] [CrossRef]
- Shirai, K.; Saika, S. Ocular surface mucins and local inflammation—Studies in genetically modified mouse lines. BMC Ophthalmol. 2015, 15, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.; Chen, Y.; Lee, S.-M.; Lee, H.S.; Hua, J.; Dohlman, T.; Shiang, T.; Dana, R. Extraorbital lacrimal gland excision: A reproducible model of severe aqueous tear-deficient dry eye disease. Cornea 2014, 33, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Mores, A.M.; Casey, S.D.; Felix, C.M.; Phuan, P.W.; Verkman, A.S.; Levin, M.H. Small-molecule CFTR activators increase tear secretion and prevent experimental dry eye disease. FASEB J. 2016, 30, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; Cotrim, A.P.; Zheng, C.; Riveros, P.P.; Baum, B.J.; Chiorini, J.A. Recovery of Radiation-Induced Dry Eye and Corneal Damage by Pretreatment with Adenoviral Vector-Mediated Transfer of Erythropoietin to the Salivary Glands in Mice. Hum. Gene Ther. 2013, 24, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, B.; Woo, I.H.; Eom, Y.; Lee, H.K.; Kim, H.M.; Song, J.S. Effects of topical mucolytic agents on the tears and ocular surface: A plausible animal model of mucin-deficient dry eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3104–3114. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, X.; Zhou, T.; Wang, Y.; Bai, L.; He, H.; Liu, Z. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol. Vis. 2011, 17, 257. [Google Scholar]
- Marques, D.L.; Alves, M.; Modulo, C.M.; Silva, L.E.C.M.d.; Reinach, P. Lacrimal osmolarity and ocular surface in experimental model of dry eye caused by toxicity. Rev. Bras. De Oftalmol. 2015, 74, 68–72. [Google Scholar] [CrossRef]
- Suwan-Apichon, O.; Rizen, M.; Rangsin, R.; Herretes, S.; Reyes, J.M.; Lekhanont, K.; Chuck, R.S. Botulinum toxin B-induced mouse model of keratoconjunctivitis sicca. Investig. Ophthalmol. Vis. Sci. 2006, 47, 133–139. [Google Scholar] [CrossRef]
- Chen, Z.-y.; Liang, Q.-f.; Yu, G.-y. Establishment of a rabbit model for keratoconjunctivitis sicca. Cornea 2011, 30, 1024–1029. [Google Scholar] [CrossRef]
- Gilbard, J.; Rossi, S.R.; Gray, K.L. A new rabbit model for keratoconjunctivitis sicca. Investig. Ophthalmol. Vis. Sci. 1987, 28, 225–228. [Google Scholar]
- Li, N.; Deng, X.; Gao, Y.; Zhang, S.; He, M.; Zhao, D. Establishment of the mild, moderate and severe dry eye models using three methods in rabbits. BMC Ophthalmol. 2013, 13, 50. [Google Scholar] [CrossRef]
- Gilbard, J.P.; Rossi, S.R.; Heyda, K.G. Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology 1989, 96, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Zheng, X.; Okamoto, S.; Ohashi, Y. Tear film stability analysis system: Introducing a new application for videokeratography. Cornea 2004, 23, S65–S70. [Google Scholar] [CrossRef] [PubMed]
- Kollias, C.M.; Huneke, R.B.; Wigdahl, B.; Jennings, S.R. Animal models of herpes simplex virus immunity and pathogenesis. J. Neurovirology 2015, 21, 8–23. [Google Scholar] [CrossRef]
- Rowe, A.M.; St Leger, A.J.; Jeon, S.; Dhaliwal, D.K.; Knickelbein, J.E.; Hendricks, R.L. Herpes keratitis. Prog. Retin. Eye Res. 2013, 32, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Al-Dujaili, L.J.; Clerkin, P.P.; Clement, C.; McFerrin, H.E.; Bhattacharjee, P.S.; Varnell, E.D.; Kaufman, H.E.; Hill, J.M. Ocular herpes simplex virus: How are latency, reactivation, recurrent disease and therapy interrelated? Future Microbiol. 2011, 6, 877–907. [Google Scholar] [CrossRef]
- Hendricks, R.L.; Yun, H.; Rowe, A.M.; Carroll, K.L. Animal Models of Herpes Keratitis. In Animal Models of Ophthalmic Diseases; Chan, C.-C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–10. [Google Scholar] [CrossRef]
- Royer, D.J.; Cohen, A.; Carr, D. The Current State of Vaccine Development for Ocular HSV-1 Infection. Expert Rev. Ophthalmol. 2015, 10, 113–126. [Google Scholar] [CrossRef]
- Hamrah, P.; Cruzat, A.; Dastjerdi, M.H.; Zheng, L.; Shahatit, B.M.; Bayhan, H.A.; Dana, R.; Pavan-Langston, D. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: An in vivo confocal microscopy study. Ophthalmology 2010, 117, 1930–1936. [Google Scholar] [CrossRef]
- Yun, H.; Rowe, A.M.; Lathrop, K.L.; Harvey, S.A.; Hendricks, R.L. Reversible nerve damage and corneal pathology in murine herpes simplex stromal keratitis. J. Virol. 2014, 88, 7870–7880. [Google Scholar] [CrossRef]
- Decman, V.; Freeman, M.L.; Kinchington, P.R.; Hendricks, R.L. Immune Control of HSV-1 Latency. Viral Immunol. 2005, 18, 466–473. [Google Scholar] [CrossRef]
- Hill, G.M.; Ku, E.S.; Dwarakanathan, S. Herpes simplex keratitis. Dis.-A-Mon. 2014, 60, 239–246. [Google Scholar] [CrossRef]
- Chentoufi, A.A.; Dhanushkodi, N.R.; Srivastava, R.; Prakash, S.; Coulon, P.-G.A.; Zayou, L.; Vahed, H.; Chentoufi, H.A.; Hormi-Carver, K.K.; BenMohamed, L. Combinatorial herpes simplex vaccine strategies: From bedside to bench and back. Front. Immunol. 2022, 13, 1606. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.S.; Neumann, D.M.; Foster, T.P.; Bouhanik, S.; Clement, C.; Vinay, D.; Thompson, H.W.; Hill, J.M. Effect of human apolipoprotein E genotype on the pathogenesis of experimental ocular HSV-1. Exp. Eye Res. 2008, 87, 122–130. [Google Scholar] [CrossRef]
- Biswas, P.S.; Banerjee, K.; Kim, B.; Rouse, B.T. Mice transgenic for IL-1 receptor antagonist protein are resistant to herpetic stromal keratitis: Possible role for IL-1 in herpetic stromal keratitis pathogenesis. J. Immunol. 2004, 172, 3736–3744. [Google Scholar] [CrossRef]
- Kim, B.; Sarangi, P.P.; Azkur, A.K.; Kaistha, S.D.; Rouse, B.T. Enhanced viral immunoinflammatory lesions in mice lacking IL-23 responses. Microbes Infect. 2008, 10, 302–312. [Google Scholar] [CrossRef]
- Haenchen, S.D.; Utter, J.A.; Bayless, A.M.; Dobrowsky, R.T.; Davido, D.J. Role of a cdk5-associated protein, p35, in herpes simplex virus type 1 replication in vivo. J. Neurovirology 2010, 16, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Laycock, K.; Lee, S.; Brady, R.; Pepose, J. Characterization of a murine model of recurrent herpes simplex viral keratitis induced by ultraviolet B radiation. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2741–2746. [Google Scholar]
- Sawtell, N.; Thompson, R. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J. Virol. 1992, 66, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Steiner, I.; Spivack, J.; Deshmane, S.; Ace, C.; Preston, C.; Fraser, N. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J. Virol. 1990, 64, 1630–1638. [Google Scholar] [CrossRef]
- Valencia, F.; Veselenak, R.L.; Bourne, N. In vivo evaluation of antiviral efficacy against genital herpes using mouse and guinea pig models. In Antiviral Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2013; pp. 315–326. [Google Scholar]
- Hill, J.M.; Nolan, N.M.; McFerrin, H.E.; Clement, C.; Foster, T.P.; Halford, W.P.; Kousoulas, K.G.; Lukiw, W.J.; Thompson, H.W.; Stern, E.M.; et al. HSV-1 latent rabbits shed viral DNA into their saliva. Virol. J. 2012, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, B.; Varnell, E.; Hill, J.; Kaufman, H. Animal models of ocular herpes simplex virus infection (rabbits, primates, mice). In Handbook of Animal Models of Infection; Academic Press: Cambridge, MA, USA, 1999; pp. 919–926. [Google Scholar]
- Chentoufi, A.A.; Dasgupta, G.; Christensen, N.D.; Hu, J.; Choudhury, Z.S.; Azeem, A.; Jester, J.V.; Nesburn, A.B.; Wechsler, S.L.; BenMohamed, L. A novel HLA (HLA-A* 0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J. Immunol. 2010, 184, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.V.; Pahar, B.; Chouljenko, V.N.; Walker, J.D.; Stanfield, B.; Kousoulas, K.G. Single dose of Glycoprotein K (gK)-deleted HSV-1 live-attenuated virus protects mice against lethal vaginal challenge with HSV-1 and HSV-2 and induces lasting T cell memory immune responses. Virol. J. 2013, 10, 317. [Google Scholar] [CrossRef]
- Desjardins, P.; Couture, C.; Germain, L.; Guérin, S.L. Contribution of the WNK1 kinase to corneal wound healing using the tissue-engineered human cornea as an in vitro model. J. Tissue Eng. Regen. Med. 2019, 13, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- McGwin, G.; Owsley, C. Incidence of emergency department–treated eye injury in the United States. Arch. Ophthalmol. 2005, 123, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Couture, C.; Desjardins, P.; Zaniolo, K.; Germain, L.; Guérin, S.L. Enhanced wound healing of tissue-engineered human corneas through altered phosphorylation of the CREB and AKT signal transduction pathways. Acta Biomater. 2018, 73, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Wahlig, S.; Peh, G.S.-L.; Lovatt, M.; Mehta, J.S. Dysfunctional Corneal Endothelium: Delivery of Cell Therapy. In Corneal Regeneration; Springer International Publishing: Cham, Switzerland, 2019; pp. 485–497. [Google Scholar]
- Heur, M.; Jiao, S.; Schindler, S.; Crump, J.G. Regenerative potential of the zebrafish corneal endothelium. Exp. Eye Res. 2013, 106, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Leonard, B.C.; Raghunathan, V.K.; Kim, S.; Li, J.Y.; Mannis, M.J.; Murphy, C.J.; Thomasy, S.M. Animal models of corneal endothelial dysfunction to facilitate development of novel therapies. Ann. Transl. Med. 2021, 9, 1271. [Google Scholar] [CrossRef] [PubMed]
- Català, P.; Thuret, G.; Skottman, H.; Mehta, J.S.; Parekh, M.; Dhubhghaill, S.N.; Collin, R.W.; Nuijts, R.M.; Ferrari, S.; LaPointe, V.L.; et al. Approaches for corneal endothelium regenerative medicine. Prog. Retin. Eye Res. 2021, 87, 100987. [Google Scholar] [CrossRef] [PubMed]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Mobaraki, M.; Abbasi, R.; Omidian Vandchali, S.; Ghaffari, M.; Moztarzadeh, F.; Mozafari, M. Corneal Repair and Regeneration: Current Concepts and Future Directions. Front. Bioeng. Biotechnol. 2019, 7, 135. [Google Scholar] [CrossRef]
- Han, S.B.; Ang, H.; Balehosur, D.; Peh, G.; Chaurasia, S.S.; Tan, D.T.; Mehta, J.S. A mouse model of corneal endothelial decompensation using cryoinjury. Mol. Vis. 2013, 19, 1222. [Google Scholar]
- Cui, Z.; Zeng, Q.; Liu, S.; Zhang, Y.; Zhu, D.; Guo, Y.; Xie, M.; Mathew, S.; Cai, D.; Zhang, J.; et al. Cell-laden and orthogonal-multilayer tissue-engineered corneal stroma induced by a mechanical collagen microenvironment and transplantation in a rabbit model. Acta Biomater. 2018, 75, 183–199. [Google Scholar] [CrossRef]
- Zhou, Z.; Long, D.; Hsu, C.-C.; Liu, H.; Chen, L.; Slavin, B.; Lin, H.; Li, X.; Tang, J.; Yiu, S.; et al. Nanofiber-reinforced decellularized amniotic membrane improves limbal stem cell transplantation in a rabbit model of corneal epithelial defect. Acta Biomater. 2019, 97, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Ma, X.; Zhao, J.; Wen, Q.; Hu, X.; Yu, H.; Shi, W. Transplantation of tissue-engineered human corneal endothelium in cat models. Mol. Vis. 2013, 19, 400. [Google Scholar] [PubMed]
- Rolev, K.; Coussons, P.; King, L.; Rajan, M. Experimental models of corneal endothelial cell therapy and translational challenges to clinical practice. Exp. Eye Res. 2019, 188, 107794. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Tsuchiya, H.; Hamuro, J.; Kinoshita, S. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am. J. Pathol. 2012, 181, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, M.; Shima, N.; Yamaguchi, M.; Hiraoka, Y.; Amano, S.; Yamagami, S. Development of a bioengineered corneal endothelial cell sheet to fit the corneal curvature. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2337–2343. [Google Scholar] [CrossRef]
- Okumura, N.; Sakamoto, Y.; Fujii, K.; Kitano, J.; Nakano, S.; Tsujimoto, Y.; Nakamura, S.-i.; Ueno, M.; Hagiya, M.; Hamuro, J. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci. Rep. 2016, 6, 26113. [Google Scholar] [CrossRef]
- Tuft, S.; Coster, D. The corneal endothelium. Eye 1990, 4, 389–424. [Google Scholar] [CrossRef]
- Valdez-Garcia, J.E.; Lozano-Ramirez, J.F.; Zavala, J. Adult white New Zealand rabbit as suitable model for corneal endothelial engineering. BMC Res. Notes 2015, 8, 28. [Google Scholar] [CrossRef]
- Rodrigues, G.N.; Laus, J.L.; Santos, J.M.; Rigueiro, M.P.; Smith, R.L. Corneal endothelial cell morphology of normal dogs in different ages. Vet. Ophthalmol. 2006, 9, 101–107. [Google Scholar] [CrossRef]
- Van Horn, D.L.; Sendele, D.D.; Seideman, S.; Buco, P.J. Regenerative capacity of the corneal endothelium in rabbit and cat. Investig. Ophthalmol. Vis. Sci. 1977, 16, 597–613. [Google Scholar]
- Doughty, M.J. The cornea and corneal endothelium in the aged rabbit. Optom. Vis. Sci. 1994, 71, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Morita, H. Specular microscopy of corneal endothelial cells in rabbits. J. Vet. Med. Sci. 1995, 57, 273–277. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Mittal, S.K.; Foulsham, W.; Elbasiony, E.; Singhania, D.; Sahu, S.K.; Chauhan, S.K. Therapeutic efficacy of different routes of mesenchymal stem cell administration in corneal injury. Ocul. Surf. 2019, 17, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Chen, X.; Cao, H.; Zheng, L.; Li, Q.; Zhang, K.; Han, Z.; Han, Z.-C.; Guo, Z.; Li, Z. Mesenchymal stem cell-derived extracellular vesicles for corneal wound repair. Stem Cells Int. 2019, 2019, 5738510. [Google Scholar] [CrossRef] [PubMed]
- Faye, P.A.; Poumeaud, F.; Chazelas, P.; Duchesne, M.; Rassat, M.; Miressi, F.; Lia, A.S.; Sturtz, F.; Robert, P.-Y.; Favreau, F. Focus on cell therapy to treat corneal endothelial diseases. Exp. Eye Res. 2021, 204, 108462. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Okumura, N. Cell based therapy for corneal endothelial regeneration. In Corneal Regeneration; Springer International Publishing: Cham, Switzerland, 2019; pp. 455–462. [Google Scholar]
- Ho, W.-T.; Liu, H.-Y.; Hu, F.-R.; Wang, I. Corneal Endothelium Regeneration: Future Prospects. In Corneal Regeneration; Springer International Publishing: Cham, Switzerland, 2019; pp. 463–473. [Google Scholar]
- Bleul, T.; Zhuang, X.; Hildebrand, A.; Lange, C.; Böhringer, D.; Schlunck, G.; Reinhard, T.; Lapp, T. Different Innate Immune Responses in BALB/c and C57BL/6 Strains following Corneal Transplantation. J. Innate Immun. 2020, 13, 49–59. [Google Scholar] [CrossRef]
- Bourne, W.; Gebhardt, B.; Sugar, A.; Meyer, R.; Kaufman, H. The effect of splenectomy on corneal graft rejection. Investig. Ophthalmol. Vis. Sci. 1976, 15, 541–542. [Google Scholar]
- Khodadoust, A.A.; Silverstein, A.M. Studies on the nature of the privilege enjoyed by corneal allografts. Invest. Ophthalmol. 1972, 11, 137–148. [Google Scholar]
- Nicholas, M.P.; Mysore, N. Corneal neovascularization. Exp. Eye Res. 2021, 202, 108363. [Google Scholar] [CrossRef]
- Ciftci, M.; Selver, O. Clinical Evaluation of Corneal Neovascularization: A Brief Review. J. Ophthalmic Res. Vis. Care 2022, 2, 6. [Google Scholar]
- Chang, J.-H.; Gabison, E.E.; Kato, T.; Azar, D.T. Corneal neovascularization. Curr. Opin. Ophthalmol. 2001, 12, 242–249. [Google Scholar] [CrossRef]
- Gonzalez, L.; Loza, R.J.; Han, K.-Y.; Sunoqrot, S.; Cunningham, C.; Purta, P.; Drake, J.; Jain, S.; Hong, S.; Chang, J.-H. Nanotechnology in Corneal Neovascularization Therapy—A Review. J. Ocul. Pharmacol. Ther. 2013, 29, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Azari, A.A.; Safapour, S. Therapeutic approaches for corneal neovascularization. Eye Vis. 2017, 4, 28. [Google Scholar] [CrossRef]
- BenEzra, D.; Griffin, B.W.; Maftzir, G.; Sharif, N.A.; Clark, A.F. Topical formulations of novel angiostatic steroids inhibit rabbit corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1954–1962. [Google Scholar]
- Ko, B.-Y.; Kim, Y.-s.; Baek, S.-g.; Lee, G.-w.; Kim, J.-M.; Jean, W.-S.; Lee, N.-S.; Kang, J. Inhibition of corneal neovascularization by subconjunctival and topical bevacizumab and sunitinib in a rabbit model. Cornea 2013, 32, 689–695. [Google Scholar] [CrossRef]
- Giacomini, C.; Ferrari, G.; Bignami, F.; Rama, P. Alkali burn versus suture-induced corneal neovascularization in C57BL/6 mice: An overview of two common animal models of corneal neovascularization. Exp. Eye Res. 2014, 121, 1–4. [Google Scholar] [CrossRef]
- Voiculescu, O.; Voinea, L.; Alexandrescu, C. Corneal neovascularization and biological therapy. J. Med. Life 2015, 8, 444. [Google Scholar]
- Hsu, C.-C.; Chang, H.-M.; Lin, T.-C.; Hung, K.-H.; Chien, K.-H.; Chen, S.-Y.; Chen, S.-N.; Chen, Y.-T. Corneal neovascularization and contemporary antiangiogenic therapeutics. J. Chin. Med. Assoc. 2015, 78, 323–330. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Wang, X.; Liang, J.; Zhang, Y. Recent drug therapies for corneal neovascularization. Chem. Biol. Drug Des. 2017, 90, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-T.; Hu, F.-R.; Kuo, K.-T.; Chen, Y.-M.; Chu, H.-S.; Lin, Y.-H.; Chen, W.-L. The different effects of early and late bevacizumab (Avastin) injection on inhibiting corneal neovascularization and conjunctivalization in rabbit limbal insufficiency. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6277–6285. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.M.; Kumar, A.; Mousa, S.; Dyskin, E.; Yalcin, M.; Ajayan, P.; Linhardt, R.J.; Mousa, S.A. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology 2009, 20, 455104. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-K.; Kang, S.; Choi, H.; Rho, C.R. Topically administered gold nanoparticles inhibit experimental corneal neovascularization in mice. Cornea 2015, 34, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Masse, F.; Ouellette, M.; Lamoureux, G.; Boisselier, E. Gold nanoparticles in ophthalmology. Med. Res. Rev. 2019, 39, 302–327. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.S.; Møller, H.U.; Aldave, A.J.; Seitz, B.; Bredrup, C.; Kivelä, T.; Munier, F.L.; Rapuano, C.J.; Nischal, K.K.; Kim, E.K. IC3D classification of corneal dystrophies—Edition 2. Cornea 2015, 34, 117–159. [Google Scholar] [CrossRef] [PubMed]
- Bourges, J.L. Corneal dystrophies. J. Français D’ophtalmologie 2017, 40, e177–e192. [Google Scholar] [CrossRef]
- Aggarwal, S.; Peck, T.; Golen, J.; Karcioglu, Z.A. Macular corneal dystrophy: A review. Surv. Ophthalmol. 2018, 63, 609–617. [Google Scholar] [CrossRef]
- Lisch, W.; Weiss, J.S. Clinical and genetic update of corneal dystrophies. Exp. Eye Res. 2019, 186, 107715. [Google Scholar] [CrossRef]
- Zhang, J.; McGhee, C.N.; Patel, D.V. The molecular basis of fuchs’ endothelial corneal dystrophy. Mol. Diagn. Ther. 2019, 23, 97–112. [Google Scholar] [CrossRef]
- Thériault, M.; Gendron, S.P.; Brunette, I.; Rochette, P.J.; Proulx, S. Function-Related Protein Expression in Fuchs Endothelial Corneal Dystrophy Cells and Tissue Models. Am. J. Pathol. 2018, 188, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Ong Tone, S.; Kocaba, V.; Böhm, M.; Wylegala, A.; White, T.L.; Jurkunas, U.V. Fuchs endothelial corneal dystrophy: The vicious cycle of Fuchs pathogenesis. Prog. Retin. Eye Res. 2021, 80, 100863. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, M.; Hribek, A.; Clahsen, T.; Bachmann, B.; Cursiefen, C.; Jun, A.S. Fuchs endothelial corneal dystrophy: Clinical, genetic, pathophysiologic, and therapeutic aspects. Annu. Rev. Vis. Sci. 2019, 5, 151–175. [Google Scholar] [CrossRef]
- Goyer, B.; Thériault, M.; Gendron, S.P.; Brunette, I.; Rochette, P.J.; Proulx, S. Extracellular matrix and integrin expression profiles in Fuchs endothelial corneal dystrophy cells and tissue model. Tissue Eng. Part A 2018, 24, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Nanda, G.G.; Alone, D.P. Current understanding of the pathogenesis of Fuchs’ endothelial corneal dystrophy. Mol. Vis. 2019, 25, 295. [Google Scholar]
- Vedana, G.; Villarreal, G., Jr.; Jun, A.S. Fuchs endothelial corneal dystrophy: Current perspectives. Clin. Ophthalmol. (Auckl. NZ) 2016, 10, 321. [Google Scholar]
- Okumura, N.; Kinoshita, S.; Koizumi, N. Cell-based approach for treatment of corneal endothelial dysfunction. Cornea 2014, 33, S37–S41. [Google Scholar] [CrossRef]
- Koizumi, N.; Okumura, N.; Ueno, M.; Nakagawa, H.; Hamuro, J.; Kinoshita, S. Rho-associated kinase inhibitor eye drop treatment as a possible medical treatment for Fuchs corneal dystrophy. Cornea 2013, 32, 1167–1170. [Google Scholar] [CrossRef]
- Koizumi, N.; Okumura, N.; Ueno, M.; Kinoshita, S. New therapeutic modality for corneal endothelial disease using Rho-associated kinase inhibitor eye drops. Cornea 2014, 33, S25–S31. [Google Scholar] [CrossRef]
- Hopfer, U.; Fukai, N.; Hopfer, H.; Wolf, G.; Joyce, N.; Li, E.; Olsen, B.R. Targeted disruption of Col8a1 and Col8a2 genes in mice leads to anterior segment abnormalities in the eye. FASEB J. 2005, 19, 1232–1244. [Google Scholar] [CrossRef]
- Jun, A.S.; Meng, H.; Ramanan, N.; Matthaei, M.; Chakravarti, S.; Bonshek, R.; Black, G.C.; Grebe, R.; Kimos, M. An alpha 2 collagen VIII transgenic knock-in mouse model of Fuchs endothelial corneal dystrophy shows early endothelial cell unfolded protein response and apoptosis. Hum. Mol. Genet. 2012, 21, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Matthaei, M.; Ramanan, N.; Grebe, R.; Chakravarti, S.; Speck, C.L.; Kimos, M.; Vij, N.; Eberhart, C.G.; Jun, A.S. L450W and Q455K Col8a2 knock-in mouse models of Fuchs endothelial corneal dystrophy show distinct phenotypes and evidence for altered autophagy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Meng, H.; Jun, A.S. Lithium treatment increases endothelial cell survival and autophagy in a mouse model of Fuchs endothelial corneal dystrophy. Br. J. Ophthalmol. 2013, 97, 1068–1073. [Google Scholar] [CrossRef]
- Kim, E.C.; Meng, H.; Jun, A.S. N-Acetylcysteine increases corneal endothelial cell survival in a mouse model of Fuchs endothelial corneal dystrophy. Exp. Eye Res. 2014, 127, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.P.; Hill, M.J.; Quantock, A.J.; Martin-Rendon, E.; Blake, D.J. The emerging roles of TCF4 in disease and development. Trends Mol. Med. 2014, 20, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Flora, A.; Garcia, J.J.; Thaller, C.; Zoghbi, H.Y. The E-protein Tcf4 interacts with Math1 to regulate differentiation of a specific subset of neuronal progenitors. Proc. Natl. Acad. Sci. USA 2007, 104, 15382. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Azizi, B.; Jurkunas, U.V. Fuchs endothelial corneal dystrophy. Ocul. Surf. 2010, 8, 173–184. [Google Scholar] [CrossRef]
- Lopez, I.A.; Rosenblatt, M.I.; Kim, C.; Galbraith, G.C.; Jones, S.M.; Kao, L.; Newman, D.; Liu, W.; Yeh, S.; Pushkin, A. Slc4a11 gene disruption in mice: Cellular targets of sensorineuronal abnormalities. J. Biol. Chem. 2009, 284, 26882–26896. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, X.; Tan, J.; Darling, D.S.; Kaplan, H.J.; Dean, D.C. Zeb1 Mutant Mice as a Model of Posterior Corneal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1843–1849. [Google Scholar] [CrossRef]
- Liu, C.; Miyajima, T.; Melangath, G.; Miyai, T.; Vasanth, S.; Deshpande, N.; Kumar, V.; Tone, S.O.; Gupta, R.; Zhu, S. Ultraviolet A light induces DNA damage and estrogen-DNA adducts in Fuchs endothelial corneal dystrophy causing females to be more affected. Proc. Natl. Acad. Sci. USA 2020, 117, 573–583. [Google Scholar] [CrossRef]
- Markoulli, M.; Flanagan, J.; Tummanapalli, S.S.; Wu, J.; Willcox, M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul. Surf. 2018, 16, 45–57. [Google Scholar] [CrossRef]
- Mansoor, H.; Tan, H.C.; Lin, M.T.-Y.; Mehta, J.S.; Liu, Y.-C. Diabetic Corneal Neuropathy. J. Clin. Med. 2020, 9, 3956. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.V. Diabetic complications in the cornea. Vis. Res. 2017, 139, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.; Dwarakanathan, S. Diabetic keratopathy. Dis.-A-Mon. 2021, 67, 101135. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Patel, D.V.; McGhee, C.N.; Alany, R.G. New therapeutic approaches in the treatment of diabetic keratopathy: A review. Clin. Exp. Ophthalmol. 2011, 39, 259–270. [Google Scholar] [CrossRef]
- Bu, Y.; Shih, K.C.; Kwok, S.S.; Chan, Y.K.; Lo, A.C.-Y.; Chan, T.C.Y.; Jhanji, V.; Tong, L. Experimental modeling of cornea wound healing in diabetes: Clinical applications and beyond. BMJ Open Diabetes Res. Care 2019, 7, e000779. [Google Scholar] [CrossRef]
- Olivares, A.M.; Althoff, K.; Chen, G.F.; Wu, S.; Morrisson, M.A.; DeAngelis, M.M.; Haider, N. Animal models of diabetic retinopathy. Curr. Diabetes Rep. 2017, 17, 93. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Whelchel, A.; Nicholas, S.; Sharif, R.; Riaz, K.; Karamichos, D. Diabetic Keratopathy: Insights and Challenges. Surv. Ophthalmol. 2020, 65, 513–529. [Google Scholar] [CrossRef]
- Kottaisamy, C.P.D.; Raj, D.S.; Prasanth Kumar, V.; Sankaran, U. Experimental animal models for diabetes and its related complications—A review. Lab. Anim. Res. 2021, 37, 23. [Google Scholar] [CrossRef]
- Hatchell, D.L.; Magolan, J.J.; Besson, M.J.; Goldman, A.I.; Pederson, H.J.; Schultz, K.J. Damage to the epithelial basement membrane in the corneas of diabetic rabbits. Arch. Ophthalmol. 1983, 101, 469–471. [Google Scholar] [CrossRef]
- Nakamura, M.; Kawahara, M.; Morishige, N.; Chikama, T.; Nakata, K.; Nishida, T. Promotion of corneal epithelial wound healing in diabetic rats by the combination of a substance P-derived peptide (FGLM-NH 2) and insulin-like growth factor-1. Diabetologia 2003, 46, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Bikbova, G.; Oshitari, T.; Baba, T.; Bikbov, M.; Yamamoto, S. Diabetic corneal neuropathy: Clinical perspectives. Clin. Ophthalmol. 2018, 12, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Macdonald Ighodaro, O.; Mohammed Adeosun, A.; Adeboye Akinloye, O. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina 2017, 53, 365–374. [Google Scholar] [CrossRef]
- Crooke, A.; Huete-Toral, F.; Martínez-Águila, A.; Colligris, B.; Pintor, J. Ocular disorders and the utility of animal models in the discovery of melatoninergic drugs with therapeutic potential. Expert Opin. Drug Discov. 2012, 7, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Culina, S.; Brezar, V.; Mallone, R. MECHANISMS IN ENDOCRINOLOGY: Insulin and type 1 diabetes: Immune connections. Eur. J. Endocrinol. 2013, 168, R19–R31. [Google Scholar] [CrossRef]
- Li, C.; Yang, M.; Wang, X.; Zhang, H.; Yao, C.; Sun, S.; Liu, Q.; Pan, H.; Liu, S.; Huan, Y.; et al. Glutazumab, a novel long-lasting GLP-1/anti-GLP-1R antibody fusion protein, exerts anti-diabetic effects through targeting dual receptor binding sites. Biochem. Pharmacol. 2018, 150, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Shevalye, H.; Yorek, M.S.; Coppey, L.J.; Holmes, A.; Harper, M.M.; Kardon, R.H.; Yorek, M.A. Effect of enriching the diet with menhaden oil or daily treatment with resolvin D1 on neuropathy in a mouse model of type 2 diabetes. J. Neurophysiol. 2015, 114, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hamid, M.; Moustafa, N. Amelioration of alloxan-induced diabetic keratopathy by beta-carotene. Exp. Toxicol. Pathol. 2014, 66, 49–59. [Google Scholar] [CrossRef]
- Davidson, E.P.; Coppey, L.J.; Shevalye, H.; Obrosov, A.; Kardon, R.H.; Yorek, M.A. Impaired corneal sensation and nerve loss in a type 2 rat model of chronic diabetes is reversible with combination therapy of menhaden oil, α-lipoic acid and enalapril. Cornea 2017, 36, 725. [Google Scholar] [CrossRef]
- Zagon, I.S.; Sassani, J.W.; McLaughlin, P.J. Re-epithelialization of the rat cornea is accelerated by blockade of opioid receptors. Brain Res. 1998, 798, 254–260. [Google Scholar] [CrossRef]
- Zagon, I.S.; Sassani, J.W.; McLaughlin, P.J. Re-epithelialization of the rabbit cornea is regulated by opioid growth factor. Brain Res. 1998, 803, 61–68. [Google Scholar] [CrossRef]
- Zagon, I.S.; Sassani, J.W.; McLaughlin, P.J. Reepithelialization of the human cornea is regulated by endogenous opioids. Investig. Ophthalmol. Vis. Sci. 2000, 41, 73–81. [Google Scholar]
- Klocek, M.S.; Sassani, J.W.; McLaughlin, P.J.; Zagon, I.S. Topically applied naltrexone restores corneal reepithelialization in diabetic rats. J. Ocul. Pharmacol. Ther. 2007, 23, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Klocek, M.S.; Sassani, J.W.; McLaughlin, P.J. Dry eye reversal and corneal sensation restoration with topical naltrexone in diabetes mellitus. Arch. Ophthalmol. 2009, 127, 1468–1473. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, M.; Masse, F.; Lefebvre-Demers, M.; Maestracci, Q.; Grenier, P.; Millar, R.; Bertrand, N.; Prieto, M.; Boisselier, É. Insights into gold nanoparticles as a mucoadhesive system. Sci. Rep. 2018, 8, 14357. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, R.; Toussaint, K.C.; Wagoner Johnson, A. The effect of keratoconus on the structural, mechanical, and optical properties of the cornea. J. Mech. Behav. Biomed. Mater. 2011, 4, 223–236. [Google Scholar] [CrossRef]
- Nielsen, K.; Hjortdal, J.; Pihlmann, M.; Corydon, T.J. Update on the keratoconus genetics. Acta Ophthalmol. 2013, 91, 106–113. [Google Scholar] [CrossRef]
- Sharif, R.; Khaled, M.L.; McKay, T.B.; Liu, Y.; Karamichos, D. Transcriptional profiling of corneal stromal cells derived from patients with keratoconus. Sci. Rep. 2019, 9, 12567. [Google Scholar] [CrossRef]
- Liu, R.; Yan, X. Oxidative stress in corneal stromal cells contributes to the development of keratoconus in a rabbit model. Eur. J. Ophthalmol. 2021, 31, 3518–3524. [Google Scholar] [CrossRef]
- Bykhovskaya, Y.; Margines, B.; Rabinowitz, Y.S. Genetics in Keratoconus: Where are we? Eye Vis. 2016, 3, 16. [Google Scholar] [CrossRef]
- Sharif, R.; Bak-Nielsen, S.; Hjortdal, J.; Karamichos, D. Pathogenesis of Keratoconus: The intriguing therapeutic potential of Prolactin-inducible protein. Prog. Retin. Eye Res. 2018, 67, 150–167. [Google Scholar] [CrossRef]
- Peiffer, R.L., Jr.; Werblin, T.P.; Patel, A.S. Keratoconus in a rhesus monkey. J. Med. Primatol. 1987, 16, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Adachi, W.; Kinoshita, S.; Kobayashi, Y.; Honma, Y.; Hiai, H.; Matsushima, Y. Androgen-dependent hereditary mouse keratoconus: Linkage to an MHC region. Investig. Ophthalmol. Vis. Sci. 2002, 43, 51–57. [Google Scholar]
- Tachibana, M.; Okamoto, M.; Sakamoto, M.; Matsushima, Y. Hereditary keratoconus-like keratopathy in Japanese wild mice mapped to mouse Chromosome 13. Mamm. Genome 2002, 13, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Moreddu, R.; Vigolo, D.; Yetisen, A.K. Contact lens technology: From fundamentals to applications. Adv. Healthc. Mater. 2019, 8, 1900368. [Google Scholar] [CrossRef]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef]
- International Organization for Standardization, ISO 9394:2012, Reviewed and Confirmed in 2023. Available online: https://www.iso.org/standard/57318.html (accessed on 31 July 2023).
- International Organization for Standardization, ISO 10993-10:2021, Revised in 2021. Available online: https://www.iso.org/standard/75279.html (accessed on 31 July 2023).
- Miziara, I.D.; Magalhães, A.T.; Santos, M.; Gomes, E.F.; Oliveira, R.A. Research ethics in animal models. Braz. J. Otorhinolaryngol. 2012, 78, 128–131. [Google Scholar] [CrossRef]
- Moutinho, S. Researchers and regulators plan for a future without lab animals. Nat. Med. 2023, 29, 2151–2154. [Google Scholar] [CrossRef]
- Wadman, M. FDA no longer has to require animal testing for new drugs. Science 2023, 379, 127–128. [Google Scholar] [CrossRef]





| Animal Models | Rodents | Rabbits | Nonhuman Primates | Pigs | Felines | Canines | Zebrafish |
|---|---|---|---|---|---|---|---|
| Average cost per animal | ●●○○○ | ●●●○○ | ●●●●● | ●●●●○ | ●●●●○ | ●●●●○ | ●○○○○ |
| Space required | ●●○○○ | ●●●○○ | ●●●●● | ●●●●○ | ●●●●○ | ●●●●○ | ●○○○○ |
| Breeding rate | ●●●●○ | ●●●○○ | ●○○○○ | ●●○○○ | ●●○○○ | ●●○○○ | ●●●●● |
| Feasibility of high-throughput screening | ●●●●○ | ●●○○○ | ●○○○○ | ●○○○○ | ●○○○○ | ●●○○○ | ●●●●● |
| Availability of mutant, transgenic, and genetically modified strains | ●●●●● | ●●●○○ | ●○○○○ | ●●○○○ | ●●○○○ | ●●○○○ | ●●●●○ |
| Eye anatomical similarity to humans | ●●○○○ | ●●●○○ | ●●●●● | ●●●●○ | ●●●●○ | ●●●●○ | ●○○○○ |
| Eye genetic similarity to humans | ●●○○○ | ●●●○○ | ●●●●● | ●●●●○ | ●●●●○ | ●●●●○ | ●●○○○ |
| Study of associated corneal pathologies | DED HSV Repair Transplantation Neovascularization FECD DK KC | DED HSV Repair Transplantation Neovascularization FECD DK KC Contact lenses | HSV Repair Transplantation FECD Contact lenses | Repair Transplantation DK | Repair Transplantation FECD DK | DED DK | Repair Corneal dystrophy Drug-related oculotoxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loiseau, A.; Raîche-Marcoux, G.; Maranda, C.; Bertrand, N.; Boisselier, E. Animal Models in Eye Research: Focus on Corneal Pathologies. Int. J. Mol. Sci. 2023, 24, 16661. https://doi.org/10.3390/ijms242316661
Loiseau A, Raîche-Marcoux G, Maranda C, Bertrand N, Boisselier E. Animal Models in Eye Research: Focus on Corneal Pathologies. International Journal of Molecular Sciences. 2023; 24(23):16661. https://doi.org/10.3390/ijms242316661
Chicago/Turabian StyleLoiseau, Alexis, Gabrielle Raîche-Marcoux, Cloé Maranda, Nicolas Bertrand, and Elodie Boisselier. 2023. "Animal Models in Eye Research: Focus on Corneal Pathologies" International Journal of Molecular Sciences 24, no. 23: 16661. https://doi.org/10.3390/ijms242316661
APA StyleLoiseau, A., Raîche-Marcoux, G., Maranda, C., Bertrand, N., & Boisselier, E. (2023). Animal Models in Eye Research: Focus on Corneal Pathologies. International Journal of Molecular Sciences, 24(23), 16661. https://doi.org/10.3390/ijms242316661






