The Relevance of Autophagy within Inner Ear in Baseline Conditions and Tinnitus-Related Syndromes
Abstract
1. General Introduction
2. A Definition of Tinnitus as Altered Mechanisms of Auditory Stimulation
3. Specific Activities of Outer Hair Cells
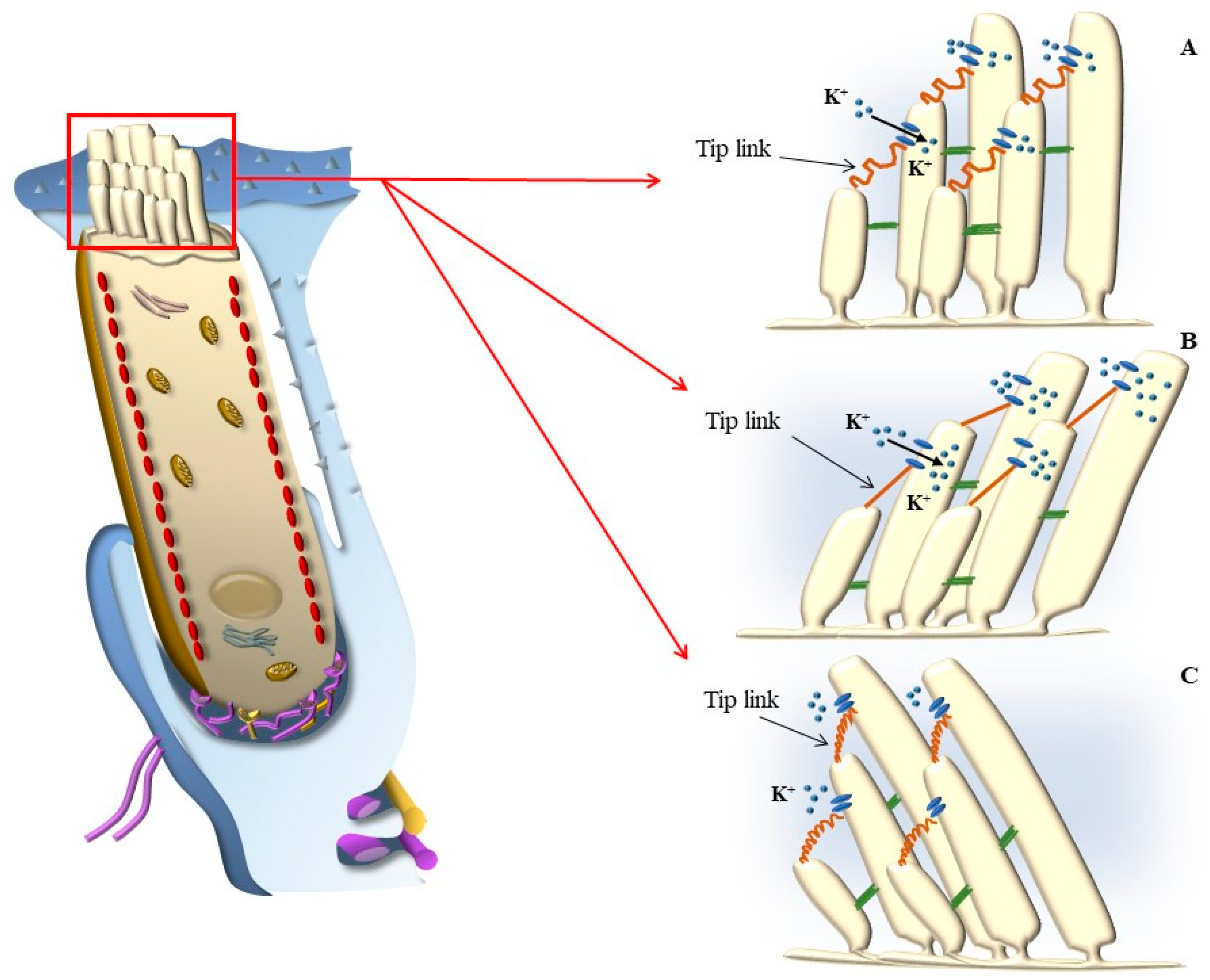
3.1. Mechano-Electrical Signaling (or Transduction, MET)
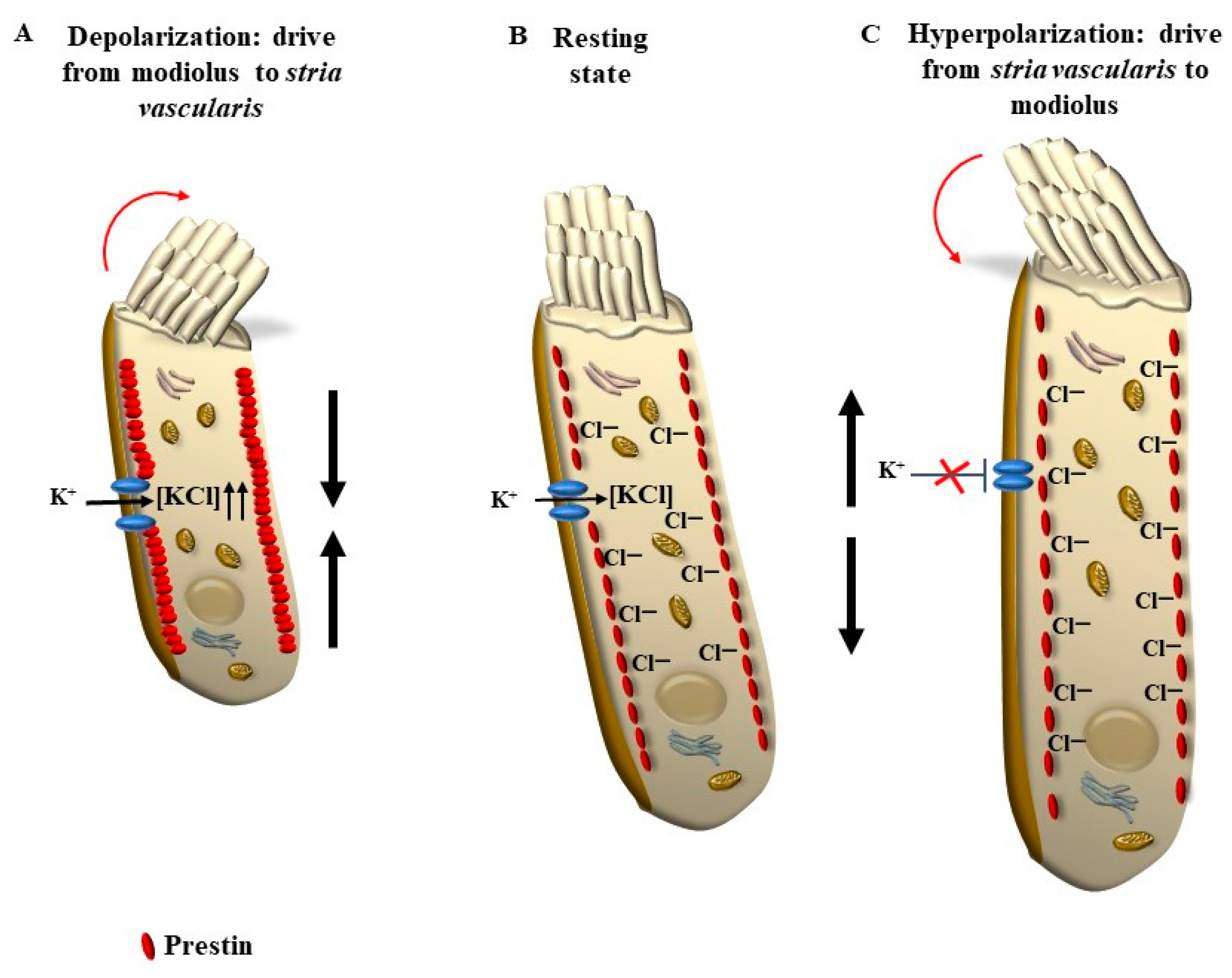
3.2. Electro-Mechanical Signaling
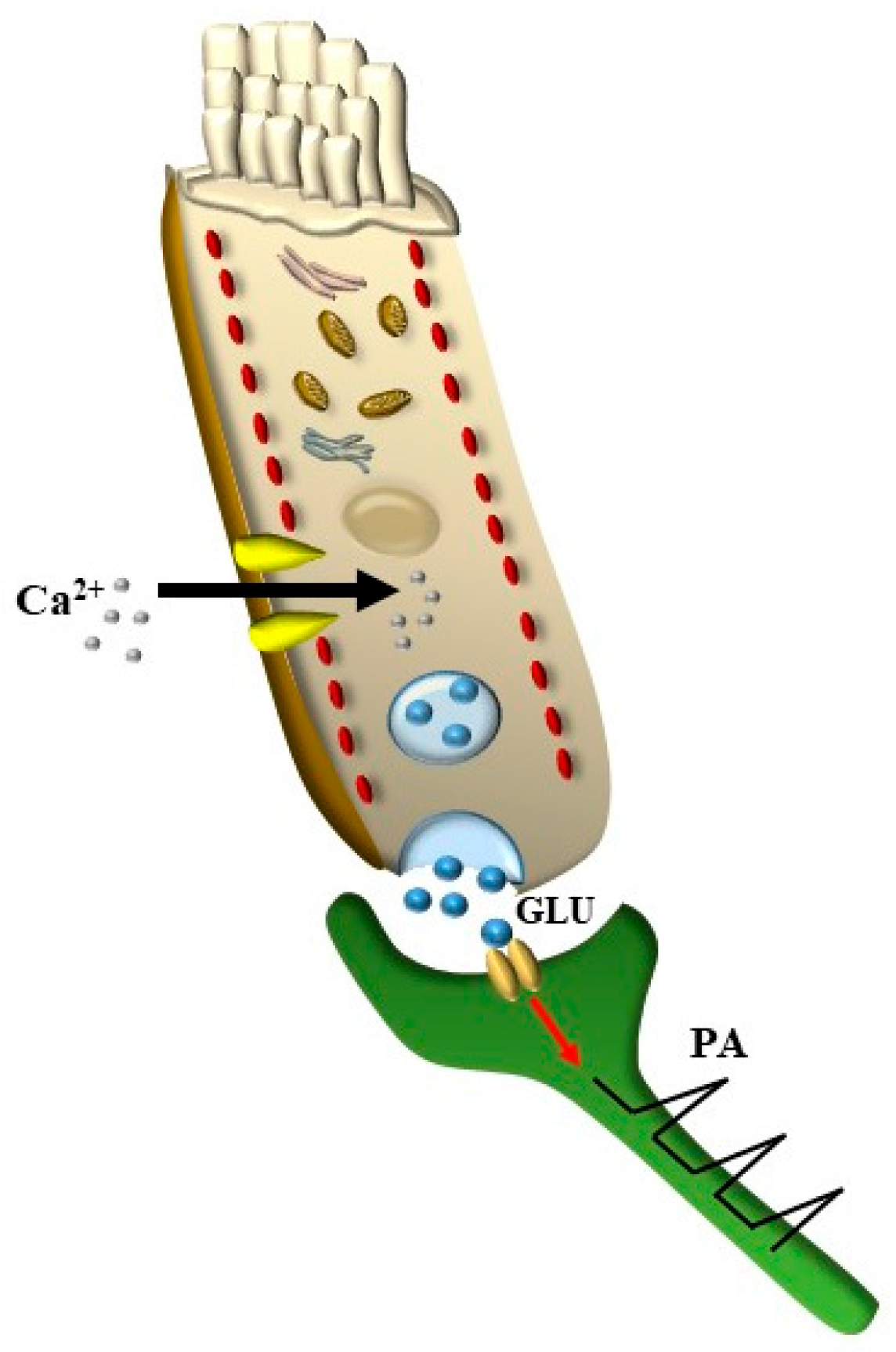
3.3. Electro-Chemical Signaling
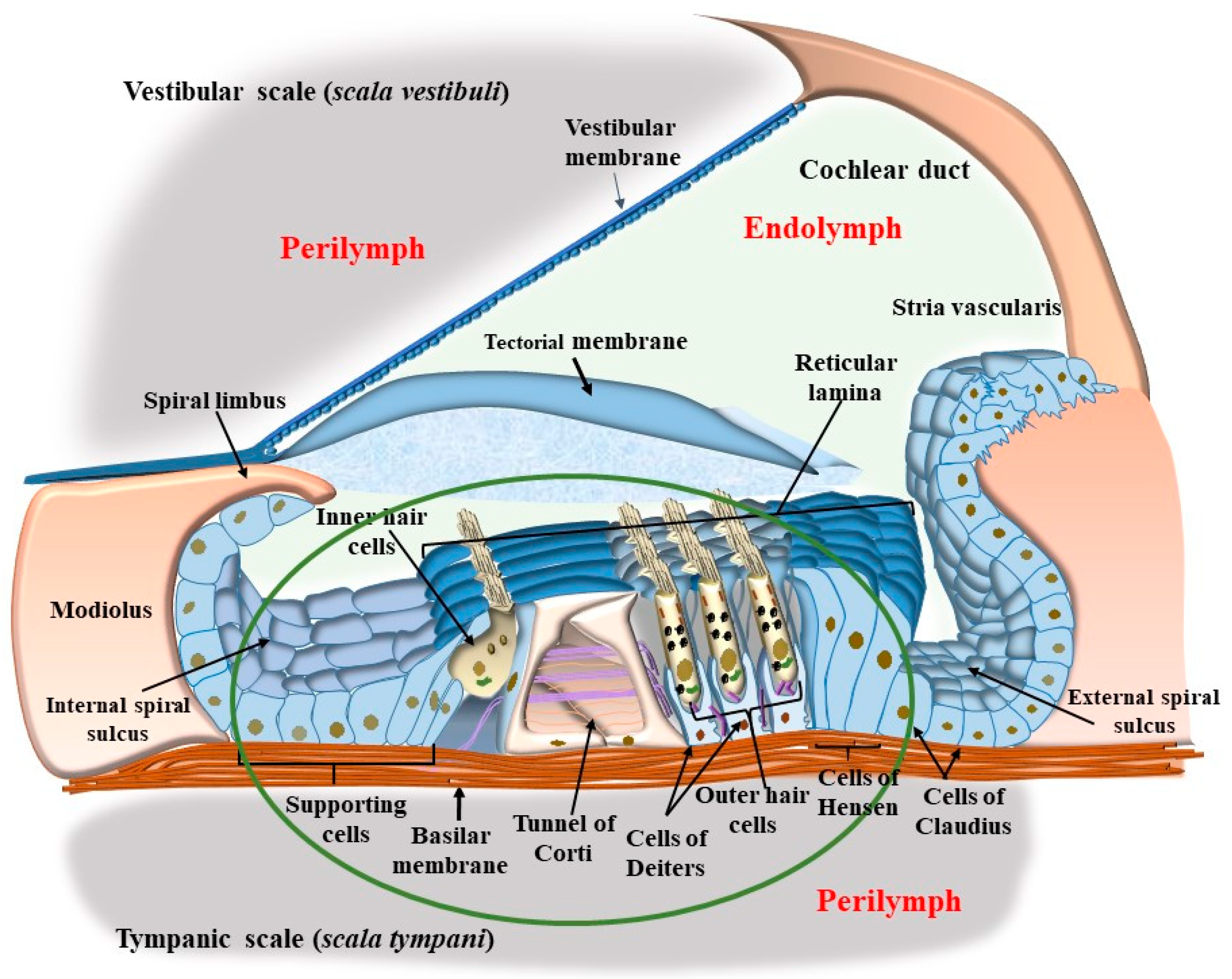
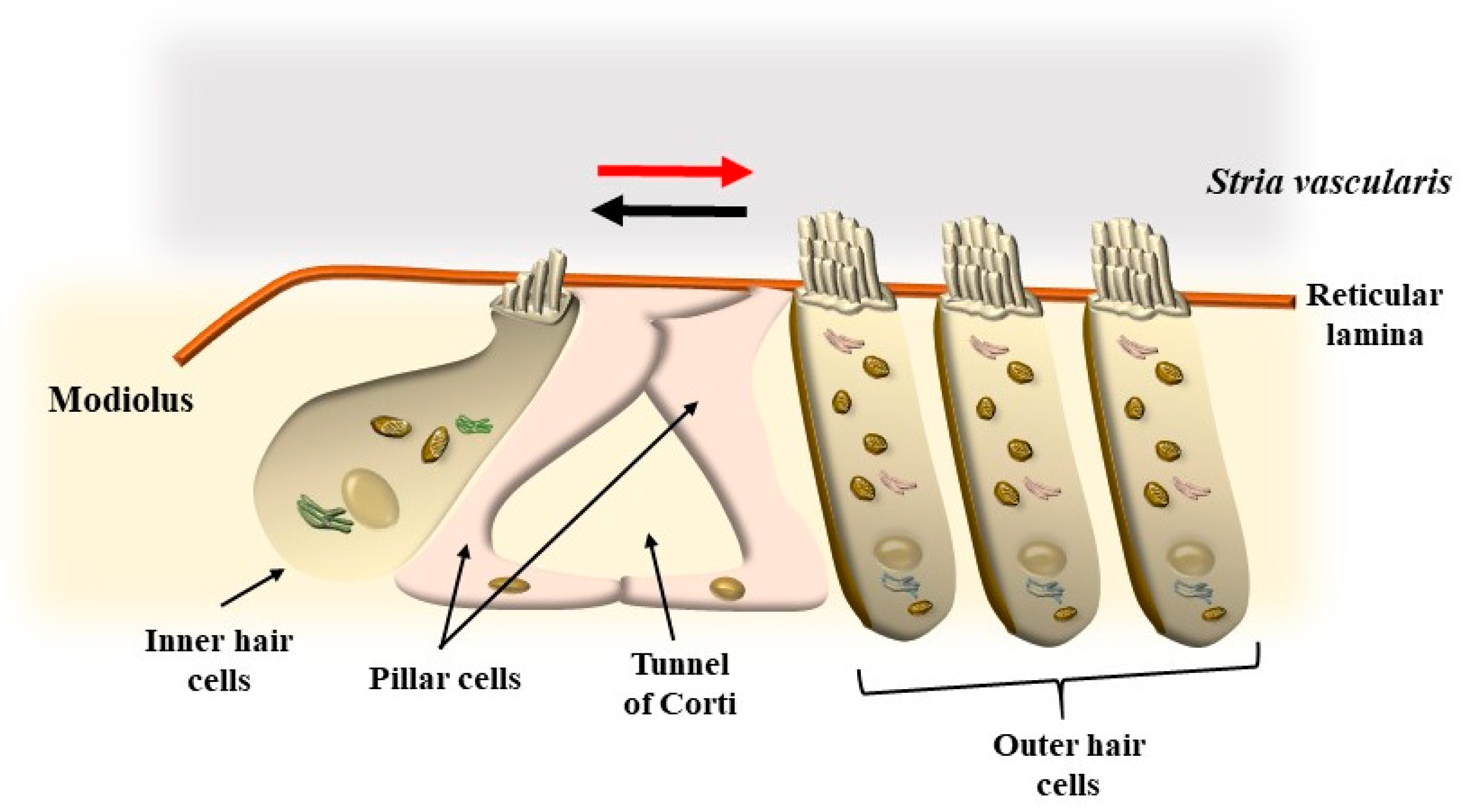
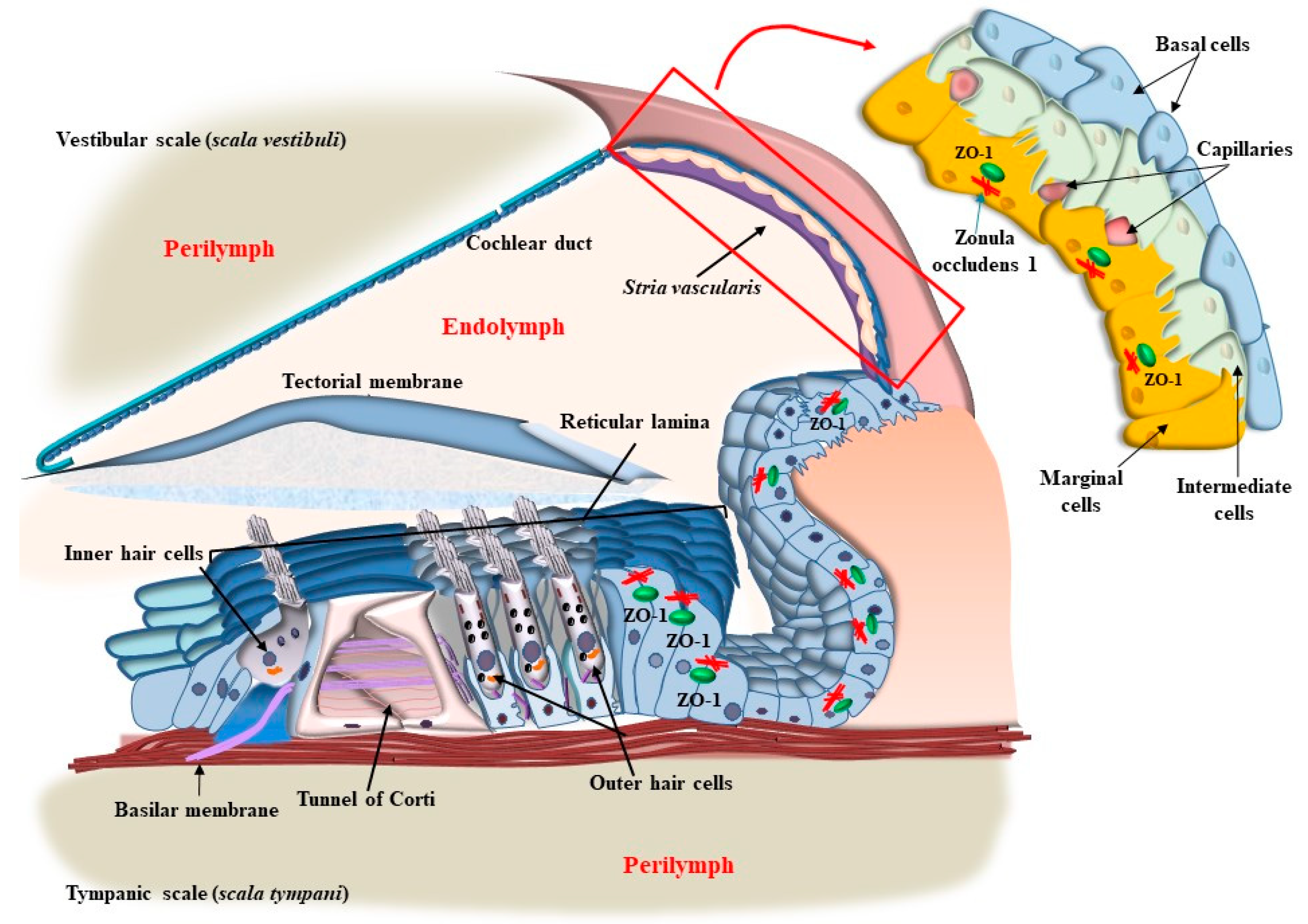
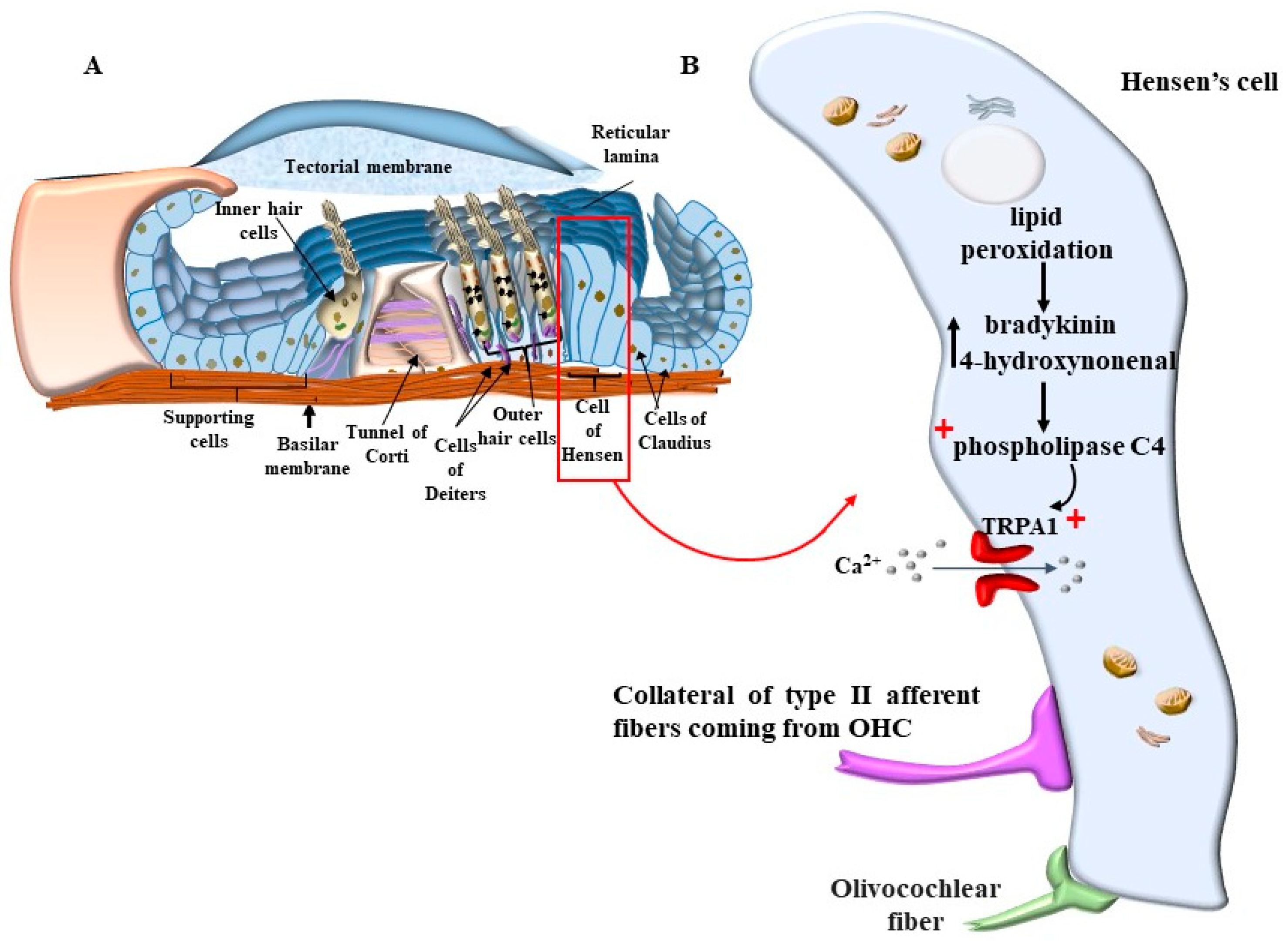
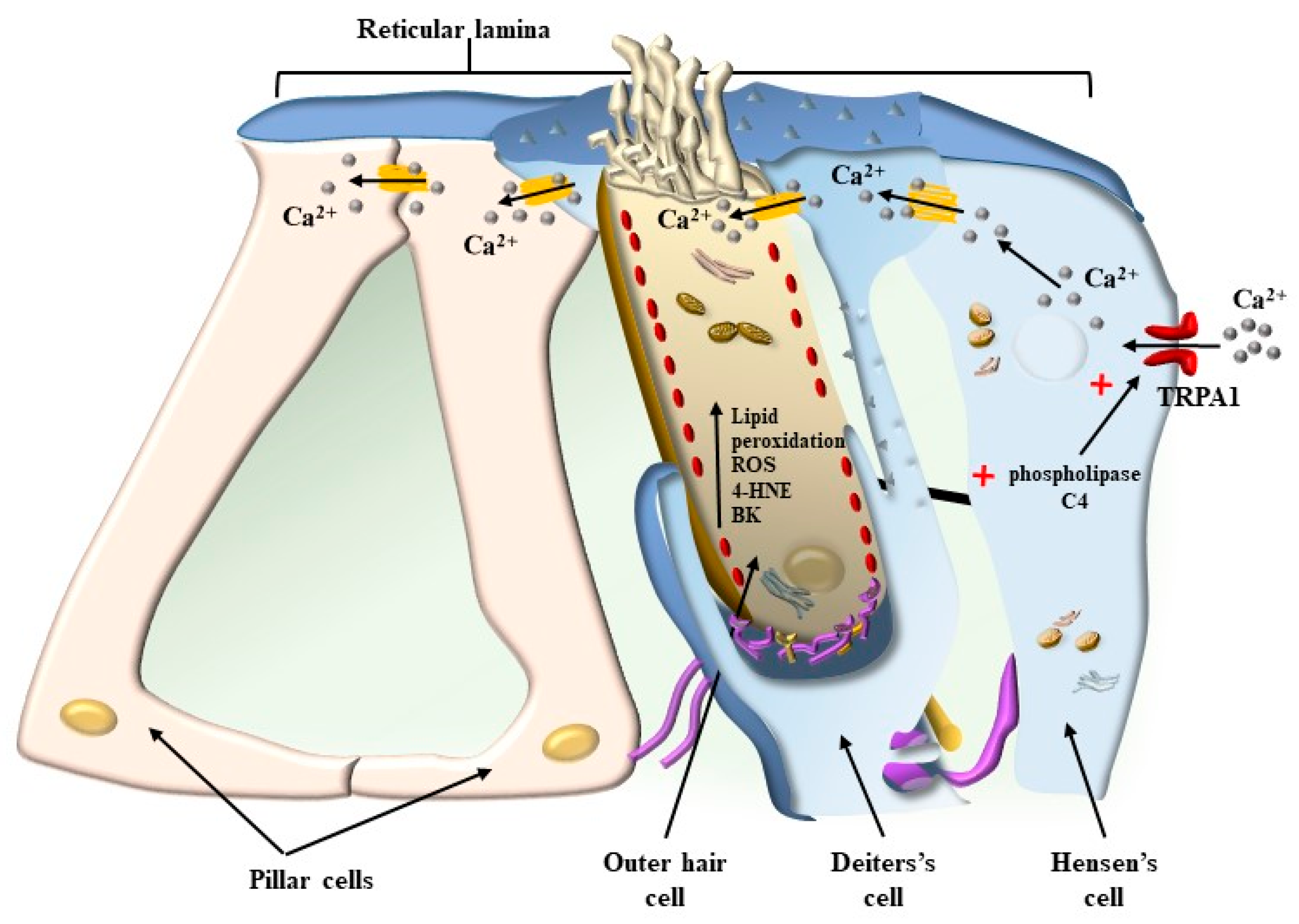
4. OHC in the Context of the Corti’s Organ
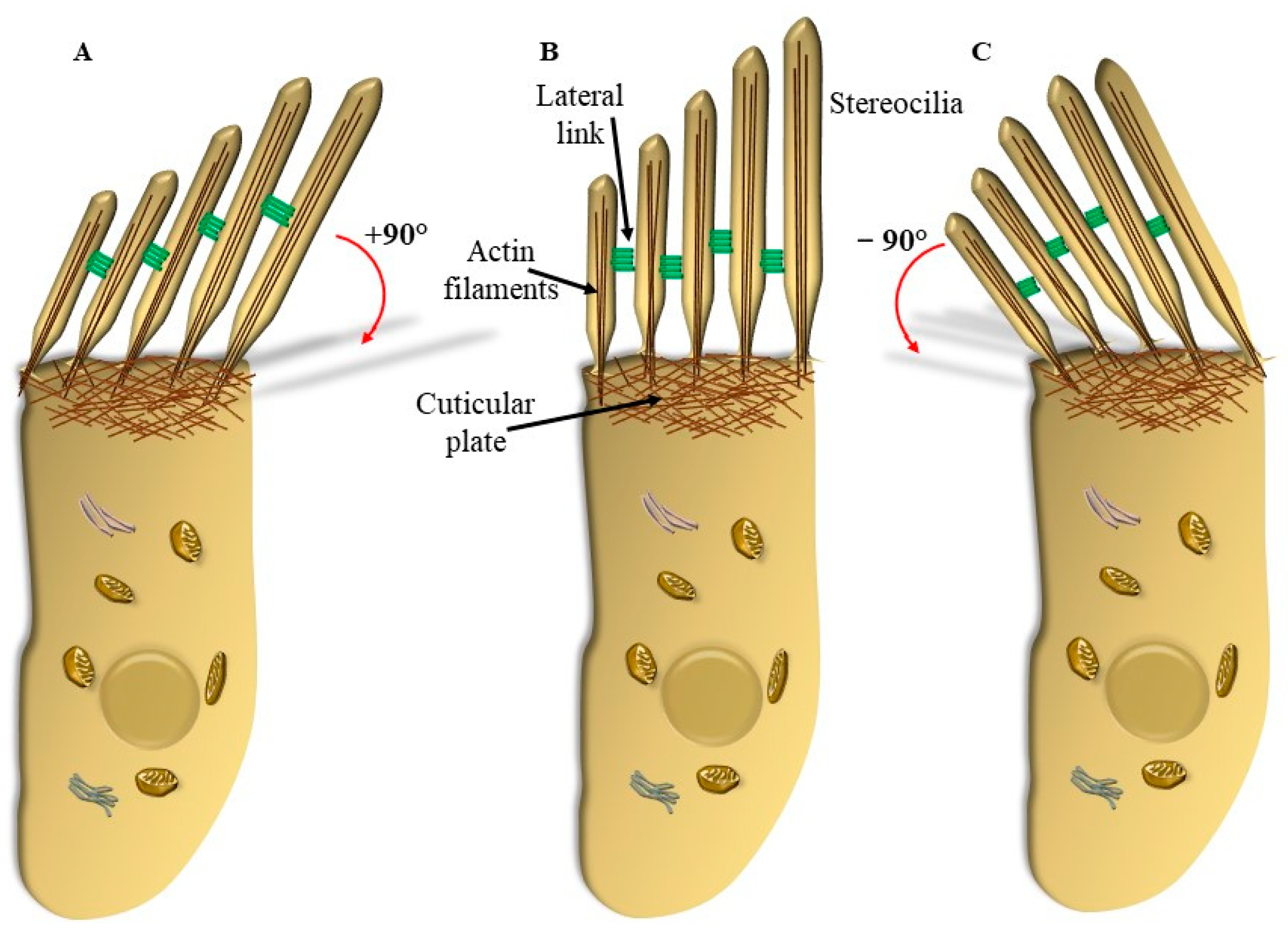
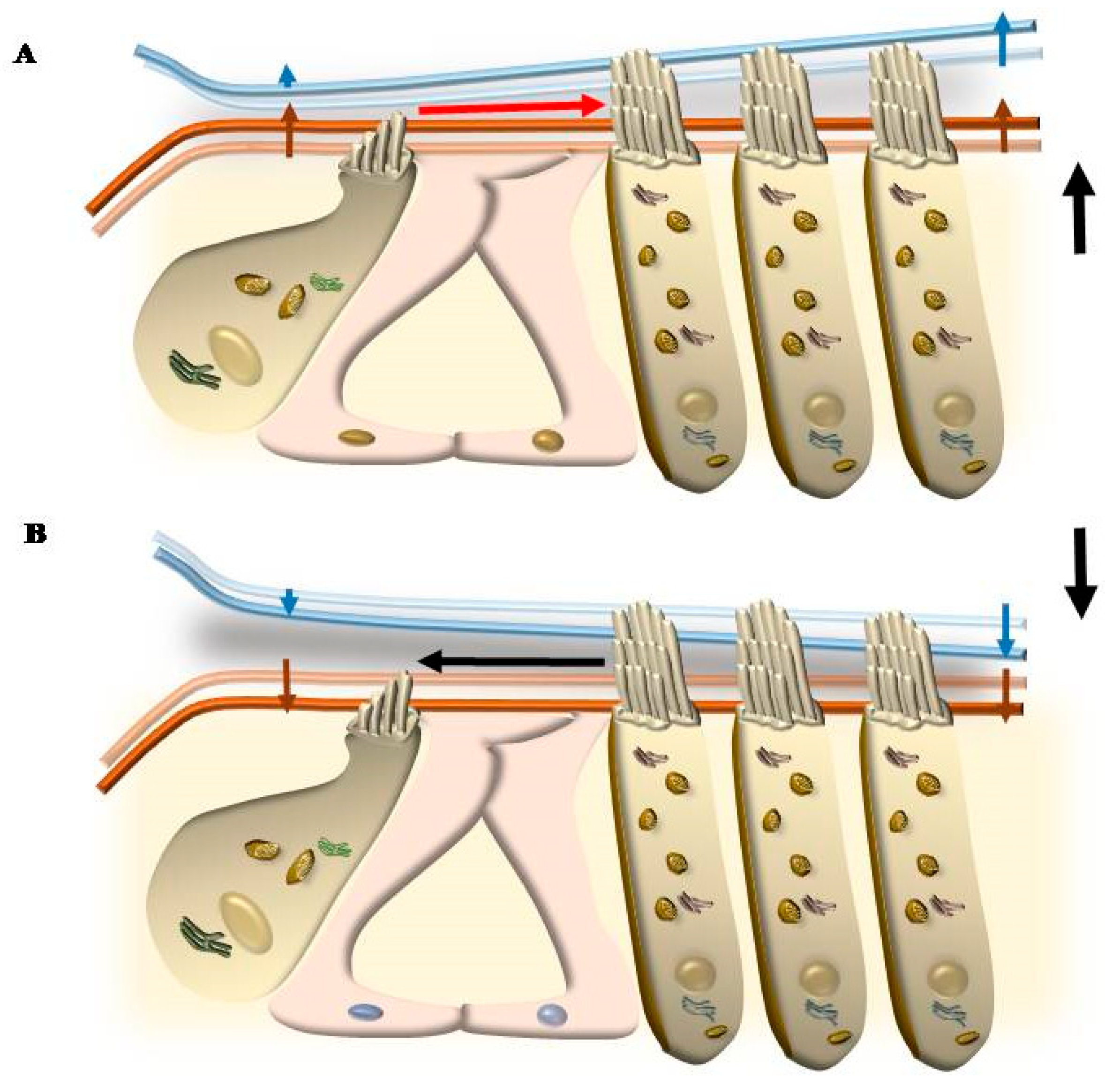
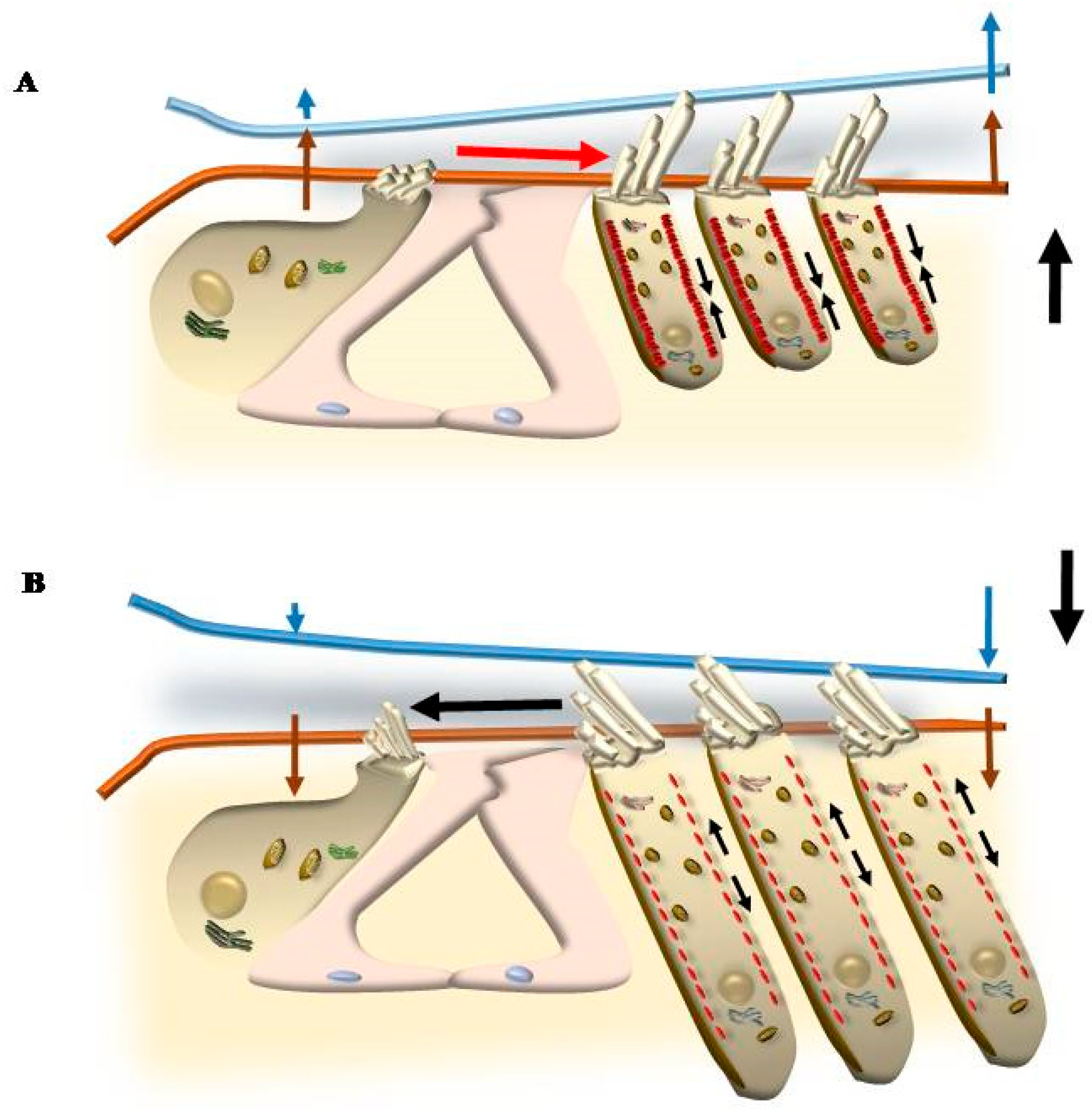
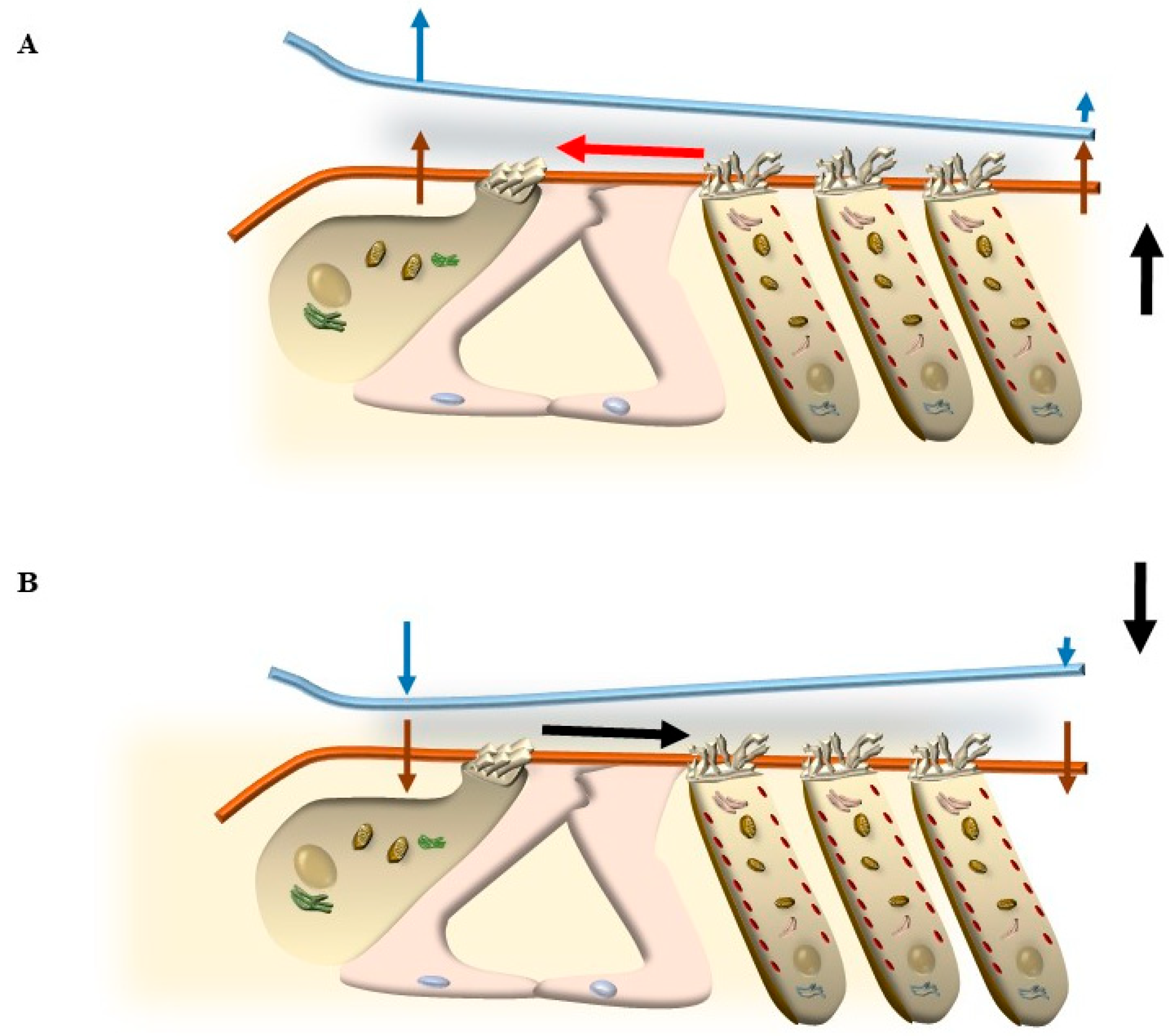
5. Mechanisms That Produce the Movement of SC
5.1. Radial Shearing
5.2. OHC Motility (Length Modulation) Adds on Radial Shearing
5.3. Further Drives to Move IHC SC
6. The Main Source of Tinnitus
6.1. The TM Push/Pull Drive in the Course of Degeneration
6.2. The Stereocilia Slant Drive in the Course of Neurodegeneration
7. Which Molecular Alterations Early Affect OHC SC to Generate Tinnitus?
7.1. The Specific Mechanisms Altering OHC SC Structure
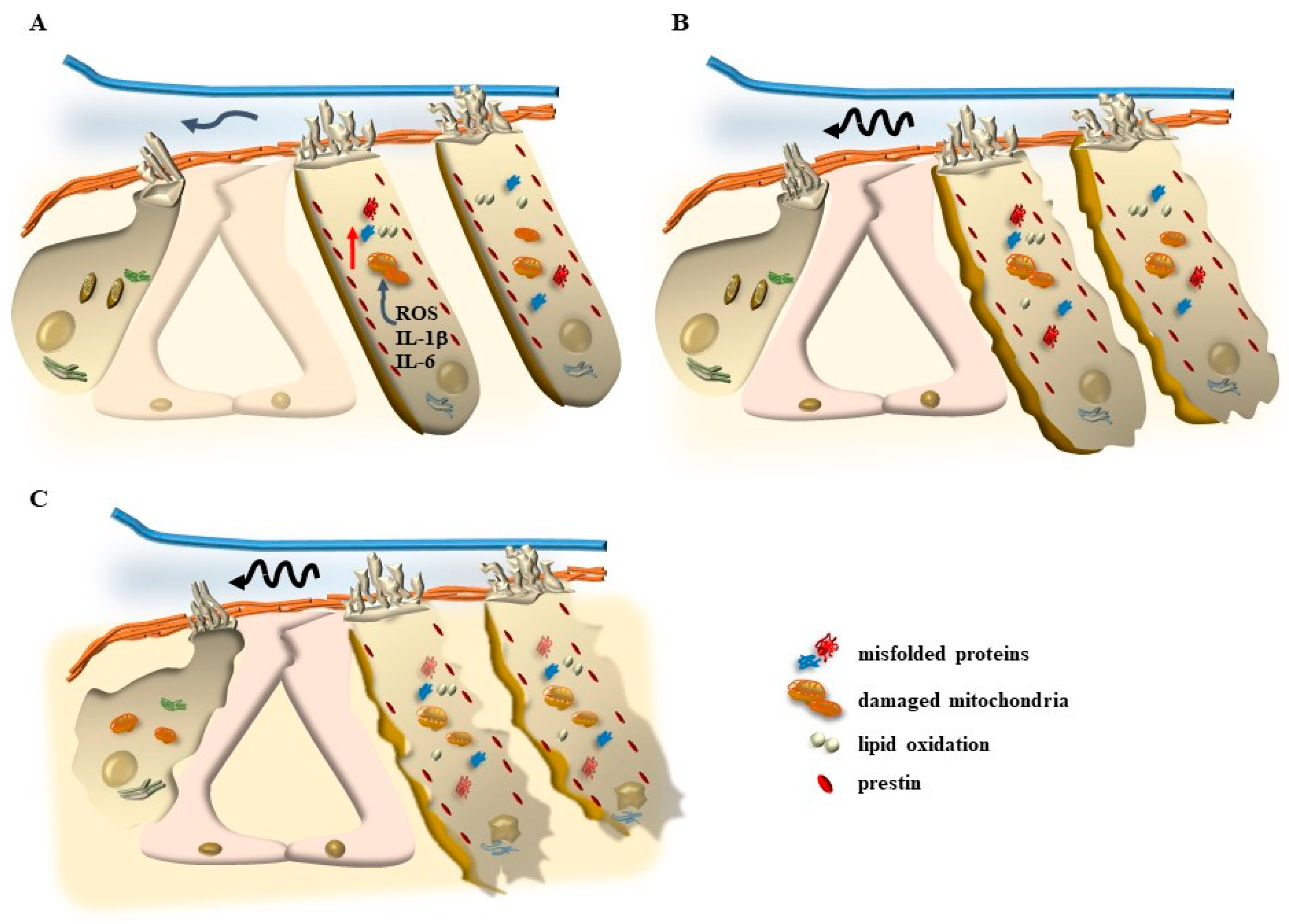
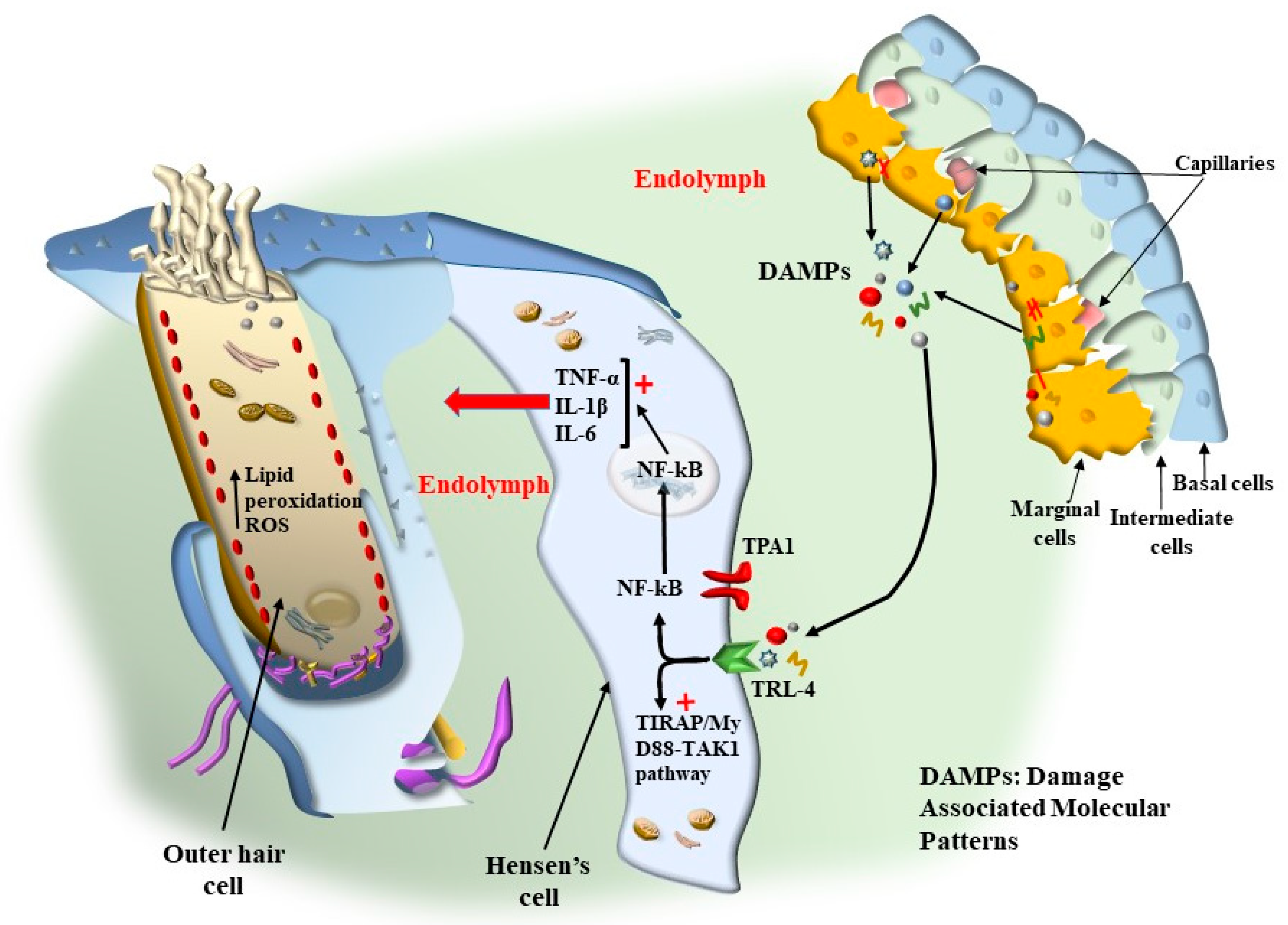
7.1.1. Oxidative Stress
7.1.2. Mitochondrial Dysfunction
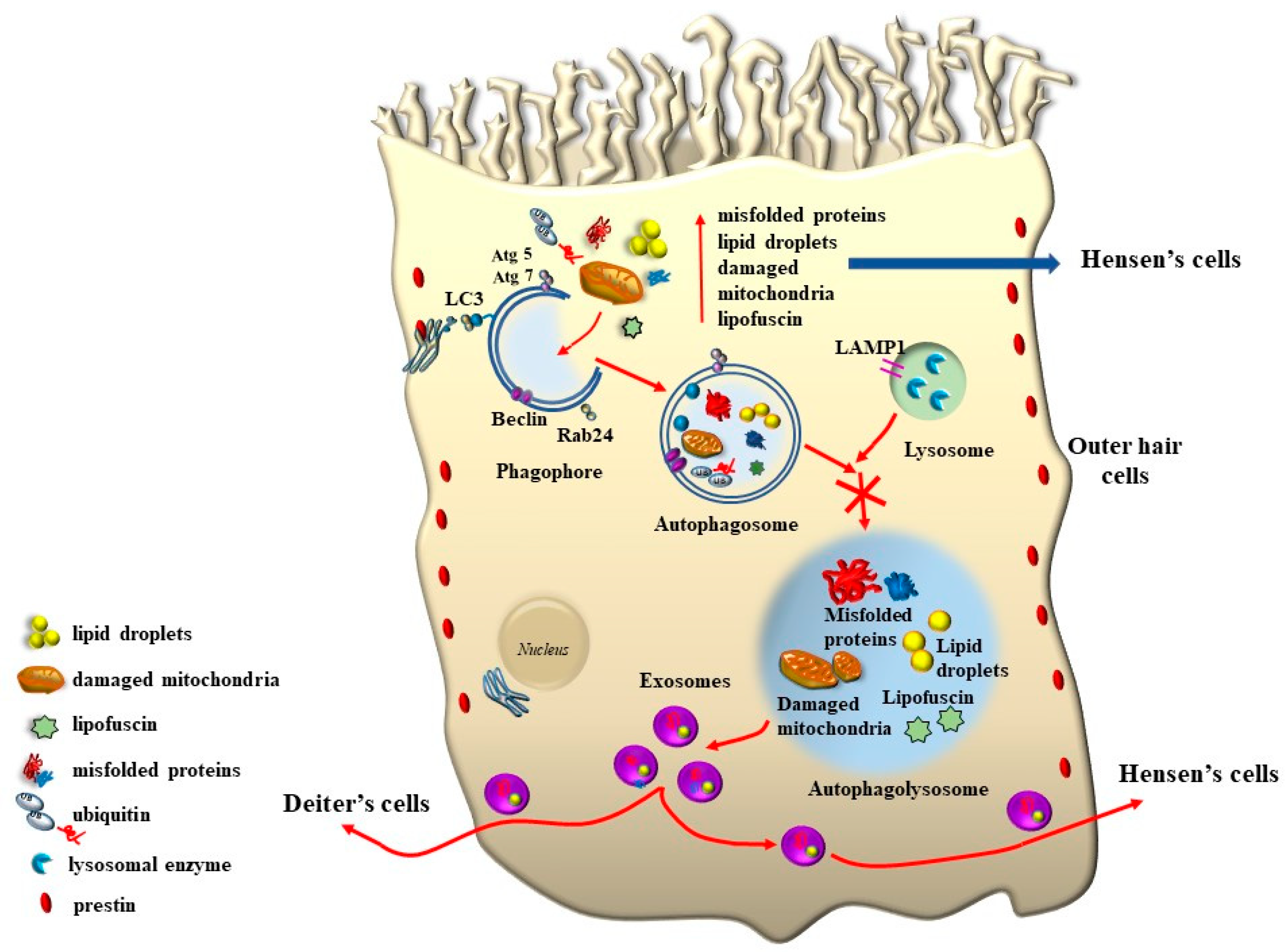
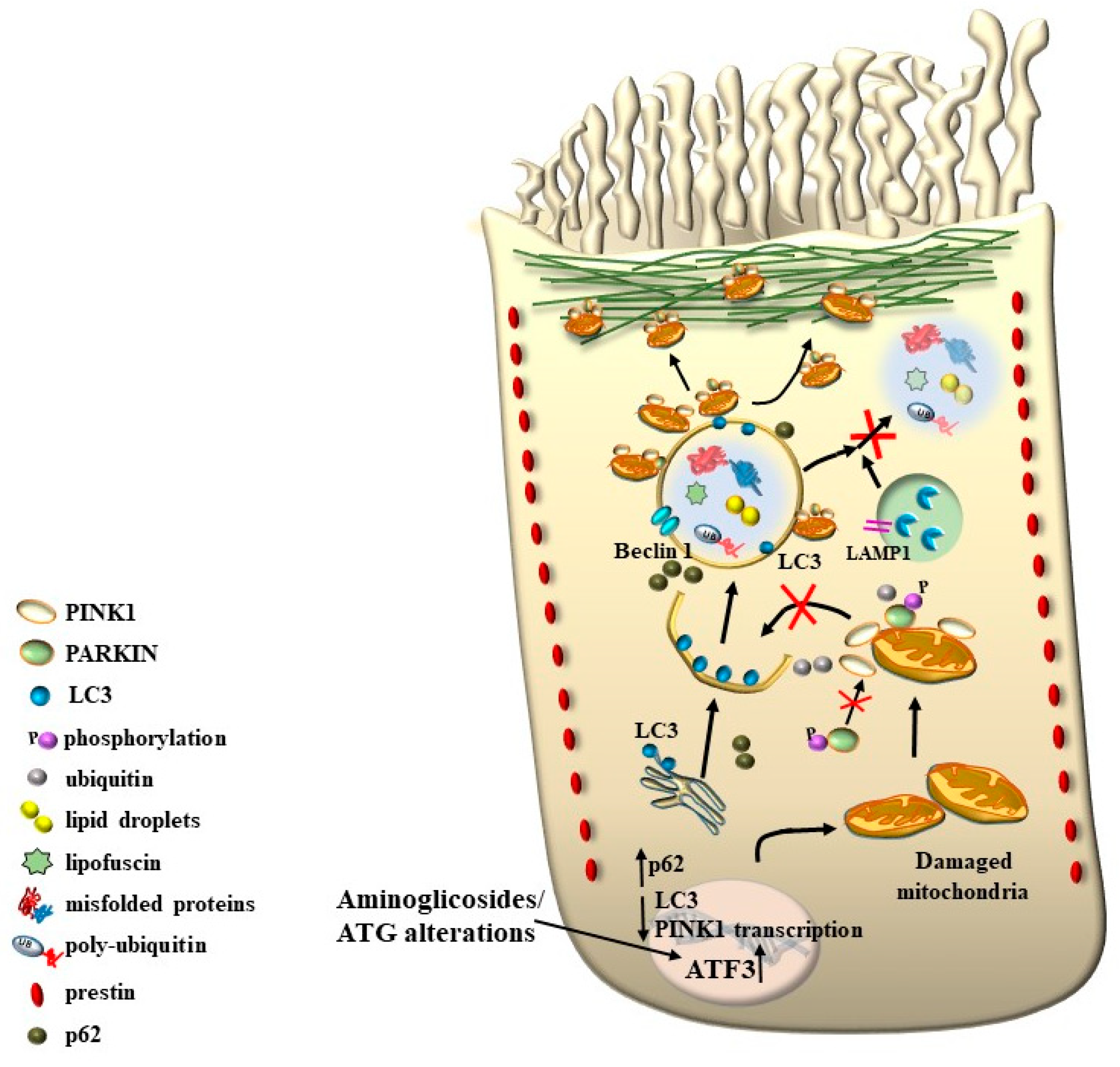
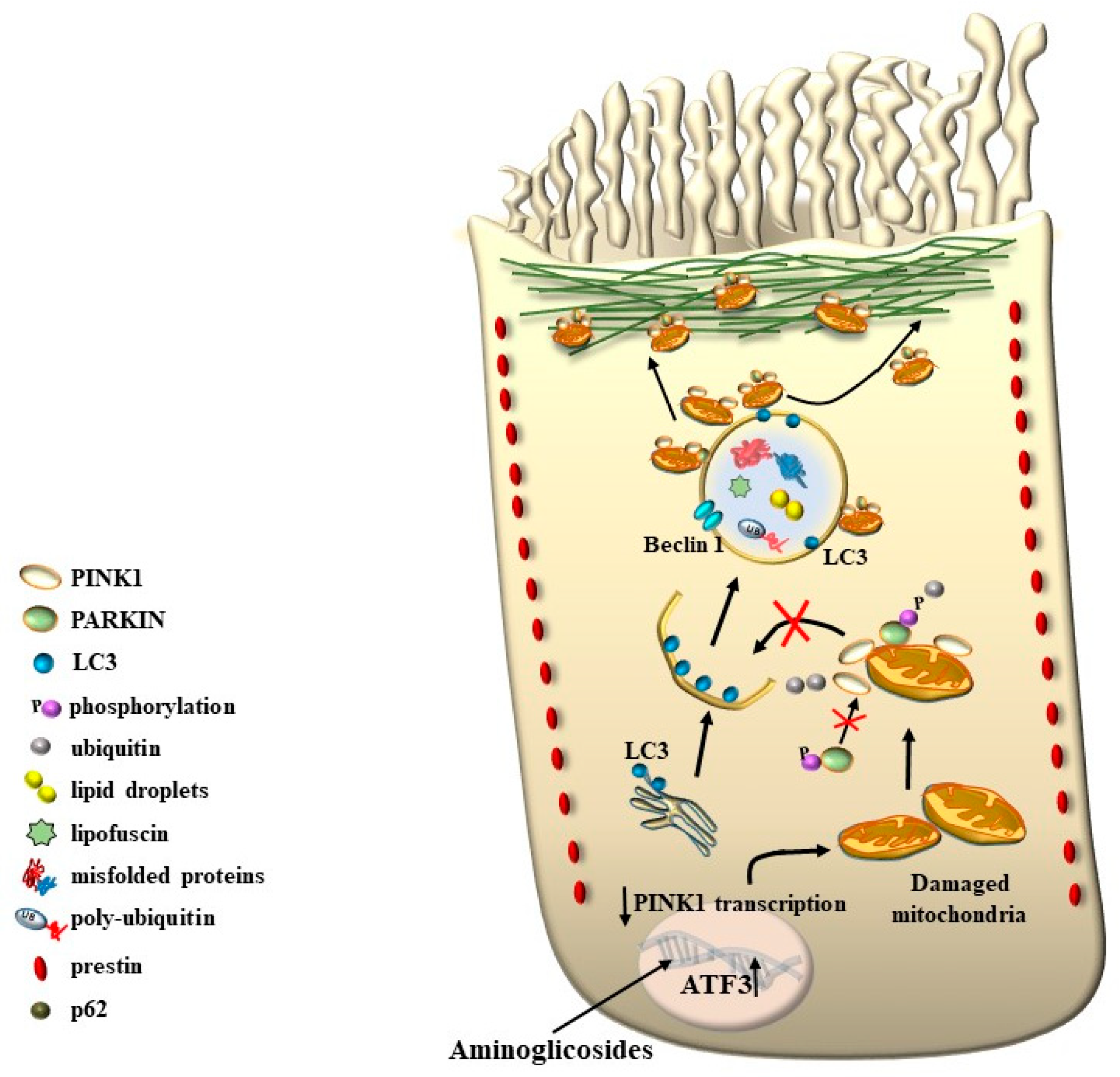
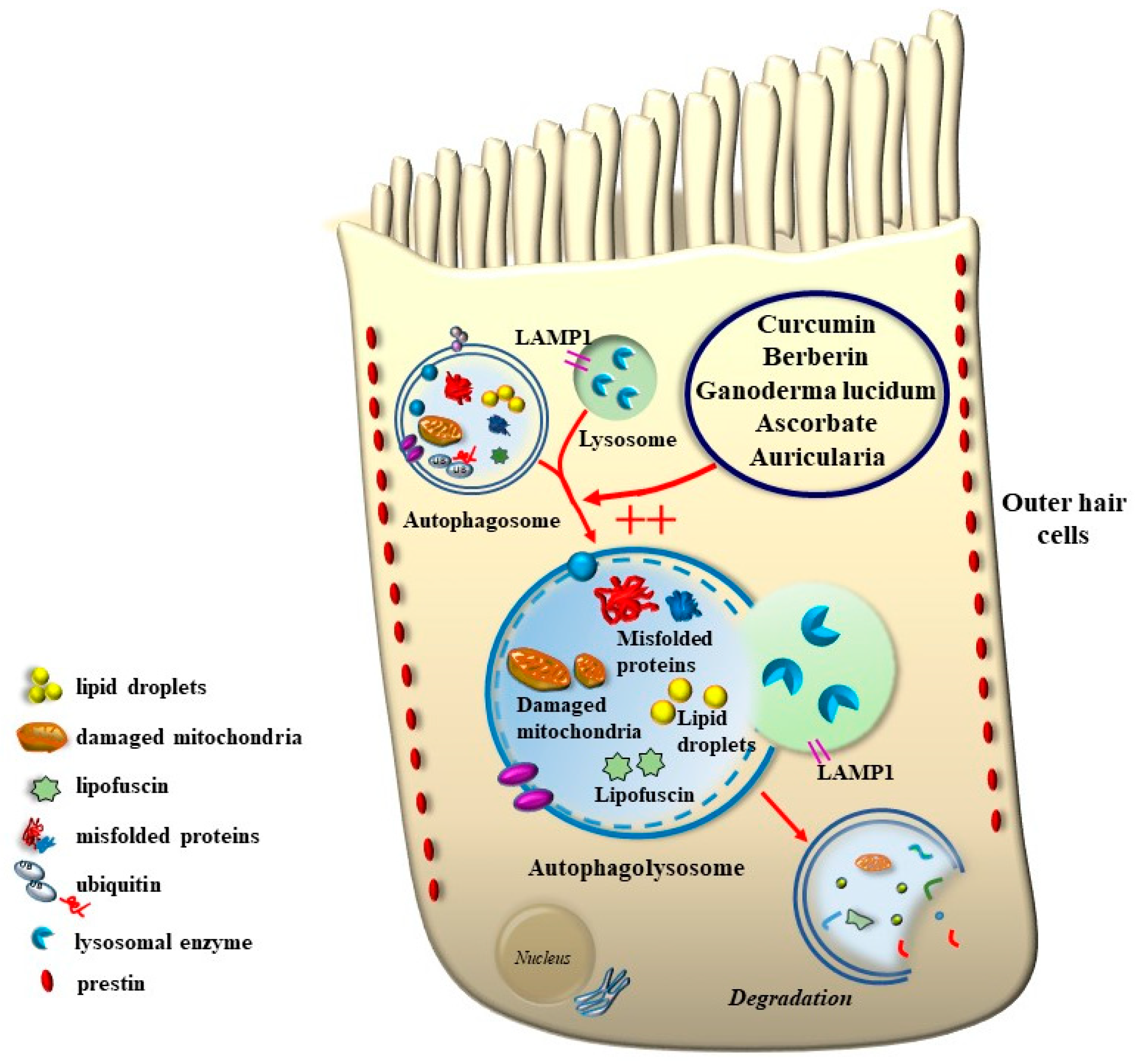
8. The Role of Autophagy in Maintaining the Structure of OHC SC, TM, and Its Failure in Tinnitus
9. The Genetics of Hearing Loss and Tinnitus May Include a Role of Autophagy and Autophagy Activators
10. The Multiple Significance of Findings Obtained Following Ototoxic Drugs
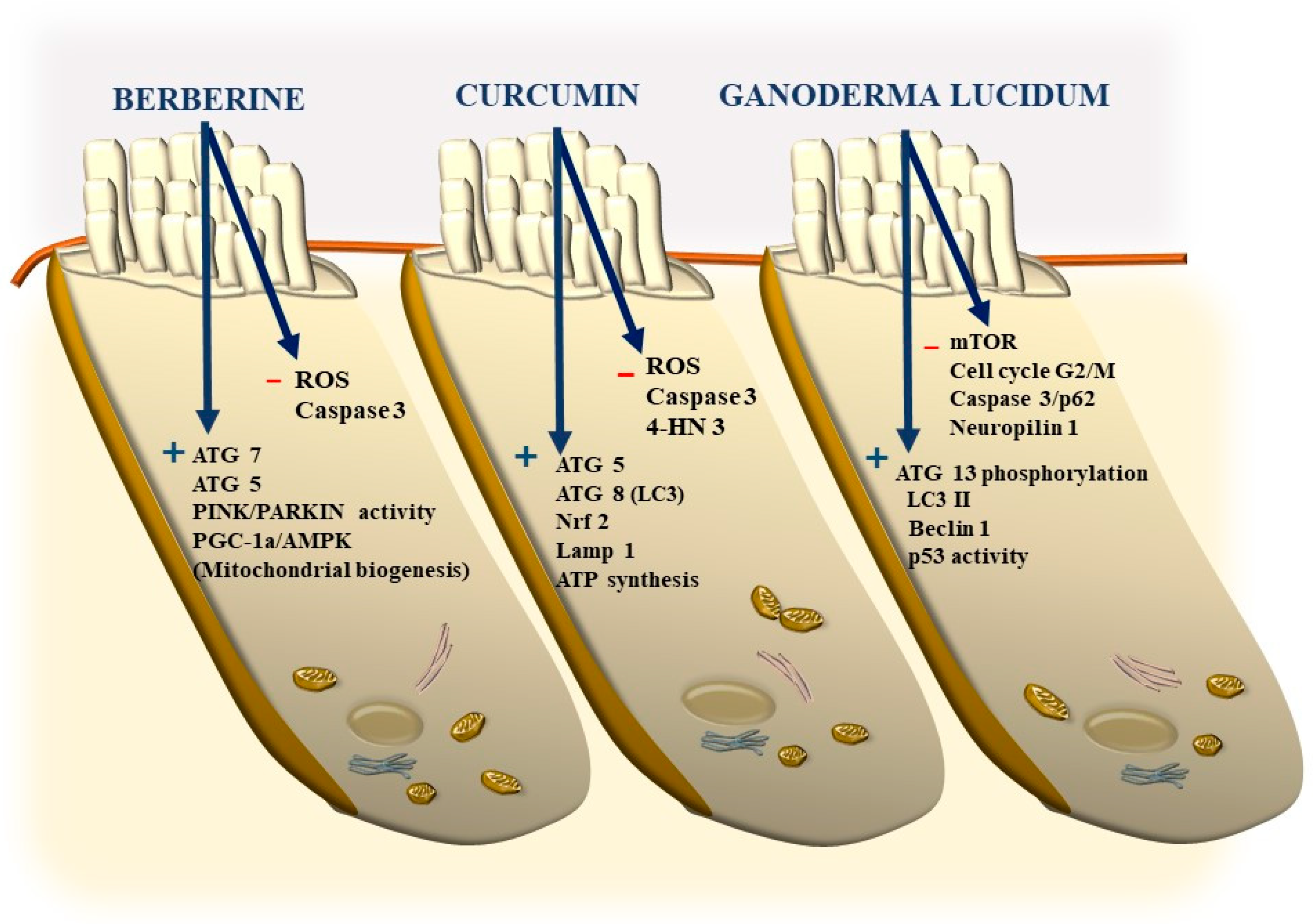
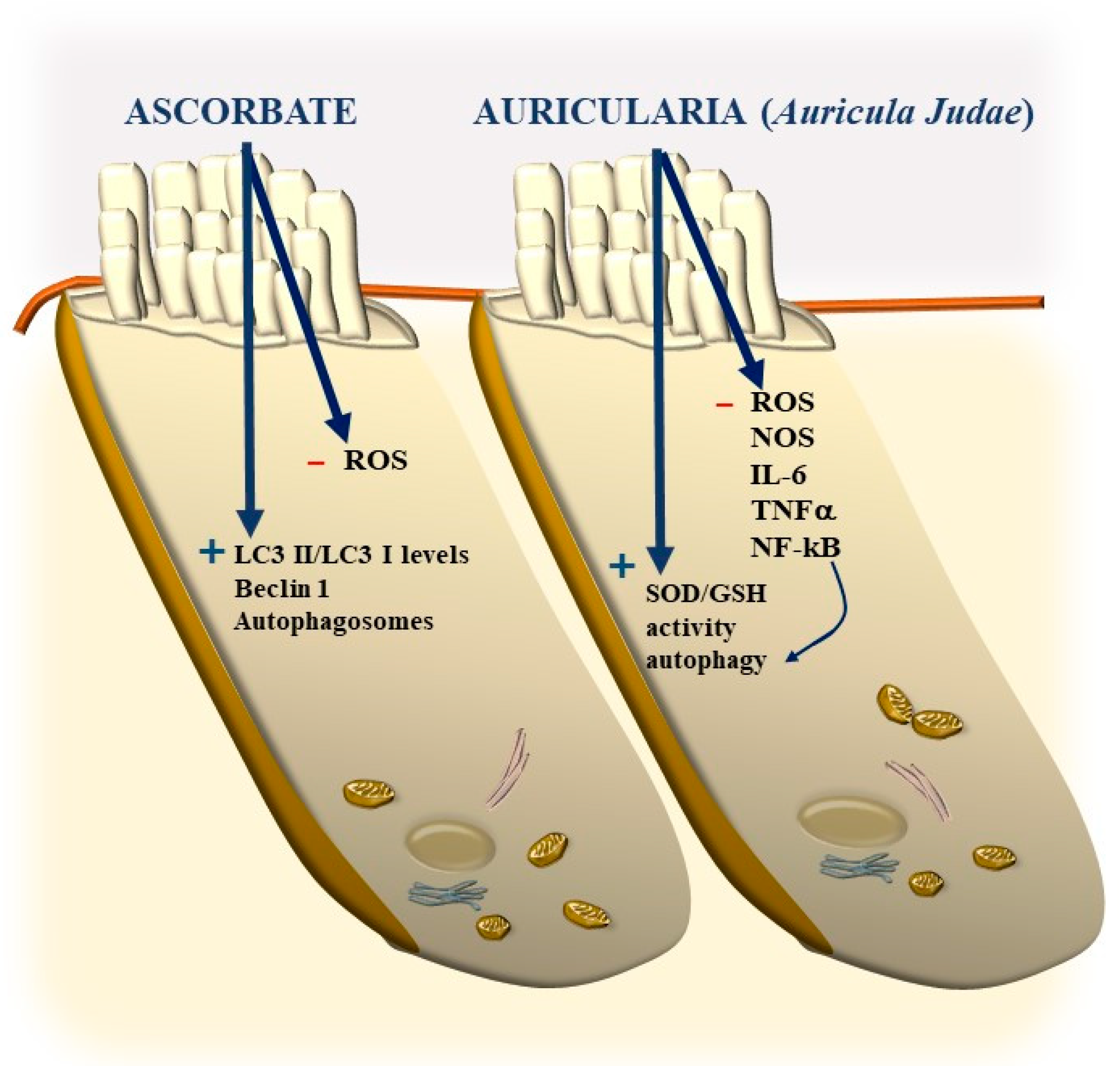
11. Potential Benefits of Nutraceuticals
11.1. Berberine
11.2. Curcumin
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Von Békésy, G. Simplified model to demonstrate the energy flow and formation of traveling waves similar to those found in the cochlea. Proc. Natl. Acad. Sci. USA 1956, 42, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Goutman, J.D.; Elgoyhen, A.B.; Gómez-Casati, M.E. Cochlear hair cells: The sound-sensing machines. FEBS Lett. 2015, 589, 3354–3361. [Google Scholar] [CrossRef] [PubMed]
- Fettiplace, R. Hair Cell Transduction, Tuning, and Synaptic Transmission in the Mammalian Cochlea. Compr. Physiol. 2017, 7, 1197–1227. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Shen, W.; He, D.Z.; Long, K.B.; Madison, L.D.; Dallos, P. Prestin is the motor protein of cochlear outer hair cells. Nature 2000, 405, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C.; Gao, J.; He, D.Z.; Wu, X.; Jia, S.; Zuo, J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 2002, 419, 300–304. [Google Scholar] [CrossRef]
- Ashmore, J.; Avan, P.; Brownell, W.E.; Dallos, P.; Dierkes, K.; Fettiplace, R.; Grosh, K.; Hackney, C.M.; Hudspeth, A.J.; Jülicher, F.; et al. The remarkable cochlear amplifier. Hear. Res. 2010, 266, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Self, T.; Sobe, T.; Copeland, N.G.; Jenkins, N.A.; Avraham, K.B.; Steel, K.P. Role of myosin VI in the differentiation of cochlear hair cells. Dev. Biol. 1999, 214, 331–341. [Google Scholar] [CrossRef]
- Vijayakumar, K.A.; Cho, G.W.; Maharajan, N.; Jang, C.H. A Review on Peripheral Tinnitus, Causes, and Treatments from the Perspective of Autophagy. Exp. Neurobiol. 2022, 31, 232–242. [Google Scholar] [CrossRef]
- Matsumoto, N.; Kalinec, F. Prestin-dependent and prestin-independent motility of guinea pig outer hair cells. Hear. Res. 2005, 208, 1–13. [Google Scholar] [CrossRef]
- Ge, J.; Elferich, J.; Dehghani-Ghahnaviyeh, S.; Zhao, Z.; Meadows, M.; von Gersdorff, H.; Tajkhorshid, E.; Gouaux, E. Molecular mechanism of prestin electromotive signal amplification. Cell 2021, 184, 4669–4679. [Google Scholar] [CrossRef]
- Guinan, J.J., Jr. How are inner hair cells stimulated? Evidence for multiple mechanical drives. Hear. Res. 2012, 292, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qian, X.; Xu, N.; Zhang, S.; Zhu, G.; Zhang, Y.; Liu, D.; Cheng, C.; Zhu, X.; Liu, Y.; et al. Disruption of Atg7-dependent autophagy causes electromotility disturbances, outer hair cell loss, and deafness in mice. Cell Death Dis. 2020, 11, 913. [Google Scholar] [CrossRef] [PubMed]
- Peixoto-Pinheiro, B.; Adel, Y.; Knipper, M.; Müller, M.; Löwenheim, H. Auditory Threshold Variability in the SAMP8 Mouse Model of Age-Related Hearing Loss: Functional Loss and Phenotypic Change Precede Outer Hair Cell Loss. Front. Aging Neurosci. 2021, 13, 708190. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Haller, P.; Bavi, N.; Faruk, N.; Perozo, E.; Sosnick, T.R. Folding of Prestin’s Anion-Binding Site and the Mechanism of Outer Hair Cell Electromotility. bioRxiv 2023. [Google Scholar] [CrossRef]
- Burwood, G.; He, W.X.; Fridberger, A.; Ren, T.Y.; Nuttall, A.L. Outer hair cell driven reticular lamina mechanical distortion in living cochleae. Hear. Res. 2022, 423, 108405. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, A.L.; Fridberger, A. Instrumentation for studies of cochlear mechanics: From von Bekesy forward. Hear. Res. 2012, 293, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Robles, L.; Ruggero, M.A. Mechanics of the mammalian cochlea. Physiol. Rev. 2001, 81, 1305–1352. [Google Scholar] [CrossRef]
- Chen, F.; Zha, D.; Fridberger, A.; Zheng, J.; Choudhury, N.; Jacques, S.L.; Wang, R.K.; Shi, X.; Nuttall, A.L. A differentially amplified motion in the ear for near-threshold sound detection. Nat. Neurosci. 2011, 14, 770–774. [Google Scholar] [CrossRef]
- Fallah, E.; Strimbu, C.E.; Olson, E.S. Nonlinearity and amplification in cochlear responses to single and multi-tone stimuli. Hear. Res. 2019, 377, 271–281. [Google Scholar] [CrossRef]
- Olson, E.S.; Strimbu, C.E. Cochlear mechanics: New Insights from Vibrometry and Optical Coherence Tomography. Curr. Opin. Physiol. 2020, 18, 56–62. [Google Scholar] [CrossRef]
- Braun, M. Impediment of basilar membrane motion reduces overload protection but not threshold sensitivity: Evidence from clinical and experimental hydrops. Hear. Res. 1996, 97, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fridberger, A.; Boutet de Monvel, J.; Ulfendahl, M. Internal Shearing within the Hearing Organ Evoked by Basilar Membrane Motion. J. Neurosci. 2002, 22, 9850–9857. [Google Scholar] [CrossRef] [PubMed]
- Fridberger, A.; Tomo, I.; Ulfendahl, M.; Boutet de Monvel, J. Stereociliary Vibration in the Guinea Pig Cochlea. In Auditory Mechanics: Processes and Models; Nuttall, A., Ed.; World Scientific: Singapore; Hackensack, NJ, USA, 2006; pp. 254–260. [Google Scholar]
- Fridberger, A.; Tomo, I.; Ulfendahl, M.; Boutet de Monvel, J. Imaging hair cell transduction at the speed of sound: Dynamic behavior of mammalian stereocilia. Proc. Natl. Acad. Sci. USA 2006, 103, 1918–1923. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Ortega, A.C.; Stepanyan, R.; Edelmann, S.E.; Torres-Gallego, S.; Park, C.; Marinkova, D.A.; Nowacki, J.S.; Sinha, G.P.; Frolenkov, G.I. TRPA1 activation in non-sensory supporting cells contributes to regulation of cochlear sensitivity after acoustic trauma. Nat. Commun. 2023, 14, 3871. [Google Scholar] [CrossRef] [PubMed]
- Fechner, F.P.; Burgess, B.J.; Adams, J.C.; Liberman, M.C.; Nadol, J.B., Jr. Dense innervation of Deiters’ and Hensen’s cells persists after chronic differentiations of guinea pig cochleas. J. Comp. Neurol. 1998, 400, 299–309. [Google Scholar] [CrossRef]
- Wright, C.G.; Preston, R.E. Efferent nerve fibers associated with the outermost supporting cells of the organ of Corti in the guinea pig. Acta Otolaryngol. 1976, 82, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Karavitaki, K.D.; Mountain, D.C. Imaging electrically evoked micro-mechanical motion within the organ of Corti of the excised gerbil cochlea. Biophys. J. 2007, 92, 3294–3316. [Google Scholar] [CrossRef]
- Hakizimana, P.; Fridberger, A. Inner hair cell stereocilia are embedded in the tectorial membrane. Nat. Commun. 2021, 12, 2604. [Google Scholar] [CrossRef]
- Verpy, E.; Weil, D.; Leibovici, M.; Goodyear, R.J.; Hamard, G.; Houdon, C.; Lefèvre, G.M.; Hardelin, J.P.; Richardson, G.P.; Avan, P.; et al. Stereocilin-deficient mice reveal the origin of cochlear waveform distortions. Nature 2008, 456, 255–258. [Google Scholar] [CrossRef]
- Strimbu, C.E.; Prasad, S.; Hakizimana, P.; Fridberger, A. Control of hearing sensitivity by tectorial membrane calcium. Proc. Natl. Acad. Sci. USA 2019, 116, 5756–5764. [Google Scholar] [CrossRef]
- Manley, G.A. Cochlear mechanisms from a phylogenetic viewpoint. Proc. Natl. Acad. Sci. USA 2000, 97, 11736–11743. [Google Scholar] [CrossRef] [PubMed]
- Zwaenepoel, I.; Mustapha, M.; Leibovici, M.; Verpy, E.; Goodyear, R.; Liu, X.Z.; Nouaille, S.; Nance, W.E.; Kanaan, M.; Avraham, K.B.; et al. Otoancorin, an inner ear protein restricted to the interface between the apical surface of sensory epithelia and their overlying acellular gels, is defective in autosomal recessive deafness DFNB22. Proc. Natl. Acad. Sci. USA 2002, 99, 6240–6245. [Google Scholar] [CrossRef] [PubMed]
- Patuzzi, R.B.; Yates, G.K.; Johnstone, B.M. Outer hair cell receptor current and sensorineural hearing loss. Hear. Res. 1989, 42, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Langguth, B.; Kreuzer, P.M.; Kleinjung, T.; De Ridder, D. Tinnitus: Causes and clinical management. Lancet Neurol. 2013, 12, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.E.; Roberts, L.E.; Langguth, B. Maladaptive plasticity in tinnitus—Triggers, mechanisms and treatment. Nat. Rev. Neurol. 2016, 12, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Roberson, D.W.; Rubel, E.W. Cell division in the gerbil cochlea after acoustic trauma. Am. J. Otol. 1994, 15, 28–34. [Google Scholar] [PubMed]
- Celik, M.; Koyuncu, İ. A Comprehensive Study of Oxidative Stress in Tinnitus Patients. Indian J. Otolaryngol. Head Neck Surg. 2018, 70, 521–526. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Kamrava, S.K.; Moore, B.C.J.; Reiter, R.J.; Ghaznavi, H.; Kamali, M.; Mehrzadi, S. Molecular Aspects of Melatonin Treatment in Tinnitus: A Review. Curr. Drug Targets 2019, 20, 1112–1128. [Google Scholar] [CrossRef]
- Ziegler, E.A.; Brieger, J.; Heinrich, U.R.; Mann, W.J. Immunohistochemical localization of cyclooxygenase isoforms in the organ of Corti and the spiral ganglion cells of guinea pig cochlea. ORL J. Otorhinolaryngol. Relat. Spec. 2004, 66, 297–301. [Google Scholar] [CrossRef]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef]
- Harris, C.; Hansen, J.M. Oxidative stress, thiols, and redox profiles. Methods Mol. Biol. 2012, 889, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, V.; Mukherjea, D.; Dhukhwa, A.; Rybak, L.P. Oxidative Stress and Inflammation Caused by Cisplatin Ototoxicity. Antioxidants 2021, 10, 1919. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Paciello, F.; Rolesi, R.; Paludetti, G.; Troiani, D. Targeting dysregulation of redox homeostasis in noise-induced hearing loss: Oxidative stress and ROS signaling. Free Radic. Biol. Med. 2019, 135, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Neri, S.; Mauceri, B.; Cilio, D.; Bordonaro, F.; Messina, A.; Malaguarnera, M.; Savastano, M.; Brescia, G.; Manci, S.; Celadini, M. Tinnitus and oxidative stress in a selected series of elderly patients. Arch. Gerontol. Geriatr. Suppl. 2002, 8, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Neri, S.; Signorelli, S.; Pulvirenti, D.; Mauceri, B.; Cilio, D.; Bordonaro, F.; Abate, G.; Interlandi, D.; Misseri, M.; Ignaccolo, L.; et al. Oxidative stress, nitric oxide, endothelial dysfunction and tinnitus. Free Radic. Res. 2006, 40, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andrè, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.A.; Gentry, C.; Moss, S.; Bevan, S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 2008, 28, 2485–2494. [Google Scholar] [CrossRef]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A gatekeeper for inflammation. Annu. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef]
- Koç, S.; Akyüz, S.; Somuk, B.T.; Soyalic, H.; Yılmaz, B.; Taskin, A.; Bilinc, H.; Aksoy, N. Paraoxonase Activity and Oxidative Status in Patients with Tinnitus. J. Audiol. Otol. 2016, 20, 17–21. [Google Scholar] [CrossRef]
- Ekinci, A.; Kamasak, K. Evaluation of serum prolidase enzyme activity and oxidative stress in patients with tinnitus. Braz. J. Otorhinolaryngol. 2020, 86, 405–410. [Google Scholar] [CrossRef]
- Oh, J.; Youn, C.K.; Jun, Y.; Jo, E.R.; Cho, S.I. Reduced mitophagy in the cochlea of aged C57BL/6J mice. Exp. Gerontol. 2020, 137, 110946. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Q.; Li, S.; Li, X.J.; Yang, W. Mitochondrial-Dependent and Independent Functions of PINK1. Front. Cell Dev. Biol. 2022, 10, 954536. [Google Scholar] [CrossRef] [PubMed]
- Kane, L.A.; Lazarou, M.; Fogel, A.I.; Li, Y.; Yamano, K.; Sarraf, S.A.; Banerjee, S.; Youle, R.J. PINK1 Phosphorylates Ubiquitin to Activate Parkin E3 Ubiquitin Ligase Activity. J. Cell Biol. 2014, 205, 143–153. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, Y.; Yin, H.; Li, H.; Zhou, M.; Sun, G.; Cao, Z.; Man, R.; Wang, H.; Li, J. PINK1 Protects Against Gentamicin-Induced Sensory Hair Cell Damage: Possible Relation to Induction of Autophagy and Inhibition of p53 Signal Pathway. Front. Mol. Neurosci. 2018, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Hu, S.; Hu, Y.; Liu, D.; Zhou, J.; Liu, X.; Ma, X.; Dong, Y. Mitophagy in ototoxicity. Front. Cell. Neurosci. 2023, 17, 1140916. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.I.; Jo, E.R.; Song, H. Mitophagy Impairment Aggravates Cisplatin-Induced Ototoxicity. BioMed Res. Int. 2021, 2021, 5590973. [Google Scholar] [CrossRef] [PubMed]
- Savas, J.N. The cochlea is built to last a lifetime. Hear. Res. 2023, 436, 108821. [Google Scholar] [CrossRef]
- Mu, Y.R.; Zou, S.Y.; Li, M.; Ding, Y.Y.; Huang, X.; He, Z.H.; Kong, W.J. Role and mechanism of FOXG1-related epigenetic modifications in cisplatin-induced hair cell damage. Front. Mol. Neurosci. 2023, 16, 1064579. [Google Scholar] [CrossRef]
- Magarinos, M.; Pulido, S.; Aburto, M.R.; de Iriarte-Rodriguez, R.; Varela-Nieto, I. Autophagy in the vertebrate inner ear. Front. Cell Dev. Biol. 2017, 5, 56. [Google Scholar] [CrossRef]
- Fujimoto, C.; Iwasaki, S.; Urata, S.; Morishita, H.; Sakamaki, Y.; Fujioka, M.; Kondo, K.; Mizushima, N.; Yamasoba, T. Autophagy is essential for hearing in mice. Cell Death Dis. 2017, 8, e2780. [Google Scholar] [CrossRef]
- Jongkamonwiwat, N.; Ramirez, M.A.; Edassery, S.; Wong, A.C.Y.; Yu, J.; Abbott, T.; Pak, K.; Ryan, A.F.; Savas, J.N. Noise Exposures Causing Hearing Loss Generate Proteotoxic Stress and Activate the Proteostasis Network. Cell Rep. 2020, 33, 108431. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, Y.; Chen, S.; Zhou, X.; Wu, X.; Kong, W.; Kong, W. Impaired unfolded protein response in the degeneration of cochlea cells in a mouse model of age-related hearing loss. Exp. Gerontol. 2015, 70, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.N.; Kim, S.G.; Lim, J.Y.; Kim, S.J.; Choe, S.K.; Park, R. Proteasome inhibitors induce auditory hair cell death through peroxisome dysfunction. Biochem. Biophys. Res. Commun. 2015, 456, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, C.; Mei, X.; Cao, Y.; Guo, Z.; Yuan, Y.; Zhao, Z.; Song, C.; Guo, Y.; Shen, Z. Berberine attenuated pro-inflammatory factors and protect against neuronal damage via triggering oligodendrocyte autophagy in spinal cord injury. Oncotarget 2017, 8, 98312–98321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, S.; Xie, R.; Liu, J. Extracellular ATP accelerates cell death and decreases tight junction protein ZO-1 in hypoxic cochlear strial marginal cells in neonatal rats. Cell. Signal. 2023, 108, 110732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, Q.; Wang, H.; Qi, J.; Sun, S.; Liao, M.; Wu, Y.; Hu, Y.; Jiang, P.; Cheng, C.; et al. Increased mitophagy protects cochlear hair cells from aminoglycoside-induced damage. Autophagy 2023, 19, 75–91. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Guo, L.; Shu, Y.; Fang, Q.; Zhou, H.; Liu, Y.; Liu, D.; Lu, L.; Zhang, X.; Ding, X.; et al. Autophagy protects auditory hair cells against neomycin-induced damage. Autophagy 2017, 13, 1884–1904. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, Y.; Cao, H.; Liu, Q.; Feng, X.; Yin, H.; Wang, B. PIN1 protects auditory hair cells from senescence via autophagy. PeerJ 2022, 10, e14267. [Google Scholar] [CrossRef]
- Xiong, H.; Pang, J.; Min, X.; Ye, Y.; Lai, L.; Zheng, Y. miR-34a/ATG9A/TFEB Signaling Modulates Autophagy in Cochlear Hair Cells and Correlates with Age-related Hearing Loss. Neuroscience 2022, 491, 98–109. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Ji, D.; Yu, W.; Zhang, Y.; Xiang, Y.; Zhou, C.; Wang, L.; Deng, P.; Pi, H.; et al. Metformin attenuates cadmium-induced degeneration of spiral ganglion neuron via restoring autophagic flux in primary culture. J. Inorg. Biochem. 2022, 234, 111901. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Q.; Tian, Y.; Jiang, Y.; Xu, M.; Wang, H.; Xiong, Y.; Fang, J.; Lu, W.; Yu, D.; et al. Trehalose protects against cisplatin-induced cochlear hair cell damage by activating TFEB-mediated autophagy. Biochem. Pharmacol. 2022, 197, 114904. [Google Scholar] [CrossRef]
- Liu, H.; Li, F.; Li, X.; Wu, Q.; Dai, C. Rapamycin ameliorates age-related hearing loss in C57BL/6J mice by enhancing autophagy in the SGNs. Neurosci. Lett. 2022, 772, 136493. [Google Scholar] [CrossRef]
- Guo, L.; Cao, W.; Niu, Y.; He, S.; Chai, R.; Yang, J. Autophagy Regulates the Survival of Hair Cells and Spiral Ganglion Neurons in Cases of Noise, Ototoxic Drug, and Age-Induced Sensorineural Hearing Loss. Front. Cell. Neurosci. 2021, 15, 760422. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hong, L.; Luo, G.; Lu, S.; Li, Y.; Wang, J.; Zhang, Y.; Zhang, L. SIRT6 promotes autophagy through direct interaction with ULK1 and competitive binding to PUMA. Genes Dis. 2022, 10, 1747–1750. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, S.; Kong, D.H.; Meng, X.; Zong, Z.H.; Liu, B.Q.; Guan, Y.; Du, Z.X.; Wang, H.Q. BAG3 is upregulated by c-Jun and stabilizes JunD. Biochim. Biophys Acta 2013, 1833, 3346–3354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, C.; Zheng, Z.; Wang, P.; He, S.; He, Y. Autophagy: A Novel Horizon for Hair Cell Protection. Neural Plast. 2021, 2021, 5511010. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, P.; Chatterton, P.; Ikeda, A.; Ikeda, S.; Corey, D.P.; Ervasti, J.M.; Perrin, B.J. Length regulation of mechanosensitive stereocilia depends on very slow actin dynamics and filament-severing proteins. Nat. Commun. 2015, 6, 6855. [Google Scholar] [CrossRef]
- Park, J.; Bird, J.E. The actin cytoskeleton in hair bundle development and hearing loss. Hear. Res. 2023, 436, 108817. [Google Scholar] [CrossRef]
- Barr-Gillespie, P.G. Assembly of hair bundles, an amazing problem for cell biology. Mol. Biol. Cell 2015, 26, 2727–2732. [Google Scholar] [CrossRef]
- Belyantseva, I.A.; Boger, E.T.; Naz, S.; Frolenkov, G.I.; Sellers, J.R.; Ahmed, Z.M.; Griffith, A.J.; Friedman, T.B. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat. Cell Biol. 2005, 7, 148–156. [Google Scholar] [CrossRef]
- Stepanyan, R.; Frolenkov, G.I. Fast adaptation and Ca2+ sensitivity of the mechanotransducer require myosin-XVa in inner but not outer cochlear hair cells. J. Neurosci. 2009, 29, 4023–4034. [Google Scholar] [CrossRef] [PubMed]
- Manor, U.; Disanza, A.; Grati, M.; Andrade, L.; Lin, H.; Di Fiore, P.P.; Scita, G.; Kachar, B. Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8. Curr. Biol. 2011, 21, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.I.; Oh, K.S.; Kim, J.A.; Noh, B.; Choi, H.J.; Joo, S.Y.; Rim, J.H.; Kim, H.Y.; Kim, D.Y.; Yu, S.; et al. OSBPL2 mutations impair autophagy and lead to hearing loss, potentially remedied by rapamycin. Autophagy 2022, 18, 2593–2614. [Google Scholar] [CrossRef] [PubMed]
- Parra-Perez, A.M.; Lopez-Escamez, J.A. Types of Inheritance and Genes Associated with Familial Meniere Disease. J. Assoc. Res. Otolaryngol. 2023, 24, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Roman-Naranjo, P.; Gallego-Martinez, A.; Soto-Varela, A.; Aran, I.; Moleon, M.D.C.; Espinosa-Sanchez, J.M.; Amor-Dorado, J.C.; Batuecas-Caletrio, A.; Perez-Vazquez, P.; Lopez-Escamez, J.A. Burden of Rare Variants in the OTOG Gene in Familial Meniere’s Disease. Ear Hear. 2020, 41, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Avan, P.; Le Gal, S.; Michel, V.; Dupont, T.; Hardelin, J.P.; Petit, C.; Verpy, E. Otogelin, otogelin-like, and stereocilin form links connecting outer hair cell stereocilia to each other and the tectorial membrane. Proc. Natl. Acad. Sci. USA 2019, 116, 25948–25957. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.S.; Oziębło, D.; Skarżyński, H.; Ołdak, M. Monogenic Causes of Low-Frequency Non-Syndromic Hearing Loss. Audiol. Neurootol. 2023, 28, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mecca, A.; Kim, J.; Caprara, G.A.; Wagner, E.L.; Du, T.T.; Petrov, L.; Xu, W.; Cui, R.; Rebustini, I.T.; et al. Myosin-VIIa is expressed in multiple isoforms and essential for tensioning the hair cell mechano-transduction complex. Nat. Commun. 2020, 11, 2066. [Google Scholar] [CrossRef]
- Roman-Naranjo, P.; Moleon, M.D.C.; Aran, I.; Escalera-Balsera, A.; Soto-Varela, A.; Bächinger, D.; Gomez-Fiñana, M.; Eckhard, A.H.; Lopez-Escamez, J.A. Rare coding variants involving MYO7A and other genes encoding stereocilia link proteins in familial meniere disease. Hear. Res. 2021, 409, 108329. [Google Scholar] [CrossRef]
- Frejo, L.; Lopez-Escamez, J.A. Recent advances in understanding molecular bases of Ménière’s disease. Fac. Rev. 2023, 12, 11. [Google Scholar] [CrossRef]
- Gallego-Martinez, A.; Escalera-Balsera, A.; Trpchevska, N.; Robles-Bolivar, P.; Roman-Naranjo, P.; Frejo, L.; Perez-Carpena, P.; Bulla, J.; Gallus, S.; Canlon, B.; et al. Using coding and non-coding rare variants to target candidate genes in patients with severe tinnitus. NPJ Genom. Med. 2022, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Skarp, S.; Korvala, J.; Kotimäki, J.; Sorri, M.; Männikkö, M.; Hietikko, E. New Genetic Variants in CYP2B6 and SLC6A Support the Role of Oxidative Stress in Familial Ménière’s Disease. Genes 2022, 13, 998. [Google Scholar] [CrossRef] [PubMed]
- Manche, S.K.; Jangala, M.; Putta, P.; Koralla, R.M.; Akka, J. Association of oxidative stress gene polymorphisms with presbycusis. Gene 2016, 593, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Altissimi, G.; Colizza, A.; Cianfrone, G.; de Vincentiis, M.; Greco, A.; Taurone, S.; Musacchio, A.; Ciofalo, A.; Turchetta, R.; Angeletti, D.; et al. Drugs inducing hearing loss, tinnitus, dizziness and vertigo: An updated guide. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7946–7952. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zou, Q.; Pu, Y.; Cai, Z.; Tang, Y. Berberine Rescues D-Ribose-Induced Alzheimer’s Pathology via Promoting Mitophagy. Int. J. Mol. Sci. 2023, 24, 5896. [Google Scholar] [CrossRef] [PubMed]
- Babolmorad, G.; Latif, A.; Domingo, I.K.; Pollock, N.M.; Delyea, C.; Rieger, A.M.; Allison, W.T.; Bhavsar, A.P. Toll-like receptor 4 is activated by platinum and contributes to cisplatin-induced ototoxicity. EMBO Rep. 2021, 22, e51280. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Böttger, E.C.; Schacht, J. The mitochondrion: A perpetrator of acquired hearing loss. Hear. Res. 2013, 303, 12–19. [Google Scholar] [CrossRef]
- Podratz, J.L.; Knight, A.M.; Ta, L.E.; Staff, N.P.; Gass, J.M.; Genelin, K.; Schlattau, A.; Lathroum, L.; Windebank, A.J. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol. Dis. 2011, 41, 661–668. [Google Scholar] [CrossRef]
- Xie, J.; Talaska, A.E.; Schacht, J. New developments in aminoglycoside therapy and ototoxicity. Hear. Res. 2011, 281, 28–37. [Google Scholar] [CrossRef]
- Fu, X.; Wan, P.; Li, P.; Wang, J.; Guo, S.; Zhang, Y.; An, Y.; Ye, C.; Liu, Z.; Gao, J.; et al. Mechanism and Prevention of Ototoxicity Induced by Aminoglycosides. Front. Cell. Neurosci. 2021, 15, 692762. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yan, X.; Huang, W.; Chu, M.; Dong, Y.; Song, H.; Peng, Y.; Shi, J.; Liu, Q. Curcumin protects against the age-related hearing loss by attenuating apoptosis and senescence via activating Nrf2 signaling in cochlear hair cells. Biochem. Pharmacol. 2023, 212, 115575. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Fernandez, A.F.; Cabrera, S.; Lundberg, Y.W.; Cabanillas, R.; Rodriguez, F.; Salvador-Montoliu, N.; Vega, J.A.; Germanà, A.; Fueyo, A.; et al. Autophagy is essential for mouse sense of balance. J. Clin. Investig. 2010, 120, 2331–2344. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.H.; Kim, Y.; Yi, J.; Chung, J.W. Antioxidant Therapy against Oxidative Damage of the Inner Ear: Protection and Preconditioning. Antioxidants 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Baggioni, A. Berberine and Its Role in Chronic Disease. Adv. Exp. Med. Biol. 2016, 928, 27–45. [Google Scholar] [CrossRef]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.T.S.; Šmejkal, K.; Malaník, M.; et al. Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics. Theranostics 2019, 9, 1923–1951. [Google Scholar] [CrossRef]
- Hou, Q.; He, W.J.; Wu, Y.S.; Hao, H.J.; Xie, X.Y.; Fu, X.B. Berberine: A Traditional Natural Product with Novel Biological Activities. Altern. Ther. Health Med. 2020, 26, 20–27. [Google Scholar]
- Kim, Y.R.; Baek, J.I.; Lee, K.Y.; Kim, U.K. Berberine chloride protects cochlear hair cells from aminoglycoside-induced ototoxicity by reducing the accumulation of mitochondrial reactive oxygen species. Free Radic. Biol. Med. 2023, 204, 177–183. [Google Scholar] [CrossRef]
- Kilic, K.; Sakat, M.S.; Sahin, A.; Yildirim, S.; Dortbudak, M.B. The effectiveness of berberine on noise-induced hearing loss: A rat model. Rev. Assoc. Med. Bras. 2022, 68, 1330–1336. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, Z.; Naveena, K.; Lei, G.; Qiu, S.; Li, X.; Li, T.; Shi, X.; Zhuang, W.; Li, Y.; et al. ROS-Responsive Nanoparticle as a Berberine Carrier for OHC-Targeted Therapy of Noise-Induced Hearing Loss. ACS Appl. Mater. Interfaces 2021, 13, 7102–7114. [Google Scholar] [CrossRef]
- Fang, X.; Wu, H.; Wei, J.; Miao, R.; Zhang, Y.; Tian, J. Research progress on the pharmacological effects of berberine targeting mitochondria. Front. Endocrinol. 2022, 13, 982145. [Google Scholar] [CrossRef] [PubMed]
- Hori, I.; Harashima, H.; Yamada, Y. Development of a Mitochondrial Targeting Lipid Nanoparticle Encapsulating Berberine. Int. J. Mol. Sci. 2023, 24, 903. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Jiang, M.; Zhao, Y.; Gong, J.; Su, H.; Yuan, F.; Fang, K.; Yuan, X.; Yu, X.; Dong, H.; et al. Berberine protects against diabetic kidney disease via promoting PGC-1α-regulated mitochondrial energy homeostasis. Br. J. Pharmacol. 2020, 177, 3646–3661. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Bucak, A.; Ozdemir, C.; Ulu, S.; Gonul, Y.; Aycicek, A.; Uysal, M.; Cangal, A. Investigation of protective role of curcumin against paclitaxel-induced inner ear damage in rats. Laryngoscope 2015, 125, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Soyalıç, H.; Gevrek, F.; Koç, S.; Avcu, M.; Metin, M.; Aladağ, İ. Intraperitoneal curcumin and vitamin E combination for the treatment of cisplatin-induced ototoxicity in rats. Int. J. Pediatr. Otorhinolaryngol. 2016, 89, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhakim, Y.M.; Abdel-Mota, S.M.; Malhat, S.M.; Mostafa, H.I.; Ibrahim, W.M.; Beheiry, R.R.; Moselhy, A.A.A.; Said, E.N. Curcumin attenuates gentamicin and sodium salicylate ototoxic effects by modulating the nuclear factor-kappaB and apoptotic pathways in rats. Environ. Sci. Pollut. Res. Int. 2022, 29, 89954–89968. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yoneyama, M.; Onaka, Y.; Imaizumi, A.; Ogita, K. Preventive effect of curcumin and its highly bioavailable preparation on hearing loss induced by single or repeated exposure to noise: A comparative and mechanistic study. J. Pharmacol. Sci. 2017, 134, 225–233. [Google Scholar] [CrossRef]
- So, H.; Kim, H.; Kim, Y.; Kim, E.; Pae, H.O.; Chung, H.T.; Kim, H.J.; Kwon, K.B.; Lee, K.M.; Lee, H.Y.; et al. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J. Assoc. Res. Otolaryngol. 2008, 9, 290–306. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, Y.; Zheng, Y. Nrf2 Pathway and Autophagy Crosstalk: New Insights into Therapeutic Strategies for Ischemic Cerebral Vascular Diseases. Antioxidants 2022, 11, 1747. [Google Scholar] [CrossRef]
- Liao, D.; Shangguan, D.; Wu, Y.; Chen, Y.; Liu, N.; Tang, J.; Yao, D.; Shi, Y. Curcumin protects against doxorubicin induced oxidative stress by regulating the Keap1-Nrf2-ARE and autophagy signaling pathways. Psychopharmacology 2023, 240, 1179–1190. [Google Scholar] [CrossRef]
- Han, J.; Pan, X.Y.; Xu, Y.; Xiao, Y.; An, Y.; Tie, L.; Pan, Y.; Li, X.J. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy 2012, 8, 812–825. [Google Scholar] [CrossRef]
- Yu, T.; Wang, L.; Zhang, L.; Deuster, P.A. Mitochondrial Fission as a Therapeutic Target for Metabolic Diseases: Insights into Antioxidant Strategies. Antioxidants 2023, 12, 1163. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzeri, G.; Biagioni, F.; Ferrucci, M.; Puglisi-Allegra, S.; Lenzi, P.; Busceti, C.L.; Giannessi, F.; Fornai, F. The Relevance of Autophagy within Inner Ear in Baseline Conditions and Tinnitus-Related Syndromes. Int. J. Mol. Sci. 2023, 24, 16664. https://doi.org/10.3390/ijms242316664
Lazzeri G, Biagioni F, Ferrucci M, Puglisi-Allegra S, Lenzi P, Busceti CL, Giannessi F, Fornai F. The Relevance of Autophagy within Inner Ear in Baseline Conditions and Tinnitus-Related Syndromes. International Journal of Molecular Sciences. 2023; 24(23):16664. https://doi.org/10.3390/ijms242316664
Chicago/Turabian StyleLazzeri, Gloria, Francesca Biagioni, Michela Ferrucci, Stefano Puglisi-Allegra, Paola Lenzi, Carla Letizia Busceti, Francesco Giannessi, and Francesco Fornai. 2023. "The Relevance of Autophagy within Inner Ear in Baseline Conditions and Tinnitus-Related Syndromes" International Journal of Molecular Sciences 24, no. 23: 16664. https://doi.org/10.3390/ijms242316664
APA StyleLazzeri, G., Biagioni, F., Ferrucci, M., Puglisi-Allegra, S., Lenzi, P., Busceti, C. L., Giannessi, F., & Fornai, F. (2023). The Relevance of Autophagy within Inner Ear in Baseline Conditions and Tinnitus-Related Syndromes. International Journal of Molecular Sciences, 24(23), 16664. https://doi.org/10.3390/ijms242316664








